The Photocatalytic Performance of Nd2O3 Doped CuO Nanoparticles with Enhanced Methylene Blue Degradation: Synthesis, Characterization and Comparative Study
Abstract
:1. Introduction
2. Experimental Techniques
2.1. Synthesis of Nd2O3 Doped CuO Nanostructured
2.2. Characterization Techniques and Devices
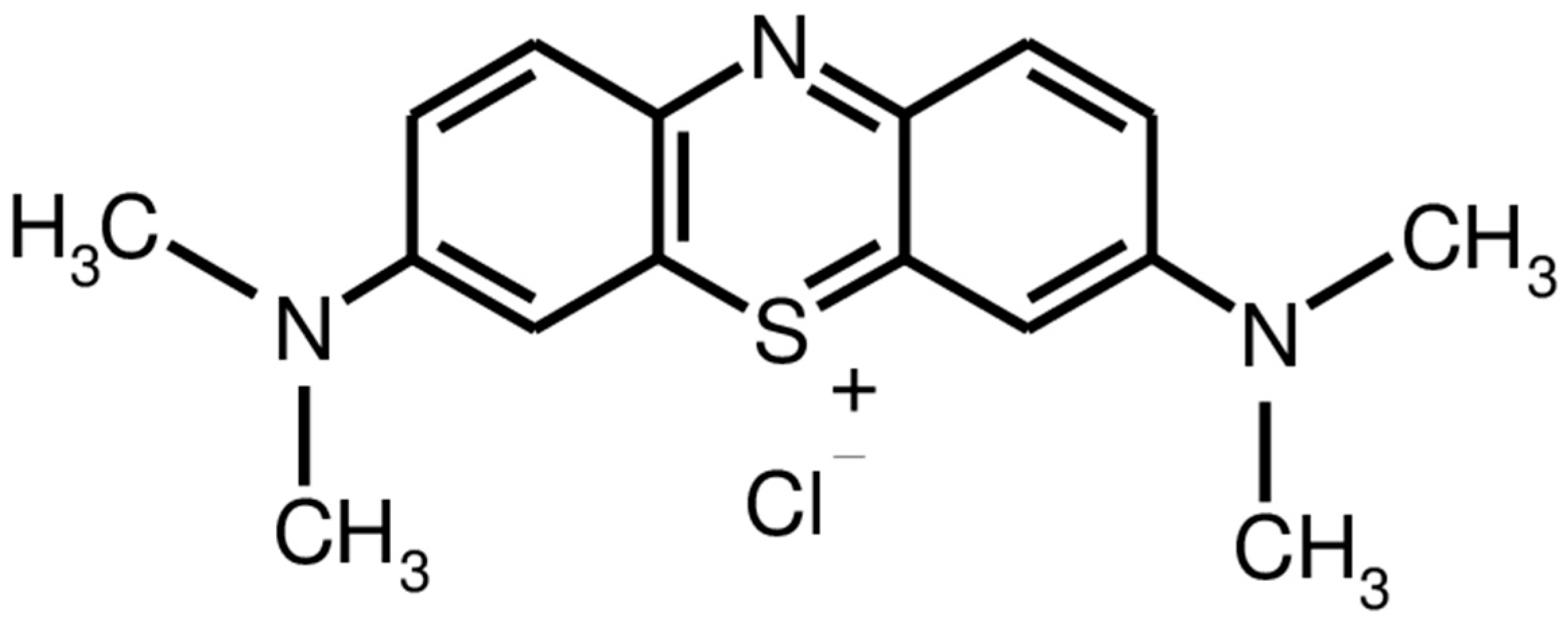
2.3. Photocatalytic Performance of Nd2O3 Doped CuO Nanopowders
3. Results and Discussion
3.1. XRD of the Prepared Nanostructured Nd2O3 Doped CuO
3.2. FESEM Analysis of Nd2O3 Doped CuO Nanostructured
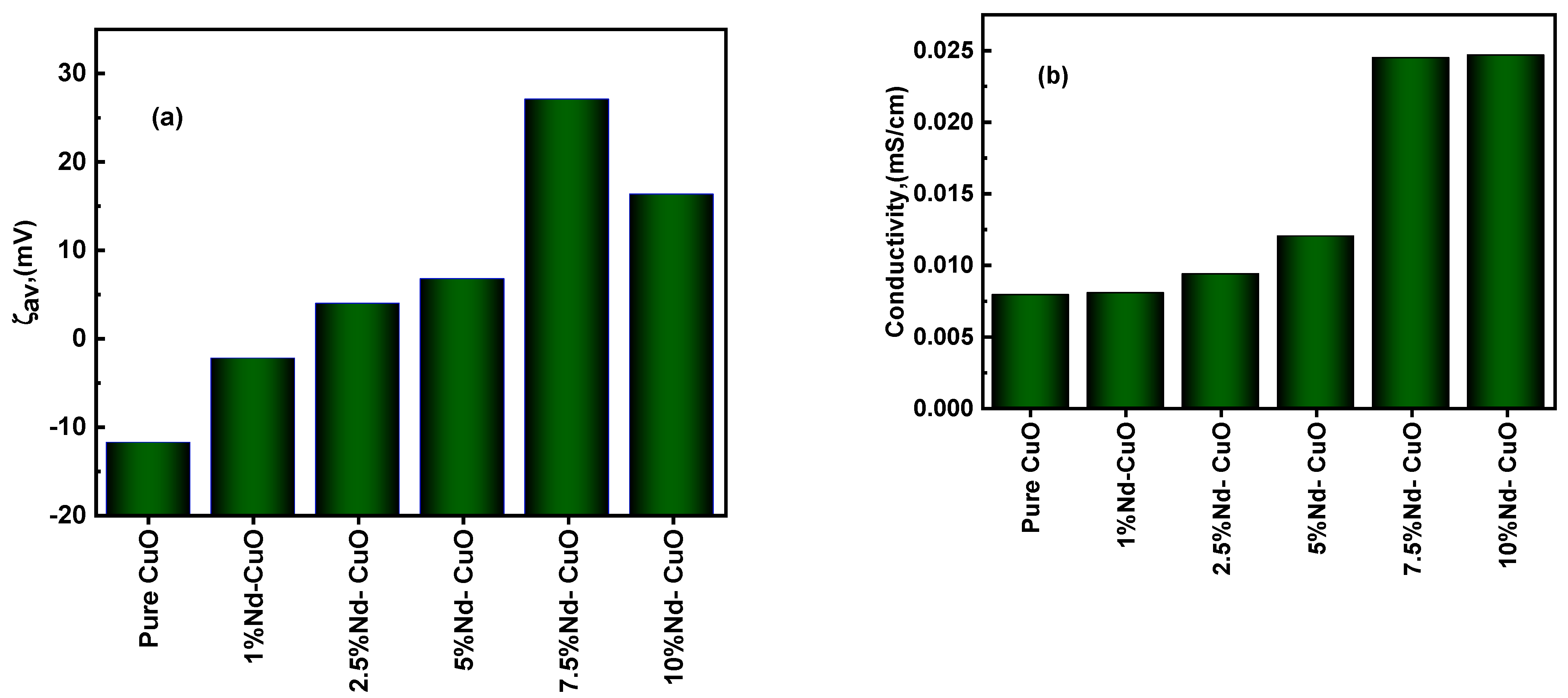
3.3. DLS of Nanostructured Nd2O3 Doped CuO
3.4. Photocatalysis Using Nanostructured Nd2O3 Doped CuO
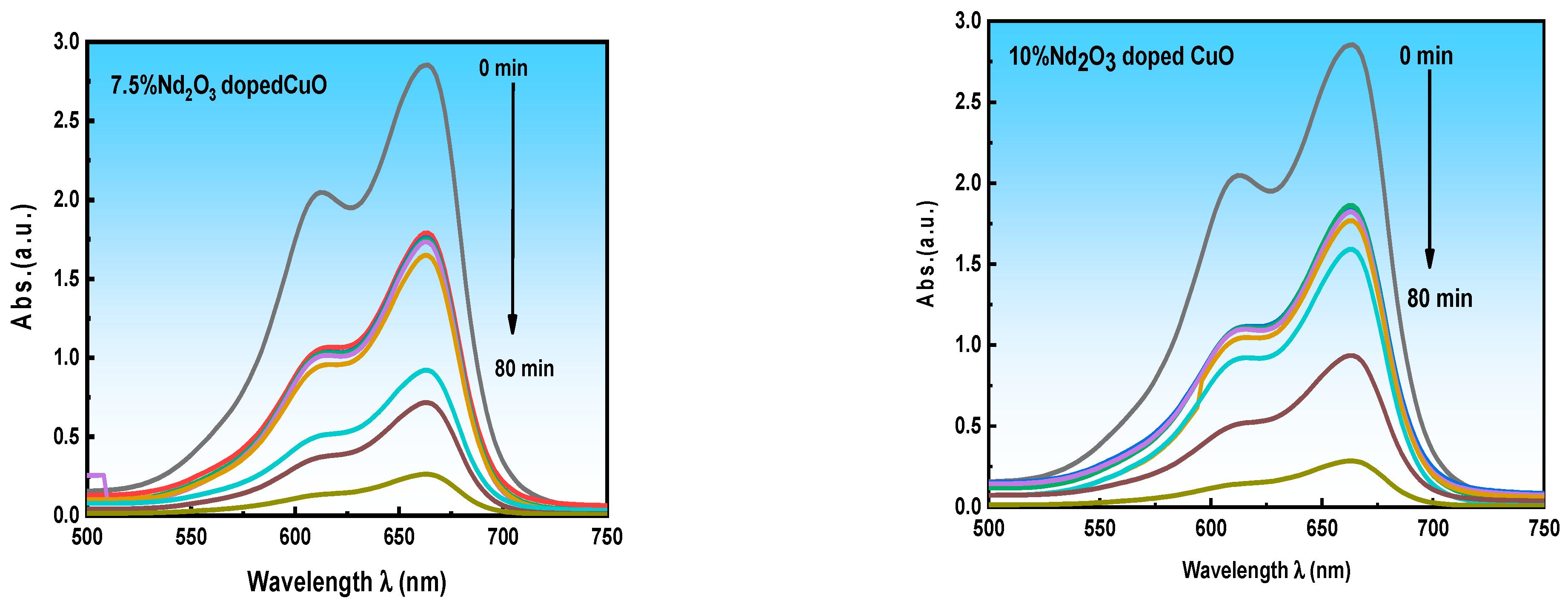
3.4.1. Effect of Nd+3 Doping
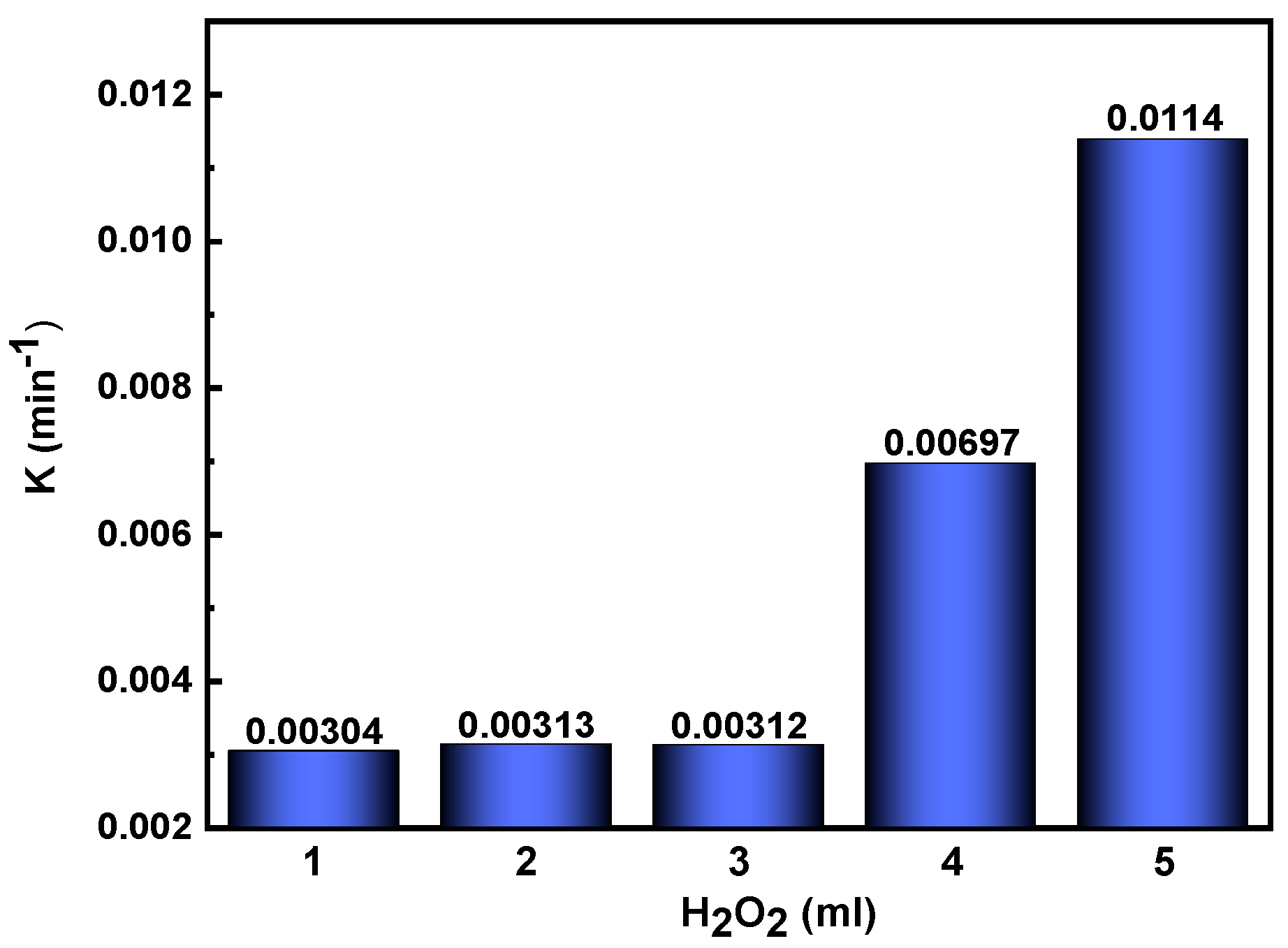
3.4.2. Influence of H2O2 Concentration of 7.5% Nd2O3 Doped CuO Nanoparticle
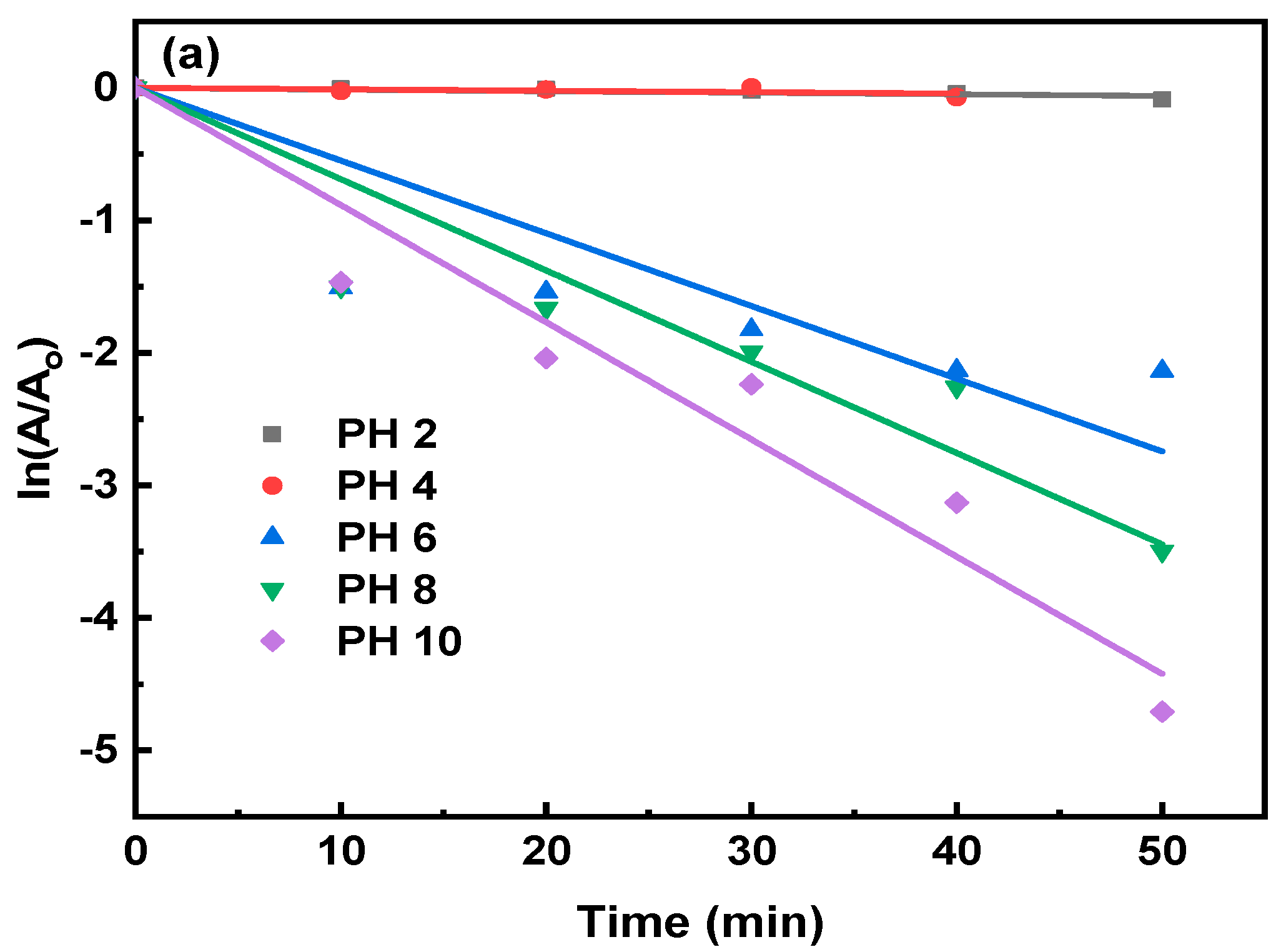
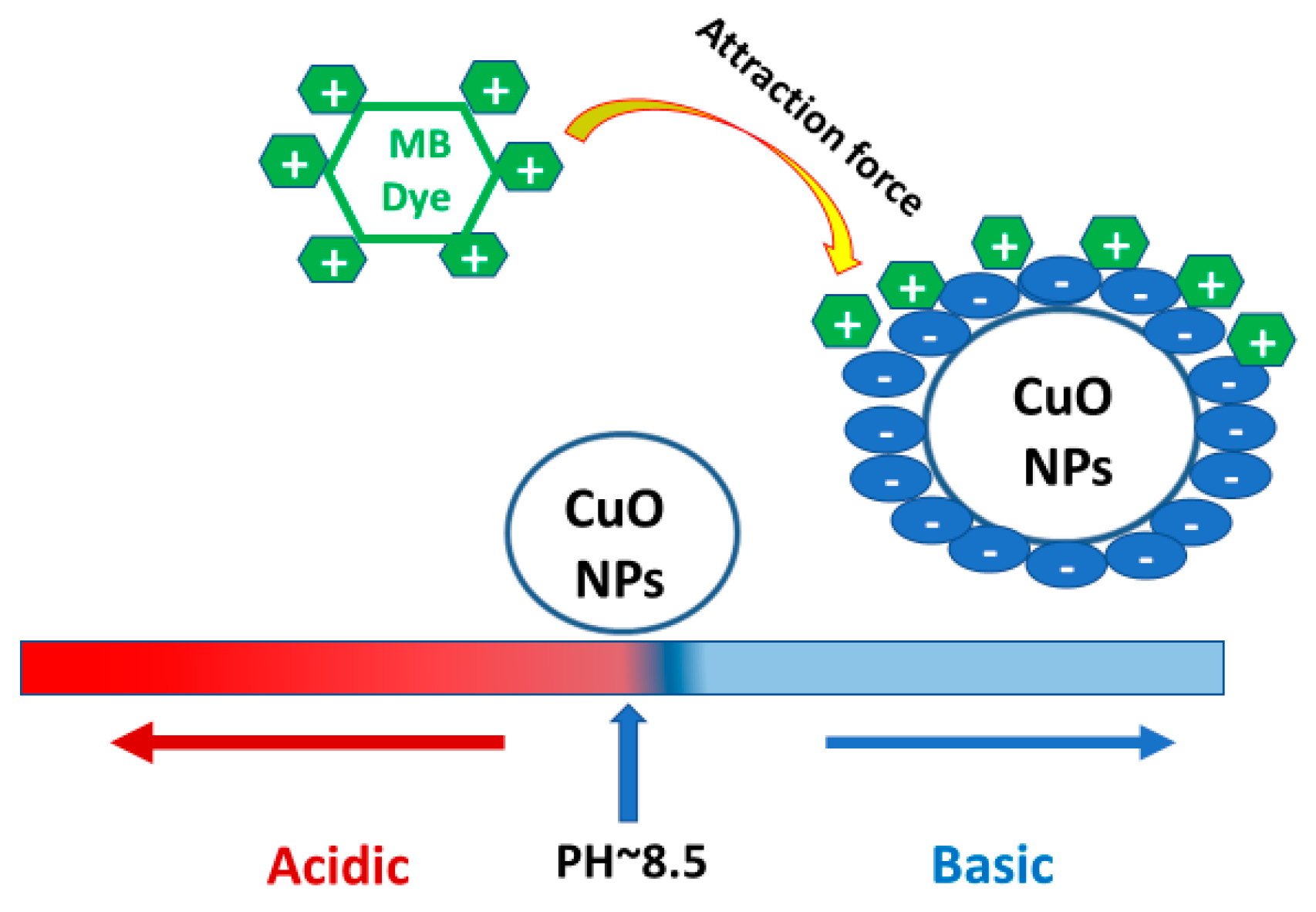
3.4.3. Effect of pH on 7.5% Nd2O3 Doped CuO Nanoparticle
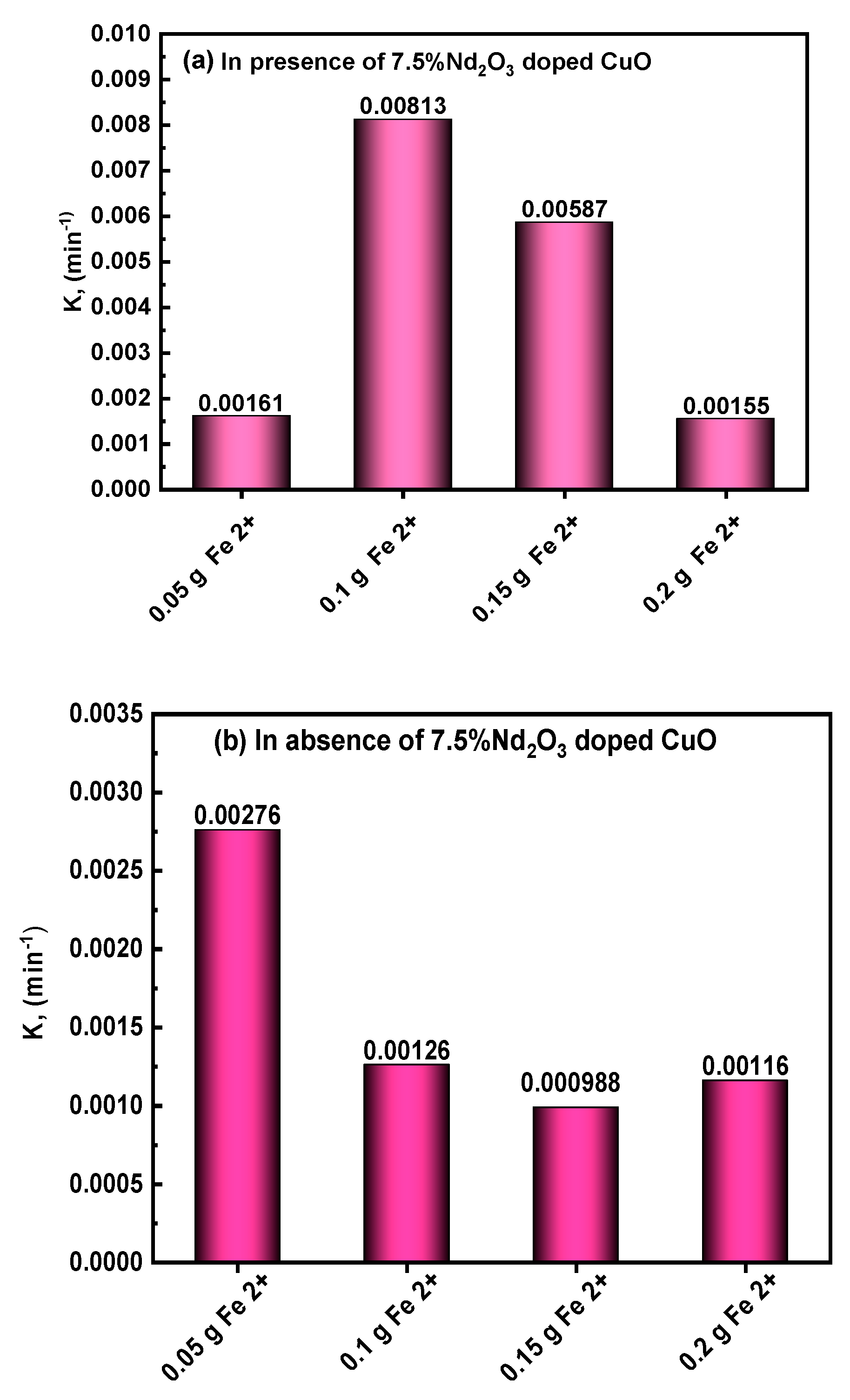
3.4.4. Effect of Fenton and Photo-Fenton on MB Photodegradation
3.4.5. Comparison of the Photocatalytic Activity of Several Compounds Based on CuO
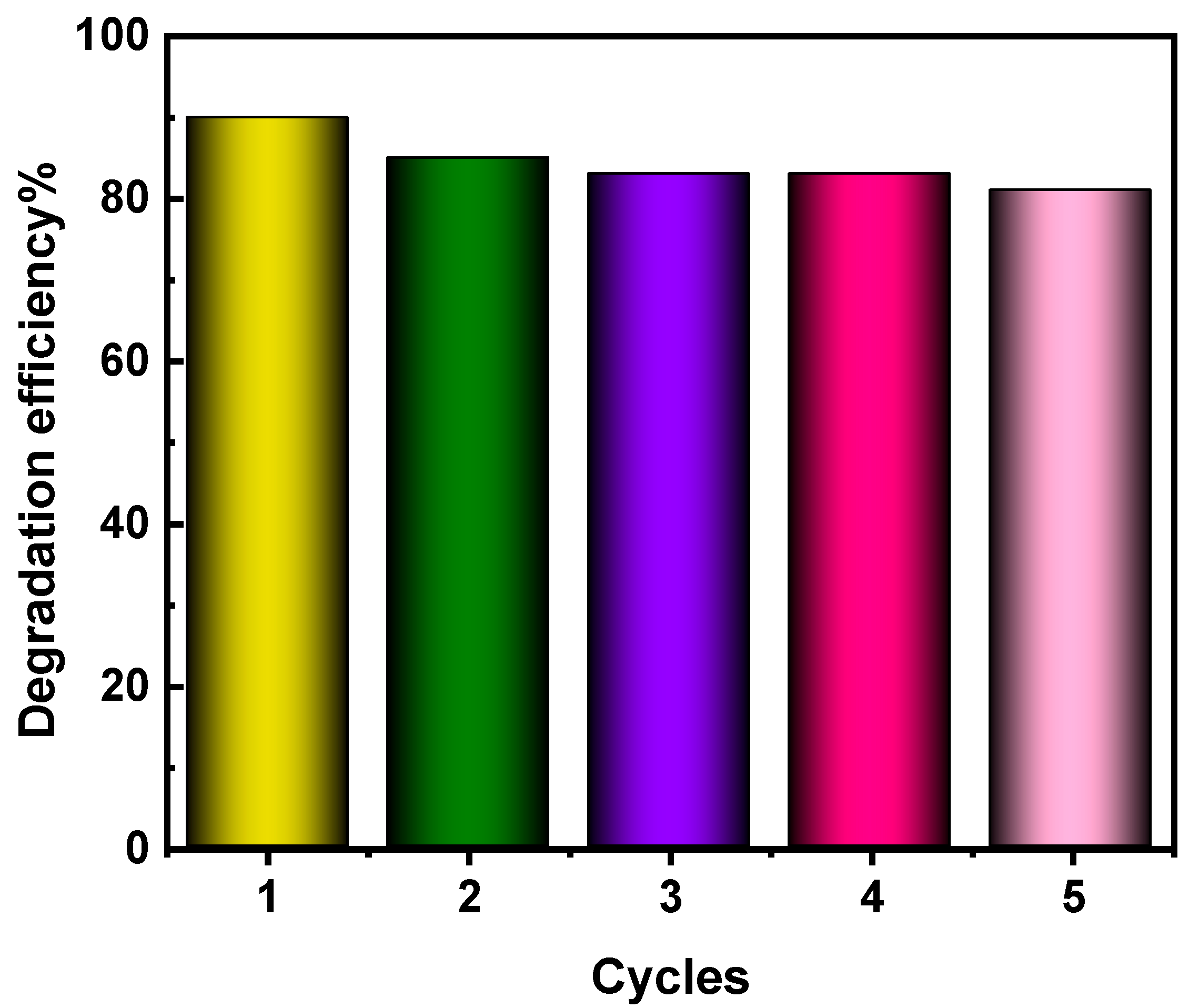
3.4.6. Reusability and Stability
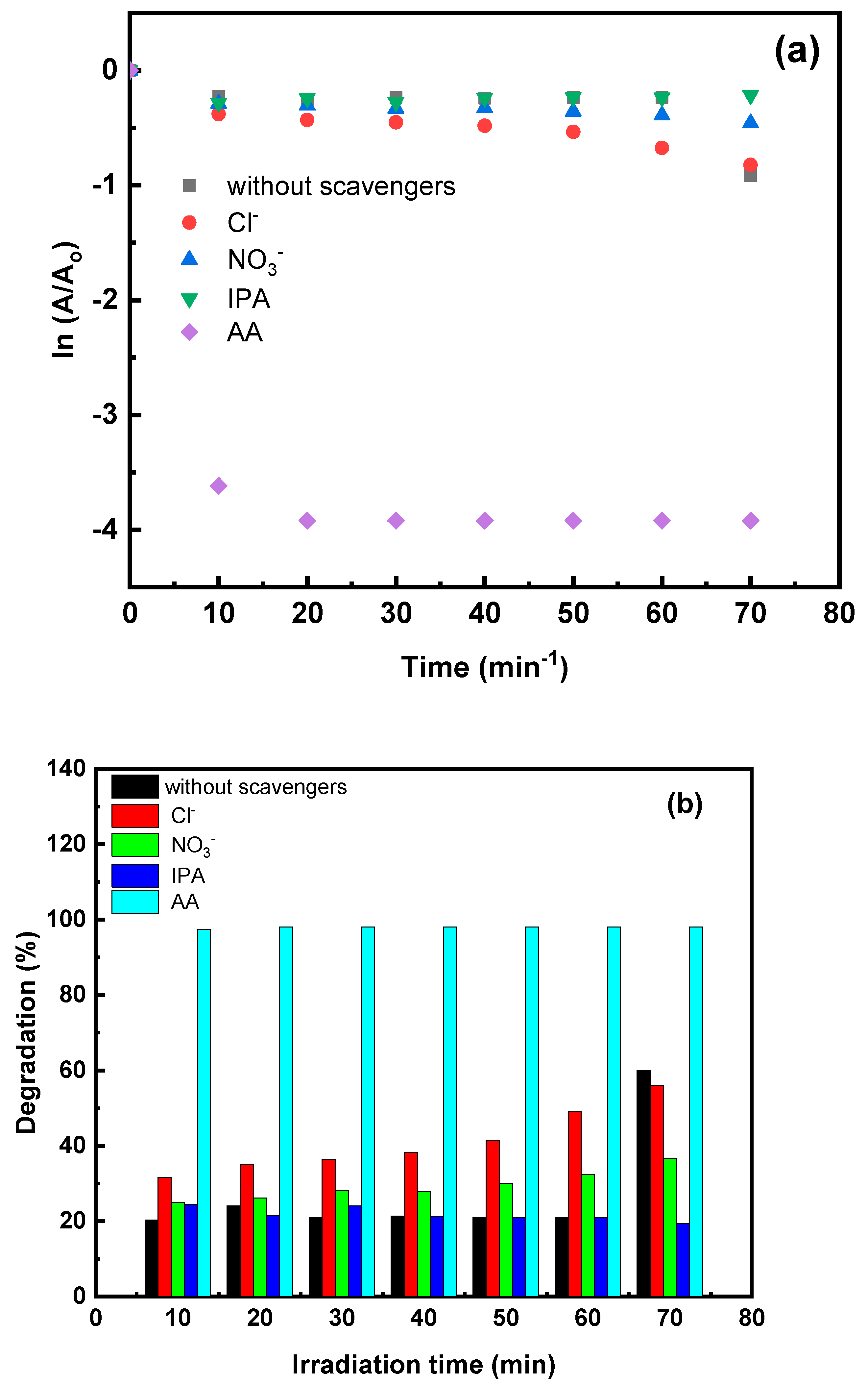
3.4.7. Influence of Radicals’ Scavengers on Photocatalytic Activity
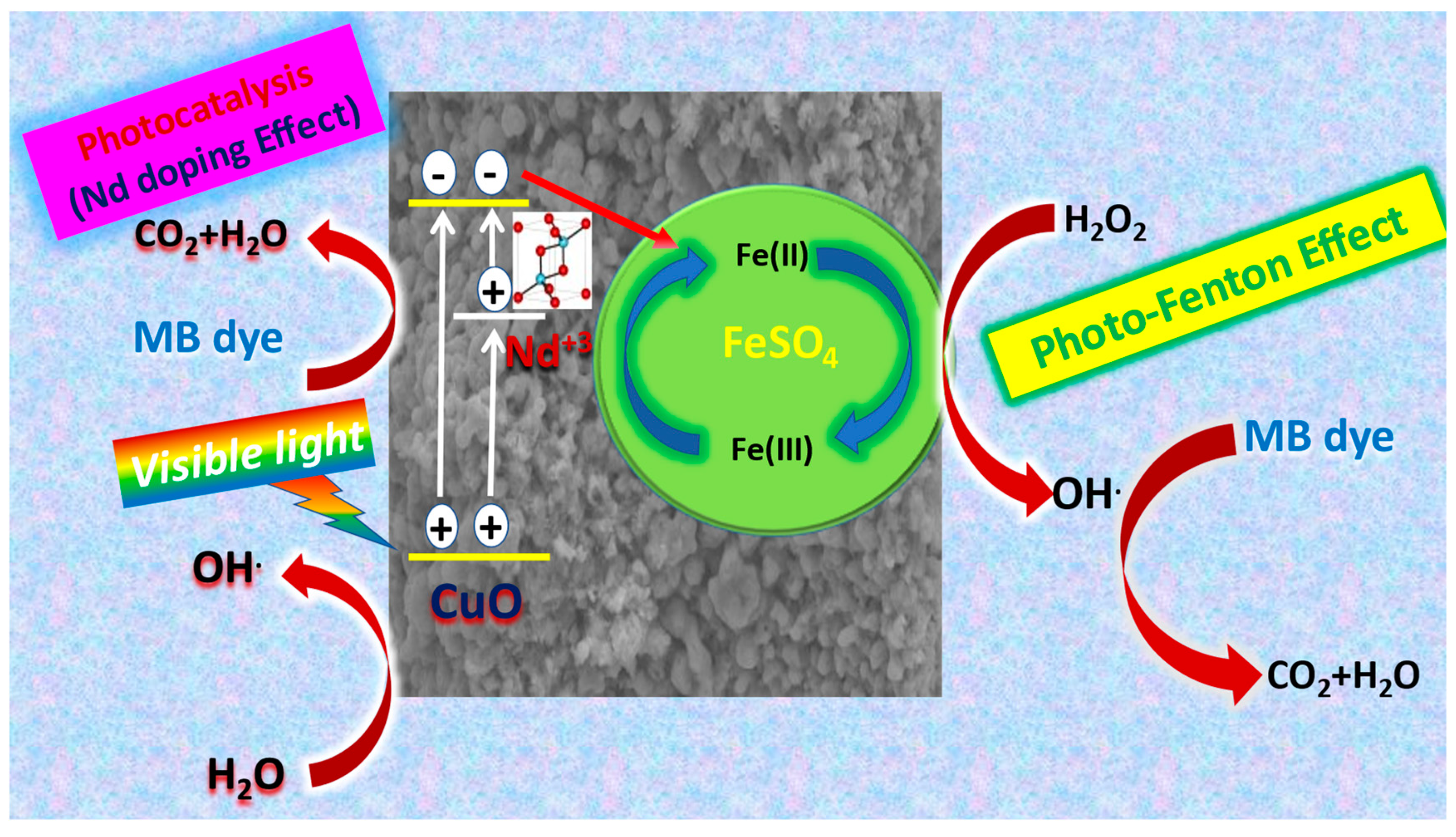
3.4.8. Mechanism of Photocatalysis for Nd2O3 Doped CuO Nanostructured
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Cho, M.H. Polythiophene nanocomposites for photodegradation applications: Past, present and future. J. Saudi Chem. Soc. 2015, 19, 494–504. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Sivakumar, V.; Tamilarasan, R. Adsorption of Victoria blue by carbon/Ba/Alignate beads: Kinetics, Thermodynamics and Isotherm studies. Carbohydr. Polym. 2013, 98, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, A.; Rejani, P.; Bhaskaran, B. CuO/polypyrrole nanocomposites as a marker of toxic lead ions for ecological remediation in contrast with CuO and polypyrrole. Main Group Met. Chem. 2015, 38, 133–143. [Google Scholar] [CrossRef]
- Vinuth, M.; Bhojya Naik, H.S.; Mahadevaswamy, M.M.; Prabhakara, M.C. Environmentally benign Fe(III)– montmorillonite for rapid adsorption of methylene blue dye in aqueous medium under ambient conditions. Fash. Text. 2017, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Pourmortazavi, S.M.; Rahimi-Nasrabadi, M.; Aghazadeh, M.; Ganjali, M.R.; Karimi, M.S.; Norouzi, P. Synthesis, characterization and photocatalytic activity of neodymium carbonate and neodymium oxide nanoparticles. J. Mol. Struct. 2017, 1150, 411–418. [Google Scholar] [CrossRef]
- Chennakesavulu, K.; Madhusudhana Reddy, M.; Ramanjaneya Reddy, G.; Rabel, A.M.; Brijitta, J.; Vinita, V. Synthesis, Characterization and Photo catalytic studies of the composites by Tantalum oxide and Zinc oxide Nanorods. J. Mol. Struct. 2015, 1091, 49–56. [Google Scholar] [CrossRef]
- Nagaveni, K.; Sivalingam, G.; Hegde, M.S.; Madras, G. Solar photocatalytic degradation of dyes high activity of combustion synthesized Nano-TiO2. Appl. Catal. B Environ. 2004, 48, 83–93. [Google Scholar] [CrossRef]
- Jamal, N.; Radhakrishnan, A.; Raghavan, R.; Bhaskaran, B. Efficient photocatalytic degradation of organic dye from aqueous solutions over zinc oxide incorporated nanocellulose under visible light irradiation. Main Group Metal Chem. 2020, 43, 84–91. [Google Scholar] [CrossRef]
- Abhilasha, J.; Ashma, A.; Marazban, K. A greener approach for the degradation of dye methylene blue by organic additive catalysed photo-fenton process. J. Chil. Chem. Soc. 2016, 61, 3043–3048. [Google Scholar] [CrossRef] [Green Version]
- Muthuvel, A.; Jothibas, M.; Manoharan, C. Synthesis of copper oxide nanoparticles by chemical and biogenic methods: Photocatalytic degradation and in vitro antioxidant activity. Nanotechnol. Environ. Eng. 2020, 5, 14. [Google Scholar] [CrossRef]
- Arunkumar, B.; Johnson Jeyakumar, S.; Jothibas, M. A sol gel approach to the synthesis of CuO nanoparticles using Lantana camara leaf extract and their photo catalytic activity. Optik 2019, 183, 698–705. [Google Scholar] [CrossRef]
- Vinothkumar, P.; Manoharan, C.; Shanmugapriya, B.; Bououdina, M. Effect of reaction time on structural, morphological, optical and photocatalytic properties of copper oxide (CuO) nanostructures. J. Mater. Sci. Mater. Electron. 2019, 30, 6249–6262. [Google Scholar] [CrossRef]
- Lembang, M.S.; Yulizar, Y.; Sudirman, S.; Apriandanu, D.O.B. A facile method for green synthesis of Nd2O3 nanoparticles using aqueous extract of Terminalia catappa leaf. AIP Conf. Proc. 2018, 2023, 020093. [Google Scholar] [CrossRef]
- Hussien, M.S.A.; Mohammed, M.I.; Yahia, I.S. Flexible photocatalytic membrane based on CdS/PMMA polymeric nanocomposite films: Multifunctional materials. Environ. Sci. Pollut. Res. Int. 2020, 27, 45225–45237. [Google Scholar] [CrossRef] [PubMed]
- Sarma, G.K.; Khan, A.; El-Toni, A.M.; Rashid, M.H. Shape-tunable CuO-Nd(OH)3 nanocomposites with excellent adsorption capacity in organic dye removal and regeneration of spent adsorbent to reduce secondary waste. J. Hazard. Mater. 2019, 380, 120838. [Google Scholar] [CrossRef]
- Shubha, L.N.; Kalpana, M.; Madhusudana Rao, P. Study of Chemically Synthesized Polyaniline/CopperOxide Nanocomposites. In Proceedings of the National Seminar on Frontiers in Chemical Research and Analysis, Hyderabad, India, 24–25 July 2015; pp. 1–4. [Google Scholar]
- Arunpandian, M.; Selvakumar, K.; Nagarajan, E.R.; Arunachalam, S. Ag/Nd2O3-Zno Nanocomposite: Visible Active Efficient Photocatalytic Degradation of Methylene Blue and Its Antibacterial Activity. Int. J. Innov. Technol. Explor. Eng. 2019, 9, 743–747. [Google Scholar] [CrossRef]
- Khan, Z.R.; Shkir, M.; Ganesh, V.; Yahia, I.S.; AlFaify, S. A facile synthesis of Au-nanoparticles decorated PbI2 single crystalline nanosheets for optoelectronic device applications. Sci. Rep. 2018, 8, 13806. [Google Scholar] [CrossRef] [Green Version]
- Zinatloo-Ajabshir, S.; Mortazavi-Derazkola, S.; Salavati-Niasari, M. Nd2O3 nanostructures: Simple synthesis, characterization and its photocatalytic degradation of methylene blue. J. Mol. Liq. 2017, 234, 430–436. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, M.S.; Chandekar, K.V.; Shkir, M.; AlFaify, S.; Ibrahim, E.H.; Ahmad, Z.; Kilany, M.; Al-Shehri, B.M.; Al-Namshah, K.S. Novel Mg@ZnO nanoparticles synthesized by facile one-step combustion route for anti-microbial, cytotoxicity and photocatalysis applications. J. Nanostruct. Chem. 2021, 11, 147–163. [Google Scholar] [CrossRef]
- Hussien, M.S.A. Facile Synthesis of Nanostructured Mn-Doped Ag3PO4 for Visible Photodegradation of Emerging Pharmaceutical Contaminants: Streptomycin Photodegradation. J. Inorg. Organomet. Polym. Mater. 2021, 31, 945–959. [Google Scholar] [CrossRef]
- Davarpanah, S.J.; Karimian, R.; Goodarzi, V.; Piri, F. Synthesis of Copper (II) Oxide (CuO) Nanoparticles and Its Application as Gas Sensor. J. Appl. Biotechnol. Rep. 2015, 2, 329–332. [Google Scholar]
- Eslami, A.; Juibari, N.M.; Hosseini, S.G.; Abbasi, M. Synthesis and Characterization of CuO Nanoparticles by the Chemical Liquid Deposition Method and Investigation of Its Catalytic Effect on the Thermal Decomposition of Ammonium Perchlorate. Cent. Eur. J. Energetic Mater. 2017, 14, 152–168. [Google Scholar] [CrossRef]
- Hussien, M.S.; Yahia, I. Fabrication progress of selective and durable Ni2+-doped Ag3PO4 for visible-light degradation of various textile dyes. J. Photochem. Photobiol. A Chem. 2019, 368, 210–218. [Google Scholar] [CrossRef]
- Omima Adly, M.I.; Hussien, M.S.A. Photo-Removal of RB5 from Textile Industrial Waste by Zn (II) Complex Nano-Powder. J. Pharm. Appl. Chem. 2018, 4, 33–40. [Google Scholar] [CrossRef]
- Chauhan, M.; Kaur, N.; Bansal, P.; Kumar, R.; Srinivasan, S.; Chaudhary, G.R. Proficient Photocatalytic and Sonocatalytic Degradation of Organic Pollutants Using CuO Nanoparticles. J. Nanomater. 2020, 2020, 6123178. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Takas, M.E. Synthesis and characterization of porous clay heterostructure intercalated with CuO nanoparticles as a visible light-driven photocatalyst. J. Iran. Chem. Soc. 2019, 16, 45–55. [Google Scholar] [CrossRef]
- Fenton, H. Oxidation of tartaric acid in the presence of iron. J. Chem. Soc. 1894, 65, 899–910. [Google Scholar]
- Haber, F.; Willstätter, R. Unparrigkit und radikalatten im reaktionsmechanismus organischer und enzymatischer vorgag. Chem. Europ. J. Inorg. Chem. 1931, 64, 2844–2856. [Google Scholar] [CrossRef]
- Arunpandian, M.; Selvakumar, K.; Raja, A.; Rajasekaran, P.; Thiruppathi, M.; Nagarajan, E.R.; Arunachalam, S. Fabrication of novel Nd2O3/ZnO-GO nanocomposite: An efficient photocatalyst for the degradation of organic pollutants. Colloids Surf. A 2019, 567, 213–227. [Google Scholar] [CrossRef]
- Chandran, D.; Nair, L.S.; Balachandran, S.; Babu, K.R.; Deepa, M. Band gap narrowing and photocatalytic studies of Nd3+ ion-doped SnO2 nanoparticles using solar energy. Bull. Mater. Sci. 2016, 39, 27–33. [Google Scholar]
- Samuel Oppong, O.B.; William Anku, W.; Sudheesh Shukla, K.; Penny Govender, P. Synthesis and characterisation of neodymium doped zinc oxide–graphene oxide nanocomposite as a highly efficient photocatalyst for enhanced degradation of indigo carmine in water under simulated solar light. Res. Chem. Intermed. 2017, 43, 481–501. [Google Scholar] [CrossRef]
- Oppong, S.O.-B.; Anku, W.W.; Shukla, S.K.; Agorku, E.S.; Govender, P.P. Photocatalytic degradation of indigo carmine using Nd-doped TiO2-decorated graphene oxide nanocomposites. J. Sol-Gel Sci. Technol. 2016, 80, 38–49. [Google Scholar] [CrossRef]
- Alam, U.; Khan, A.; Ali, D.; Bahnemann, D.; Muneer, M. Comparative photocatalytic activity of sol–gel derived rare earth metal (La, Nd, Sm and Dy)-doped ZnO photocatalysts for degradation of dyes. RSC Adv. 2018, 8, 17582–17594. [Google Scholar] [CrossRef] [Green Version]
- Sonali Chaudhari, P.; Anjali Bodade, B.; Prashant Jolhe, D.; Satish Meshram, P.; Gajanan Chaudhari, N. PEG-200 Assisted Sonochemical Synthesis of Cerium(Ce3+) Doped Copper Oxide (CuO) Nano-Composites and Their Photocatalytic Activities. Am. J. Mater. Synth. Proc. 2017, 2, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Pan, F.; Zheng, L.; Zhang, R.; Zhang, W.-Y. Nd -doped TiO2-C hybrid aerogels and their photocatalytic properties. New Carb. Mater. 2018, 33, 116–124. [Google Scholar] [CrossRef]
- Reda Mohamed, M.; Adel Ismail, A.; Mohammad Kadi, W.; Ajayb Alresheedi, S.; Ibraheem Mkhalid, A. Photocatalytic performance mesoporous Nd2O3 modified ZnO nanoparticles with enhanced degradation of tetracycline. Catal. Today 2020, 380, 259–267. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Derazkolab, S.M.; Niasarib, M.S. Nd2O3-SiO2 nanocomposites: A simple sonochemical preparation, characterization and photocatalytic activity. Ultrason. Sonochem. 2018, 42, 171–182. [Google Scholar] [CrossRef]
- Li, K.; Lu, X.; Zhang, Y.; Liu, K.; Huanga, Y.; Liu, H. Bi3TaO7/Ti3C2 heterojunctions for enhanced photocatalytic removal of water-borne contaminant. Environ. Res. 2020, 185, 109409. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, H.; Yang, H.; Lin, Y.; Liu, H.; Tong, Y. Efficient Charges Separation Using Advanced BiOI-Based Hollow Spheres Decorated with Palladium and Manganese Dioxide Nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 2751–2757. [Google Scholar] [CrossRef]


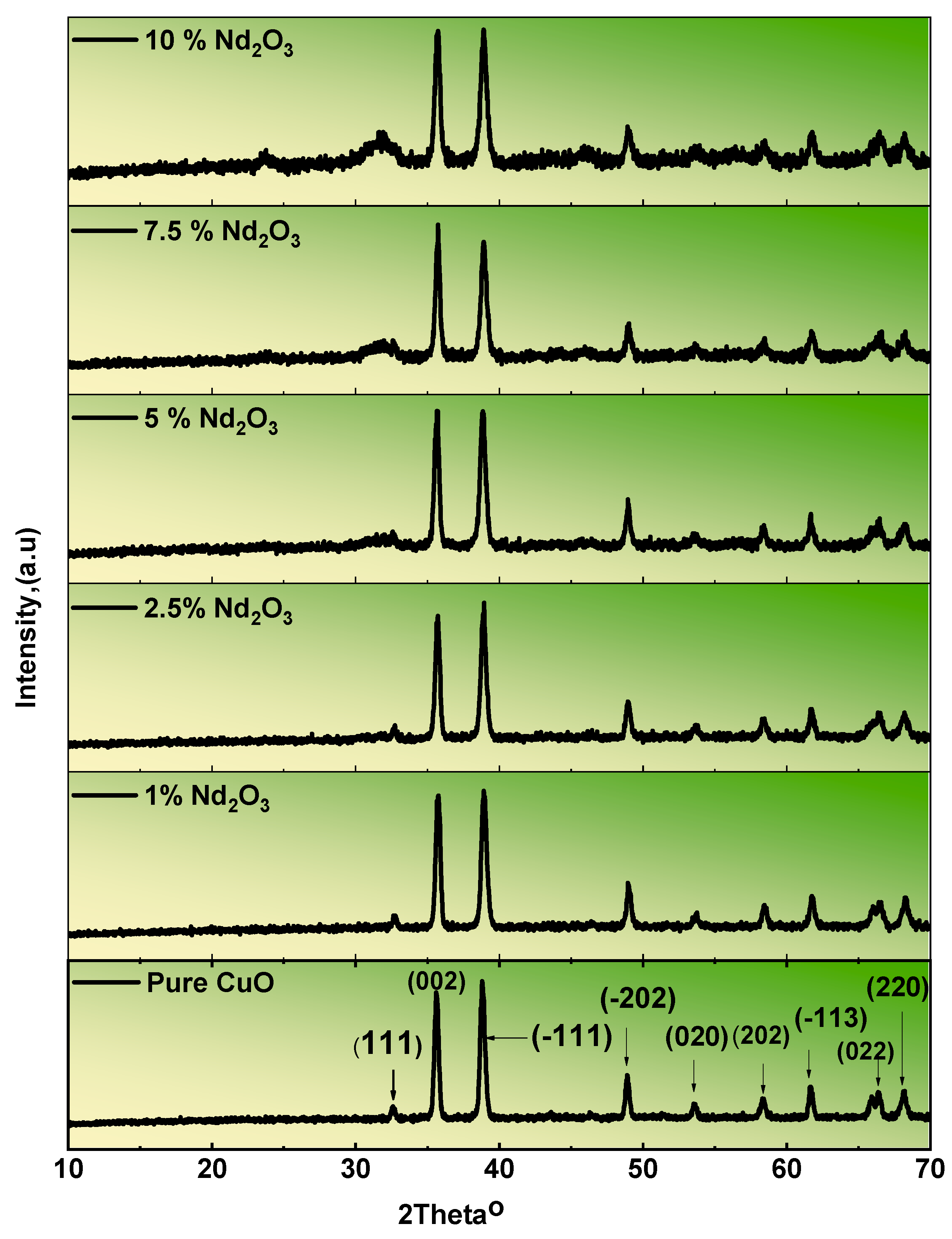




| Sample | Line | Position, 2θ | Intensity (Counts) | Line | Position, 2θ | Intensity (Counts) |
|---|---|---|---|---|---|---|
| Pure CuO | 1 | 35.6 | 901 | 5 | 58.3152 | 149 |
| 1% Nd2O3-doped CuO | 784 | 119 | ||||
| 2.5% Nd2O3-doped CuO | 580 | 88 | ||||
| 5% Nd2O3-doped CuO | 507 | 64 | ||||
| 7.5% Nd2O3-doped CuO | 364 | 51 | ||||
| 10% Nd2O3-doped CuO | 286 | 40 | ||||
| Pure CuO | 2 | 38.7 | 982 | 6 | 61.6 | 245 |
| 1% Nd2O3-doped CuO | 803 | 197 | ||||
| 2.5% Nd2O3-doped CuO | 624 | 148 | ||||
| 5% Nd2O3-doped CuO | 507 | 121 | ||||
| 7.5% Nd2O3-doped CuO | 350 | 76 | ||||
| 10% Nd2O3-doped CuO | 281 | 67 | ||||
| Pure CuO | 3 | 48.8 | 311 | 7 | 66.3 | 211 |
| 1% Nd2O3-doped CuO | 241 | 152 | ||||
| 2.5% Nd2O3-doped CuO | 182 | 116 | ||||
| 5% Nd2O3-doped CuO | 156 | 90 | ||||
| 7.5% Nd2O3-doped CuO | 96 | 41 | ||||
| 10% Nd2O3-doped CuO | 72 | 53 | ||||
| Pure CuO | 4 | 53.5 | 104 | 8 | 68.0 | 203 |
| 1% Nd2O3-doped CuO | 71 | 158 | ||||
| 2.5% Nd2O3-doped CuO | 47 | 109 | ||||
| 5% Nd2O3-doped CuO | 32 | 77 | ||||
| 7.5% Nd2O3-doped CuO | 25 | 54 | ||||
| 10% Nd2O3-doped CuO | 10 | 52 |
| Samples | Phases | Mean Values of the Grain Size, (nm) | Mean Values of Dislocation Density, (nm)2 | Mean Values of Lattice Strain |
|---|---|---|---|---|
| Pure CuO | Phase1, Pure CuO | 29.687 | 1.262 × 10−3 | 1.213 × 10−3 |
| 1% Nd-doped CuO | Phase1, Pure CuO | 22.089 | 2.089 × 10−3 | 1.579 × 10−3 |
| Phase 2, Nd2O3 | 36.744 | 1.187 × 10−3 | 1.124 × 10−3 | |
| 2.5% Nd-doped CuO | Phase1, Pure CuO | 30.969 | 1.582 × 10−3 | 1.305 × 10−3 |
| Phase 2, Nd2O3 | 27.180 | 1.636 × 10−3 | 1.356 × 10−3 | |
| 5% Nd-doped CuO | Phase1, Pure CuO | 26.809 | 1.679 × 10−3 | 1.384 × 10−3 |
| Phase 2, Nd2O3 | 35.557 | 9.307 × 10−3 | 1.033 × 10−3 | |
| 7.5% Nd-doped CuO | Phase 1, Pure CuO | 30.109 | 1.439 × 10−3 | 1.267 × 10−3 |
| Phase 2, Nd2O3 | 30.154 | 1.315 × 10−3 | 1.218 × 10−3 | |
| 10% Nd-doped CuO | Phase1, Pure CuO | 27.295 | 2.141 × 10−3 | 1.522 × 10−3 |
| Phase 2, Nd2O3 | 36.609 | 1.518 × 10−3 | 1.208 × 10−3 | |
| Sample | Particle Size Average Value (nm) |
|---|---|
| CuO | 19 |
| 1%Nd2O3 | 22 |
| 2.5%Nd2O3 | 39 |
| 5%Nd2O3 | 36 |
| 7.5%Nd2O3 | 17 |
| 10%Nd2O3 | 26 |
| Samples | K, (min−1) |
|---|---|
| Pure CuO | 0.01683 |
| 1% Nd2O3-CuO | 0.01743 |
| 2.5% Nd2O3-CuO | 0.02122 |
| 5% Nd2O3-CuO | 0.01672 |
| 7.5% Nd2O3-CuO | 0.02274 |
| 10% Nd2O3-CuO | 0.01935 |
| 7.5% Nd2O3-CuO, 1 mL H2O2 | 0.00304 |
| 7.5% Nd2O3-CuO, 2 mL H2O2 | 0.00313 |
| 7.5% Nd2O3-CuO, 3 mL H2O2 | 0.00312 |
| 7.5% Nd2O3-CuO, 4 mL H2O2 | 0.00697 |
| 7.5% Nd2O3-CuO, 5 mL H2O2 | 0.0114 |
| 7.5% Nd2O3-CuO, 2 mL H2O2, pH = 2 | 0.00161 |
| 7.5% Nd2O3-CuO, 2 mL H2O2, pH = 4 | 0.00115 |
| 7.5% Nd2O3-CuO, 2 mL H2O2, pH = 6 | 0.03666 |
| 7.5% Nd2O3-CuO, 2 mL H2O2, pH = 8 | 0.05734 |
| 7.5% Nd2O3-CuO, 2 mL H2O2, pH = 10 | 0.08209 |
| Photocatalysts | Method of Preparation | Organic Solution | Irradiation Time | Lamp Source | % Degradation | k, (min−1) | Refs. |
|---|---|---|---|---|---|---|---|
| 7.5% Nd-doped CuO | Combustion | MB | 80 min | Visible light | 90.8% | 0.0227 | Present work |
| 7.5% Nd-doped CuO at pH 4 | Combustion | MB | 80 min | Visible light | 99% | 0.082 | Present work |
| Nd2O3-doped ZnO | Hydrothermal method | MB | 60 min | UV-light | 96% | 0.3145 | [30] |
| Ag/Nd2O3-ZnO | Hydrothermal method | MB | 30 min | Visible-light | 98.12% | ------- | [17] |
| Nd2O3-doped SnO2 | Sol gel method | MB | 4 h | Visible-light | 93.1% | 0.615 h−1 | [31] |
| Nd2O3-doped ZnO | Co-precipitation | indigo carmine | 210 min | Visible-light | 74% | 3.87 × 10−3 | [32] |
| Nd2O3-ZnO-GO (0.3% Nd) | Co-precipitation | indigo carmine | 210 min | Visible-light | 95% | 1.36 × 10−2 | [32] |
| Nd–TiO2–GO (0.6% Nd) | Sol gel method | indigo carmine | 180 min | Visible-light | 92% | 13.42 × 10−3 | [33] |
| Nd-doped ZnO | Sol-gel method | MB | 25 min | UV-light | 98% | ------- | [34] |
| 3 mol%Ce3+-doped CuO | Sonochemical method | MB | 180 min | Visible-light | 98% | ------- | [35] |
| Nd-TiO2-C | Sol-gel and impregnation method | MB | 160 min | UV-light | 100% | -------- | [36] |
| Nd-doped ZnO | sol-gel method | TC | 120 min | Visible-light | 100% | -------- | [37] |
| CuO | Microwave | VB(10−5 M) DR(10−5 M) | ------- | UV-visible irradiation and US radiations | 100% | 0.09861 0.10587 | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, F.; Hussien, M.S.A.; Mohammed, M.I.; Ganesh, V.; AlAbdulaal, T.H.; Zahran, H.Y.; Yahia, I.S.; Hegazy, H.H.; Abdel-wahab, M.S.; Shkir, M.; et al. The Photocatalytic Performance of Nd2O3 Doped CuO Nanoparticles with Enhanced Methylene Blue Degradation: Synthesis, Characterization and Comparative Study. Nanomaterials 2022, 12, 1060. https://doi.org/10.3390/nano12071060
El-Sayed F, Hussien MSA, Mohammed MI, Ganesh V, AlAbdulaal TH, Zahran HY, Yahia IS, Hegazy HH, Abdel-wahab MS, Shkir M, et al. The Photocatalytic Performance of Nd2O3 Doped CuO Nanoparticles with Enhanced Methylene Blue Degradation: Synthesis, Characterization and Comparative Study. Nanomaterials. 2022; 12(7):1060. https://doi.org/10.3390/nano12071060
Chicago/Turabian StyleEl-Sayed, Fatma, Mai S. A. Hussien, Mervat I. Mohammed, Vanga Ganesh, Thekrayat H. AlAbdulaal, Heba Y. Zahran, Ibrahim S. Yahia, Hosam H. Hegazy, Mohamed Sh. Abdel-wahab, Mohd. Shkir, and et al. 2022. "The Photocatalytic Performance of Nd2O3 Doped CuO Nanoparticles with Enhanced Methylene Blue Degradation: Synthesis, Characterization and Comparative Study" Nanomaterials 12, no. 7: 1060. https://doi.org/10.3390/nano12071060







