Preparation of 6-Mercaptopurine Loaded Liposomal Formulation for Enhanced Cytotoxic Response in Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of 6-MP Liposomal Formulations
2.3. Characterization of the Prepared 6-MP Liposomal Formulations
2.4. MTT Assay
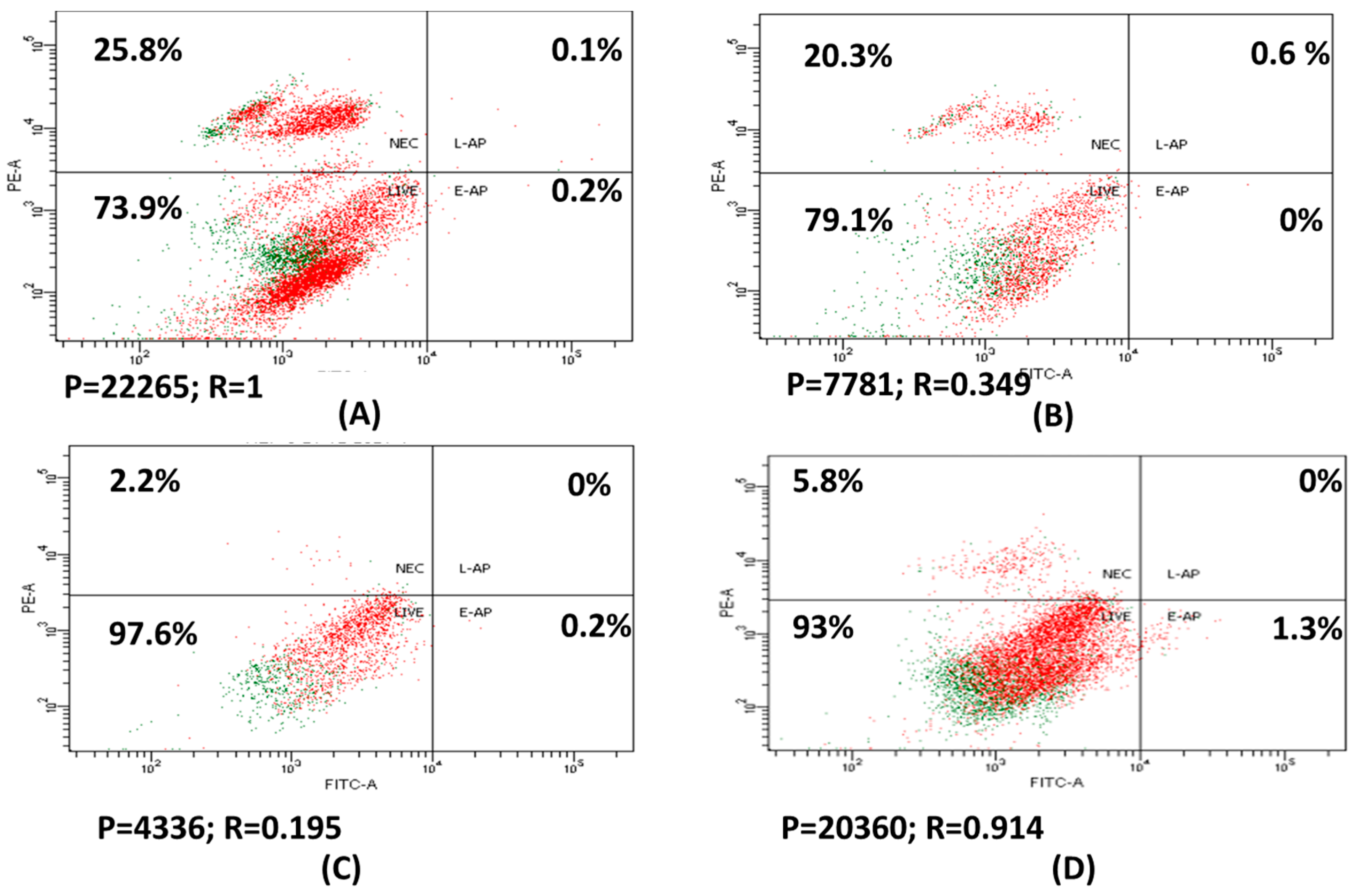
2.5. Apoptosis Assay
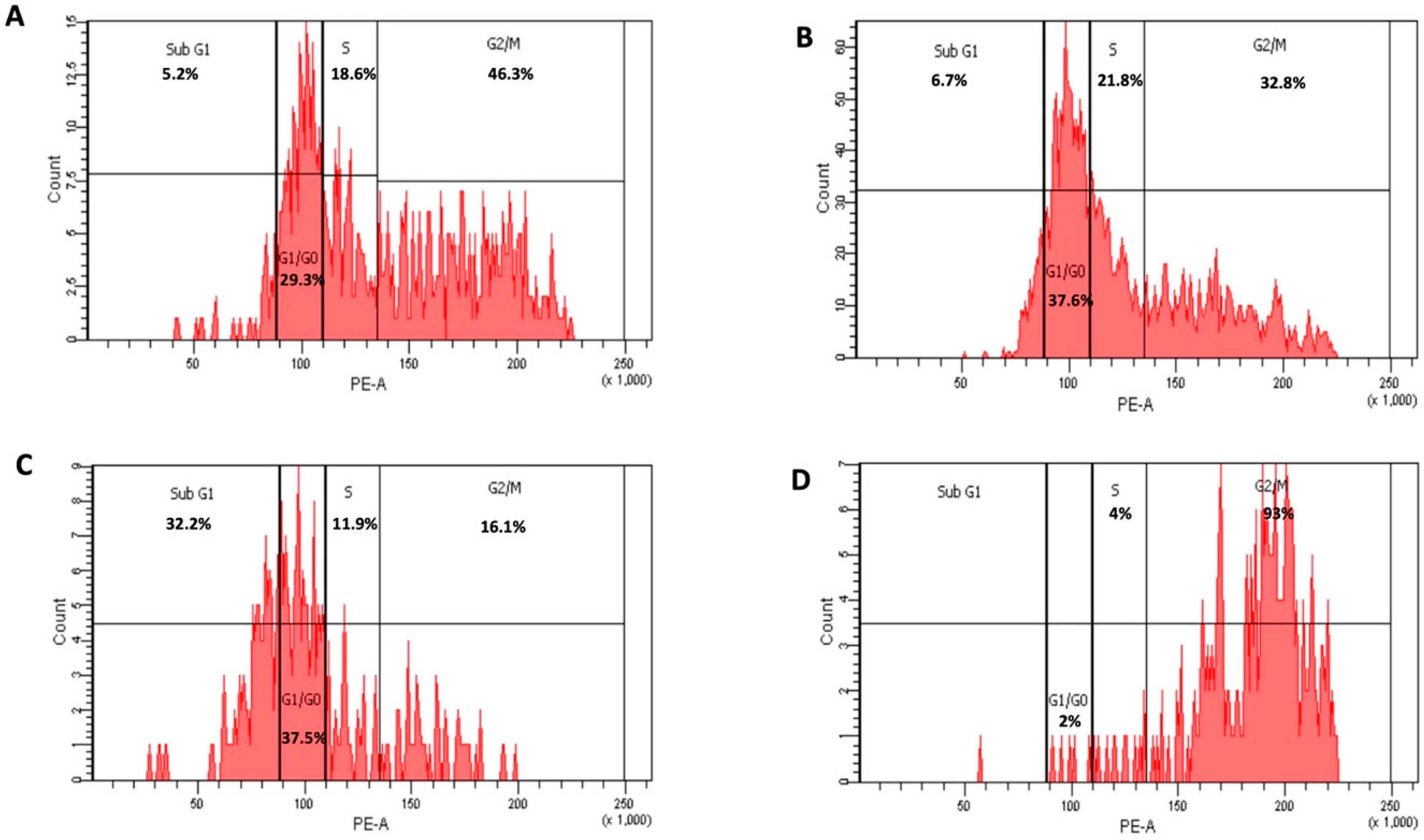
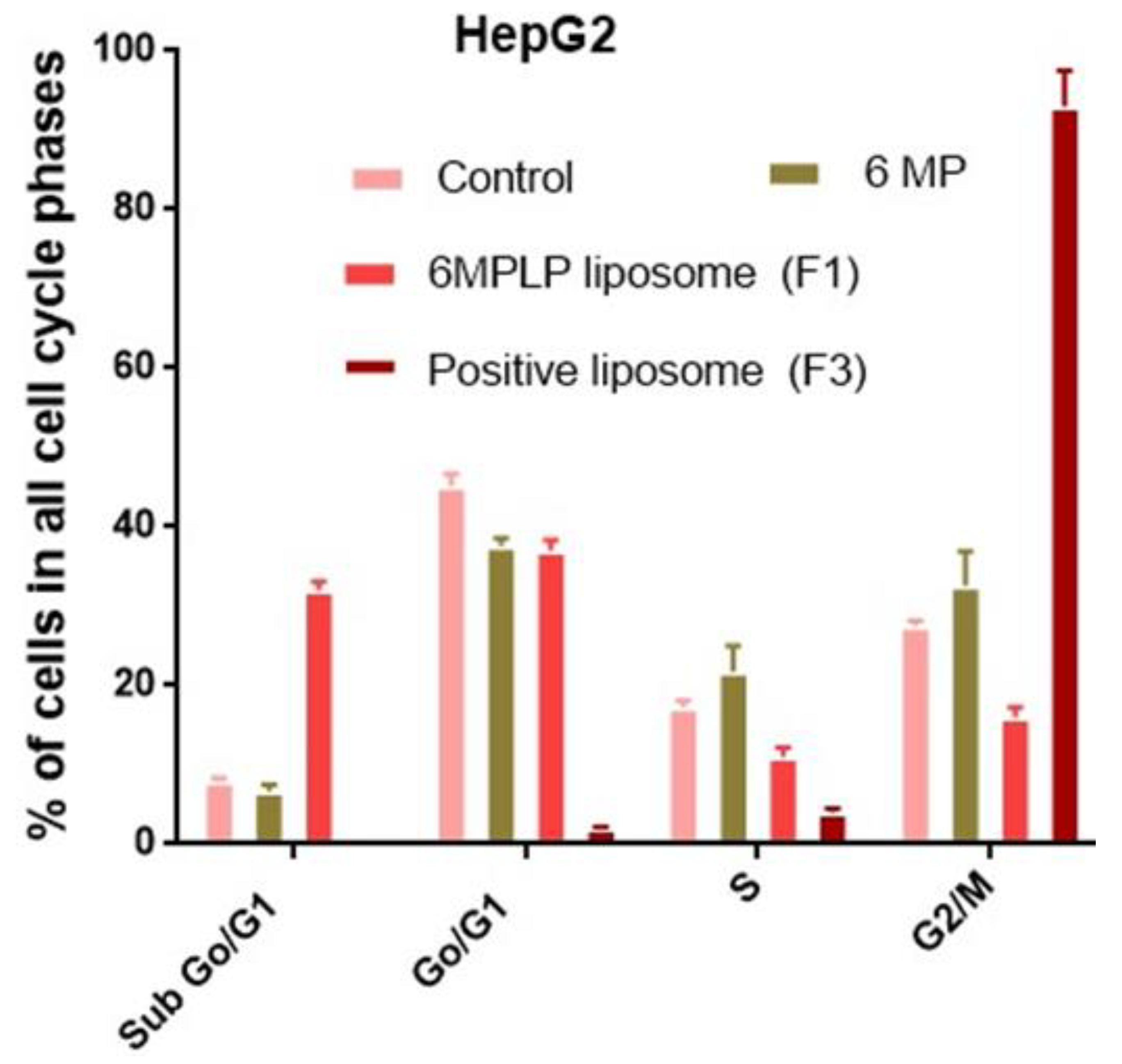
2.6. Cell Cycle Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Prepared 6-MP Liposomal Formulations
3.2. Anti-Tumor Activity of the Prepared Liposomal Formulations against HepG 2, HCT116, and MCF-7 Cells
3.3. Apoptosis and Necrosis of HepG2 Treated with Drug Loaded Positive Liposome (F1)
3.4. Cell Cycle Analysis of HepG2 Treated with Free 6-MP and Liposomal Formulation (F1)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ramos, A.A.; Marchetti-Laurent, C.; Poindessous, V.; Antonio, S.; Laurent-Puig, P.; Bortoli, S.; Loriot, M.A.; Pallet, N. 6-mercaptopurine promotes energetic failure in proliferating T cells. Oncotarget 2017, 8, 43048–43060. [Google Scholar] [CrossRef] [PubMed]
- Luengo, A.; Gui, D.Y.; Vander Heiden, M.G. Targeting Metabolism for Cancer Therapy. Cell Chem. Biol. 2017, 24, 1161–1180. [Google Scholar] [CrossRef] [PubMed]
- Shaye, O.A.; Yadegari, M.; Abreu, M.T.; Poordad, F.; Simon, K.; Martin, P.; Papadakis, K.A.; Ippoliti, A.; Vasiliauskas, E.; Tran, T.T. Hepatotoxicity of 6-mercaptopurine (6-MP) and Azathioprine (AZA) in adult IBD patients. Am. J. Gastroenterol. 2007, 102, 2488–2494. [Google Scholar] [CrossRef]
- Van Laar, J.M. Chapter 62—Immunosuppressive Drugs. In Kelley and Firestein’s Textbook of Rheumatology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 983–998.e4. [Google Scholar]
- Ozpolat, B.; Sood, A.K.; Lopez-Berestein, G. Liposomal siRNA nanocarriers for cancer therapy. Adv. Drug Deliv. Rev. 2014, 66, 110–116. [Google Scholar] [CrossRef]
- Ahmed, T.A. Development of rosuvastatin flexible lipid-based nanoparticles: Promising nanocarriers for improving intestinal cells cytotoxicity. BMC Pharmacol. Toxicol. 2020, 21, 14. [Google Scholar] [CrossRef]
- Ahmed, T.A. Preparation of transfersomes encapsulating sildenafil aimed for transdermal drug delivery: Plackett–Burman design and characterization. J. Liposome Res. 2015, 25, 1–10. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Alzahrani, M.M.; Sirwi, A.; Alhakamy, N.A. Study the Antifungal and Ocular Permeation of Ketoconazole from Ophthalmic Formulations Containing Trans-Ethosomes Nanoparticles. Pharmaceutics 2021, 13, 151. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Aldawsari, H. Intranasal optimized solid lipid nanoparticles loaded in situ gel for enhancing trans-mucosal delivery of simvastatin. J. Drug Deliv. Sci. Technol. 2018, 48, 499–508. [Google Scholar] [CrossRef]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Patki, M.; Vartak, R.; Jablonski, J.; Mediouni, S.; Gandhi, T.; Fu, Y.; Cetindag, E.; Dave, R.; Valente, S.T.; Patel, K. Efavirenz nanomicelles loaded vaginal film (EZ film) for preexposure prophylaxis (PrEP) of HIV. Colloids Surfaces B Biointerfaces 2020, 194, 111174. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Ma, G.; Kampf, N.; Yuan, Z.; Chen, S. Development of Long-Circulating Zwitterionic Cross-Linked Micelles for Active-Targeted Drug Delivery. Biomacromolecules 2016, 17, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Bardsley, R.; Gardner, G.; Tse, H.M.; Fraker, C.A. Optical sensor arrays designed for guided manufacture of perfluorocarbon nanoemulsions with a non-synthetic stabilizer. Acta Biomater. 2021, 136, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Thomas, C.; Ahsan, F. Dendrimers as a Carrier for Pulmonary Delivery of Enoxaparin, a Low-Molecular Weight Heparin. J. Pharm. Sci. 2007, 96, 2090–2106. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters With Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 748. [Google Scholar] [CrossRef]
- Zheng, H.; Rao, Y.; Yin, Y.; Xiong, X.; Xu, P.; Lu, B. Preparation, characterization, and in vitro drug release behavior of 6-mercaptopurine-carboxymethyl chitosan. Carbohydr. Polym. 2011, 83, 1952–1958. [Google Scholar] [CrossRef]
- Dorniani, D.; Bin Hussein, M.Z.; Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Preparation and characterization of 6-mercaptopurine-coated magnetite nanoparticles as a drug delivery system. Drug Des. Dev. Ther. 2013, 7, 1015. [Google Scholar] [CrossRef]
- Ghanbarzadeh, S.; Valizadeh, H.; Zakeri-Milani, P. Application of response surface methodology in development of sirolimus liposomes prepared by thin film hydration technique. BioImpacts 2013, 3, 75–81. [Google Scholar] [CrossRef]
- Alomrani, A.; Badran, M.; Harisa, G.I.; ALshehry, M.; Alhariri, M.; Alshamsan, A.; Alkholief, M. The use of chitosan-coated flexible liposomes as a remarkable carrier to enhance the antitumor efficacy of 5-fluorouracil against colorectal cancer. Saudi Pharm. J. 2019, 27, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A. Study the pharmacokinetics, pharmacodynamics and hepatoprotective activity of rosuvastatin from drug loaded lyophilized orodispersible tablets containing transfersomes nanoparticles. J. Drug Deliv. Sci. Technol. 2021, 63, 102489. [Google Scholar] [CrossRef]
- Ong, S.G.M.; Ming, L.C.; Lee, K.S.; Yuen, K.H. Influence of the Encapsulation Efficiency and Size of Liposome on the Oral Bioavailability of Griseofulvin-Loaded Liposomes. Pharmaceutics 2016, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Rudra, A.; Deepa, R.M.; Ghosh, M.K.; Ghosh, S.; Mukherjee, B. Doxorubicin-loaded phosphatidylethanolamine-conjugated nanoliposomes: In vitro characterization and their accumulation in liver, kidneys, and lungs in rats. Int. J. Nanomed. 2010, 5, 811–823. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.M.; Elashkar, A.A.; El-Kassas, H.Y.; Salim, E.I. Methotrexate loaded on magnetite iron nanoparticles coated with chitosan: Biosynthesis, characterization, and impact on human breast cancer MCF-7 cell line. Int. J. Biol. Macromol. 2018, 120, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Saneja, A.; Panda, A.K. An Annexin V-FITC—Propidium Iodide-Based Method for Detecting Apoptosis in a Non-Small Cell Lung Cancer Cell Line. Methods Mol. Biol. 2021, 2279, 213–223. [Google Scholar] [CrossRef]
- Fried, J.; Perez, A.G.; Clarkson, B.D. Flow cytofluorometric analysis of cell cycle distributions using propidium iodide: Properties of the method and mathematical analysis of the data. J. Cell Biol. 1976, 71, 172–181. [Google Scholar] [CrossRef]
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymers 2021, 13, 1027. [Google Scholar] [CrossRef]
- Voinea, M.; Simionescu, M. Designing of “intelligent” liposomes for efficient delivery of drugs. J. Cell. Mol. Med. 2002, 6, 465–474. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Sinani, V.A.; Bahng, J.H.; Kam, N.W.S.; Lee, J.; Kotov, N.A. Gold nanoparticles enhance the anti-leukemia action of a 6-mercaptopurine chemotherapeutic agent. Langmuir 2008, 24, 568–574. [Google Scholar] [CrossRef]
- Zou, Y.; Mei, D.; Yuan, J.; Han, J.; Xu, J.; Sun, N.; He, H.; Yang, C.; Zhao, L. Preparation, Characterization, Pharmacokinetic, and Therapeutic Potential of Novel 6-Mercaptopurine-Loaded Oral Nanomedicines for Acute Lymphoblastic Leukemia. Int. J. Nanomed. 2021, 16, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, S.A.; McGarrity, S.; Sahota, K.; Berry, P.; Redfern, C.P.F. Three Faces of Mercaptopurine Cytotoxicity In Vitro: Methylation, Nucleotide Homeostasis, and Deoxythioguanosine in DNA. Drug Metab. Dispos. 2018, 46, 1191–1199. [Google Scholar] [CrossRef]
- Karran, P. Thiopurines, DNA damage, DNA repair and therapy-related cancer. Br. Med. Bull. 2006, 79, 153–170. [Google Scholar] [CrossRef]
- Yan, T.; Berry, S.E.; Desai, A.B.; Kinsella, T.J. DNA Mismatch Repair (MMR) Mediates 6-Thioguanine Genotoxicity by Introducing Single-strand Breaks to Signal a G2-M Arrest in MMR-proficient RKO Cells1. Clin. Cancer Res. 2003, 9, 2327–2334. [Google Scholar] [PubMed]
- Plesca, D.; Mazumder, S.; Almasan, A. DNA Damage Response and Apoptosis. Methods Enzymol. 2008, 446, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Zannini, L.; Delia, D.; Buscemi, G. CHK2 kinase in the DNA damage response and beyond. J. Mol. Cell Biol. 2014, 6, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xie, Y.; Wang, F. Application and Analysis of 6-Mercaptopurine Nanomedicine in the Treatment of Leukemia. J. Nanosci. Nanotechnol. 2021, 21, 1001–1007. [Google Scholar] [CrossRef] [PubMed]


| Run | Ingredients | EE (%) | Drug Loading (%) | Size (nm) | PDI | ZP (mV) | |
|---|---|---|---|---|---|---|---|
| Drug | Stearyl Amine | ||||||
| F1 | √ | √ | 42.11 ± 2.07 | 3.56 ± 0.07 | 574.67 ± 37.29 | 0.543 ± 0.031 | +12.4 ± 1.31 |
| F2 | √ | - | 94.44 ± 0.56 | 9.42 ± 0.21 | 660.47 ± 44.32 | 0.667 ± 0.046 | −0.48 ± 0.13 |
| F3 | - | √ | - | - | 429.47 ± 24.79 | 0.465 ± 0.052 | +9.61 ± 0.69 |
| F4 | - | - | - | - | 538.80 ± 49.73 | 0.711 ± 0.049 | −0.74 ± 0.24 |
| Cells | IC50 (Range/Value) | Pure Drug | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|---|
| HepG2 | IC50 range Value of IC50 (µg/mL) | 8.0–11.6 9.6 | 2.8–7.6 4.6 | 8.9–12.1 10.35 | 14.18–19.68 16.7 | 27.8–41.4 33.9 |
| HCT116 | IC50 range Value of IC50 (µg/mL) | 14.0–19.9 16.7 | 4.0–5.6 4.70 | 12.4–17.2 14.6 | 13.7–18.9 16.12 | 32.1–42.9 37.1 |
| MCF7 | IC50 range Value of IC50 (µg/mL) | 10.7–15.2 12.8 | 3.8–6.4 4.9 | 10.3–13.2 11.6 | 19.0–24.4 21.5 | 36.0–48.9 41.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamal, A.; Asseri, A.H.; Ali, E.M.M.; El-Gowily, A.H.; Khan, M.I.; Hosawi, S.; Alsolami, R.; Ahmed, T.A. Preparation of 6-Mercaptopurine Loaded Liposomal Formulation for Enhanced Cytotoxic Response in Cancer Cells. Nanomaterials 2022, 12, 4029. https://doi.org/10.3390/nano12224029
Jamal A, Asseri AH, Ali EMM, El-Gowily AH, Khan MI, Hosawi S, Alsolami R, Ahmed TA. Preparation of 6-Mercaptopurine Loaded Liposomal Formulation for Enhanced Cytotoxic Response in Cancer Cells. Nanomaterials. 2022; 12(22):4029. https://doi.org/10.3390/nano12224029
Chicago/Turabian StyleJamal, Alam, Amer H. Asseri, Ehab M. M. Ali, Afnan H. El-Gowily, Mohamed Imran Khan, Salman Hosawi, Reem Alsolami, and Tarek A. Ahmed. 2022. "Preparation of 6-Mercaptopurine Loaded Liposomal Formulation for Enhanced Cytotoxic Response in Cancer Cells" Nanomaterials 12, no. 22: 4029. https://doi.org/10.3390/nano12224029





