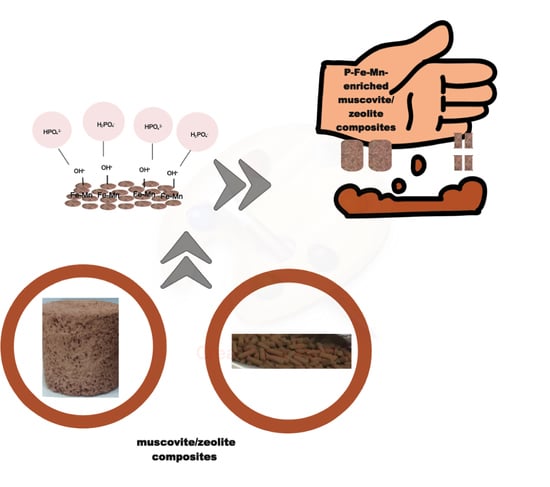
Fe3+/Mn2+ (Oxy)Hydroxide Nanoparticles Loaded onto Muscovite/Zeolite Composites (Powder, Pellets and Monoliths): Phosphate Carriers from Urban Wastewater to Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clay Collection and Pre-Treatment
2.2. Obtaining of Fe3+/Mn2+ (Oxy)Hydroxide Nanoparticles and Loading onto Muscovite/Sodalite Composite
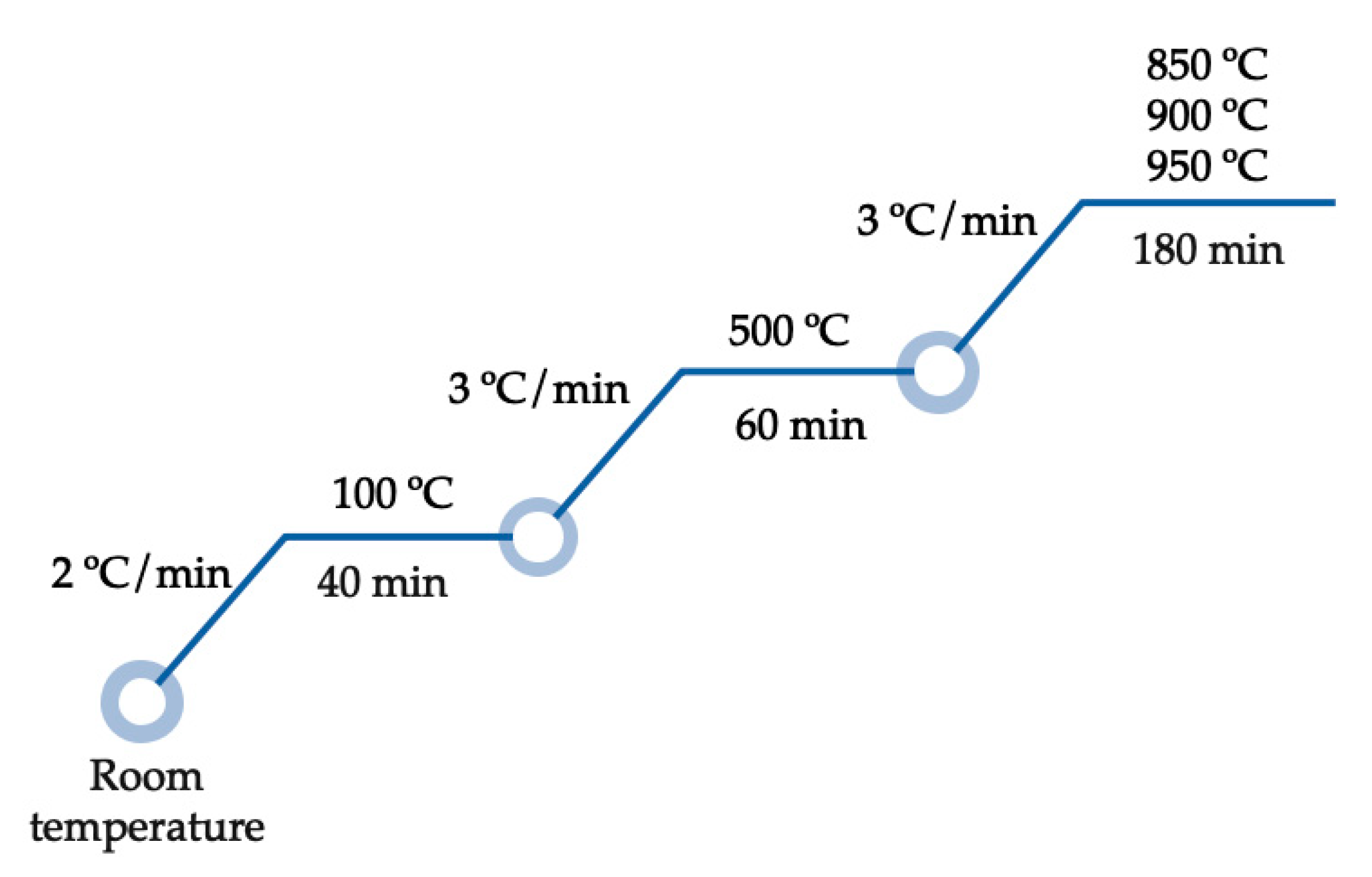
2.3. Obtaining of Fe3+/Mn2+ (Oxy)Hydroxide Nanoparticles Loaded onto Muscovite/Analcime Ceramic Composites (Monoliths and Pellets)
2.4. Materials Characterization
2.5. Phosphate Adsorption Assays in Bath Mode
2.5.1. Effect of the Calcination Temperature of Composites
2.5.2. Effect of the pH
2.5.3. Equilibrium Adsorption Capacity
2.5.4. Phosphate Adsorption Kinetic
2.5.5. Phosphate Fractioning
2.5.6. Regeneration of Phosphate Saturated Adsorbents
2.6. Phosphate Adsorption in Continuous Mode
3. Results
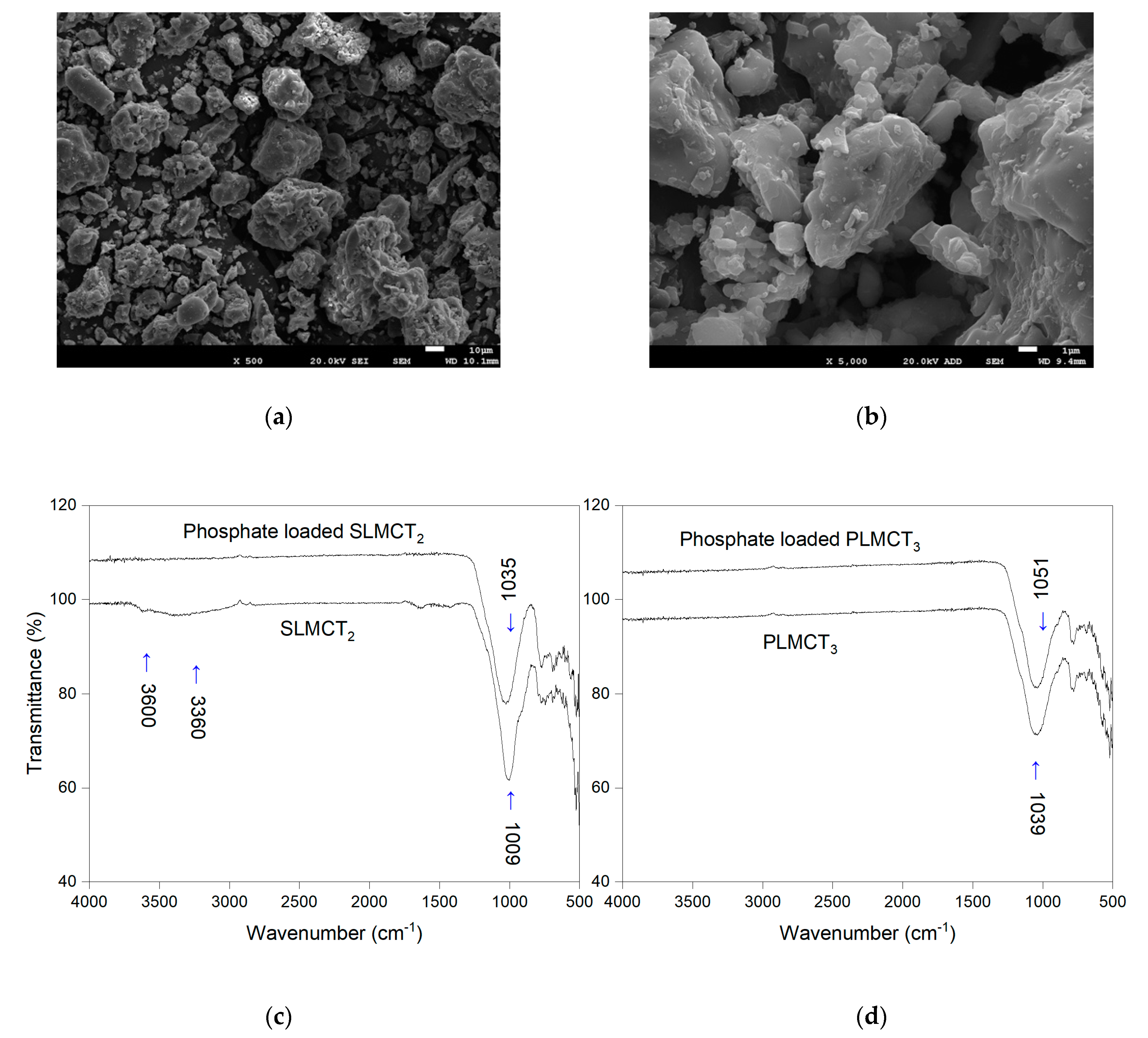
3.1. Physicochemical Propierties of Materials
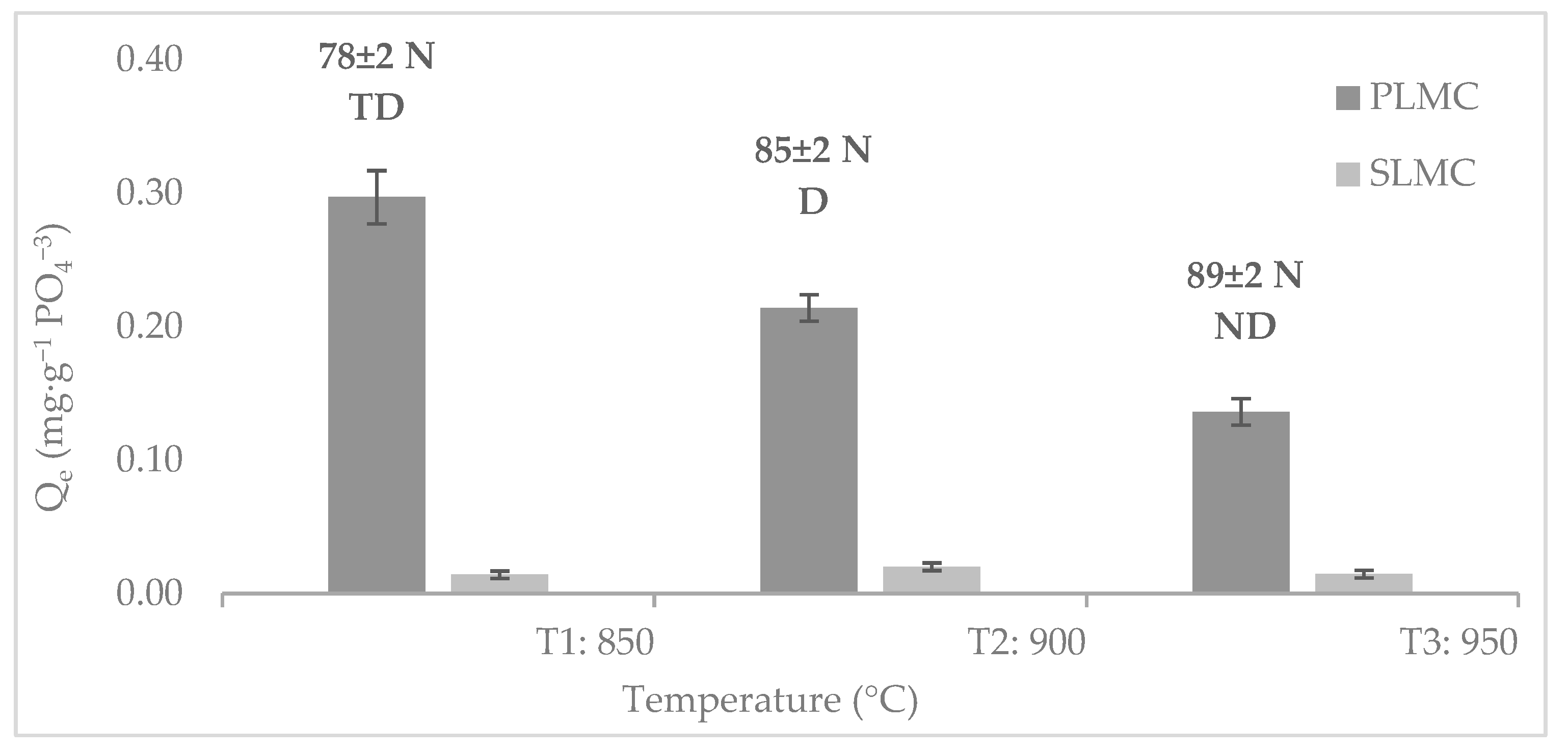
3.2. Influence of the Calcination Temperature on the Phosphate Adsorption
3.3. Effect of the pH on Phosphate Removal
3.4. Isotherms of Phosphate Adsorption onto the Adsorbents
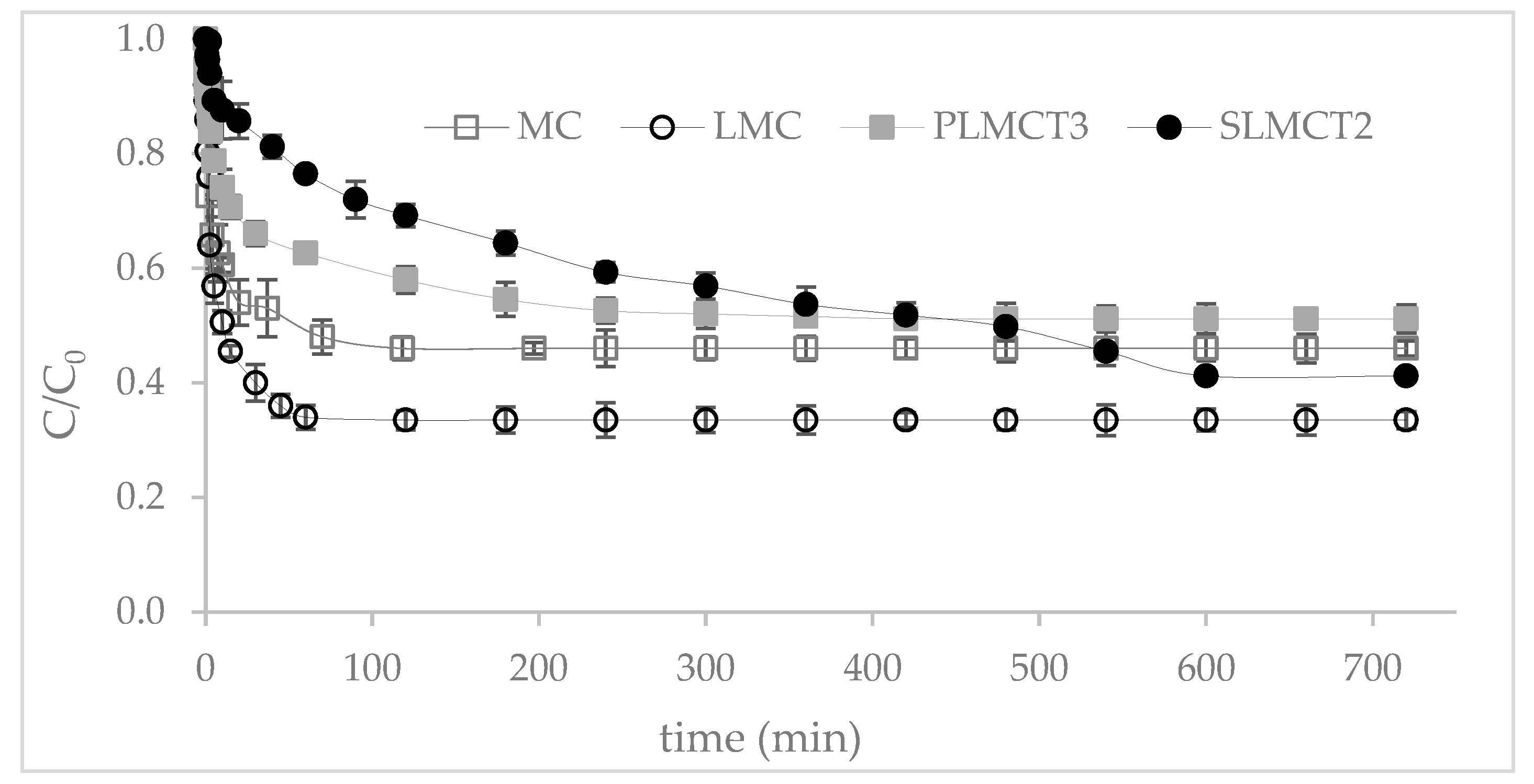
3.5. Kinetic of Phosphate Adsorption onto Adsorbents
3.6. Phosphate Fractioning from Doped Adsorbents
3.7. Regeneration of Adsorbents
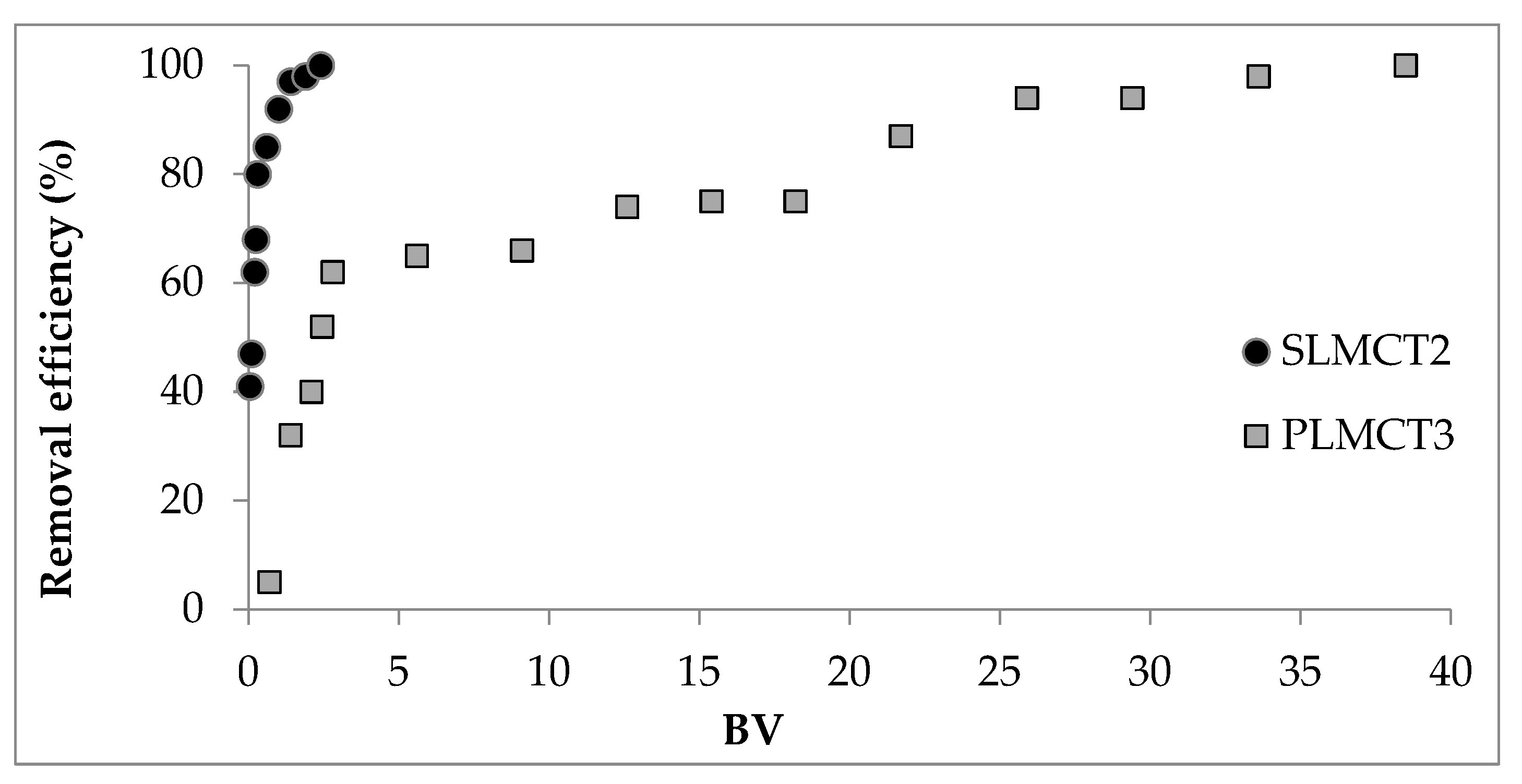
3.8. Phosphate Adsorption in Continuos Mode
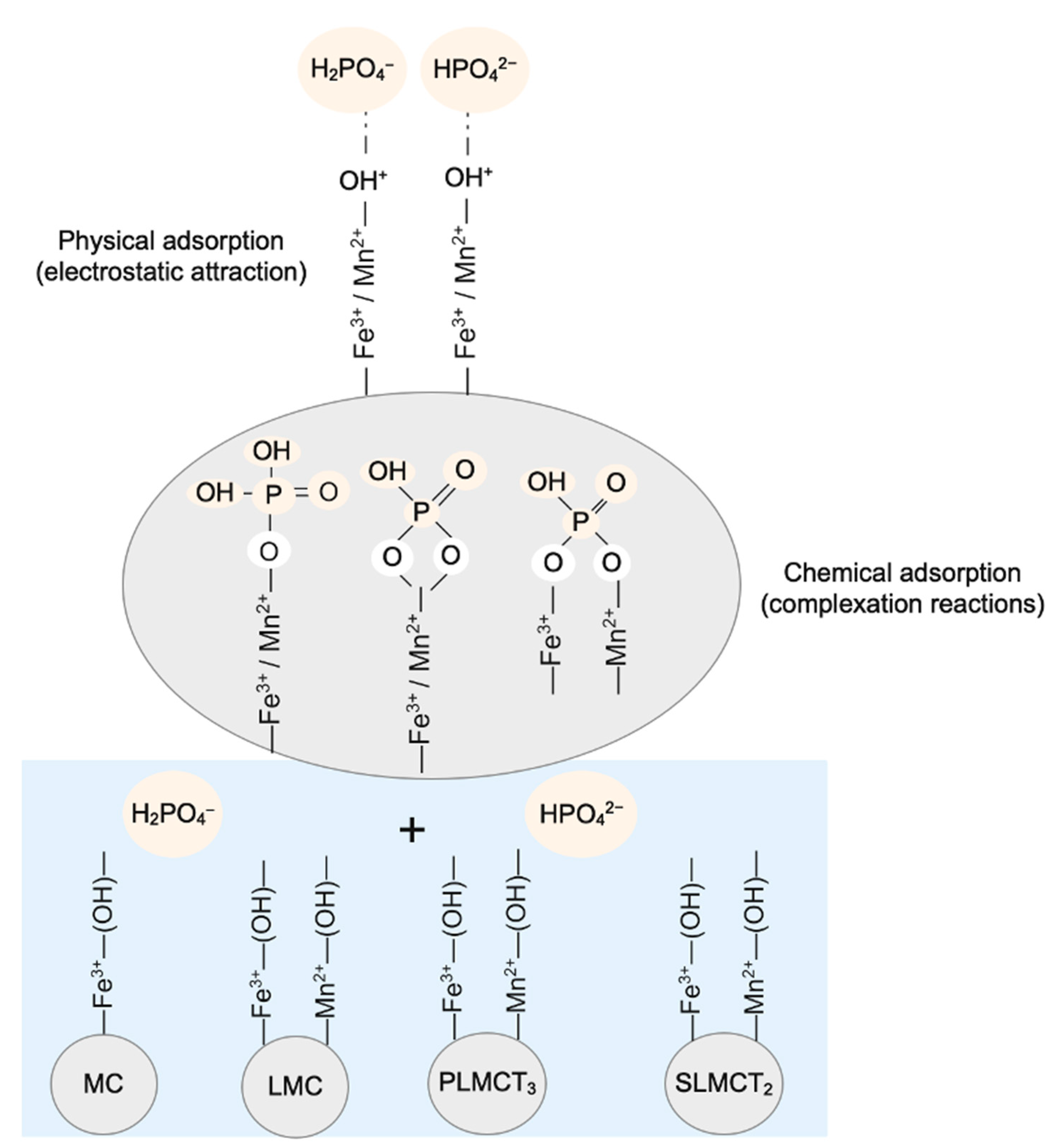
3.9. Implications of Phosphate Adsorption Using the Fe3+/Mn2+ Muscovite/Sodalite Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guaya, D.; Valderrama, C.; Farran, A.; Sauras, T.; Cortina, J.L. Valorisation of N and P from Waste Water by Using Natural Reactive Hybrid Sorbents: Nutrients (N,P,K) Release Evaluation in Amended Soils by Dynamic Experiments. Sci. Total Environ. 2018, 612, 728–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppelaar, R.H.E.M.; Weikard, H.P. Assessing phosphate rock depletion and phosphorus recycling options. Glob. Environ. Change. 2013, 23, 1454–1466. [Google Scholar] [CrossRef]
- di Capua, F.; de Sario, S.; Ferraro, A.; Petrella, A.; Race, M.; Pirozzi, F.; Fratino, U.; Spasiano, D. Phosphorous Removal and Recovery from Urban Wastewater: Current Practices and New Directions. Sci. Total Environ. 2022, 823, 153750. [Google Scholar] [CrossRef] [PubMed]
- European Union. Regulation of the European parliament and of the council laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union. 2019, 170, 1–14. [Google Scholar]
- Guaya, D.; Jiménez, R.; Sarango, J.; Valderrama, C.; Cortina, J.L. Iron-Doped Natural Clays: Low-Cost Inorganic Adsorbents for Phosphate Recovering from Simulated Urban Treated Wastewater. J. Water Process Eng. 2021, 43, 102274. [Google Scholar] [CrossRef]
- United Nations Sustainable Development Goals. Available online: http://www.undp.org/content/undp/en/home/sustainable-development-goals.html (accessed on 24 September 2022).
- Guaya, D.; Valderrama, C.; Farran, A.; Cortina, J.L. Simultaneous Nutrients (N,P) Removal by Using a Hybrid Inorganic Sorbent Impregnated with Hydrated Manganese Oxide. J. Environ. Chem. Eng. 2017, 5, 1516–1525. [Google Scholar] [CrossRef]
- Dittmann, E.; Wiegand, C. Cyanobacterial Toxins—Occurrence, Biosynthesis and Impact on Human Affairs. Mol. Nutr. Food Res. 2006, 50, 7–17. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Armijos, C.; Cortina, J.L. Simultaneous Phosphate and Ammonium Removal from Aqueous Solution by a Hydrated Aluminum Oxide Modified Natural Zeolite. Chem. Eng. J. 2015, 271, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Guaya, D.; Valderrama, C.; Farran, A.; Cortina, J.L. Modification of a Natural Zeolite with Fe(III) for Simultaneous Phosphate and Ammonium Removal from Aqueous Solutions. J. Chem. Technol. Biotechnol. 2016, 91, 1737–1746. [Google Scholar] [CrossRef]
- Awual, M.R. Efficient Phosphate Removal from Water for Controlling Eutrophication Using Novel Composite Adsorbent. J. Clean. Prod. 2019, 228, 1311–1319. [Google Scholar] [CrossRef]
- Guaya, D.; Cobos, H.; Valderrama, C.; Cortina, J.L. Effect of Mn2+/Zn2+/Fe3+ Oxy(Hydroxide) Nanoparticles Doping onto Mg-Al-LDH on the Phosphate Removal Capacity from Simulated Wastewater. Nanomaterials 2022, 12, 3680. [Google Scholar] [CrossRef] [PubMed]
- Awual, M.R.; Shenashen, M.A.; Jyo, A.; Shiwaku, H.; Yaita, T. Preparing of Novel Fibrous Ligand Exchange Adsorbent for Rapid Column-Mode Trace Phosphate Removal from Water. J. Ind. Eng. Chem. 2014, 20, 2840–2847. [Google Scholar] [CrossRef]
- Guaya, D.; Cobos, H.; Camacho, J.; López, C.M.; Valderrama, C.; Cortina, J.L. LTA and FAU-X Iron-Enriched Zeolites: Use for Phosphate Removal from Aqueous Medium. Materials 2022, 15, 5418. [Google Scholar] [CrossRef] [PubMed]
- Abukhadra, M.R.; Mostafa, M. Effective Decontamination of Phosphate and Ammonium Utilizing Novel Muscovite/Phillipsite Composite; Equilibrium Investigation and Realistic Application. Sci. Total Environ. 2019, 667, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Zhou, Z.; Huang, R.; Zou, C.; Xu, M.; Cheng, W.; Li, R. Synthesis and Swelling Properties of Microcrystal Muscovite Composite Superabsorbent. Appl. Clay Sci. 2014, 101, 199–204. [Google Scholar] [CrossRef]
- Salam, M.A.; Adlii, A.; Eid, M.H.; Abukhadra, M.R. Effective Decontamination of Ca2+ and Mg2+ Hardness from Groundwater Using Innovative Muscovite Based Sodalite in Batch and Fixed-Bed Column Studies; Dynamic and Equilibrium Studies. J. Contam. Hydrol. 2021, 241, 103817. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Peng, T.; You, H.; Qin, Y.; Zeng, L. Effects of Muscovite Matrix on Photocatalytic Degradation in TiO2/Muscovite Nanocomposites. Appl. Clay. Sci. 2019, 179, 105155. [Google Scholar] [CrossRef]
- Rui, J.; Deng, N.; Zhao, Y.; Tao, C.; Zhou, J.; Zhao, Z.; Huang, X. Activation of Persulfate via Mn Doped Mg/Al Layered Double Hydroxide for Effective Degradation of Organics: Insights from Chemical and Structural Variability of Catalyst. Chemosphere 2022, 302, 134849. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, N.; Fan, Z.; Hu, Z.-T.; Fan, L.; Zhou, J.; Huang, X. On-Site H2O2 Electro-Generation Process Combined with Ultraviolet: A Promising Approach for Odorous Compounds Purification in Drinking Water System. Chem. Eng. J. 2022, 430, 132829. [Google Scholar] [CrossRef]
- Zhu, S.; Xia, M.; Chu, Y.; Khan, M.A.; Lei, W.; Wang, F.; Muhmood, T.; Wang, A. Adsorption and Desorption of Pb(II) on l-Lysine Modified Montmorillonite and the Simulation of Interlayer Structure. Appl. Clay. Sci. 2019, 169, 40–47. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Ferreira, T.O.; Barcellos, D.; Nóbrega, G.N.; Antelo, J.; Otero, X.L.; Bernardino, A.F. From Sinks to Sources: The Role of Fe Oxyhydroxide Transformations on Phosphorus Dynamics in Estuarine Soils. J. Environ. Manage 2021, 278, 111575. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kang, L.; Zhang, J.; Jung, E.; Lee, S.; Jun, S.C. Structural Engineering and Surface Modification of MOF-Derived Cobalt-Based Hybrid Nanosheets for Flexible Solid-State Supercapacitors. Energy Storage Mater 2020, 32, 167–177. [Google Scholar] [CrossRef]
- Chaudhary, M.; Bhattacharya, P.; Maiti, A. Synthesis of Iron Oxyhydroxide Nanoparticles and Its Application for Fluoride Removal from Water. J. Environ. Chem. Eng. 2016, 4, 4897–4903. [Google Scholar] [CrossRef]
- Jo, J.-Y.; Kim, J.-G.; Tsang, Y.F.; Baek, K. Removal of Ammonium, Phosphate, and Sulfonamide Antibiotics Using Alum Sludge and Low-Grade Charcoal Pellets. Chemosphere 2021, 281, 130960. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; An, T.; Li, G.; Li, Y.; Yu, J.C.; Wong, P.K. Free-Standing Red Phosphorous/Silver Sponge Monolith as an Efficient and Easily Recyclable Macroscale Photocatalyst for Organic Pollutant Degradation under Visible Light Irradiation. J. Colloid Interface Sci. 2018, 518, 130–139. [Google Scholar] [CrossRef]
- Hosseini, S.; Moghaddas, H.; Masoudi Soltani, S.; Kheawhom, S. Technological Applications of Honeycomb Monoliths in Environmental Processes: A Review. Process Saf. Environ. Prot. 2020, 133, 286–300. [Google Scholar] [CrossRef]
- Guaya, D.; Hermassi, M.; Valderrama, C.; Farran, A.; Cortina, J.L. Recovery of Ammonium and Phosphate from Treated Urban Wastewater by Using Potassium Clinoptilolite Impregnated Hydrated Metal Oxides as N-P-K Fertilizer. J. Environ. Chem. Eng. 2016, 4, 3519–3526. [Google Scholar] [CrossRef]
- McCrady, M.H. Standard Methods for the Examination of Water and Wastewater, 12th ed.; American Public Health Association: Washington, DC, USA, 2011. [Google Scholar]
- Hieltjes, A.H.M.; Lijklema, L. Fractionation of Inorganic Phosphates in Calcareous Sediments. J. Environ. Qual. 1980, 9, 405–407. [Google Scholar] [CrossRef]
- Bao, T.; Damtie, M.M.; Hosseinzadeh, A.; Frost, R.L.; Yu, Z.M.; Jin, J.; Wu, K. Catalytic Degradation of P-Chlorophenol by Muscovite-Supported Nano Zero Valent Iron Composite: Synthesis, Characterization, and Mechanism Studies. Appl. Clay Sci. 2020, 195, 105735. [Google Scholar] [CrossRef]
- Jia, F.; Su, J.; Song, S. Can Natural Muscovite Be Expanded? Colloids Surf. A Physicochem. Eng. Asp. 2015, 471, 19–25. [Google Scholar] [CrossRef]
- Ma, B.; Fernandez-Martinez, A.; Mancini, A.; Lothenbach, B. Spectroscopic Investigations on Structural Incorporation Pathways of FeIII into Zeolite Frameworks in Cement-Relevant Environments. Cem. Concr. Res. 2021, 140, 106304. [Google Scholar] [CrossRef]
- Klima, K.M.; Schollbach, K.; Brouwers, H.J.H.; Yu, Q. Enhancing the Thermal Performance of Class F Fly Ash-Based Geopolymer by Sodalite. Constr. Build Mater 2022, 314, 125574. [Google Scholar] [CrossRef]
- Sathupunya, M.; Gulari, E.; Wongkasemjit, S. ANA and GIS Zeolite Synthesis Directly from Alumatrane and Silatrane by Sol-Gel Process and Microwave Technique. J. Eur. Ceram. Soc. 2002, 22, 2305–2314. [Google Scholar] [CrossRef]
- Zhu, S.; Cui, H.; Jia, Y.; Zhu, X.; Tong, H.; Ma, L. Occurrence, Composition, and Origin of Analcime in Sedimentary Rocks of Non-Marine Petroliferous Basins in China. Mar. Pet. Geol. 2020, 113, 104164. [Google Scholar] [CrossRef]
- Bortolini, H.R.; Lima, D.S.; Perez-Lopez, O.W. Hydrothermal Synthesis of Analcime without Template. J. Cryst. Growth 2020, 532, 125424. [Google Scholar] [CrossRef]
- Azizi, S.N.; Ehsani Tilami, S. Framework-Incorporated Mn and Co Analcime Zeolites: Synthesis and Characterization. J. Solid State Chem. 2013, 198, 138–142. [Google Scholar] [CrossRef]
- Kumar, M.M.; Jena, H. Direct Single-Step Synthesis of Phase Pure Zeolite Na–P1, Hydroxy Sodalite and Analcime from Coal Fly Ash and Assessment of Their Cs+ and Sr2+ Removal Efficiencies. Microporous Mesoporous Mater. 2022, 333, 111738. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, R.; Lu, X.; Li, J.; Wang, T. Reprint of Genesis of Halloysite from the Weathering of Muscovite: Insights from Microscopic Observations of a Weathered Granite in the Gaoling Area, Jingdezhen, China. Appl. Clay Sci. 2016, 119, 59–66. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Salam, M.A.; Abukhadra, M.R. Effective Retention of Inorganic Selenium Ions (Se (VI) and Se (IV)) Using Novel Sodalite Structures from Muscovite; Characterization and Mechanism. J. Taiwan Inst. Chem. Eng. 2021, 120, 116–126. [Google Scholar] [CrossRef]
- Robben, L.; Gesing, T.M. Temperature-Dependent Framework–Template Interaction of |Na6(H2O)8|[ZnPO4]6 Sodalite. J. Solid State Chem. 2013, 207, 13–20. [Google Scholar] [CrossRef]
- Mofrad, A.M.; Schellenberg, P.S.; Peixoto, C.; Hunt, H.K.; Hammond, K.D. Calculated Infrared and Raman Signatures of Ag+, Cd2+, Pb2+, Hg2+, Ca2+, Mg2+, and K+ Sodalites. Microporous Mesoporous Mater. 2020, 296, 109983. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, J.; Ma, H.; Liu, C. Crystal Structural Transformation and Kinetics of NH4+/Na+ Ion-Exchange in Analcime. Microporous Mesoporous Mater. 2016, 222, 202–208. [Google Scholar] [CrossRef]
- Eden, C.L.; Daramola, M.O. Evaluation of Silica Sodalite Infused Polysulfone Mixed Matrix Membranes during H2/CO2 Separation. Mater Today Proc. 2021, 38, 522–527. [Google Scholar] [CrossRef]
- Vigil de la Villa Mencía, R.; Goiti, E.; Ocejo, M.; Giménez, R.G. Synthesis of Zeolite Type Analcime from Industrial Wastes. Microporous Mesoporous Mater. 2020, 293, 109817. [Google Scholar] [CrossRef]
- Nasseh, S.; Mehranbod, N.; Eslamloueyan, R. Optimization of Ceramic Foam Fabrication for Removal of Aluminium Ion from Aqueous Solutions. J. Environ. Chem. Eng. 2019, 7, 103513. [Google Scholar] [CrossRef]
- Kosmulski, M. The PH Dependent Surface Charging and Points of Zero Charge. VII. Update. Adv. Colloid Interface Sci. 2018, 251, 115–138. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Asiri, A.M.; Rahman, M.M. Cleaning the Arsenic(V) Contaminated Water for Safe-Guarding the Public Health Using Novel Composite Material. Compos. B Eng. 2019, 171, 294–301. [Google Scholar] [CrossRef]
- Awual, M.R.; Yaita, T.; Suzuki, S.; Shiwaku, H. Ultimate Selenium(IV) Monitoring and Removal from Water Using a New Class of Organic Ligand Based Composite Adsorbent. J. Hazard Mater. 2015, 291, 111–119. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Khaleque, M.A. Efficient Selenium(IV) Detection and Removal from Water by Tailor-Made Novel Conjugate Adsorbent. Sens. Actuators B Chem. 2015, 209, 194–202. [Google Scholar] [CrossRef]
- Albayati, T.; Sabri, A.; Abed, D. Functionalized SBA-15 by Amine Group for Removal of Ni(II) Heavy Metal Ion in the Batch Adsorption System. Desalination Water Treat. 2020, 174, 301–310. [Google Scholar] [CrossRef]
- Kadhum, S.T.; Alkindi, G.Y.; Albayati, T.M. Eco Friendly Adsorbents for Removal of Phenol from Aqueous Solution Employing Nanoparticle Zero-Valent Iron Synthesized from Modified Green Tea Bio-Waste and Supported on Silty Clay. Chin. J. Chem. Eng. 2021, 36, 19–28. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Manjaiah, K.M.; Datta, S.C.; Yadav, R.K.; Sarkar, B. Inorganically Modified Clay Minerals: Preparation, Characterization, and Arsenic Adsorption in Contaminated Water and Soil. Appl. Clay. Sci. 2017, 147, 1–10. [Google Scholar] [CrossRef]
- Albayati, T.; Sabri, A.; Abed, D. Adsorption of Binary and Multi Heavy Metals Ions from Aqueous Solution by Amine Functionalized SBA-15 Mesoporous Adsorbent in a Batch System. Desalination Water Treat. 2019, 151, 315–321. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption carbon from solutions. J. Sanit. Eng. Div. Proc. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Valderrama, C.; Barios, J.I.; Caetano, M.; Farran, A.; Cortina, J.L. Kinetic Evaluation of Phenol/Aniline Mixtures Adsorption from Aqueous Solutions onto Activated Carbon and Hypercrosslinked Polymeric Resin (MN200). React. Funct. Polym. 2010, 70, 142–150. [Google Scholar] [CrossRef]
- Moussavi, G.; Talebi, S.; Farrokhi, M.; Sabouti, R.M. The Investigation of Mechanism, Kinetic and Isotherm of Ammonia and Humic Acid Co-Adsorption onto Natural Zeolite. Chem. Eng. J. 2011, 171, 1159–1169. [Google Scholar] [CrossRef]
- Onyango, M.S.; Kuchar, D.; Kubota, M.; Matsuda, H. Adsorptive Removal of Phosphate Ions from Aqueous Solution Using Synthetic Zeolite. Ind. Eng. Chem. Res. 2007, 46, 894–900. [Google Scholar] [CrossRef]
- Awual, M.R.; Jyo, A.; El-Safty, S.A.; Tamada, M.; Seko, N. A Weak-Base Fibrous Anion Exchanger Effective for Rapid Phosphate Removal from Water. J. Hazard. Mater. 2011, 188, 164–171. [Google Scholar] [CrossRef]
- Zamparas, M.; Gianni, A.; Stathi, P.; Deligiannakis, Y.; Zacharias, I. Removal of Phosphate from Natural Waters Using Innovative Modified Bentonites. Appl. Clay. Sci. 2012, 62–63, 101–106. [Google Scholar] [CrossRef]
- Moharami, S.; Jalali, M. Removal of Phosphorus from Aqueous Solution by Iranian Natural Adsorbents. Chem. Eng. J. 2013, 223, 328–339. [Google Scholar] [CrossRef]
- Yaghoobi-Rahni, S.; Rezaei, B.; Mirghaffari, N. Bentonite Surface Modification and Characterization for High Selective Phosphate Adsorption from Aqueous Media and Its Application for Wastewater Treatments. J. Water Reuse Desalination 2017, 7, 175–186. [Google Scholar] [CrossRef]
- Yan, L.G.; Xu, Y.Y.; Yu, H.Q.; Xin, X.D.; Wei, Q.; Du, B. Adsorption of Phosphate from Aqueous Solution by Hydroxy-Aluminum, Hydroxy-Iron and Hydroxy-Iron-Aluminum Pillared Bentonites. J. Hazard Mater. 2010, 179, 244–250. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Guaya, D.; Farran, A.; Valderrama, C.; Cortina, J.L. Phosphate Removal from Aqueous Solution Using a Hybrid Impregnated Polymeric Sorbent Containing Hydrated Ferric Oxide (HFO). J. Chem. Technol. Biotechnol. 2016, 91, 693–704. [Google Scholar] [CrossRef]
- You, X.; Farran, A.; Guaya, D.; Valderrama, C.; Soldatov, V.; Cortina, J.L. Phosphate Removal from Aqueous Solutions Using a Hybrid Fibrous Exchanger Containing Hydrated Ferric Oxide Nanoparticles. J. Environ. Chem. Eng. 2016, 4, 388–397. [Google Scholar] [CrossRef]

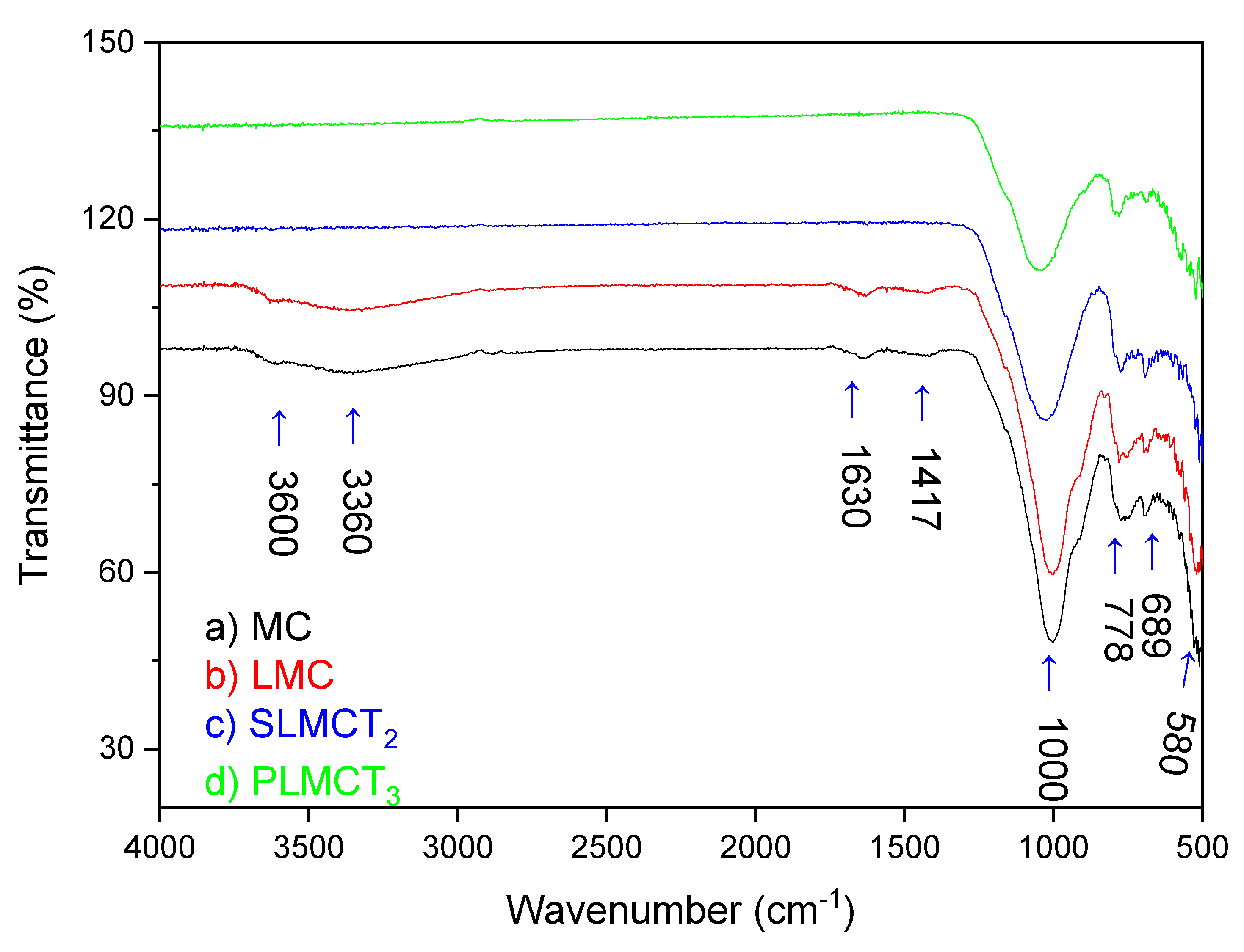
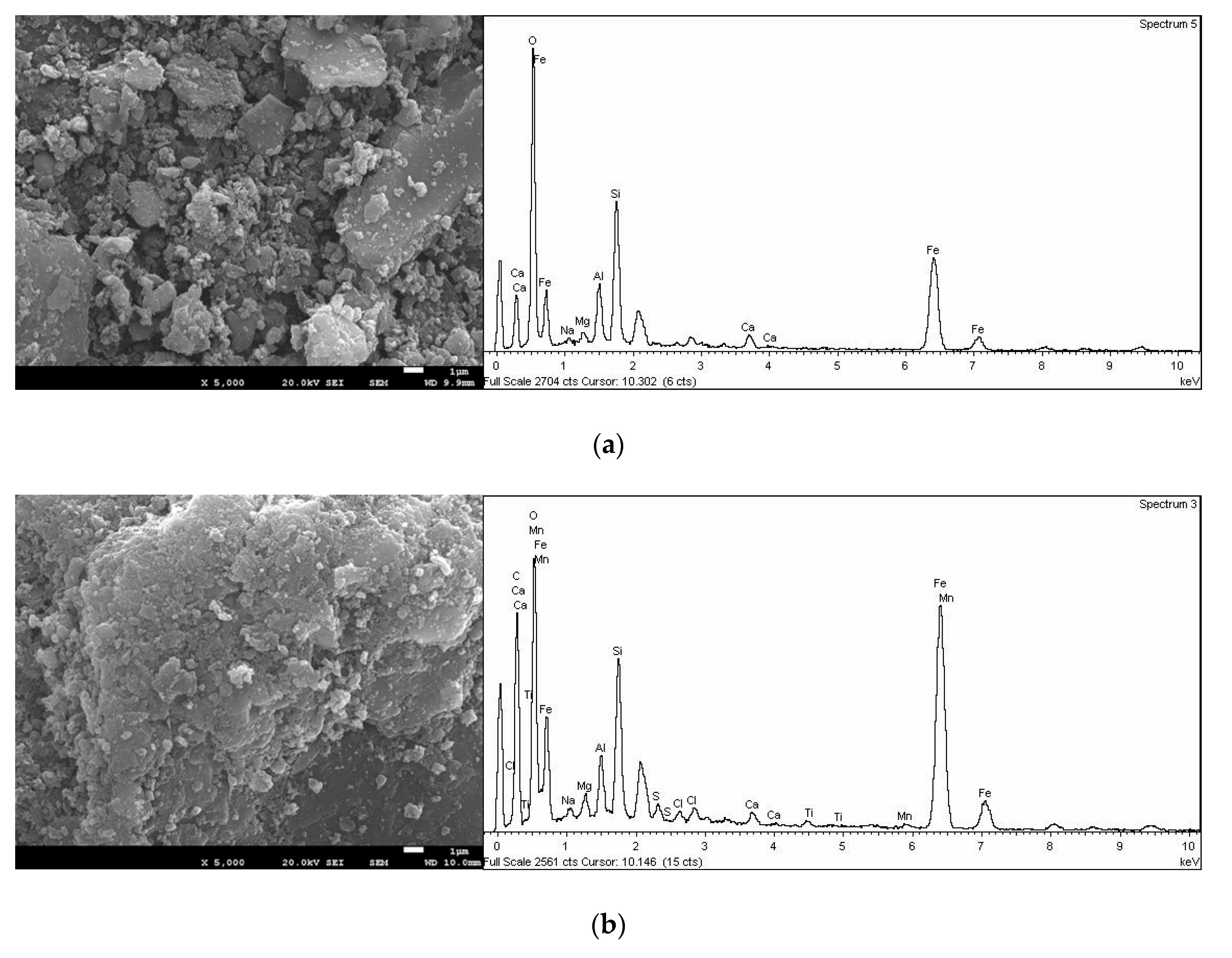


| Adsorbent Material | SiO2 (%) | Al2O3 (%) | MgO (%) | K2O (%) | CaO (%) | TiO2 (%) | Fe2O3 (%) | SnO2 (%) | MnO (%) | SA (m2.g−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| MC | 72 ± 0.5 | 12 ± 0.5 | 6 ± 0.2 | 2.5 ± 0.3 | 2.1 ± 0.4 | 0.6 ± 0.1 | 4 ± 0.2 | 0.5 ± 0.1 | 0.1 ± 0.1 | 7.0 |
| LMC | 68 ± 0.4 | 11 ± 0.5 | 1 ± 0.3 | 2.2 ± 0.4 | 0.4 ± 0.1 | 0.4 ± 0.1 | 12 ± 0.5 | 0.4 ± 0.1 | 3.5 ± 0.2 | 74.0 |
| PLMCT3 | 66 ± 0.5 | 10 ± 0.3 | 1 ± 0.2 | 2.2 ± 0.3 | 0.2 ± 0.0 | 0.4 ± 0.1 | 12 ± 0.5 | - | 3.4 ± 0.1 | 1.0 |
| SLMCT2 | 67 ± 0.5 | 10 ± 0.5 | 1 ± 0.2 | 2.2 ± 0.4 | 0.2 ± 0.0 | 0.4 ± 0.1 | 12 ± 0.4 | - | 3.4 ± 0.1 | 2.0 |
| Mg2+ (mg∙L−1) | K+ (mg∙L−1) | Na+ (mg∙L−1) | Ca2+ (mg∙L−1) |
|---|---|---|---|
| 6 | 0.3 | 1 | 2 |
| Zeolite | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| (mg·g−1) | (L·mg−1) | R2 | (mg·g−1) | R2 | ||
| MC | 2.1 | 0.03 | 0.99 | 1.37 | 0.11 | 0.79 |
| LMC | 6.0 | 0.02 | 0.99 | 1.73 | 0.16 | 0.90 |
| PLMCT3 | 0.9 | 0.14 | 0.99 | 0.52 | 0.12 | 0.74 |
| SLMCT2 | 0.2 | 0.04 | 0.99 | 0.02 | 0.42 | 0.77 |
| Kinetic Model | Kinetic Parameter | MC | LMC | PLMCT3 | SLMCT2 |
|---|---|---|---|---|---|
| HPDF Film diffusion | Df (m2·s−1) | 5.4 × 10−11 | 3.1 × 10−15 | 5.2 × 10−7 | 2.4 × 10−10 |
| R2 | 0.96 | 0.95 | 0.96 | 0.97 | |
| HPDM Particle diffusion | Dp (m2·s−1) | 2.8 × 10−12 | 5.6 × 10−13 | 3.4 × 10−9 | 2.9 × 10−10 |
| R2 | 0.95 | 0.97 | 0.96 | 0.97 |
| Adsorbent Material | Qe (mg·g−1) | LB-P | Metal-P | Alkaline-P | Residual-P | ||||
|---|---|---|---|---|---|---|---|---|---|
| (mg·g−1) | % | (mg·g−1) | % | (mg·g−1) | % | (mg·g−1) | % | ||
| MC | 0.14 ± 0.0 | 0.04 ± 0.0 | 30 ± 1 | 0.05 ± 0.0 | 39 ± 1 | 0.01 ± 0.0 | 9 ± 1 | 0.03 ± 0.0 | 22 ± 2 |
| LMC | 0.35 ± 0.0 | 0.12 ± 0.0 | 35 ± 1 | 0.17 ± 0.0 | 48 ± 1 | 0.02 ± 0.0 | 7 ± 1 | 0.04 ± 0.0 | 10 ± 2 |
| PLMCT3 | 0.14 ± 0.0 | 0.05 ± 0.0 | 33 ± 1 | 0.06 ± 0.0 | 43 ± 1 | 0.01 ± 0.0 | 6 ± 1 | 0.02 ± 0.0 | 17 ± 2 |
| SLMCT2 | 0.05 ± 0.0 | 0.016 ± 0.0 | 31 ± 1 | 0.02 ± 0.0 | 40 ± 1 | 0.004 ± 0.1 | 8 ± 1 | 0.01 ± 0.0 | 21 ± 2 |
| Adsorbent Material | Qe (mg·g−1) | Qd | Desorption % |
|---|---|---|---|
| (mg·g−1) | |||
| MC | 0.12 ± 0.0 | 0.03 ± 0.0 | 21 ± 1 |
| LMC | 0.33 ± 0.0 | 0.10 ± 0.0 | 30 ± 1 |
| PLMCT3 | 0.13 ± 0.0 | 0.05 ± 0.0 | 41 ± 1 |
| SLMCT2 | 0.11 ± 0.0 | 0.03 ± 0.0 | 51 ± 1 |
| Adsorbent | Description | Qm (mg·g−1) | Ref. | |
|---|---|---|---|---|
| Loaded Fe3+/Mn2+ (oxy)hydroxide nanoparticles onto muscovite/zeolite composite | Muscovite used as raw material for the synthesis of zeolite composites | MC | 2.1 | This study |
| LMC | 6.0 | |||
| PLMCT3 | 0.9 | |||
| SLMCT2 | 0.2 | |||
| Natural clays | Natural form | C1 | 21.4 | [5] |
| C2 | 20.9 | |||
| Modified form | C1-Fe | 38.0 | ||
| C2-Fe | 37.6 | |||
| Modified bentonite | Zn-containing bentonite clay | 4.12 | [61] | |
| Pillared bentonite by Fe | 11.15 | |||
| Natural clays | Bentonite from Iran | 0.369 | [62] | |
| Zeolite from Iran | 0.627 | |||
| Kaolinite from Iran | 0.624 | |||
| Modified bentonite | Pillared bentonite by Fe/Al | 8.33 | [63] | |
| Na-Bentonites | Pillared bentonite with Al | 12.7 | [64] | |
| Pillared bentonite with Fe | 11.2 | |||
| Pillared bentonite with Fe-Al | 10.5 | |||
| Synthetic zeolites | Hydrothermally synthesized | LTA-Fe | 18.5 | [14] |
| FAU-X-Fe | 17.5 | |||
| Natural zeolites | Clinoptilolite | ZN | 0.6 | [9] |
| Z-Al | 7.0 | |||
| Z-Fe | 3.4 | [10] | ||
| Z-Mn | 5.6 | [7] | ||
| Layered double hydroxide | Mn2+/Zn2+/Fe3+ Oxy(Hydroxide) layered double hydroxide | Mn2+/Zn2+/Fe3+/Mg-Al-LDH | 82.3 | [12] |
| Polymeric sorbent ion exchanger | Impregnated nanoparticles of hydrated ferric oxide | Lewatit FO36—HAIX | 91.30 | [65] |
| Fibrous ion exchanger | Impregnated nanoparticles of hydrated ferric oxide | FIBAN- As—FAS | 161.9 | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guaya, D.; Maza, L.; Angamarca, A.; Mendoza, E.; García, L.; Valderrama, C.; Cortina, J.L. Fe3+/Mn2+ (Oxy)Hydroxide Nanoparticles Loaded onto Muscovite/Zeolite Composites (Powder, Pellets and Monoliths): Phosphate Carriers from Urban Wastewater to Soil. Nanomaterials 2022, 12, 3848. https://doi.org/10.3390/nano12213848
Guaya D, Maza L, Angamarca A, Mendoza E, García L, Valderrama C, Cortina JL. Fe3+/Mn2+ (Oxy)Hydroxide Nanoparticles Loaded onto Muscovite/Zeolite Composites (Powder, Pellets and Monoliths): Phosphate Carriers from Urban Wastewater to Soil. Nanomaterials. 2022; 12(21):3848. https://doi.org/10.3390/nano12213848
Chicago/Turabian StyleGuaya, Diana, Luz Maza, Adriana Angamarca, Eda Mendoza, Luis García, César Valderrama, and José Luis Cortina. 2022. "Fe3+/Mn2+ (Oxy)Hydroxide Nanoparticles Loaded onto Muscovite/Zeolite Composites (Powder, Pellets and Monoliths): Phosphate Carriers from Urban Wastewater to Soil" Nanomaterials 12, no. 21: 3848. https://doi.org/10.3390/nano12213848