First-Principles Insights on the Formation Mechanism of Innermost Layers of Solid Electrolyte Interphases on Carbon Anodes for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Computation Methods
3. Results
3.1. Isolated Molecules and Pristine Surfaces
3.1.1. EC Molecules
3.1.2. CH2CH2OCO2Li Molecule
3.1.3. (CH2OCO2Li)2 Molecule
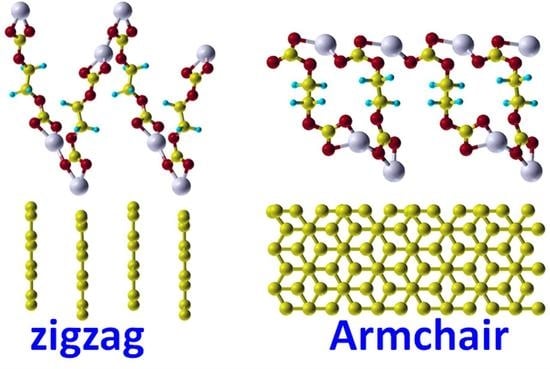
3.1.4. Graphite Surfaces
3.2. Molecule Adsorption on Graphite Surfaces
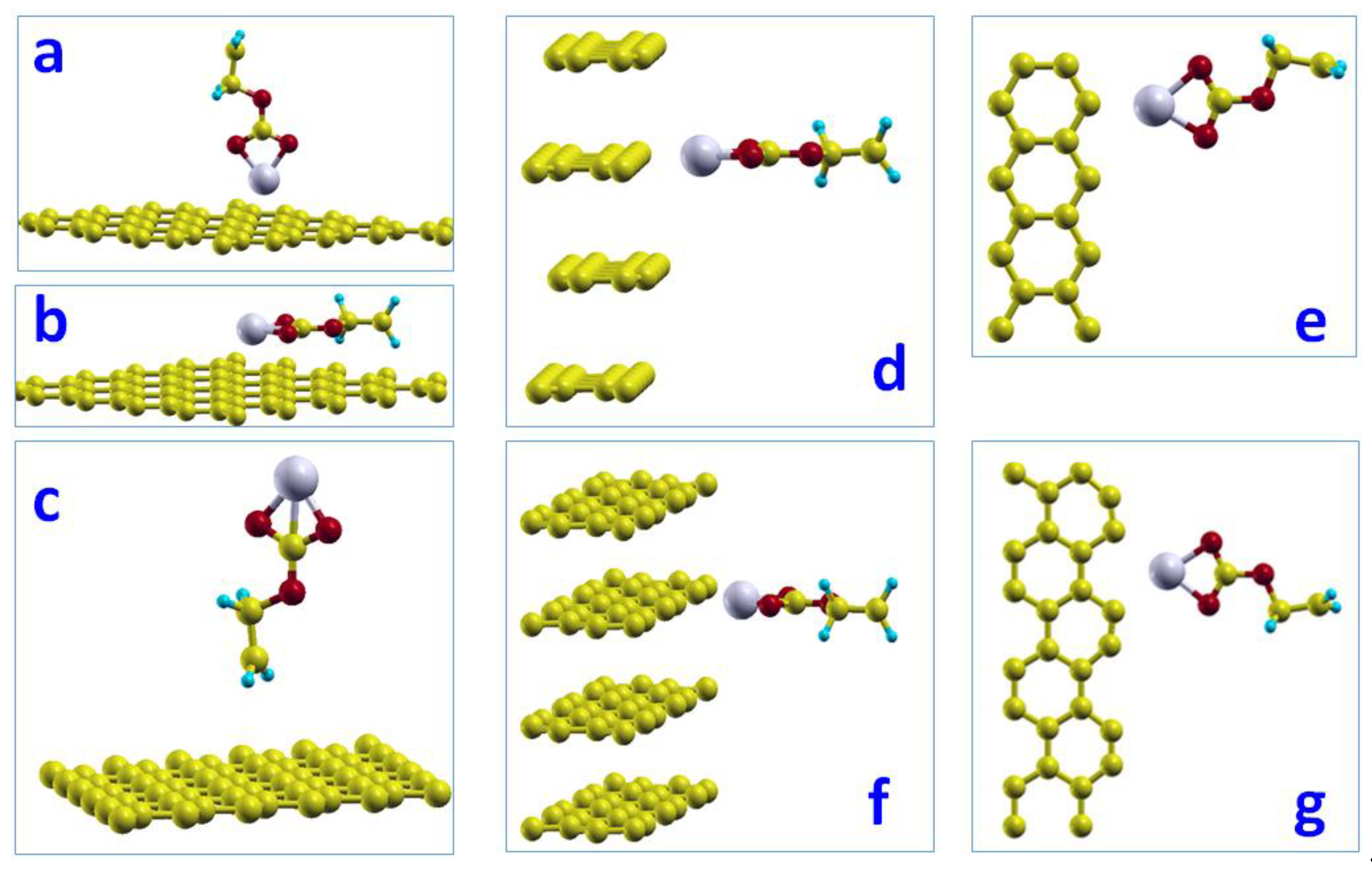
3.2.1. Adsorption of CH2CH2OCO2Li Molecule on Graphite Surfaces
Basal Plane Surface
Edge Surfaces
3.2.2. Adsorption of (CH2OCO2Li)2 Molecule on Graphite Surfaces
Basal Plane Surface
Edge Surface
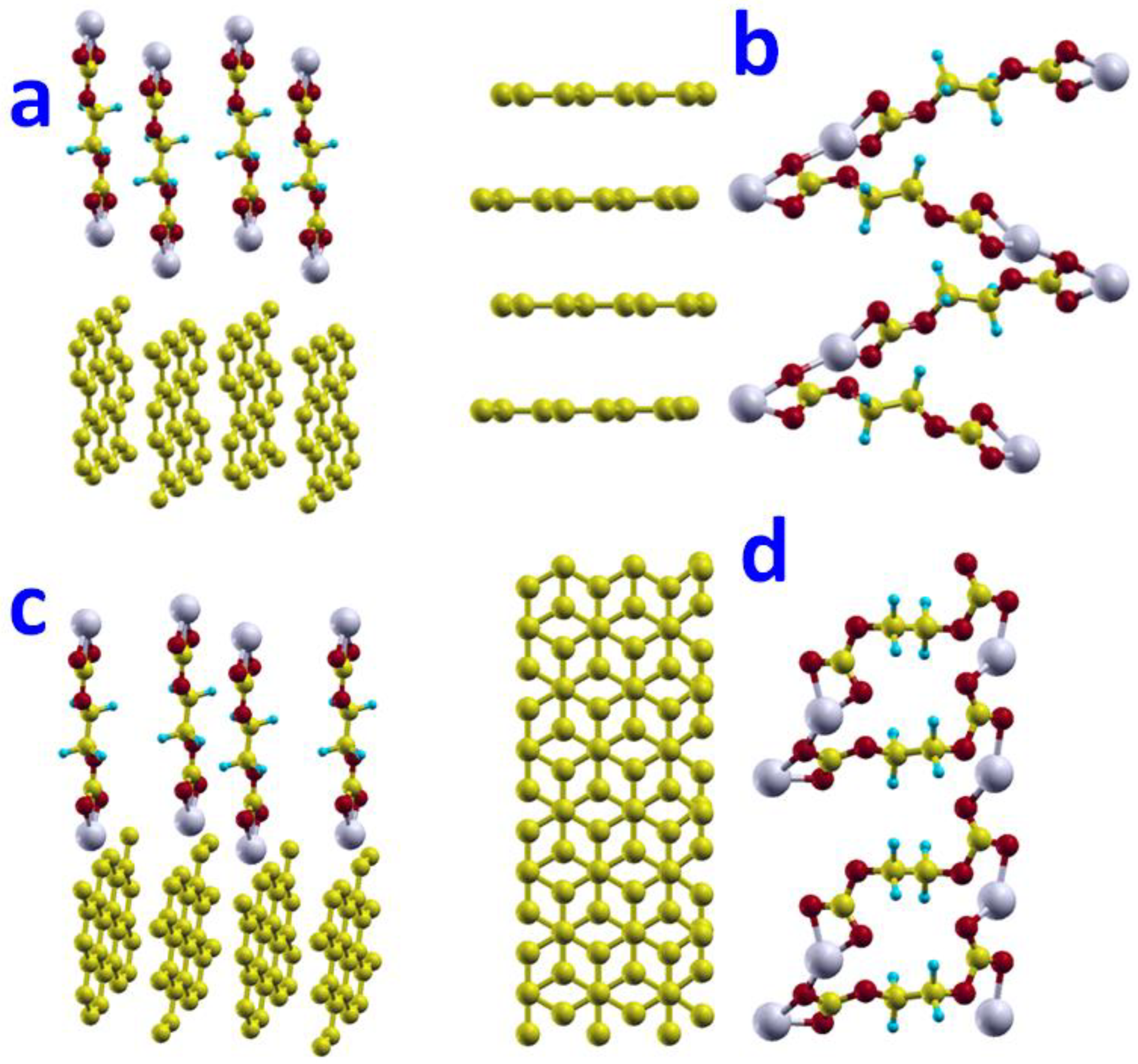
3.3. Networking Adsorption on Graphite Surfaces
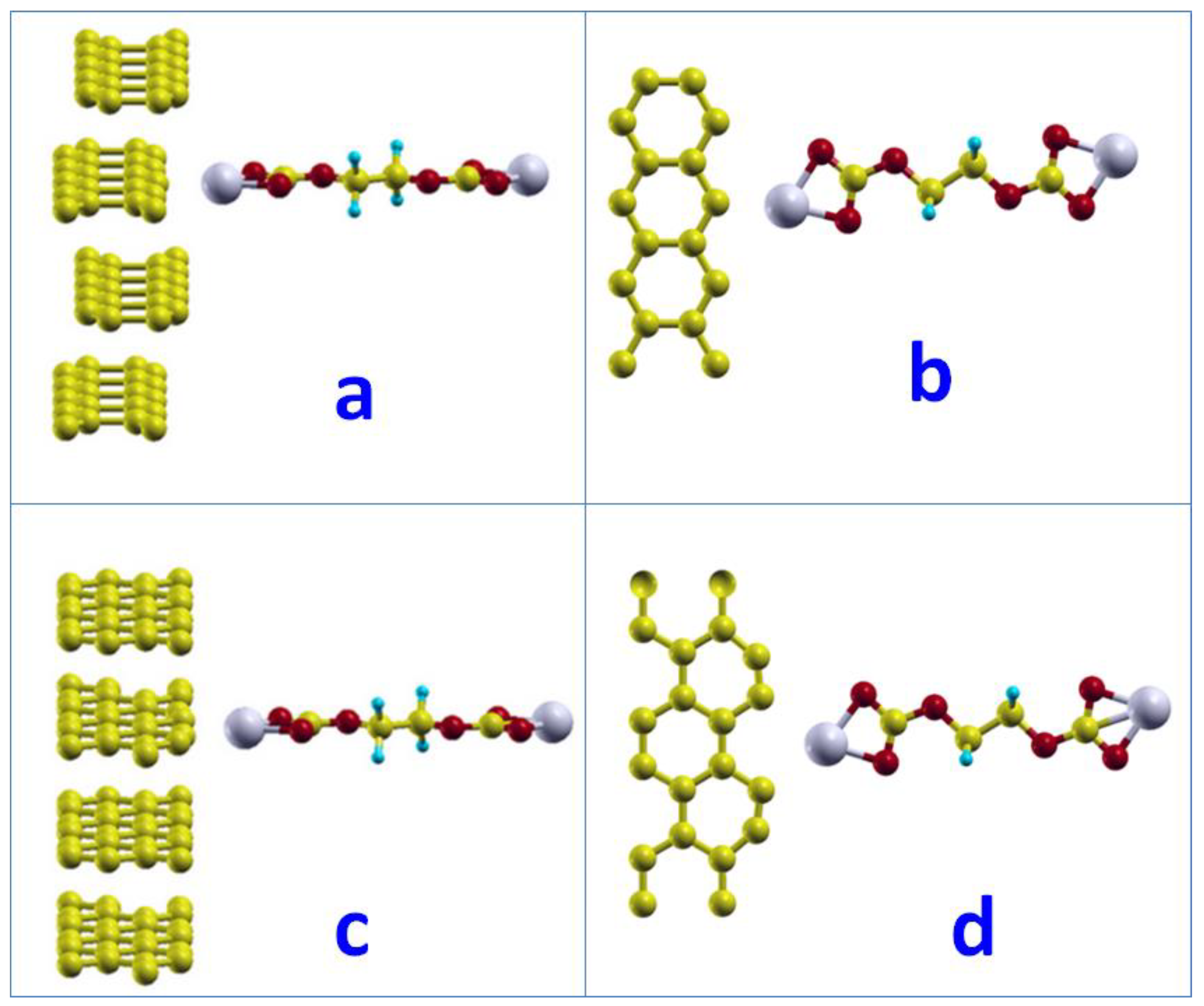
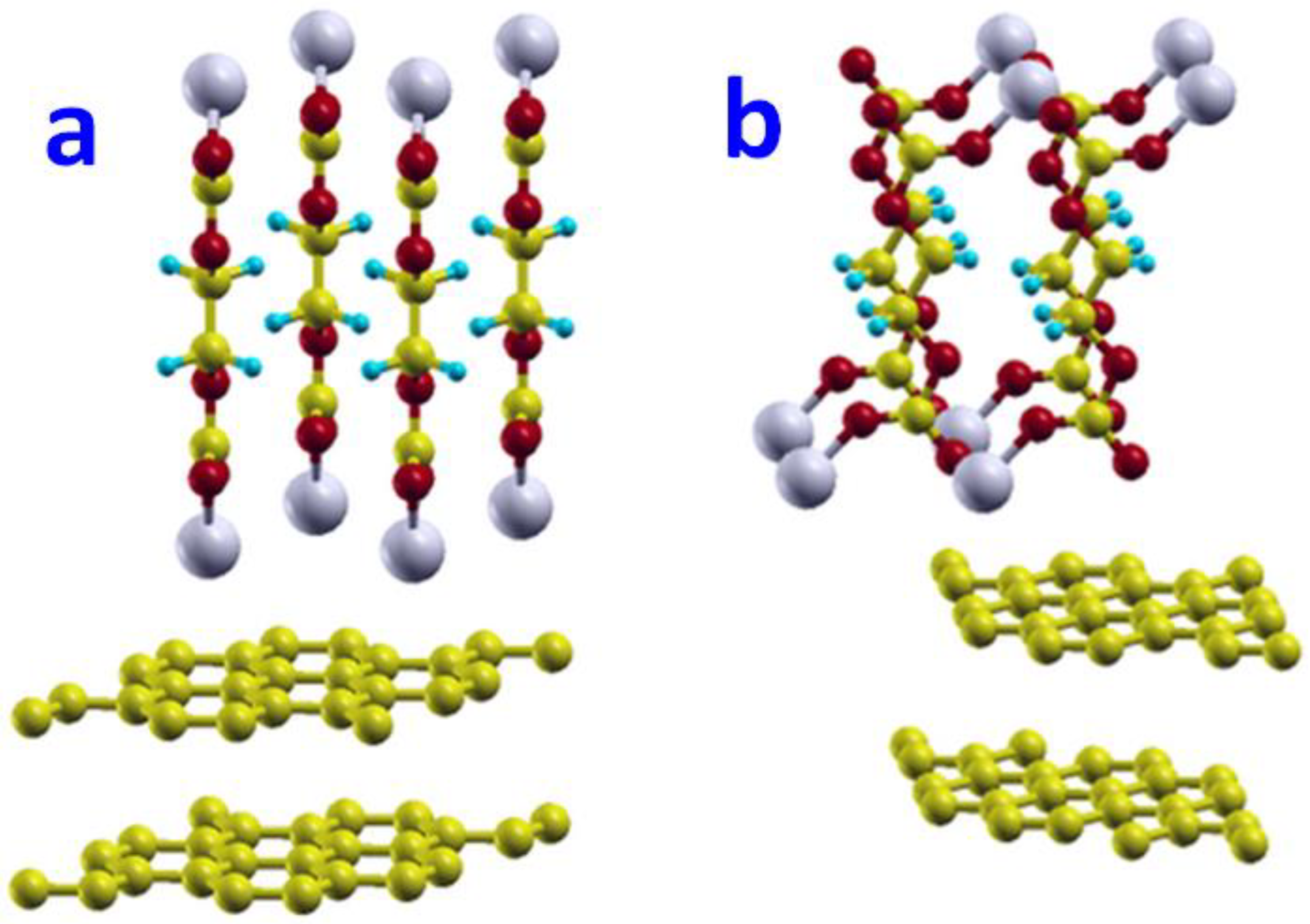
3.3.1. Network on Basal Surface
3.3.2. Network on Zigzag Edge Surface
3.3.3. Network on Armchair Edge Surface
4. Discussions
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Balbuena, P.B.; Wang, Y. Lithium Battery Solid Electrolyte Interphase; Balbuena, P.B., Wang, Y.X., Eds.; Imperial College Press: London, UK, 2004. [Google Scholar]
- Wang, Y.; Liu, B.; Li, Q.; Cartmell, S.; Ferrara, S.; Deng, Z.D.; Xiao, J. Lithium and lithium ion batteries for applications in microelectronic devices: A review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhu, Z.; Yan, Q.; Yu, S.; He, X.; Chen, Y.; Zhang, R.; Ma, L.; Liu, T.; Li, M.; et al. A disordered rock salt anode for fast-charging lithium-ion batteries. Nature 2020, 585, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Lu, J.; Wu, T.; Amine, K. State-of-the-art characterization techniques for advanced lithium-ion batteries. Nat. Energy 2017, 2, 17011. [Google Scholar] [CrossRef]
- Balbuena, P. Lithium-Ion Batteries: Solid-Electrolyte Interphase; Imperial College Press: London, UK, 2004. [Google Scholar]
- Winter, M. The solid electrolyte interphase—The most important and the least understood solid electrolyte in rechargeable Li batteries. Z. Fur Phys. Chem. 2009, 223, 1395–1406. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. NPJ Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Horstmann, B.; Single, F.; Latz, A. Review on multi-scale models of solid-electrolyte interphase formation. Curr. Opin. Electrochem. 2019, 13, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Heiskanen, S.K.; Kim, J.; Lucht, B.L. Generation and Evolution of the Solid Electrolyte Interphase of Lithium-Ion Batteries. Joule 2019, 3, 2322–2333. [Google Scholar] [CrossRef]
- Arora, P. Capacity Fade Mechanisms and Side Reactions in Lithium-Ion Batteries. J. Electrochem. Soc. 1998, 145, 3647–3667. [Google Scholar] [CrossRef] [Green Version]
- Smart, M.C.; Ratnakumar, B.V.; Surampudi, S.; Wang, Y.; Zhang, X.; Greenbaum, S.G.; Hightower, A.; Ahn, C.C.; Fultz, B. Irreversible Capacities of Graphite in Low-Temperature Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 2019, 146, 3963–3969. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, M.S.; Xu, K.; Allen, J.; Jow, T.R. Understanding solid electrolyte interface film formation on graphite electrodes. Electrochem. Solid-State Lett. 2001, 4, A206. [Google Scholar] [CrossRef]
- Yan, C.; Yao, Y.X.; Cai, W.L.; Xu, L.; Kaskel, S.; Park, H.S.; Huang, J.Q. The influence of formation temperature on the solid electrolyte interphase of graphite in lithium ion batteries. J. Energy Chem. 2020, 49, 335–338. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 2015, 3, 1500213. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, R.; Chen, X.; Cheng, X.B.; Li, X.; Zhang, Q. The Failure of Solid Electrolyte Interphase on Li Metal Anode: Structural Uniformity or Mechanical Strength? Adv. Energy Mater. 2020, 10, 1903645. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, Z.; Gray, J.L.; He, X.; Wang, D.; Chen, T.; Huang, Q.; Li, Y.C.; Wang, H.; Kim, S.H.; et al. Polymer-inorganic solid-electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 2019, 18, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Abate, I.I.; Sivonxay, E.; Shyam, B.; Jia, C.; Moritz, B.; Devereaux, T.P.; Persson, K.A.; Steinrück, H.G.; Toney, M.F. Solid Electrolyte Interphase on Native Oxide-Terminated Silicon Anodes for Li-Ion Batteries. Joule 2019, 3, 762–781. [Google Scholar] [CrossRef] [Green Version]
- Shu, Z.X.; McMillan, R.S.; Murray, J.J. Electrochemical Intercalation of Lithium into Graphite. J. Electrochem. Soc. 2019, 140, 922–927. [Google Scholar] [CrossRef]
- Aurbach, D.; Ein-Eli, Y.; Chusid (Youngman), O.; Carmeli, Y.; Babai, M.; Yamin, H. The Correlation Between the Surface Chemistry and the Performance of Li-Carbon Intercalation Anodes for Rechargeable ‘Rocking-Chair’ Type Batteries. J. Electrochem. Soc. 2019, 141, 603–611. [Google Scholar] [CrossRef]
- Aurbach, D.; Levi, M.D.; Levi, E.; Schechter, A. Failure and stabilization mechanisms of graphite electrodes. J. Phys. Chem. B 1997, 101, 2195–2206. [Google Scholar] [CrossRef]
- Peled, E. An Advanced Tool for the Selection of Electrolyte Components for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1998, 145, 3482–3486. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Weissman, I.; Levi, E.; Ein-Eli, Y. On the correlation between surface chemistry and performance of graphite negative electrodes for Li ion batteries. Electrochim. Acta 1999, 45, 67–86. [Google Scholar] [CrossRef]
- Aurbach, D.; Moshkovich, M.; Cohen, Y.; Schechter, A. Study of surface film formation on noble-metal electrodes in alkyl carbonates/Li salt solutions, using simultaneous in situ AFM, EQCM, FTIR, and EIS. Langmuir 1999, 15, 2947–2960. [Google Scholar] [CrossRef]
- Endo, E.; Ata, M.; Tanaka, K.; Sekai, K. Electron Spin Resonance Study of the Electrochemical Reduction of Electrolyte Solutions for Lithium Secondary Batteries. J. Electrochem. Soc. 2019, 145, 3757–3764. [Google Scholar] [CrossRef]
- Endo, E.; Tanaka, K.; Sekai, K. Initial Reaction in the Reduction Decomposition of Electrolyte Solutions for Lithium Batteries. J. Electrochem. Soc. 2000, 147, 4029. [Google Scholar] [CrossRef]
- Li, T.; Balbuena, P.B. Theoretical studies of the reduction of ethylene carbonate. Chem. Phys. Lett. 2000, 317, 421–429. [Google Scholar] [CrossRef]
- Márquez, A.; Balbuena, P.B. Molecular Dynamics Study of Graphite/Electrolyte Interfaces. J. Electrochem. Soc. 2001, 148, A624. [Google Scholar] [CrossRef]
- Wang, Y.; Nakamura, S.; Ue, M.; Balbuena, P.B. Theoretical studies to understand surface chemistry on carbon anodes for lithium-ion batteries: Reduction mechanisms of ethylene carbonate. J. Am. Chem. Soc. 2001, 123, 11708–11718. [Google Scholar] [CrossRef]
- Wang, Y.X.; Balbuena, P.B. Associations of lithium alkyl dicarbonates through O···Li···O interactions. J. Phys. Chem. A 2002, 106, 9582–9594. [Google Scholar] [CrossRef]
- Wang, Y.; Balbuena, P.B. Theoretical insights into the reductive decompositions of propylene carbonate and vinylene carbonate: Density functional theory studies. J. Phys. Chem. B 2002, 106, 4486–4495. [Google Scholar] [CrossRef]
- Wang, Y.; Nakamura, S.; Tasaki, K.; Balbuena, P.B. Theoretical studies to understand surface chemistry on carbon anodes for lithium-ion batteries: How does vinylene carbonate play its role as an electrolyte additive? J. Am. Chem. Soc. 2002, 124, 4408–4421. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kong, W.; Li, H.; Huang, X.; Chen, L. Experimental and theoretical studies on reduction mechanism of vinyl ethylene carbonate on graphite anode for lithium ion batteries. Electrochem. Commun. 2004, 6, 126–131. [Google Scholar] [CrossRef]
- Han, Y.K.; Lee, S.U.; Ok, J.H.; Cho, J.J.; Kim, H.J. Theoretical studies of the solvent decomposition by lithium atoms in lithium-ion battery electrolyte. Chem. Phys. Lett. 2002, 360, 359–366. [Google Scholar] [CrossRef]
- Han, Y.K.; Lee, S.U. Performance of density functional for calculation of reductive ring-opening reaction energies of Li+-EC and Li+-VC. Theor. Chem. Acc. 2004, 112, 106–112. [Google Scholar] [CrossRef]
- Wang, Y.; Balbuena, P.B. Theoretical studies on cosolvation of Li ion and solvent reductive decomposition in binary mixtures of aliphatic carbonates. Int. J. Quantum Chem. 2005, 102, 724–733. [Google Scholar] [CrossRef]
- Aurbach, D. Review of selected electrode-solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 2000, 89, 206–218. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef]
- Aurbach, D.; Ein-Ely, Y.; Zaban, A. The Surface Chemistry of Lithium Electrodes in Alkyl Carbonate Solutions. J. Electrochem. Soc. 2019, 141, L1–L3. [Google Scholar] [CrossRef]
- Wang, L.; Menakath, A.; Han, F.; Wang, Y.; Zavalij, P.Y.; Gaskell, K.J.; Borodin, O.; Iuga, D.; Brown, S.P.; Wang, C.; et al. Identifying the components of the solid—Electrolyte interphase in Li-ion batteries. Nat. Chem. 2019, 11, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, K.; Kanda, K.; Kobayashi, T.; Nakamura, S.; Ue, M. Theoretical Studies on the Reductive Decompositions of Solvents and Additives for Lithium-Ion Batteries near Lithium Anodes. J. Electrochem. Soc. 2006, 153, A2192. [Google Scholar] [CrossRef]
- Tasaki, K. Solvent decompositions and physical properties of decomposition compounds in Li-ion battery electrolytes studied by DFT calculations and molecular dynamics simulations. J. Phys. Chem. B 2005, 109, 2920–2933. [Google Scholar] [CrossRef]
- Zhuang, G.V.; Xu, K.; Yang, H.; Jow, T.R.; Ross, P.N. Lithium ethylene dicarbonate identified as the primary product of chemical and electrochemical reduction of EC in 1.2 M LiPF 6/EC:EMC electrolyte. J. Phys. Chem. B 2005, 109, 17567–17573. [Google Scholar] [CrossRef]
- Hardwick, L.J.; Marcinek, M.; Beer, L.; Kerr, J.B.; Kostecki, R. An Investigation of the Effect of Graphite Degradation on Irreversible Capacity in Lithium-ion Cells. J. Electrochem. Soc. 2008, 155, A442. [Google Scholar] [CrossRef] [Green Version]
- Leung, K. Predicting the voltage dependence of interfacial electrochemical processes at lithium-intercalated graphite edge planes. Phys. Chem. Chem. Phys. 2015, 17, 1637–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haruyama, J.; Ikeshoji, T.; Otani, M. Analysis of Lithium Insertion/Desorption Reaction at Interfaces between Graphite Electrodes and Electrolyte Solution Using Density Functional + Implicit Solvation Theory. J. Phys. Chem. C 2018, 122, 9804–9810. [Google Scholar] [CrossRef]
- Borodin, O.; Bedrov, D. Interfacial structure and dynamics of the lithium alkyl dicarbonate SEI components in contact with the lithium battery electrolyte. J. Phys. Chem. C 2014, 118, 18362–18371. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metalamorphous-semiconductor transition in germanium. Phys. Rev. B Condens. Matter 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Ouatani, L.; Dedryvère, R.; Siret, C.; Biensan, P.; Reynaud, S.; Iratçabal, P.; Gonbeau, D. The Effect of Vinylene Carbonate Additive on Surface Film Formation on Both Electrodes in Li-Ion Batteries. J. Electrochem. Soc. 2009, 156, A103. [Google Scholar] [CrossRef]
- Matias, P.M.; Jeffrey, G.A.; Wingert, L.M.; Ruble, J.R. Single crystal neutron diffraction analysis (15 K) and ab initio molecular orbital calculations for ethylene carbonate. J. Mol. Struct. THEOCHEM 1989, 184, 247–260. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Shechter, A.; Ein-Eli, Y.; Cohen, H. A Comparative Study of Synthetic Graphite and Li Electrodes in Electrolyte Solutions Based on Ethylene Carbonate-Dimethyl Carbonate Mixtures. J. Electrochem. Soc. 2019, 143, 3809–3820. [Google Scholar] [CrossRef]
- Cao, Q.; Geng, X.; Wang, H.; Wang, P.; Liu, A.; Lan, Y.; Peng, Q. A review of current development of graphene mechanics. Crystals 2018, 8, 357. [Google Scholar] [CrossRef] [Green Version]
- Deng, B.; Hou, J.; Zhu, H.; Liu, S.; Liu, E.; Shi, Y.; Peng, Q. The normal-auxeticity mechanical phase transition in graphene. 2D Mater. 2017, 4, 021020. [Google Scholar] [CrossRef]
- Hou, J.; Deng, B.; Zhu, H.; Lan, Y.; Shi, Y.; De, S.; Liu, L.; Chakraborty, P.; Gao, F.; Peng, Q. Magic auxeticity angle of graphene. Carbon 2019, 149, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Zamiri, A.R.; Ji, W.; De, S. Elastic properties of hybrid graphene/boron nitride monolayer. Acta Mech. 2012, 223, 2591–2596. [Google Scholar] [CrossRef]
- Peng, Q.; Liang, C.; Ji, W.; De, S. A theoretical analysis of the effect of the hydrogenation of graphene to graphane on its mechanical properties. Phys. Chem. Chem. Phys. 2013, 15, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Chen, X.J.; Ji, W.; De, S. Chemically Tuning Mechanics of Graphene by BN. Adv. Eng. Mater. 2013, 15, 718–727. [Google Scholar] [CrossRef]
- Peng, Q.; Han, L.; Lian, J.; Wen, X.; Liu, S.; Chen, Z.; Koratkar, N.; De, S. Mechanical degradation of graphene by epoxidation: Insights from first-principles calculations. Phys. Chem. Chem. Phys. 2015, 17, 19484–19490. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Peng, Q.; Dai, Y.; Qian, Z.; Liu, S. Temperature dependence of Raman spectra of graphene on copper foil substrate. J. Mater. Sci. Mater. Electron. 2016, 27, 3888–3893. [Google Scholar] [CrossRef]
- Shi, T.; Peng, Q.; Bai, Z.; Gao, F.; Jovanovic, I. Proton irradiation of graphene: Insights from atomistic modeling. Nanoscale 2019, 11, 20754–20765. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, S.; Xie, L.; Zhu, P.; Li, R.; Peng, Q. Grain size and hydroxyl-coverage dependent tribology of polycrystalline graphene. Nanotechnology 2019, 30, 385701. [Google Scholar] [CrossRef]
- Mackay, A.L. Essentials of Crystallography Oxford Blackwell Scientific Publications 1986. viii+435 pp. Price: Cloth £25·00, limp £12·95; McKie, D., McKie, C., Eds.; Blackwell Science: Oxford, UK, 1988; Volume 52. [Google Scholar]
- Jia, X.; Hofmann, M.; Meunier, V.; Sumpter, B.G.; Campos-Delgado, J.; Romo-Herrera, J.M.; Son, H.; Hsieh, Y.P.; Reina, A.; Kong, J.; et al. Controlled formation of sharp zigzag and armchair edges in graphitic nanoribbons. Science 2009, 323, 1701–1705. [Google Scholar] [CrossRef]
- Enoki, T.; Kobayashi, Y.; Fukui, K.I. Electronic structures of graphene edges and nanographene. Int. Rev. Phys. Chem. 2007, 26, 609–645. [Google Scholar] [CrossRef]
- Nakada, K.; Fujita, M.; Dresselhaus, G.; Dresselhaus, M.S. Edge state in graphene ribbons: Nanometer size effect and edge shape dependence. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 17954–17961. [Google Scholar] [CrossRef] [Green Version]
- Zaghib, K.; Nadeau, G.; Kinoshita, K. Influence of edge and basal plane sites on the electrochemical behavior of flake-like natural graphite for Li-ion batteries. J. Power Sources 2001, 97–98, 97–103. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Zhu, Y.; Marin, T.W.; Abraham, D. Reduction of carbonate electrolytes and the formation of solid-electrolyte interface (SEI) in lithium-ion batteries. 1. Spectroscopic observations of radical intermediates generated in one-electron reduction of carbonates. J. Phys. Chem. C 2013, 117, 19255–19269. [Google Scholar] [CrossRef]


| Basal Surface | Zigzag Edge Surface | Armchair Edge Surface | |
|---|---|---|---|
| CH2CH2OCO2Li | −0.24 | −0.91 | −0.54 |
| (CH2OCO2Li)2 | −0.17 | −0.49 | −0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Q. First-Principles Insights on the Formation Mechanism of Innermost Layers of Solid Electrolyte Interphases on Carbon Anodes for Lithium-Ion Batteries. Nanomaterials 2022, 12, 3654. https://doi.org/10.3390/nano12203654
Peng Q. First-Principles Insights on the Formation Mechanism of Innermost Layers of Solid Electrolyte Interphases on Carbon Anodes for Lithium-Ion Batteries. Nanomaterials. 2022; 12(20):3654. https://doi.org/10.3390/nano12203654
Chicago/Turabian StylePeng, Qing. 2022. "First-Principles Insights on the Formation Mechanism of Innermost Layers of Solid Electrolyte Interphases on Carbon Anodes for Lithium-Ion Batteries" Nanomaterials 12, no. 20: 3654. https://doi.org/10.3390/nano12203654