Valorization of Sugarcane By-Products through Synthesis of Biogenic Amorphous Silica Microspheres for Sustainable Cosmetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of SCBA
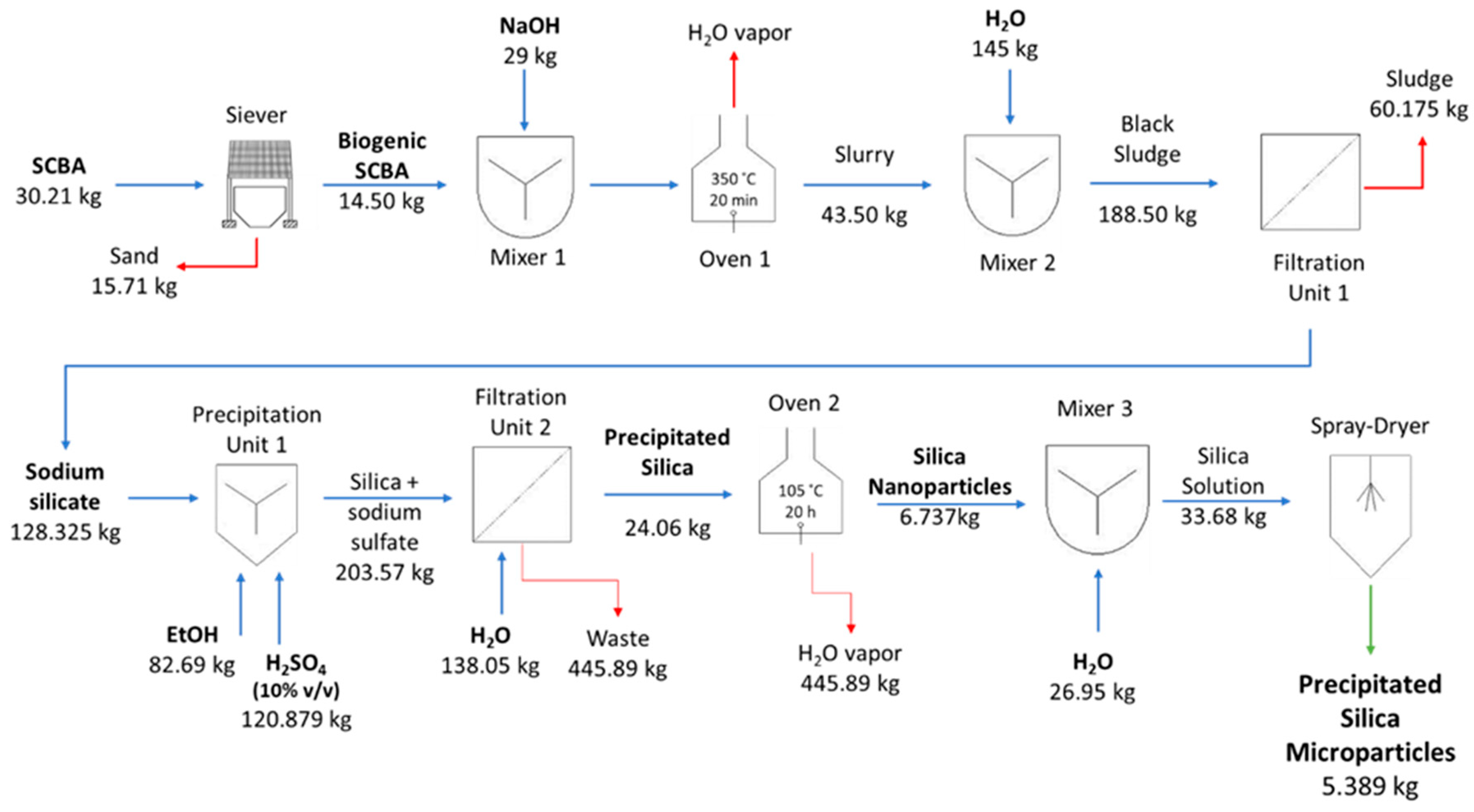
2.3. Production of Biogenic Silica Microparticles
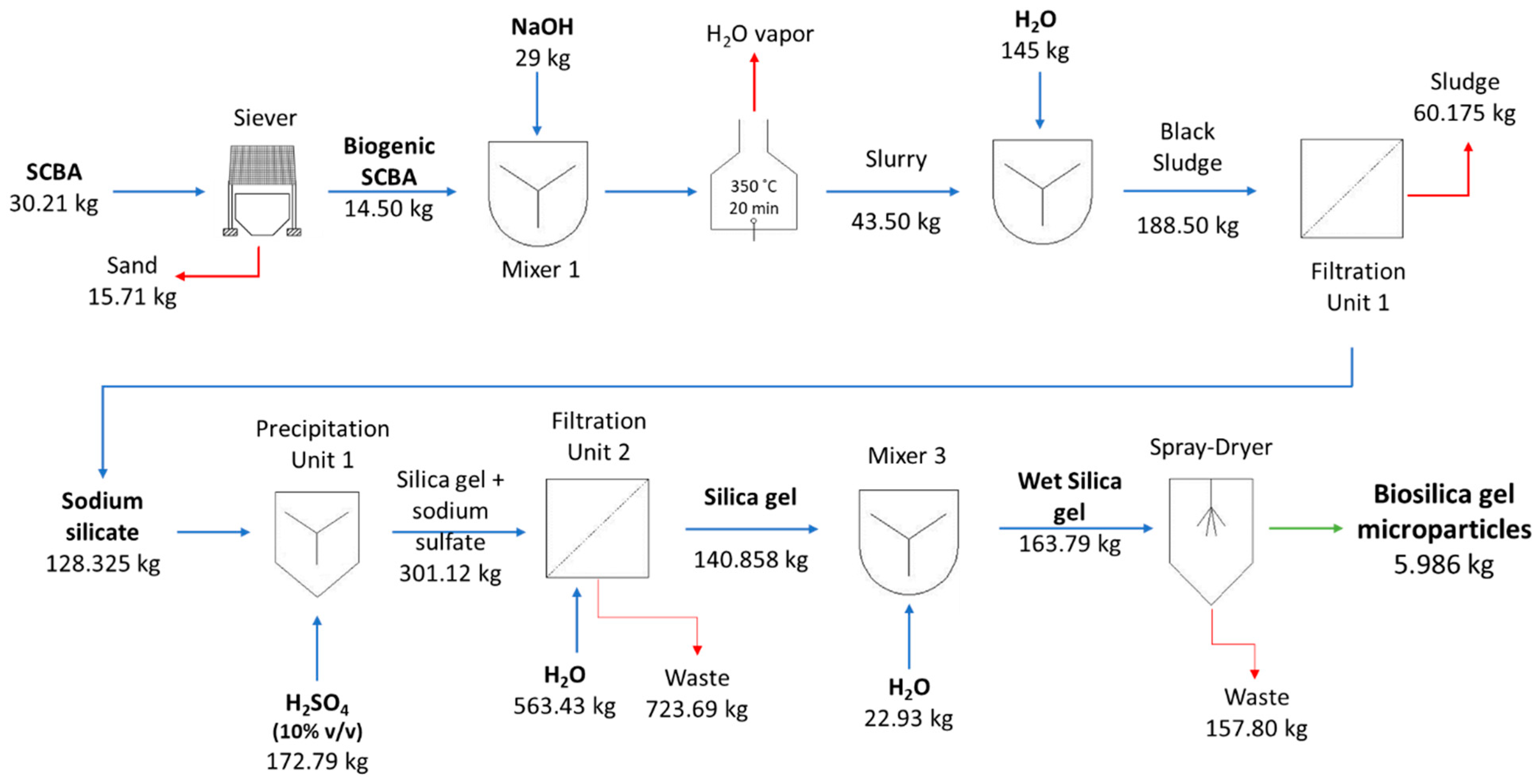
2.3.1. Production of Na2SiO3 from SCBA
2.3.2. Production of Silica Gel Microparticles
2.3.3. Production of Precipitated Silica Microparticles
2.4. Characterization of Silica Microparticles
2.4.1. Chemical Composition
2.4.2. Powder X-ray Diffraction
2.4.3. Fourier Transform Infrared Spectroscopy
2.4.4. Morphology
2.4.5. Particle Size Distribution
2.5. Oil and Water Absorption Capacity
2.6. Production Cost Estimation
2.7. Statistical Analysis
3. Results
3.1. SCBA Characterization
3.2. Characterization of Biogenic Silica Prototypes
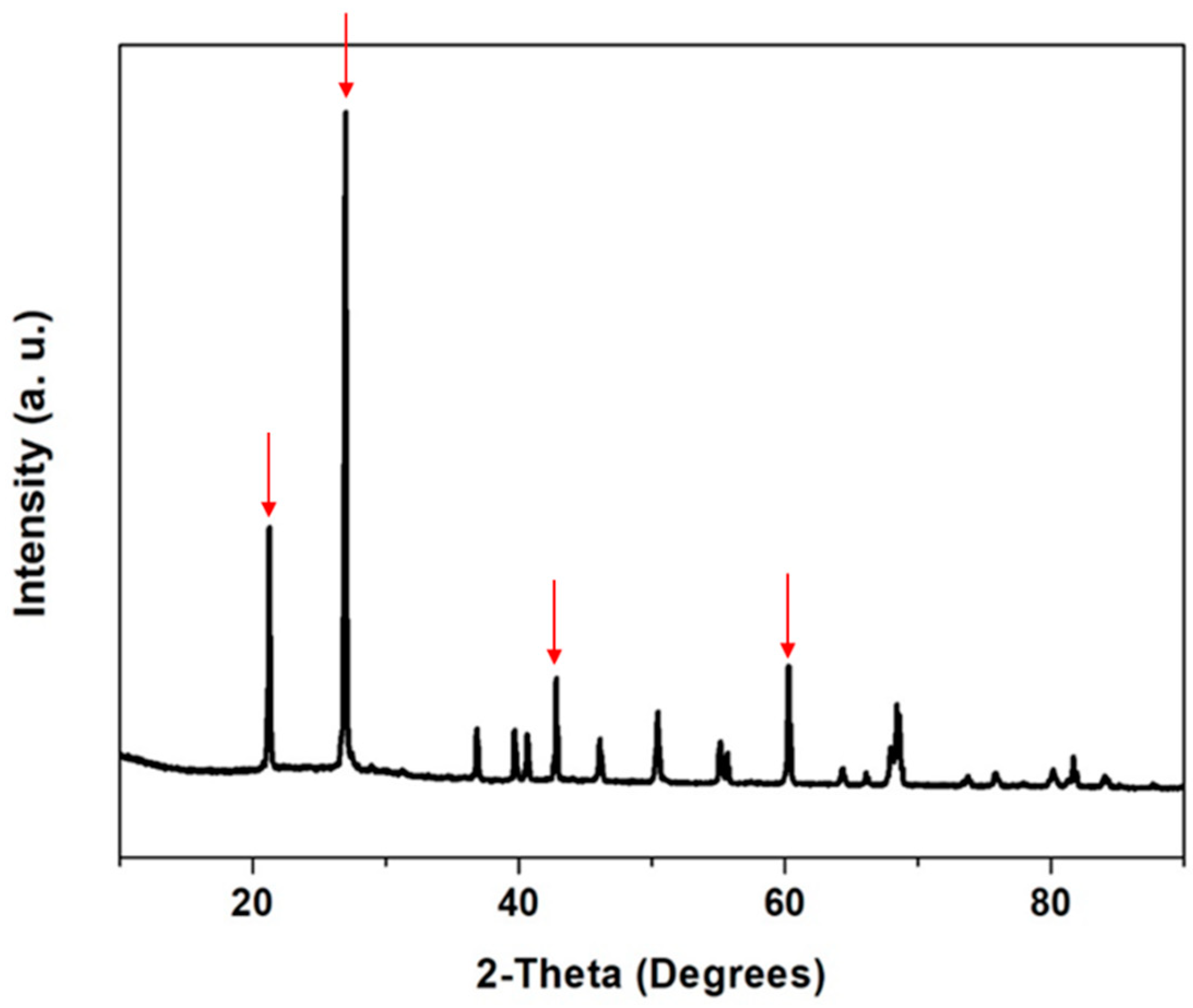
3.2.1. Powder X-ray Diffraction
3.2.2. FT-IR Spectroscopy
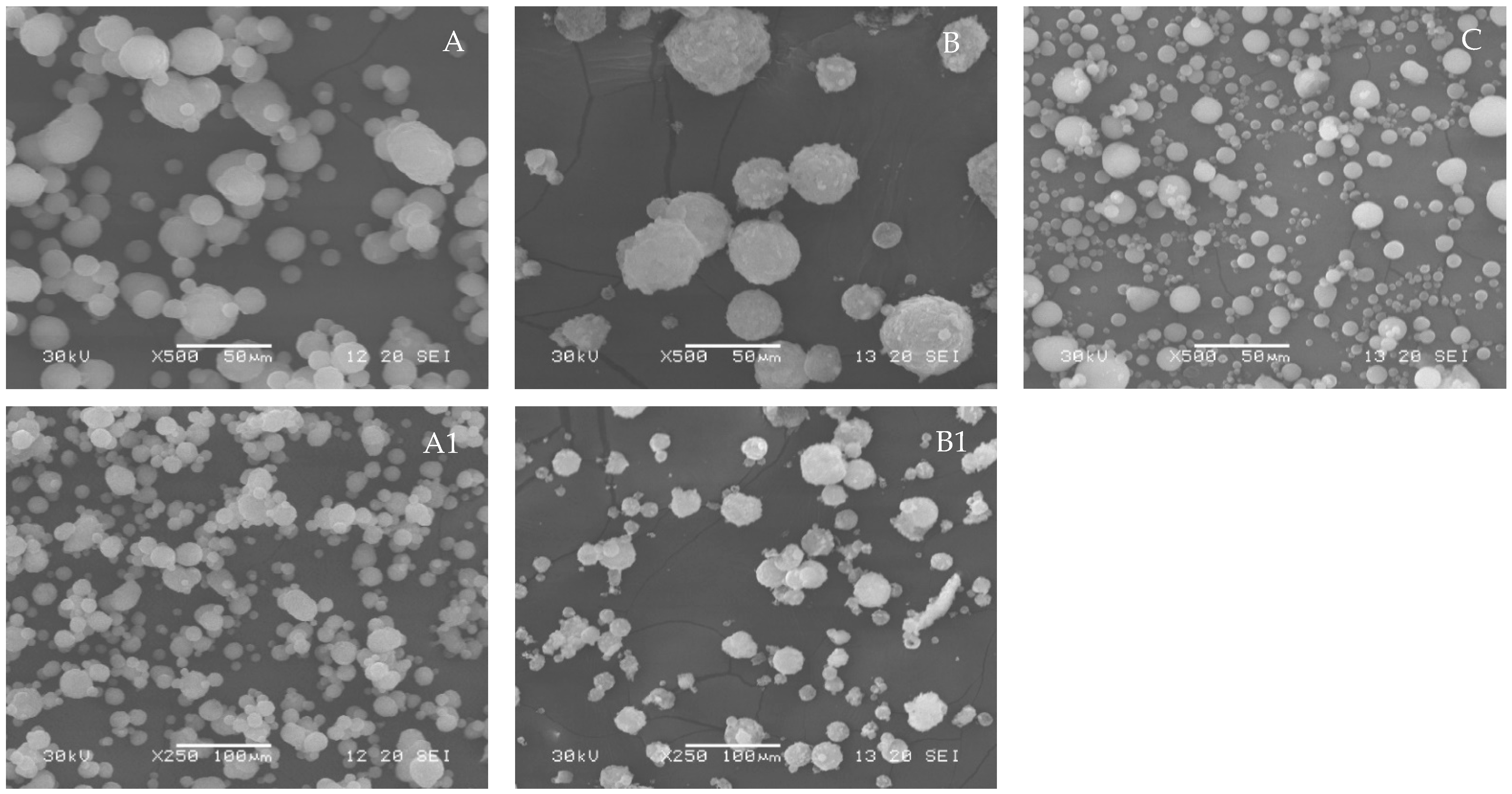
3.2.3. Particle Morphology
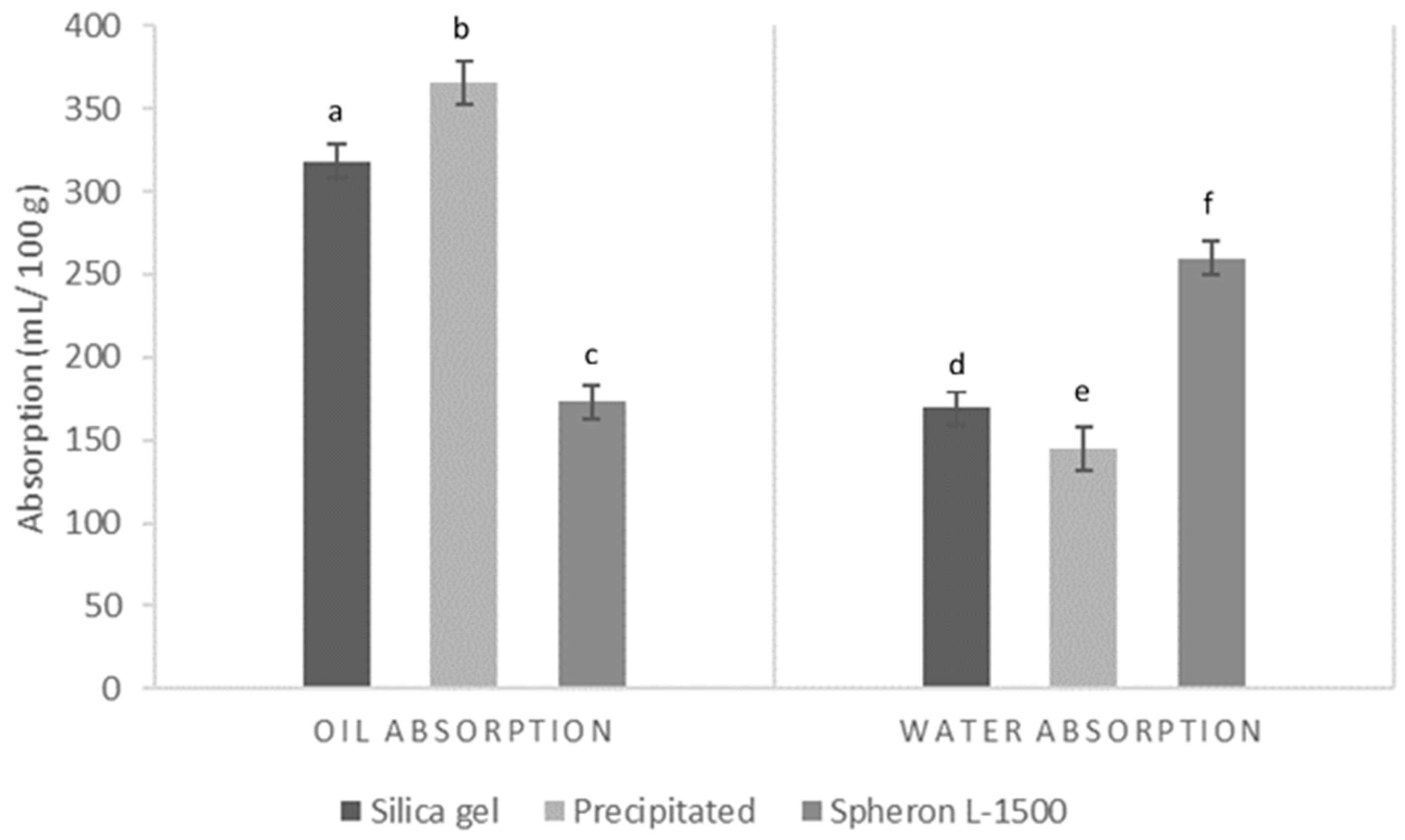
3.3. Oil and Water Absorption Capacities
3.4. Production Cost Estimation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira-Leitão, V.; Gottschalk, L.M.F.; Ferrara, M.A.; Nepomuceno, A.L.; Molinari, H.B.C.; Bon, E.P.S. Biomass Residues in Brazil: Availability and Potential Uses. Waste Biomass Valoriz. 2010, 1, 65–75. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Batra, V.S. Valorization of solid waste in sugar factories with possible applications in India: A review. J. Environ. Manag. 2011, 92, 2886–2891. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, T.; Gao, S.; Yang, Z.; Wu, N. Characteristics and Applications of Sugar Cane Bagasse Ash Waste in Cementitious Materials. Materials 2019, 12, 39. [Google Scholar] [CrossRef] [Green Version]
- Le Blond, J.S.; Horwell, C.J.; Williamson, B.J.; Oppenheimer, C. Generation of crystalline silica from sugarcane burning. JEM 2010, 12, 1459–1470. [Google Scholar] [CrossRef]
- Norsuraya, S.; Fazlena, H.; Norhasyimi, R. Sugarcane Bagasse as renewable source of silica to synthesize Santa Barbara Amorphous-15 (SBA-15). Procedia Eng. 2016, 148, 839–846. [Google Scholar] [CrossRef] [Green Version]
- Almeida, F.C.R.; Sales, A.; Moretti, J.P.; Mendes, P.C.D. Sugarcane bagasse ash sand (SBAS): Brazilian agroindustrial by-product for use in mortar. Constr. Build Mater. 2015, 82, 31–38. [Google Scholar] [CrossRef]
- Misran, H.; Singh, R.; Begum, S.; Yarmo, M.A. Processing of mesoporous silica materials (MCM-41) from coal fly ash. J. Mater. Process. Technol. 2007, 186, 8–13. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Reinosa, J.J.; de la Rubia, M.A.; Reyes, E.; Alonso Peralto, F.; Fernández, J.F. Critical aspects in the handling of reactive silica in cementitious materials: Effectiveness of rice husk ash vs. nano-silica in mortar dosage. Constr. Build Mater. 2019, 223, 360–367. [Google Scholar] [CrossRef]
- Megawati, D.; Putri, R.; Fianti, O.; Simalando, A.F.; Akhir, A.E. Synthesis of Silica Powder from Sugar Cane Bagasse Ash and Its Application as Adsorbent in Adsorptive-distillation of Ethanol-water Solution. MATEC Web Conf. 2020, 237, 02002. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Eco-production of silica from sugarcane bagasse ash for use as a photochromic pigment filler. Sci. Rep. 2020, 10, 9890. [Google Scholar] [CrossRef]
- Affandi, S.; Setyawan, H.; Winardi, S.; Purwanto, A.; Balgis, R. A facile method for production of high-purity silica xerogels from bagasse ash. Adv. Powder Technol. 2009, 20, 468–472. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Review. Amended Safety Assessment of Silica and Silicates as Used in Cosmetics. Available online: https://www.cir-safety.org/sites/default/files/Silica%20&%20Silicates.pdf (accessed on 27 December 2018).
- Amberg, N.; Fogarassy, C. Green Consumer Behavior in the Cosmetics Market. Resources 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Statista. Demand for “Natural or Organic Ingredients” or “Sustainable” Beauty Products Worldwide as of September 2019. Available online: https://www.statista.com (accessed on 9 August 2021).
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Solids and Moisture in Flour, Method Number 925.10. In Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Isah, S.; Oshodi, A.A.; Atasie, V.N. Physicochemical properties of cross linked acha (digitaria exilis) starch with citric acid. Chem. Int. 2017, 3, 150–157. [Google Scholar]
- Green, D.W.; Perry, R.H. (Eds.) Perry’s Chemical Engineers’ Handbook, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Eurostat. Electricity Prices for Non-Household Consumers. Available online: https://ec.europa.eu/eurostat/ (accessed on 15 February 2020).
- Alves, R.H.; Reis, T.; Rovani, S.; Fungaro, D. Green Synthesis and Characterization of Biosilica Produced from Sugarcane Waste Ash. Hindawi J. Chem. 2017, 2017, 6129035. [Google Scholar] [CrossRef] [Green Version]
- Biswas, R.K.; Khan, P.; Mukherjee, S.; Mukhopadhyay, A.K.; Ghosh, J.; Muraleedharan, K. Study of short range structure of amorphous Silica from PDF using Ag radiation in laboratory XRD system, RAMAN and NEXAFS. J. Non-Cryst. Solids 2018, 488, 1–9. [Google Scholar] [CrossRef]
- Kalapathy, U.; Proctor, A.; Shultz, J. A simple method for production of pure silica from rice hull ash. Bioresour. Technol. 2000, 73, 257–262. [Google Scholar] [CrossRef]
- Rangaraj, S.; Venkatachalam, R. A lucrative chemical processing of bamboo leaf biomass to synthesize biocompatible amorphous silica nanoparticles of biomedical importance. Appl. Nanosci. 2017, 7, 145–153. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food & Drug Administration (FDA). Available online: http://www.fda.gov/cosmetics (accessed on 22 June 2021).
- Setyawan, N.; Hoerudin; Wulanawati, A. Simple extraction of silica nanoparticles from rice husk using technical grade solvent: Effect of volume and concentration. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Kuta, Bali, Indonesia, 29–31 August 2018; Volume 309. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Pomastowski, P.; Hornowska, M.; Król, A.; Rafinska, K.; Buszewski, B. Naturally organic functionalized 3D biosilica from diatom microalgae. Mater. Des. 2017, 132, 22–29. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R′′Si(OR′)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Kortesuo, P.; Ahola, M.; Kangas, M.; Jokinen, M.; Leino, T.; Vuorilehto, L.; Laakso, S.; Kiesvaara, J.; Yli-Urpo, A.; Marvola, M. Effect of synthesis parameter of the sol-gel processed spray-dried silica gel microparticles on the release rate of dexmedetomiidine. Biomaterials 2002, 23, 2795–2801. [Google Scholar] [CrossRef]
- SCCS (Scientific Committee on Consumer Safety). Guidance on the Safety Assessment of Nanomaterials in Cosmetics, 30–31 October 2019, SCCS/1611/19. Available online: https://health.ec.europa.eu/system/files/2020-10/sccs_o_233_0.pdf (accessed on 20 July 2022).
- Bamford, E.; Grahn, A.; Arhammar, C.; Ajaxon, I.; Anneren, C. Mesoporous magnesium carbonate for use in powder cosmetics. Int. J. Cosmet. Sci. 2021, 43, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; McLaughlin, E.; Pfeffer, R.; Lin, Y.S. Adsorption of oils from pure liquid and oil oil-water emulsion on hydrophobic silica aerogels. Sep. Purif. Technol. 2012, 99, 28–35. [Google Scholar] [CrossRef]
- Jesionowski, T. Preparation of colloidal silica from sodium metasilicate solution and sulphuric acid in emulsion medium. Colloids Surf. 2001, 190, 153–165. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.; Hilonga, A.; Kim, H.T. Production of low-density sodium silicate-based hydrophobic silica aerogel beads by a novel fast gelation process and ambient pressure drying process. Solid State Sci. 2010, 12, 911–918. [Google Scholar] [CrossRef]
- Chevallier, Y. Silica with a High Oil Absorption Capability and a Controlled Primary Structure and Process for the Production Thereof. U.S. Patent No. 4,842,838, 27 June 1989. Available online: https://patentimages.stoage.gooleapis.com/7e/de/bc/8375bab7a4dc72/US4842838.pdf (accessed on 20 July 2022).
- Nishi, S. Highly Oil Absorbing Amorphous Silica Particles. U.S. Patent No. 2007/0125269 A1, 7 June 2007. Available online: https://patentimages.storage.googleapis.com/96/78/2b/195ac3d646c89b/US20070125269A1.pdf (accessed on 20 July 2022).


| Energy price (EUR/kW) Water (EUR/kg) | 0.14 1 |
| 0.002 2 | |
| Sodium hydroxide (EUR/kg) Absolute ethanol (EUR/kg) Sulfuric acid (EUR/kg) | 0.34 0.48 0.25 |
| Sugarcane ash cost | Not considered |
| Overheads | 80% of chemicals and energy costs |
| Element | Concentration (g.100 g−1) | Element | Concentration (ppm) |
|---|---|---|---|
| Silicon | 70.26 ± 0.03 | Manganese | 843.7 ± 2.38 |
| Potassium | 7.23 ± 0.023 | Sodium | 248 ± 0.7 |
| Iron | 1.72 ± 0.005 | Aluminum | 121.0 ± 0.34 |
| Calcium | 1.64 ± 0.005 | Zinc | 94.98 ± 0.27 |
| Magnesium | 0.83 ± 0.002 | Boron | 50.65 ± 0.14 |
| Phosphorous | 0.44 ± 0.001 | Copper | 44.44 ± 0.13 |
| Nickel | 7.8 ± 0.02 | ||
| Lead | 3.54 ± 0.01 | ||
| Cadmium | 2.29 ± 0.01 |
| D10 | D50 | D90 | Span | |
|---|---|---|---|---|
| Silica gel | 2.4 μm | 3.9 μm | 8.1 μm | 1.46 |
| Precipitated silica | 4.9 μm | 6.9 μm | 13.0 μm | 1.17 |
| Spheron L-1500 | 3.1 μm | 4.8 μm | 9.3 μm | 1.29 |
| Silica Gel (EUR/kg Silica) | Precipitated Silica (EUR/kg Silica) | |
|---|---|---|
| Chemicals Sodium Hydroxide | 3.18 1.65 | 10.30 1.83 |
| Sulfuric acid | 1.25 | 0.95 |
| Absolute ethanol | - | 7.36 |
| Water | 0.29 | 0.13 |
| Energy Siever Mixer 1 + Oven 1 + Mixer 2 | 2.87 0.002 1.27 | 5.79 0.002 1.41 |
| Filtration Unit 1 Precipitation Unit 1 Filtration Unit 2 Oven Mixer 3 Spray-Dryer | 0.05 0.13 0.09 - 0.04 1.30 | |
| Overheads | 4.84 | 12.86 |
| Total Production Cost | 10.89 | 28.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.R.; Capeto, A.P.; Pereira, C.F.; Pedrosa, S.S.; Mota, I.F.; Burgal, J.d.S.; Pintado, A.I.; Pintado, M.E.; Oliveira, C.S.S.; Costa, P.; et al. Valorization of Sugarcane By-Products through Synthesis of Biogenic Amorphous Silica Microspheres for Sustainable Cosmetics. Nanomaterials 2022, 12, 4201. https://doi.org/10.3390/nano12234201
Costa JR, Capeto AP, Pereira CF, Pedrosa SS, Mota IF, Burgal JdS, Pintado AI, Pintado ME, Oliveira CSS, Costa P, et al. Valorization of Sugarcane By-Products through Synthesis of Biogenic Amorphous Silica Microspheres for Sustainable Cosmetics. Nanomaterials. 2022; 12(23):4201. https://doi.org/10.3390/nano12234201
Chicago/Turabian StyleCosta, Joana R., Ana Paula Capeto, Carla F. Pereira, Sílvia S. Pedrosa, Inês F. Mota, João da Silva Burgal, Ana I. Pintado, Manuela E. Pintado, Catarina S. S. Oliveira, Patrícia Costa, and et al. 2022. "Valorization of Sugarcane By-Products through Synthesis of Biogenic Amorphous Silica Microspheres for Sustainable Cosmetics" Nanomaterials 12, no. 23: 4201. https://doi.org/10.3390/nano12234201







