Fabrication and Optical Properties of Transparent P(VDF-TrFE) Ultrathin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Films Preparation
2.3. Characterization
3. Results and Discussions
3.1. Deposition Mechanism of Ultrathin Films
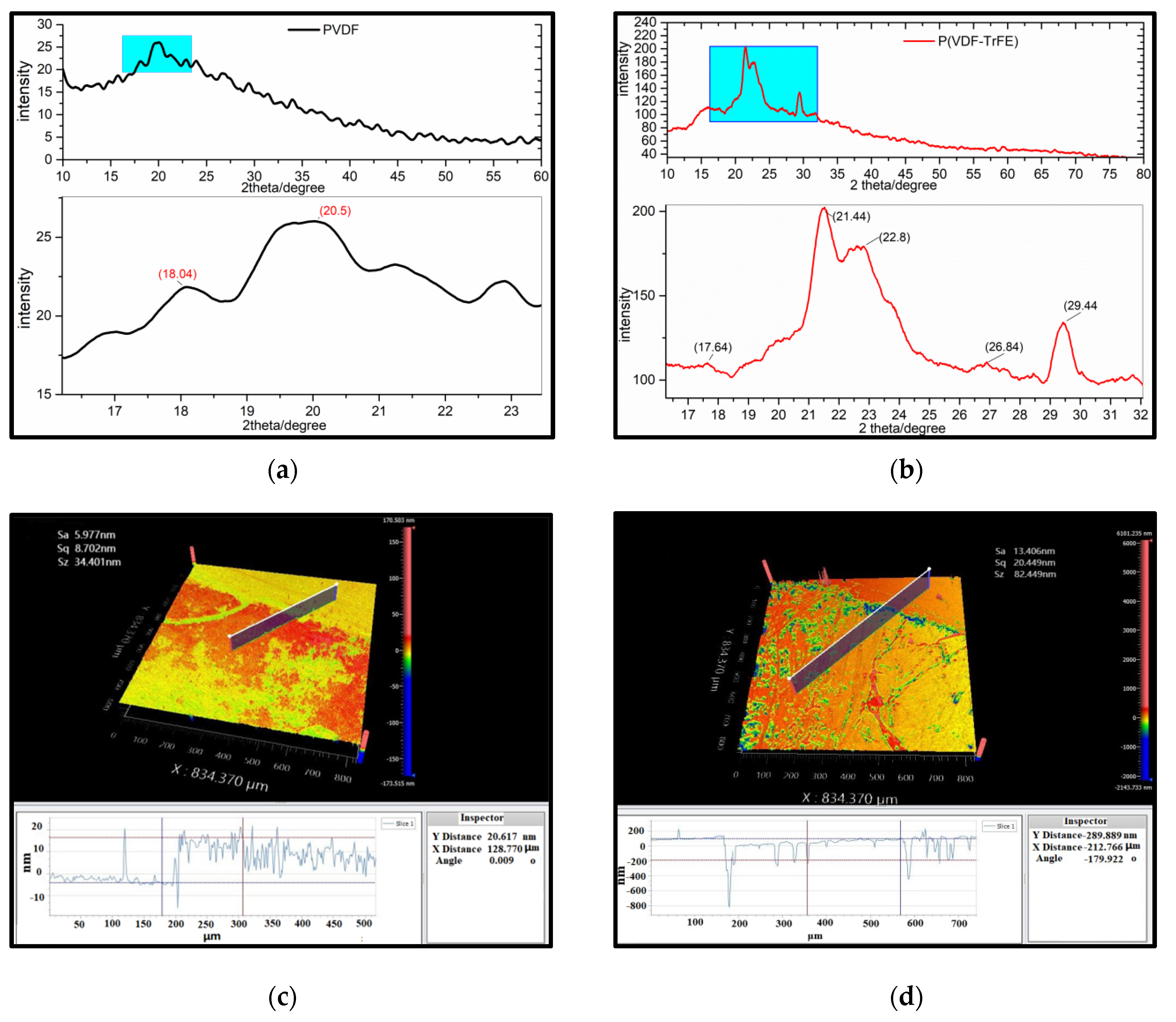
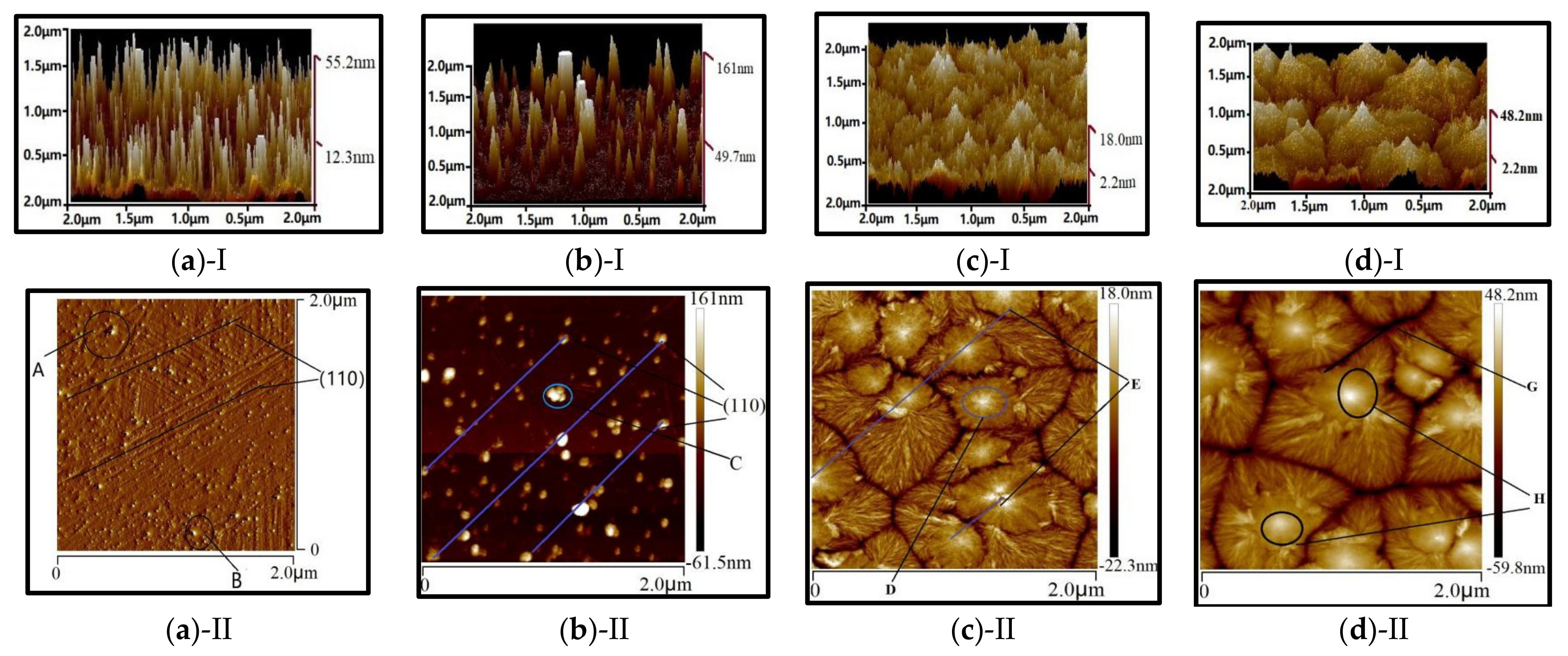
3.2. Structure and Surface Morphology
3.3. Optical Properties
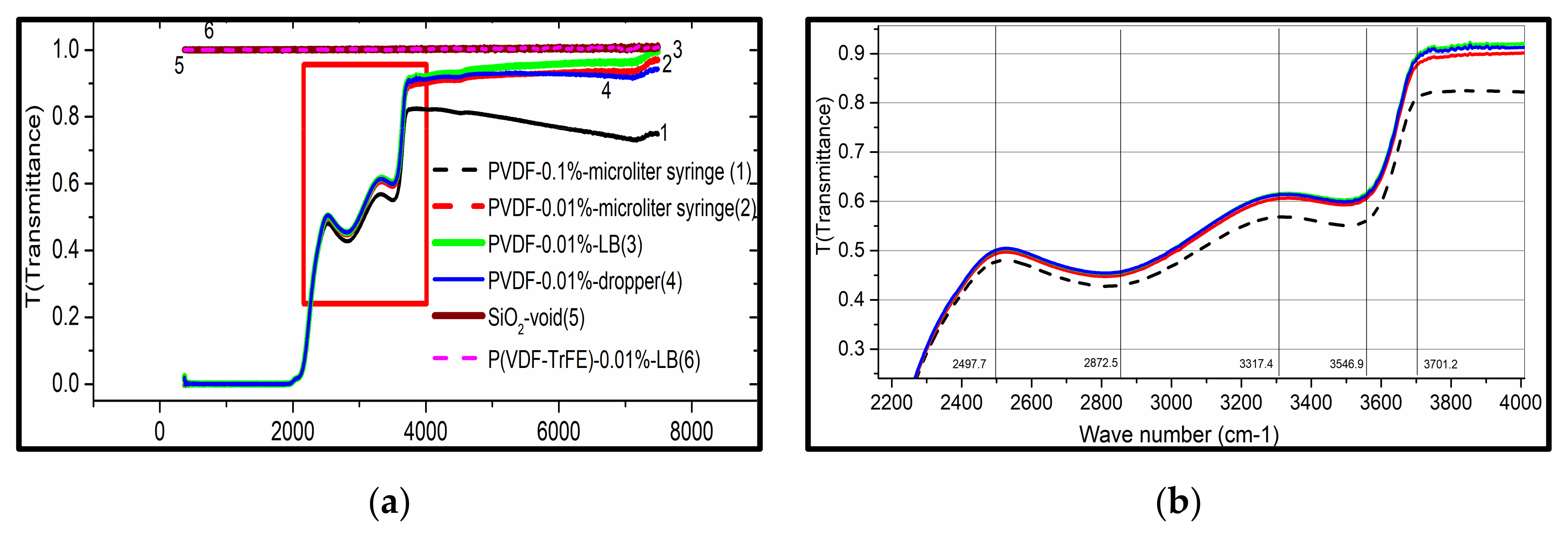
3.3.1. FTIR Spectroscopy
3.3.2. UV-Vis Spectroscopy
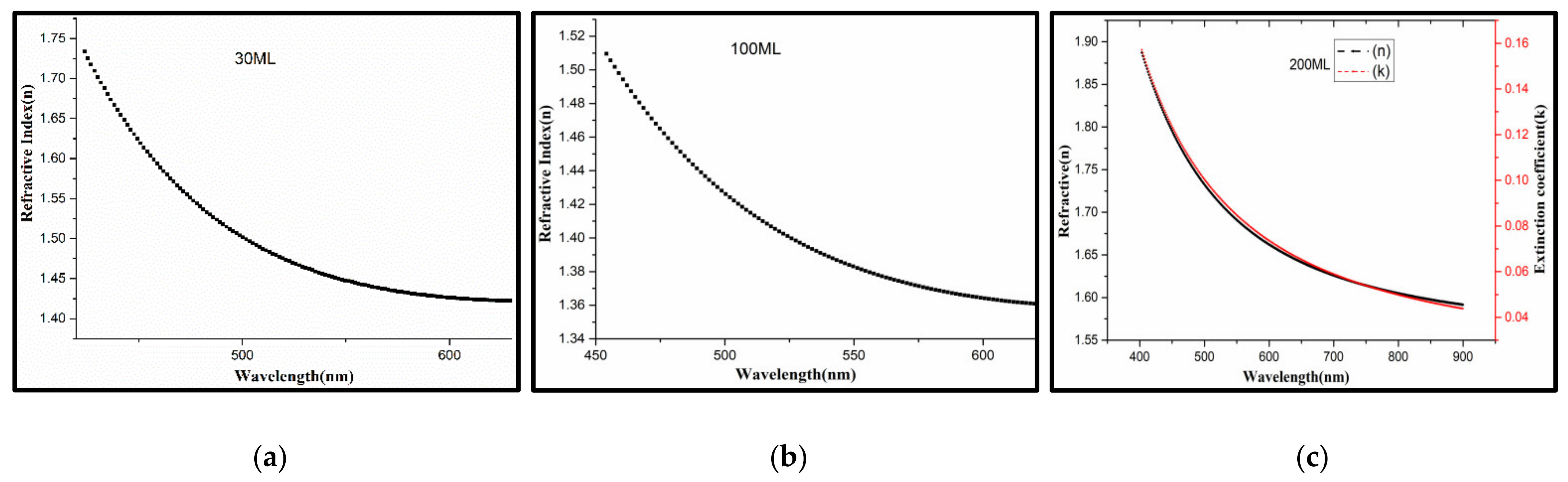
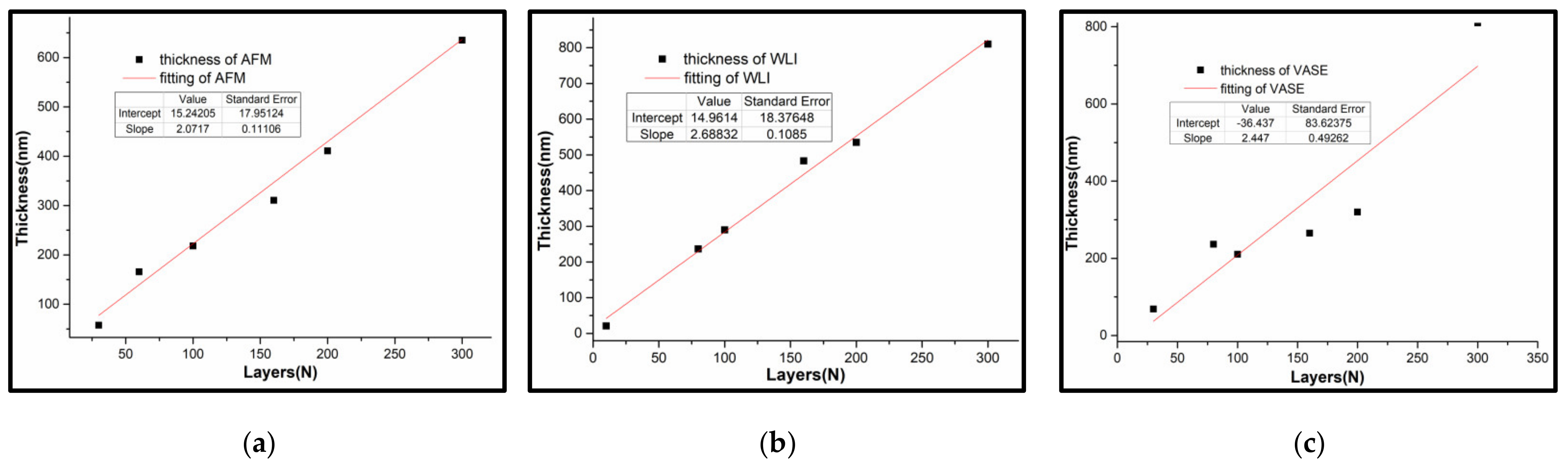
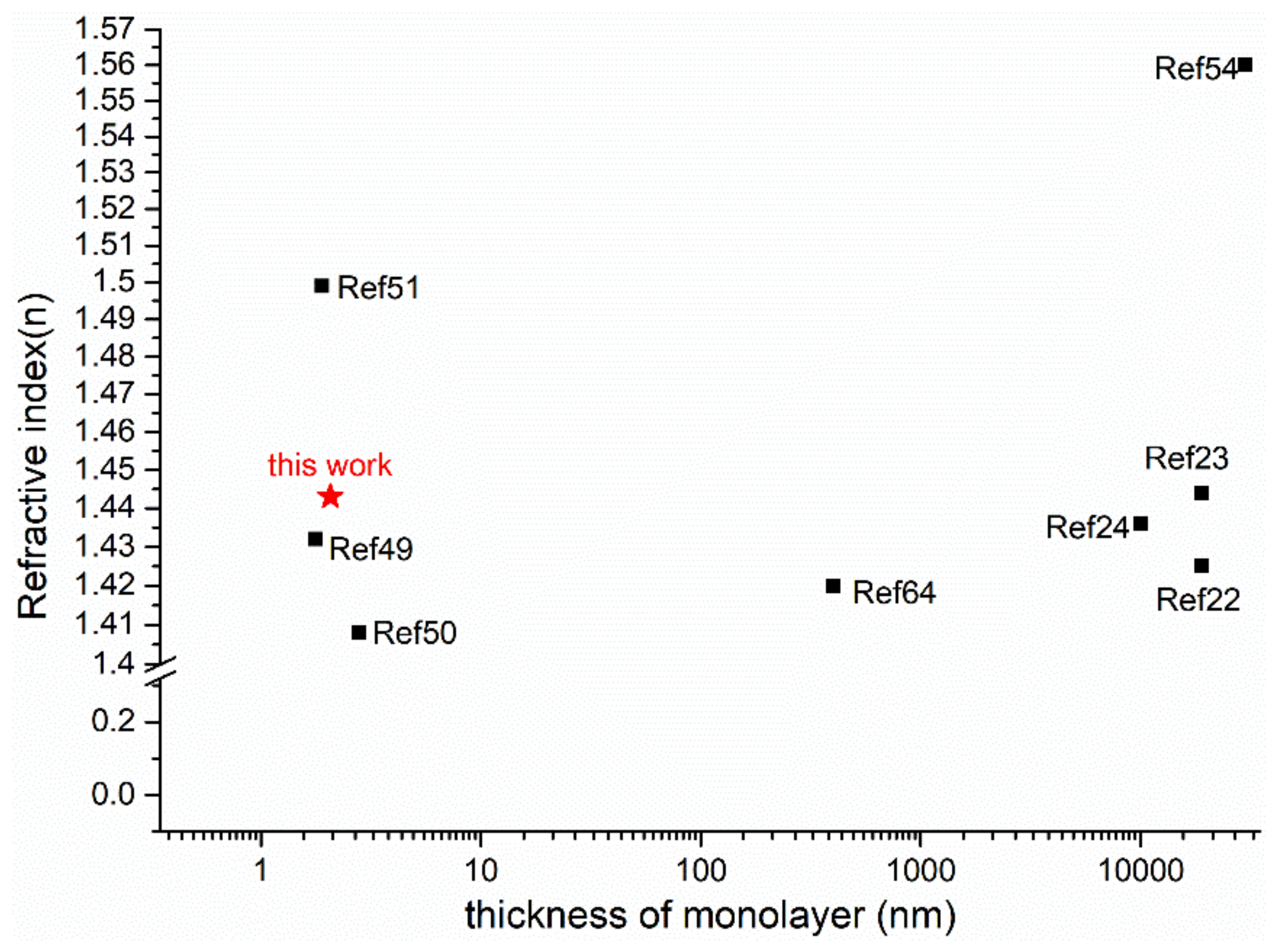
3.3.3. Optical Constants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, C.L.; Cao, X.L.; Wu, X.J.; He, Q.Y.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 9, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.R.; Ryu, H.; Xu, K.; Liu, J.L.; Cai, Y.L.; Yan, Y.L.; Zhu, W.J. Nanoscale Devices Based on Two-dimensional Materials and Ferroelectric Materials. In Proceedings of the 14th IEEE International Conference on Solid-State and Integrated Circuit Technology (ICSICT), Qingdao, China, 31 October 2018. [Google Scholar]
- Gong, W.; Tao, L.; Sun, X.C.; Pu, L.; Xu, Y.S.; Hua, J.G.; Yu, Y.H.; Li, S.X.; Dai, Y.Z.; Song, X.Y. Flexible pressure sensor based on PVDF nanofiber. Sens. Actuators A Phys. 2018, 280, 319–325. [Google Scholar] [CrossRef]
- Sandilands, L.J.; Reijnders, A.A.; Su, A.H.; Baydina, V.; Xu, Z.; Yang, A.; Gu, G.; Pedersen, T.; Borondics, F.; Burch, K.S. Two-dimensional atomic crystals. Phys. Rev. B. 2014, 90, 081402. [Google Scholar] [CrossRef] [Green Version]
- King, D.; Tuna, F.; Mcinnes, E.; Mcmaster, J.; Lewis, W.; Blake, A.; Liddle, S. Isolation and characterization of a uranium(vi)-nitride triple bond. Nat. Chem. 2013, 5, 482–488. [Google Scholar] [CrossRef]
- Koski, K.J.; Yi, C. The New Skinny in Two-Dimensional Nanomaterials. ACS Nano 2013, 7, 3739–3743. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z.; Lin, D.B.; Liu, W.G.; Zhou, X.; Ge, S.B.; Su, Y.M.; Peng, C.; Zhang, L. Effects of particle size of dielectric fillers on the output performance of piezoelectric and triboelectric nanogenerators. J. Adv. Ceram. 2021, 10, 991–1000. [Google Scholar] [CrossRef]
- Zhou, S.; Lin, D.B.; Su, Y.M.; Zhang, L.; Liu, W.G. Enhanced dielectric, ferroelectric, and optical properties in rare earth elements doped PMN-PT thin films. J. Adv. Ceram. 2021, 10, 98–107. [Google Scholar] [CrossRef]
- Cai, C.L.; Zhang, D.Q.; Liu, W.G.; Wang, J.; Zhou, S.; Su, Y.M.; Sun, X.P.; Lin, D.B. Synthesis, Giant Dielectric, and Pyroelectric Response of [001]-Oriented Pr3+ Doped Pb (Mg1/3Nb2/3) O3-PbTiO3 Ferroelectric Nano-Films Grown on Si Substrates. Materials 2018, 11, 2392. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, F.; Fu, W.; Fang, Z.; Zhou, W.; Liu, Z. Two-dimensional heterostructures: Fabrication, characterization, and application. Nanoscale 2014, 6, 12250–12272. [Google Scholar] [CrossRef]
- Kawai, H. The piezoelectricity of poly (vinylidene fluoride). Jpn. J. Appl. Phys. 1969, 8, 975. [Google Scholar] [CrossRef]
- Kepler, R.G.; Anderson, R. Ferroelectric polymers. Adv. Phys. 1992, 41, 1–57. [Google Scholar] [CrossRef]
- Ruan, L.; Yao, X.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Properties and applications of the β phase poly (vinylidene fluoride). Polymers 2018, 10, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, C.; Costa, C.M.; Correia, D.M.; Nunes-Pereira, J.; Oliveira, J.; Martins, P.; Goncalves, R.; Cardoso, V.F.; Lanceros-Mendez, S. Electroactive poly (vinylidene fluoride)-based structures for advanced applications. Nat. Protoc. 2018, 13, 681–704. [Google Scholar] [CrossRef] [PubMed]
- Doll, W.; Lando, J. The polymorphism of poly (vinylidene fluoride) IV. The structure of high-pressure-crystallized poly (vinylidene fluoride). J. Macromol. Sci. B 1970, 4, 889–896. [Google Scholar] [CrossRef]
- Lovinger, A.J. Annealing of poly (vinylidene fluoride) and formation of a fifth phase. Macromolecules 1982, 15, 40–44. [Google Scholar] [CrossRef]
- Naegele, D.; Yoon, D.; Broadhurst, M. Formation of a new crystal form (αp) of poly (vinylidene fluoride) under electric field. Macromolecules 1978, 11, 1297–1298. [Google Scholar] [CrossRef]
- Bachmann, M.; Gordon, W.; Weinhold, S.; Lando, J. The crystal structure of phase IV of poly (vinylidene fluoride). Appl. Phys. 1980, 51, 5095–5099. [Google Scholar] [CrossRef]
- Zhang, S.T.; Qi, A. Progress on the Design and Fabrication of High Performance Piezoelectric Flexible Materials Based on Polyvinylidene Fluoride. Chem. J. Chin. Univ. 2021, 42, 1114–1145. [Google Scholar] [CrossRef]
- Adohi, B.; Haidar, B.; Costa, L.; Laur, V.; Brosseau, C. Assessing the role of graphene content in the electromagnetic response of graphene polymer nanocomposites. Eur. Phys. J. B 2015, 88, 280. [Google Scholar] [CrossRef]
- Wang, T.; Peng, R.C.; Peng, W.J.; Zhou, C.; Yang, S.; Zhou, Z.Y.; Liu, M. 2-2 Type PVDF-Based Composites Interlayered by Epitaxial (111)-Oriented BTO Films for High Energy Storage Density. Adv. Funct. Mater. 2021, 2108496. [Google Scholar] [CrossRef]
- McFee, J.; Bergman, J., Jr.; Crane, G. Pyroelectric and nonlinear optical properties of poled polyvinylidene fluoride films. Ferroelectrics 1972, 3, 305–313. [Google Scholar] [CrossRef]
- Cakmak, M.; Wang, Y. The intrinsic birefringence of the α, β, and γ forms of polyvinylidene fluoride and the estimation of orientation in fibers and films. J. Appl. Polym. Sci. 1989, 37, 977–985. [Google Scholar] [CrossRef]
- Broussoux, D.; Micheron, F. Electro-optic and elasto-optic effects in polyvinylidene fluoride. J. Appl. Phys. 1980, 51, 2020–2023. [Google Scholar] [CrossRef]
- Berge, B.; Wicker, A.; Lajzerowicz, J.; Legrand, J.F. Second-Harmonic Generation of Light and Evidence of Phase Matching in Thin Films of P(VDF-TrFE) Copolymers. Europhys. Lett. 1989, 9, 657–662. [Google Scholar] [CrossRef]
- Gaabour, L.H.; Hamam, K.A. The Change of Structural, Optical and Thermal Properties of a PVDF/PVC Blend Containing ZnO Nanoparticles. Silicon 2017, 10, 1403–1409. [Google Scholar] [CrossRef]
- Mohammed, M.I. Optical properties of ZnO nanoparticles dispersed in PMMA/PVDF blend. J. Mol. Struct. 2018, 1169, 9–17. [Google Scholar] [CrossRef]
- Shanshool, H.M.; Yahaya, M.; Wan, M.M.Y.; Abdullah, I.Y. Influence of CuO nanoparticles on third order nonlinearity and optical limiting threshold of polymer/ZnO nanocomposites. Opt. Quantum. Electron. 2017, 49, 18. [Google Scholar] [CrossRef]
- Av, A.; Gweb, C.; Nd, A. Study of the optoelectronic and piezoelectric properties of ZrO2 doped PVDF from quantum chemistry calculations. Chin. J. Phys. 2020, 63, 213–219. [Google Scholar] [CrossRef]
- Veved, A.; Ejuh, G.W.; Djongyang, N. Effect of HfO2 on the dielectric, optoelectronic and energy harvesting properties of PVDF. Opt. Quantum. Electron. 2019, 51, 330. [Google Scholar] [CrossRef]
- Bosshard, C.; Sutter, K.; Prêtre, P.; Hulliger, J.; Flörsheimer, M.; Kaatz, P.; Günter, P. Organic Nonlinear Optical Materials, 1st ed.; CRC Press: Boca Raton, FL, USA, 1995; ISBN 9780429114090. [Google Scholar]
- Ismail, A.M.; Mohammed, M.I.; Fouad, S.S. Optical and structural properties of polyvinylidene fluoride (PVDF)/reduced graphene oxide (RGO) nanocomposites. J. Mol. Struct. 2018, 1170, 51–59. [Google Scholar] [CrossRef]
- Baibarac, M.; Daescu, M.; Matei, E.; Nastac, D.; Cramariuc, O. Optical Properties of Composites Based on Poly(o-phenylenediamine), Poly(vinylenefluoride) and Double-Wall Carbon Nanotubes. Int. J. Mol. Sci. 2021, 22, 8260. [Google Scholar] [CrossRef] [PubMed]
- Verkhovskaya, K.A.; Chumakova, S.P.; Savelev, V.V.; Krivenko, T.V. The Photorefractive and Photovoltaic Properties of a Composite Based on Ferroelectric Polymer Doped with Carbon Nanotubes. Crystallogr. Rep. 2018, 63, 802–805. [Google Scholar] [CrossRef]
- Badawi, A.; Alharthi, S.S.; Mostafa, N.Y.; Althobaiti, M.G.; Altalhi, T. Effect of carbon quantum dots on the optical and electrical properties of polyvinylidene fluoride polymer for optoelectronic applications. Appl. Phys. A 2019, 125, 858. [Google Scholar] [CrossRef]
- Bodkhe, S.; Rajesh, P.S.M.; Kamle, S.; Verma, V. Beta-phase enhancement in polyvinylidene fluoride through filler addition: Comparing cellulose with carbon nanotubes and clay. J. Polym. Res. 2014, 21, 434. [Google Scholar] [CrossRef]
- El-Metwally, E.G.; Nasrallah, D.A.; Fadel, M. The effect of Li4Ti5O12 nanoparticles on structural, linear and third order nonlinear optical properties of PVDF films. Mater. Res. Express 2019, 6, 085312. [Google Scholar] [CrossRef]
- Palto, S.; Blinov, L.; Bune, A.; Dubovik, E.; Fridkin, V.; Petukhova, N. Ferroelectric langmuir-blodgett films. Ferroelectr. Lett. 1995, 19, 65–68. [Google Scholar] [CrossRef]
- Palto, S.; Blinov, L.; Dubovik, E.; Fridkin, V.; Petukhova, N.; Sorokin, A.; Zlatkin, A. Ferroelectric Langmuir-Blodgett films showing bistable switching. Europhys. Lett. 1996, 34, 465–470. [Google Scholar] [CrossRef]
- Jiang, Y.D.; Ye, Y.; Yu, J.S.; Yang, Y.J.; Xu, J.H.; Wu, Z.M. A study on ferroelectric PVDF ultrathin films prepared by LB technique. Integr. Ferroelectr. 2007, 88, 21–26. [Google Scholar] [CrossRef]
- Aktsipetrov, O.; Misuryaev, T.; Murzina, T.; Blinov, L.; Fridkin, V.; Palto, S. Optical second-harmonic-generation probe of two-dimensional ferroelectricity. Opt. Lett. 2000, 25, 411–413. [Google Scholar] [CrossRef] [Green Version]
- Aktsipetrov, O.A.; Blinov, L.M.; Fridkin, V.M.; Misuryaev, T.V.; Murzina, T.V.; Palto, S.P.; Yudin, S.G. Two-dimensional ferroelectricity and second harmonic generation in PVDF Langmuir-Blodgett films. Surf. Sci. 2000, 454, 1016–1020. [Google Scholar] [CrossRef]
- Zhu, H.E.; Yamamoto, S.; Matsui, J.; Miyashita, T.; Mitsuishi, M. Ferroelectricity of poly (vinylidene fluoride) homopolymer Langmuir–Blodgett nanofilms. J. Mater. Chem. C. 2014, 2, 6727–6731. [Google Scholar] [CrossRef]
- Zhu, H.E.; Yamamoto, S.; Matsui, J.; Miyashita, T.; Mitsuishi, M. Highly oriented poly (vinylidene fluoride-co-trifluoroethylene) ultrathin films with improved ferroelectricity. RSC Adv. 2016, 6, 32007–32012. [Google Scholar] [CrossRef]
- He, S.; Guo, M.F.; Dan, Z.; Dan, Z.K.; Lan, S.; Ren, W.B.; Zhou, L.; Wang, Y.; Liang, Y.H.; Zheng, Y.P.; et al. Large-area atomic-smooth polyvinylidene fluoride Langmuir-Blodgett film exhibiting significantly improved ferroelectric and piezoelectric responses. Sci. Bull. 2021, 66, 1080–1090. [Google Scholar] [CrossRef]
- Chikkonda, R.; Ravindran, A.; Saikia, S.; Thankamani Sathyanathan, A.R.; Chelvane, A.; Subramanian, A.; Chinnayya, J.R.K.; Gangineni, R.B. Low-frequency ferroelectric switching studies in PVDF thin films across Cu or (Ag/Cu)/PVDF/Cu capacitor structures. J. Appl. Polym. Sci. 2021, 138, 50018. [Google Scholar] [CrossRef]
- Blinov, L.M.; Fridkin, V.M.; Palto, S.P.; Bune, A.; Dowben, P.A.; Ducharme, S. Two-dimensional ferroelectrics. Phys-USP. 2000, 43, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Bai, M.; Poulsen, M.; Sorokin, A.; Ducharme, S.; Herzinger, C.; Fridkin, V. Infrared spectroscopic ellipsometry study of vinylidene fluoride (70%)-trifluoroethylene (30%) copolymer Langmuir-Blodgett films. J. Appl. Phys. 2003, 94, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Bai, M.; Sorokin, A.; Thompson, D.W.; Poulsen, M.; Ducharme, S.; Herzinger, C.; Palto, S.; Fridkin, V.; Yudin, S.; Savchenko, V. Determination of the optical dispersion in ferroelectric vinylidene fluoride (70%) /trifluoroethylene (30%) copolymer Langmuir-Blodgett films. J. Appl. Phys. 2004, 95, 3372–3377. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.L.; Gao, Y.Q.; Huang, Z.M.; Meng, X.J.; Yuan, S.Z.; Yang, J.; Sun, J.L.; Chu, J.H. The optical dispersion of Langmuir-Blodgett terpolymer films. Ferroelectrics 2010, 405, 120–125. [Google Scholar] [CrossRef]
- Duan, C.G.; Mei, W.N.; Yin, W.G.; Liu, J.; Hardy, J.; Bai, M.; Ducharme, S. Theoretical study on the optical properties of polyvinylidene fluoride crystal. J. Phys. Condens. Matter. 2003, 15, 3805. [Google Scholar] [CrossRef] [Green Version]
- Li, J.C.; Wang, C.L.; Yang, K.; Wang, X.Y.; Zhao, M.L.; Zhang, J.L. Electronic Structure of α and β-phase of Poly (Vinylidene Fluoride). Integr. Ferroelectr. 2006, 78, 27–33. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Wang, Y.K.; Huang, M.; Zhang, Q.M.; Kavarnos, G.J.; Bauer, F. Electro-optical response of the ferroelectric relaxor poly (vinylidene fluoride-trifluoroethylene-chlorofluoroethylene) terpolymer. J. Appl. Phys. 2004, 96, 316–319. [Google Scholar] [CrossRef]
- Cheng, H.P.; Chen, G.H.; Qin, R.; Dan, J.K.; Huang, Z.M.; Peng, H.; Chen, T.N.; Lei, J.B. Electronic Structures and Optical Properties of Poly (vinylidene fluoride) Crystals. Acta Phys. Chim. Sin. 2014, 30, 281–288. [Google Scholar] [CrossRef]
- Coyle, J.D. Introduction to Organic Ohotochemistry, 1st ed.; John Wiley & Sons: Singapore, 1986; ISBN 9780471909750. [Google Scholar]
- He, P.S. Polymerization in Two-Dimensional State Polymerization in Monolayer and Langmuir–Blodgett Films; Press of University of Chinese Science and Technology: Hefei, China, 2008; ISBN 9787312022074. [Google Scholar]
- Gedde, U.W. Polymer Physics, 1st ed.; Kluwer Academic Publisher: London, UK, 1995; ISBN 0412626403. [Google Scholar]
- Furukawa, T. Ferroelectric properties of vinylidene fluoride copolymers. Phase Transit. 1989, 18, 143–211. [Google Scholar] [CrossRef]
- Baumeister, P.W. Optical Coating Technology, 1st ed.; SPIE Press: Bellingham, WA, USA, 2004; Volume 137, ISBN 0819453137. [Google Scholar]
- Nalwa, H.S.; Silva, S. Handbook of Thin Film Materials, 1st ed.; Academic Press: New York, NY, USA, 2002; ISBN 0125129084. [Google Scholar]
- Macleod, H.A. Thin-Film Optical Filters, 4th ed.; CRC Press: New York, NY, USA, 2010; ISBN 1138198242. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E.; Bhatia, A.B.; Clemmow, P.C.; Gabor, D.; Stokes, A.R.; Taylor, A.M.; Wayman, P.A.; Wilcock, W.L. Principles of Optics, 6th ed.; PERGAMON Press: Beijing, China, 1980; ISBN 0080264816. [Google Scholar]
- Schnabel, W. Polymers and Light: Fundamentals and Technical Applications, 1st ed.; John Wiley & Sons: Berlin, Germany, 2007; ISBN 3527318666. [Google Scholar]
- Ben-David, M.; Engel, L.; Shacham-Diamand, Y. Spectroscopic ellipsometry study of spin coated P(VDF-TrFE-CTFE) thin films and P(VDF-TrFE-CTFE)/PMMA blends. Microelectron Eng. 2017, 171, 37–43. [Google Scholar] [CrossRef]
- Choi, J.; Dowben, P.A.; Pebley, S.; Bune, A.; Ducharme, S.; Fridkin, V.; Palto, S.; Petukhova, N. Changes in metallicity and electronic structure across the surface ferroelectric transition of ultrathin crystalline poly (vinylidene fluoride-trifluoroethylene) copolymers. Phys. Rev. Lett. 1998, 80, 1328. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Shen, H.; Zhao, H. Dispersion of the common optical thin films in the visible spectral range. Laser Technol. 1988, 12, 29–33. [Google Scholar]
- Bune, A.; Zhu, C.; Ducharme, S.; Blinov, L.M.; Fridkin, V.M.; Palto, S.P.; Petukhova, N.; Yudin, S.G. Piezoelectric and pyroelectric properties of ferroelectric Langmuir-Blodgett polymer films. J. Appl. Phys. 1999, 85, 7869–7873. [Google Scholar] [CrossRef] [Green Version]

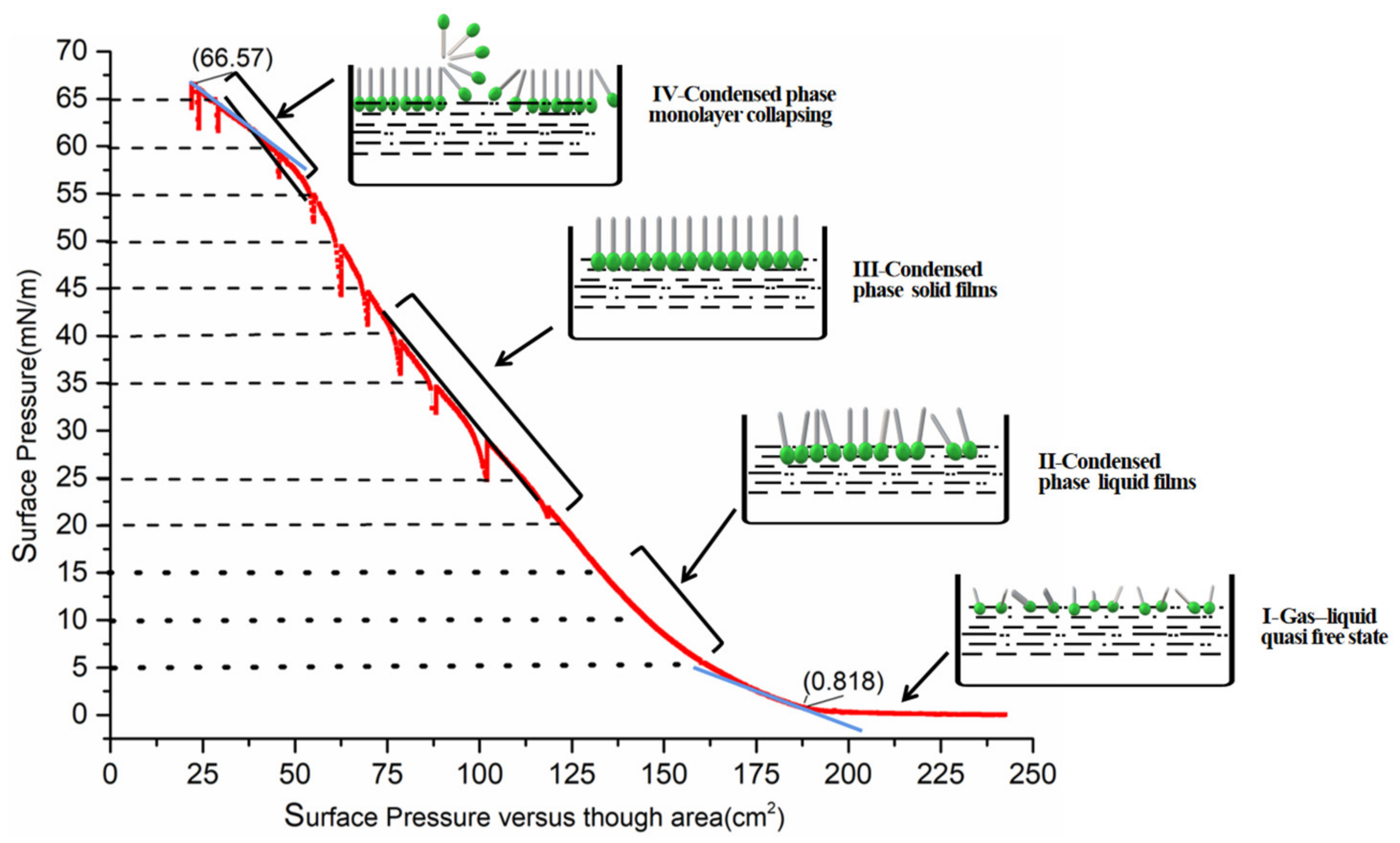

| Collapse Pressure (mN/m) | Surface Pressure for Dipping (mN/m) | Solvent | Solution Concentration | Dipping Mode | Reference |
|---|---|---|---|---|---|
| 60–70 | 15 | DMF | 0.01 wt%P(VDF-TrFE) | Horizontal | This article |
| 6 | 15 | DMSO | 0.01 wt%P(VDF-TrFE) | Horizontal | 38 |
| 5–20 | 5 | DMSO | 0.01 wt%P(VDF-TrFE) | Horizontal | 49 |
| 60 | 40 | DMF | 0.1 wt%-PVDF | Y | 40 |
| -- | 5 | DMF | P(VDF-TrFE-CFE) 0.01wt% | Horizontal | 50 |
| Number of ML | Fitting Parameters | |||||
|---|---|---|---|---|---|---|
| An | Bn | Cn | Ak | Bk | Ck | |
| 30 | 1.5978 | 0.1476 | 0.0309 | 0.1178 | 0 | 0 |
| 60 | 1.4736 | −0.1022 | 0.0226 | 0.1382 | 0 | 0 |
| 160 | 1.5499 | 0.0286 | 0.0043 | 0.1601 | 0.7523 | 0 |
| 200 | 1.443 | 0.0074 | 0.0092 | 0.0124 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, W.-G.; Lin, D.-B.; Niu, X.-L.; Zhou, S.; Zhang, J.; Ge, S.-B.; Zhu, Y.-C.; Meng, X.; Chen, Z.-L. Fabrication and Optical Properties of Transparent P(VDF-TrFE) Ultrathin Films. Nanomaterials 2022, 12, 588. https://doi.org/10.3390/nano12040588
Liu Y, Liu W-G, Lin D-B, Niu X-L, Zhou S, Zhang J, Ge S-B, Zhu Y-C, Meng X, Chen Z-L. Fabrication and Optical Properties of Transparent P(VDF-TrFE) Ultrathin Films. Nanomaterials. 2022; 12(4):588. https://doi.org/10.3390/nano12040588
Chicago/Turabian StyleLiu, Yong, Wei-Guo Liu, Da-Bin Lin, Xiao-Ling Niu, Shun Zhou, Jin Zhang, Shao-Bo Ge, Ye-Chuan Zhu, Xiao Meng, and Zhi-Li Chen. 2022. "Fabrication and Optical Properties of Transparent P(VDF-TrFE) Ultrathin Films" Nanomaterials 12, no. 4: 588. https://doi.org/10.3390/nano12040588







