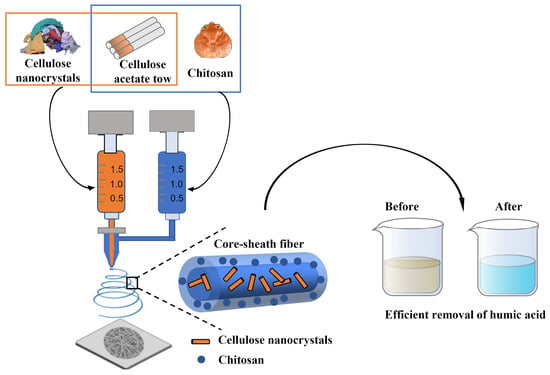
Electrospun Cellulose-Acetate/Chitosan Fibers for Humic-Acid Removal: Improved Efficiency and Robustness with a Core-Sheath Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Core-Sheath CS/CA–CNC-Fiber Fabrication
2.3. Core-Sheath CS/CA–CNC-Fiber Characterization
2.4. Adsorption Experiment
2.5. Statistical Analysis
3. Results and Discussion
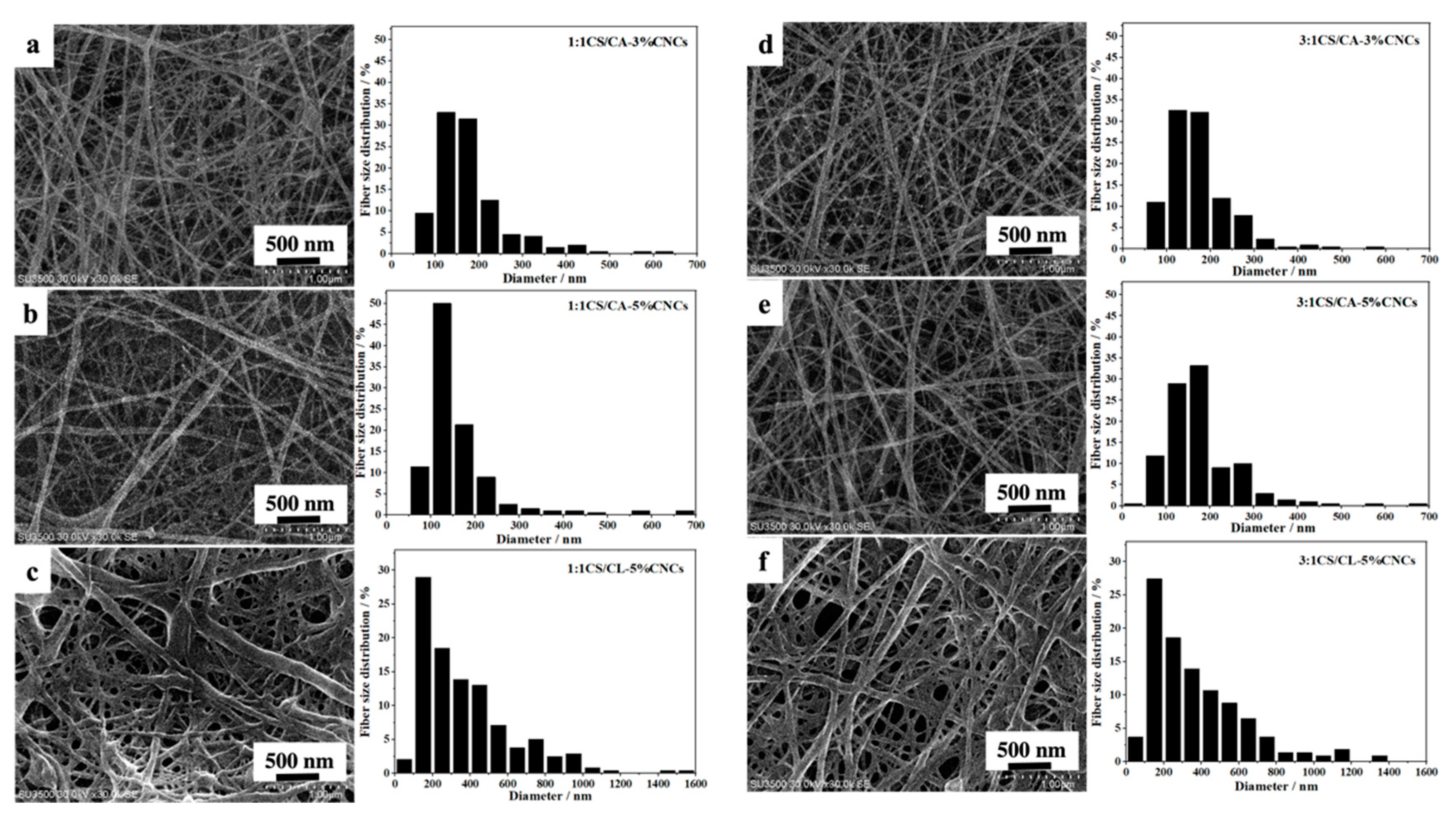
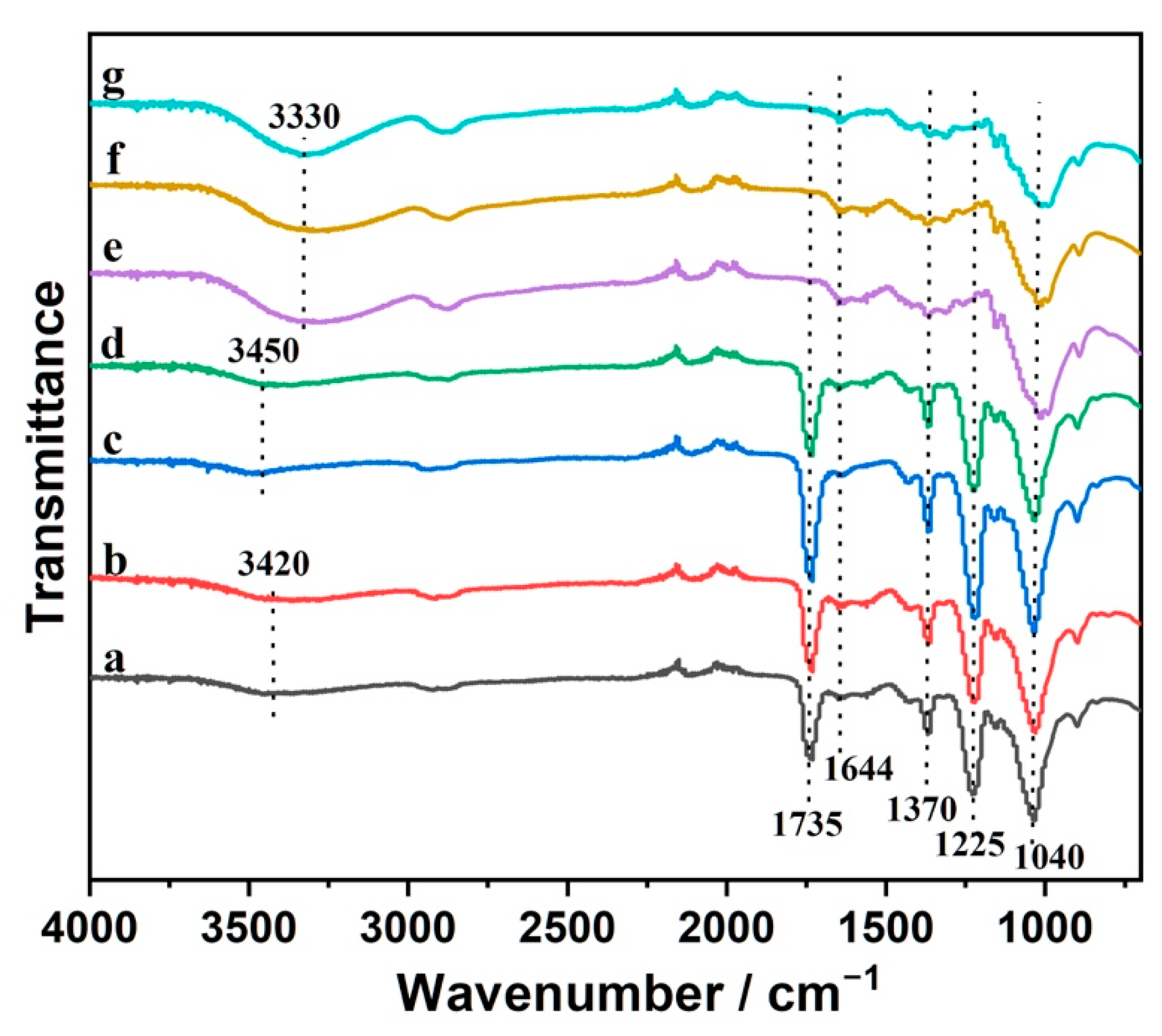
3.1. Core-Sheath CS/CA–CNC-Fiber Structure
3.2. Core-Sheath CS/CA–CNC-Fiber Mechanical Properties
3.3. Core-Sheath CS/CA–CNC-Fiber Adsorption Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osman, A.I.; Abdelkader, A.; Farrell, C.; Rooney, D.; Morgan, K. Reusing, recycling and up-cycling of biomass: A review of practical and kinetic modelling approaches. Fuel Process. Technol. 2019, 192, 179–202. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Recent progress in the conversion of biomass wastes into functional materials for value-added applications. Sci. Technol. Adv. Mater. 2020, 21, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.R.; Aloui, H.; Khomlaem, C.; Negi, A.; Yun, J.-H.; Kim, H.-S.; Kim, B.S. Biodegradable films based on chitosan and defatted Chlorella biomass: Functional and physical characterization. Food Chem. 2021, 337, 127777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, L.; Zhang, S.; Lv, Z.; Yang, D.; Liu, J.; Chen, Y.; Tian, X.; Jin, H.; Song, W. Biomass chitosan derived cobalt/nitrogen doped carbon nanotubes for the electrocatalytic oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 5740–5745. [Google Scholar] [CrossRef]
- Fico, D.; Rizzo, D.; Casciaro, R.; Corcione, C.E. A Review of Polymer-Based Materials for Fused Filament Fabrication (FFF): Focus on Sustainability and Recycled Materials. Polymers 2022, 14, 465. [Google Scholar] [CrossRef]
- Government of Canada, Statistics Canada. Results of the Bioproducts Production and Development Survey 2015 (Catalogue no. 18-001 18-001 X). 2017. Available online: https://www150.statcan.gc.ca/n1/pub/18-001-x/18-001-x2017001-eng.htm (accessed on 20 December 2021).
- Hamed, O.A.; Jodeh, S.; Al-Hajj, N.; Hamed, E.M.; Abo-Obeid, A.; Fouad, Y. Cellulose acetate from biomass waste of olive industry. J. Wood Sci. 2015, 61, 45–52. [Google Scholar] [CrossRef]
- Cao, L.; Luo, G.; Tsang, D.C.; Chen, H.; Zhang, S.; Chen, J. A novel process for obtaining high quality cellulose acetate from green landscaping waste. J. Clean. Prod. 2018, 176, 338–347. [Google Scholar] [CrossRef]
- Namboodiri, M.M.T.; Pakshirajan, K. Chapter 10—Valorization of waste biomass for chitin and chitosan production. In Waste Biorefinery; Bhaskar, T., Pandey, A., Rene, E.R., Tsang, D.C.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 241–266. [Google Scholar]
- Zhang, Y.; Wang, F.; Wang, Y. Recent developments of electrospun nanofibrous materials as novel adsorbents for water treatment. Mater. Today Commun. 2021, 27, 102272. [Google Scholar] [CrossRef]
- Hamad, A.A.; Hassouna, M.S.; Shalaby, T.; Elkady, M.F.; Elkawi, M.A.A.; Hamad, H.A. Electrospun cellulose acetate nanofiber incorporated with hydroxyapatite for removal of heavy metals. Int. J. Biol. Macromol. 2020, 151, 1299–1313. [Google Scholar] [CrossRef]
- Su, H.; Li, H.; Lin, H.; Shi, X.; Du, Y.; Luo, Y.; Deng, H. Highly sensitive formaldehyde sensors based on CuO/ZnO composite nanofibrous mats using porous cellulose acetate fibers as templates. Int. J. Biol. Macromol. 2022, 206, 653–660. [Google Scholar] [CrossRef]
- Hokmabad, V.R.; Davaran, S.; Aghazadeh, M.; Rahbarghazi, R.; Salehi, R.; Ramazani, A. Fabrication and characterization of novel ethyl cellulose-grafted-poly (ɛ-caprolactone)/alginate nanofibrous/macroporous scaffolds incorporated with nano-hydroxyapatite for bone tissue engineering. J. Biomater. Appl. 2019, 33, 1128–1144. [Google Scholar] [CrossRef] [PubMed]
- Hokmabad, V.R.; Davaran, S.; Aghazadeh, M.; Alizadeh, E.; Salehi, R.; Ramazani, A. Effect of incorporating Elaeagnus angustifolia extract in PCL-PEG-PCL nanofibers for bone tissue engineering. Front. Chem. Sci. Eng. 2019, 13, 108–119. [Google Scholar] [CrossRef]
- Goetz, L.A.; Jalvo, B.; Rosal, R.; Mathew, A.P. Superhydrophilic anti-fouling electrospun cellulose acetate membranes coated with chitin nanocrystals for water filtration. J. Membr. Sci. 2016, 510, 238–248. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Wang, Y. Electrospun Cellulose Acetate/Chitosan Fibers for Humic Acid Removal: Construction Guided by Intermolecular Interaction Study. ACS Appl. Polym. Mater. 2021, 3, 5022–5029. [Google Scholar] [CrossRef]
- Pavithra, S.; Thandapani, G.; Sugashini, S.; Sudha, P.N.; Alkhamis, H.H.; Alrefaei, A.F.; Almutairi, M.H. Batch adsorption studies on surface tailored chitosan/orange peel hydrogel composite for the removal of Cr(VI) and Cu(II) ions from synthetic wastewater. Chemosphere 2021, 271, 129415. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wang, J.; Sun, X.; Chang, Z. 3D hierarchical carbon nanofibers/TiO2@MoS2 core-shell heterostructures by electrospinning, hydrothermal and in-situ growth for flexible electrode materials. Mater. Des. 2020, 189, 108503. [Google Scholar] [CrossRef]
- Lan, T.; Shao, Z.-Q.; Wang, J.-Q.; Gu, M.-J. Fabrication of hydroxyapatite nanoparticles decorated cellulose triacetate nanofibers for protein adsorption by co-axial electrospinning. Chem. Eng. J. 2015, 260, 818–825. [Google Scholar] [CrossRef]
- Wen, H.-F.; Yang, C.; Yu, D.-G.; Li, X.-Y.; Zhang, D.-F. Electrospun zein nanoribbons for treatment of lead-contained wastewater. Chem. Eng. J. 2016, 290, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Anka, F.H.; Balkus, K.J. Novel Nanofiltration Hollow Fiber Membrane Produced via Electrospinning. Ind. Eng. Chem. Res. 2013, 52, 3473–3480. [Google Scholar] [CrossRef]
- Merkle, V.; Zeng, L.; Teng, W.; Slepian, M.; Wu, X. Gelatin shells strengthen polyvinyl alcohol core–shell nanofibers. Polymer 2013, 54, 6003–6007. [Google Scholar] [CrossRef]
- Tu, H.; Dai, F.; Cheng, G.; Yuan, M.; Zhou, X.; Wang, Y.; Zhang, R.; Zheng, Y.; Cheng, Y.; Deng, H. Incorporation of Layered Rectorite into Biocompatible Core-Sheath Nanofibrous Mats for Sustained Drug Delivery. ACS Biomater. Sci. Eng. 2021, 7, 4509–4520. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Tao, R.; Ismail, A.; Wang, Y. Cellulose Nanocrystals Derived from Textile Waste through Acid Hydrolysis and Oxidation as Reinforcing Agent of Soy Protein Film. Polymers 2020, 12, 958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yang, J.; Du, R.; Chen, L. Transition Metal Ions Enable the Transition from Electrospun Prolamin Protein Fibers to Nitrogen-Doped Freestanding Carbon Films for Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 23731–23740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, H.-J.; Lim, J.S.; Hwang, J.Y.; Kim, M.; Jeong, H.S.; Park, M.S. Carboxymethlyated cellulose nanofibrils (CMCNFs) embedded in polyurethane foam as a modular adsorbent of heavy metal ions. Carbohydr. Polym. 2018, 195, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Huang, J.; Xue, Z.; Wang, X. Electrospun graphene oxide/carbon composite nanofibers with well-developed mesoporous structure and their adsorption performance for benzene and butanone. Chem. Eng. J. 2016, 306, 99–106. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, W.; Wang, D.; Ni, X.; Han, G. Electrospun polyvinylidene fluoride-based fibrous nanocomposite membranes reinforced by cellulose nanocrystals for efficient separation of water-in-oil emulsions. J. Membr. Sci. 2018, 575, 71–79. [Google Scholar] [CrossRef]
- Jiang, L.; Li, K.; Yang, H.; Liu, X.; Li, W.; Xu, W.; Deng, B. Improving mechanical properties of electrospun cellulose acetate nanofiber membranes by cellulose nanocrystals with and without polyvinylpyrrolidone. Cellulose 2020, 27, 955–967. [Google Scholar] [CrossRef]
- Dodero, A.; Brunengo, E.; Alloisio, M.; Sionkowska, A.; Vicini, S.; Castellano, M. Chitosan-based electrospun membranes: Effects of solution viscosity, coagulant and crosslinker. Carbohydr. Polym. 2020, 235, 115976. [Google Scholar] [CrossRef]
- Ni, X.; Cheng, W.; Huan, S.; Wang, D.; Han, G. Electrospun cellulose nanocrystals/poly(methyl methacrylate) composite nanofibers: Morphology, thermal and mechanical properties. Carbohydr. Polym. 2019, 206, 29–37. [Google Scholar] [CrossRef]
- Patiño Vidal, C.; Velásquez, E.; José Galotto, M.; López de Dicastillo, C. Development of an antibacterial co-axial bionanocomposite based on electrospun core/shell fibers loaded with ethyl lauroyl arginate and cellulose nanocrystals for active food packaging. Food Packag. Shelf Life 2022, 31, 100802. [Google Scholar] [CrossRef]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Głąb, M.; Kędzierska, M.; Jaromin, A.; Mierzwiński, D.; Tyliszczak, B. Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe Vera Designed for Biomedical Use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.-N.; Lee, H.; Huang, B.; Mukai, Y.; Kim, I.-S. Fabrication of electrospun chitosan/cellulose nanofibers having adsorption property with enhanced mechanical property. Cellulose 2019, 26, 1781–1793. [Google Scholar] [CrossRef]
- Monisha, S.; Selvasekarapandian, S.; Mathavan, T.; Benial, A.M.F.; Manoharan, S.; Karthikeyan, S. Preparation and characterization of biopolymer electrolyte based on cellulose acetate for potential applications in energy storage devices. J. Mater. Sci. Mater. Electron. 2016, 27, 9314–9324. [Google Scholar] [CrossRef]
- Khatri, Z.; Wei, K.; Kim, B.-S.; Kim, I.-S. Effect of deacetylation on wicking behavior of co-electrospun cellulose acetate/polyvinyl alcohol nanofibers blend. Carbohydr. Polym. 2012, 87, 2183–2188. [Google Scholar] [CrossRef]
- Song, J.; Birbach, N.L.; Hinestroza, J. Deposition of silver nanoparticles on cellulosic fibers via stabilization of carboxymethyl groups. Cellulose 2012, 19, 411–424. [Google Scholar] [CrossRef]
- Liu, C.; Bai, R. Preparation of chitosan/cellulose acetate blend hollow fibers for adsorptive performance. J. Membr. Sci. 2005, 267, 68–77. [Google Scholar] [CrossRef]
- Zhou, C.; Girouard, F.; O’Brien, B.; Ronholm, J.; Wang, Y. Construction of chevaux-de-frise from cellulose nanocrystals to enable mechano-bactericidal activity on recycled waste cotton films. Green Chem. 2022, 24, 1109–1130. [Google Scholar] [CrossRef]
- Xie, L.; Lu, Q.; Mao, X.; Wang, J.; Han, L.; Hu, J.; Lu, Q.; Wang, Y.; Zeng, H. Probing the intermolecular interaction mechanisms between humic acid and different substrates with implications for its adsorption and removal in water treatment. Water Res. 2020, 176, 115766. [Google Scholar] [CrossRef]
- De Mesquita, J.P.; Donnici, C.L.; Pereira, F.V. Biobased Nanocomposites from Layer-by-Layer Assembly of Cellulose Nanowhiskers with Chitosan. Biomacromolecules 2010, 11, 473–480. [Google Scholar] [CrossRef]
- Tahmasebi, F.; Alimohammadi, M.; Nabizadeh, R.; Khoobi, M.; Karimian, K.; Zarei, A. Performance evaluation of graphene oxide coated on cotton fibers in removal of humic acid from aquatic solutions. Korean J. Chem. Eng. 2019, 36, 894–902. [Google Scholar] [CrossRef]
- Thuyavan, Y.L.; Anantharaman, N.; Arthanareeswaran, G.; Ismail, A.F. Adsorptive Removal of Humic Acid by Zirconia Embedded in a Poly(ether sulfone) Membrane. Ind. Eng. Chem. Res. 2014, 53, 11355–11364. [Google Scholar] [CrossRef]
- Zulfikar, M.A.; Afrita, S.; Wahyuningrum, D.; Ledyastuti, M. Preparation of Fe3O4-chitosan hybrid nano-particles used for humic acid adsorption. Environ. Nanotechnol. Monit. Manag. 2016, 6, 64–75. [Google Scholar] [CrossRef]
- Ngah, W.W.; Hanafiah, M.; Yong, S. Adsorption of humic acid from aqueous solutions on crosslinked chitosan–epichlorohydrin beads: Kinetics and isotherm studies. Colloids Surf. B Biointerfaces 2008, 65, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhan, Y. Adsorption of humic acid from aqueous solution onto unmodified and surfactant-modified chitosan/zeolite composites. Chem. Eng. J. 2012, 200–202, 202–213. [Google Scholar] [CrossRef]


| Samples | Core | Sheath | Electrospinning Conditions: | ||
|---|---|---|---|---|---|
| CA Content (wt%) | CNC Content (wt% of CA Dry Weight) | Total Solid Content (wt%) | CS/CA Ratio | Applied Voltage (kV), Tip-to-Collector Distance (cm), Flow Rate (mL/h) | |
| 1:1CS/CA–5%CNCs | 12 | 5 | 7 | 1:1 | 22, 11.5, 0.8 (sheath)-0.4 (core) |
| 1:1CS/CA–3%CNCs | 12 | 3 | 7 | 1:1 | 22, 11.5, 0.8 (sheath)-0.4 (core) |
| 3:1CS/CA–5%CNCs | 12 | 5 | 5 | 3:1 | 30, 9.5, 0.8 (sheath)-0.4 (core) |
| 3:1CS/CA–3%CNCs | 12 | 3 | 5 | 3:1 | 30, 9.5, 0.8 (sheath)-0.4 (core) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Y. Electrospun Cellulose-Acetate/Chitosan Fibers for Humic-Acid Removal: Improved Efficiency and Robustness with a Core-Sheath Design. Nanomaterials 2022, 12, 1284. https://doi.org/10.3390/nano12081284
Zhang Y, Wang Y. Electrospun Cellulose-Acetate/Chitosan Fibers for Humic-Acid Removal: Improved Efficiency and Robustness with a Core-Sheath Design. Nanomaterials. 2022; 12(8):1284. https://doi.org/10.3390/nano12081284
Chicago/Turabian StyleZhang, Yirong, and Yixiang Wang. 2022. "Electrospun Cellulose-Acetate/Chitosan Fibers for Humic-Acid Removal: Improved Efficiency and Robustness with a Core-Sheath Design" Nanomaterials 12, no. 8: 1284. https://doi.org/10.3390/nano12081284