Recent Advancements of Polyaniline/Metal Organic Framework (PANI/MOF) Composite Electrodes for Supercapacitor Applications: A Critical Review
Abstract
:1. Introduction
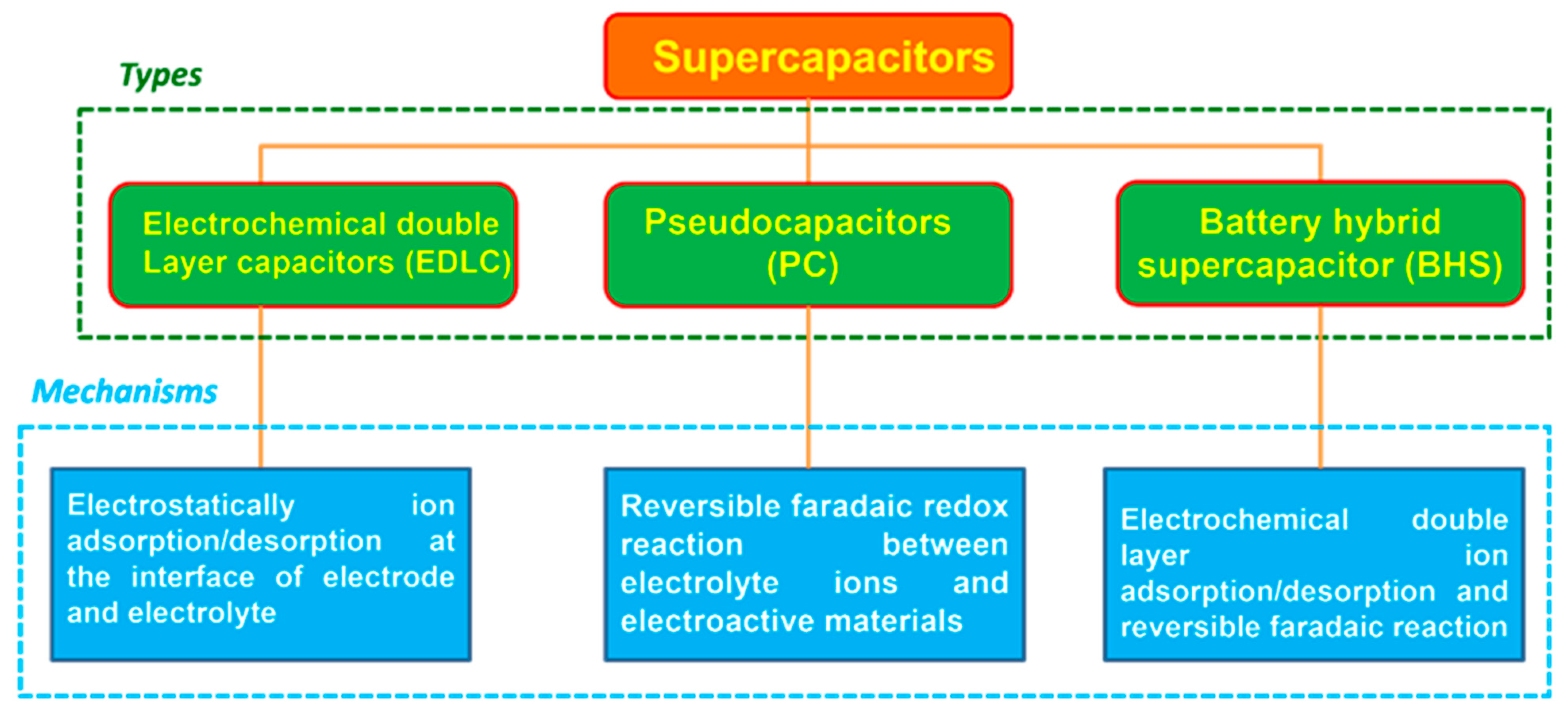
1.1. Classification of Supercapacitors
1.1.1. EDLCs
1.1.2. PCs
1.1.3. Hybrid Supercapacitors
Composite
Asymmetric
Battery Type
2. Conducting Polymers (CPs)
PANI
3. MOFs
4. PANI/MOF Composite Electrode Material for Supercapacitor Applications
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodenough, J.B. Electrochemical energy storage in a sustainable modern society. Energy Environ. Sci. 2014, 7, 14–18. [Google Scholar] [CrossRef]
- Vinodh, R.; Sasikumar, Y.; Kim, H.-J.; Atchudan, R.; Yi, M. Chitin and chitosan based biopolymer derived electrode materials for supercapacitor applications: A critical review. J. Ind. Eng. Chem. 2021, 104, 155–171. [Google Scholar] [CrossRef]
- Vinodh, R.; Gopi, C.V.V.M.; Kummara, V.G.R.; Atchudan, R.; Ahamad, T.; Sambasivam, S.; Yi, M.; Obaidat, I.M.; Kim, H.-J. A review on porous carbon electrode material derived from hypercross-linked polymers for supercapacitor applications. J. Energy Storage 2020, 32, 101831. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Kim, H.-J. Recent progress of advanced energy storage materials for flexible and wearable supercapacitor: From design and development to applications. J. Energy Storage 2020, 27, 101035. [Google Scholar]
- Kim, H.-J.; Krishna, T.; Zeb, K.; Vinodh, R.; Gopi, C.V.V.M.; Sambasivam, S.; Raghavendra, K.V.G.; Obaidat, I.M. A comprehensive review of Li-ion battery materials and their recycling techniques. Electronics 2020, 9, 1161. [Google Scholar] [CrossRef]
- Kim, I.; Vinodh, R.; Gopi, C.V.V.M.; Kim, H.-J.; Babu, R.S.; Deviprasath, C.; Devendiran, M.; Kim, S. Novel porous carbon electrode derived from hypercross-linked polymer of poly(divinylbenzene-co-vinyl benzyl chloride) for supercapacitor applications. J. Energy Storage 2021, 43, 103287. [Google Scholar] [CrossRef]
- Atchudan, R.; Samikannu, K.; Suguna, P.; Edison, T.N.J.I.; Vinodh, R.; Lee, Y.R. Aesculus turbinata biomass-originated nanoporous carbon for energy storage applications. Mater. Lett. 2022, 309, 131445. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Suguna, P.; Vinodh, R.; Babu, R.S.; Sundramoorthy, A.K.; Renita, A.A.; Lee, Y.R. Facile synthesis of nitrogen-doped porous carbon materials using waste biomass for energy storage applications. Chemosphere 2022, 289, 133225. [Google Scholar] [CrossRef]
- Vinodh, R.; Rana, P.J.S.; Gopi, C.V.V.M.; Yang, Z.; Atchudan, R.; Venkatachalam, K.; Kim, H.-J. Polyaniline-13X zeolite composite-supported platinum electrocatalysts for direct methanol fuel cell applications. Polym. Int. 2019, 68, 929–935. [Google Scholar] [CrossRef]
- Vinodh, R.; Deviprasath, C.; Gopi, C.V.V.M.; Kummara, V.G.R.; Atchudan, R.; Ahamad, T.; Kim, H.-J.; Yi, M. Novel 13X Zeolite/PANI electrocatalyst for hydrogen and oxygen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 28337–28349. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.G.; Yang, Z.; Lemmon, J.P.; Imhoff, C.; Graff, G.L.; Li, L.; Hu, J.; Wang, C.; Xiao, J.; et al. Materials science and materials chemistry for large scale electrochemical energy storage: From transportation to electrical grid. Adv. Funct. Mater. 2012, 23, 929–946. [Google Scholar] [CrossRef]
- Lin, M.-C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.-Y.; Guan, M.; Angel, M.; Chen, C.; Yang, J.; Hwang, B.-J.; et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef]
- Merlet, C.; Rotenberg, B.; Madden, P.A.; Taberna, P.-L.; Simon, P.; Gogotsi, Y.; Salanne, M. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 2012, 11, 306. [Google Scholar] [CrossRef] [Green Version]
- Farhadi, M.; Mohammed, O. Real-time operation and harmonic analysis of isolated and non-isolated hybrid DC microgrid. IEEE Trans. Ind. Appl. 2014, 50, 2900–2909. [Google Scholar] [CrossRef]
- Inthamoussou, F.A.; Pegueroles-Queralt, J.; Bianchi, F.D. Control of a Supercapacitor Energy Storage System for Microgrid Applications. IEEE Trans. Energy Convers. 2013, 28, 690–697. [Google Scholar] [CrossRef]
- Nippon Chemi-Con. Stanley Electric and Tamura Announce the Development of “Super CaLeCS,” an Environment-Friendly EDLC-Powered LED Street Lamp; Press Release Nippon Chemi-Con Corp.: Tokyo, Japan, 2010. [Google Scholar]
- Kotz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Jaafar, A.; Sareni, B.; Roboam, X.; Thiounn-Guermeur, M. Sizing of a hybrid locomotive based on accumulators and ultracapacitors. In Proceedings of the IEEE Vehicle Power and Propulsion Conference, Lille, France, 1–3 September 2010; pp. 1–6. [Google Scholar]
- Fröhlich, M.; Klohr, M.; Pagiela, S. Energy storage system with ultracaps on board of railway vehicles. In Proceedings of the 8th World Congress on Railway Research, Seoul, Korea, 18–22 May 2008. [Google Scholar]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Şahin, M.E.; Blaabjerg, F.; Sangwongwanich, A. A Comprehensive Review on Supercapacitor Applications and Developments. Energies 2022, 15, 674. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells and supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ma, Q.; Wang, S.; Liu, X.; Li, L. Poly (vinyl alcohol)-assisted fabrication of hollow carbon spheres/reduced graphene oxide nanocomposites for high-performance lithium-ion battery anodes. ACS Nano 2018, 12, 4824–4834. [Google Scholar] [CrossRef]
- Wu, F.; Yang, H.; Bai, Y.; Wu, C. Paving the path toward reliable cathode materials for aluminum-ion batteries. Adv. Mater. 2019, 31, 1806510. [Google Scholar] [CrossRef]
- Gummow, R.J.; Vamvounis, G.; Kannan, M.B.; He, Y. Calcium-ion batteries: Current state-of-the-art and future perspectives. Adv. Mater. 2018, 30, 1801702. [Google Scholar] [CrossRef]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and future perspective on electrode materials for rechargeable zinc-ion batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Palomares, V.; Casas-Cabanas, M.; Castillo-Martínez, E.; Han, M.H.; Rojo, T. Update on Na-based battery materials. A growing research path. Energy Environ. Sci. 2013, 6, 2312–2337. [Google Scholar] [CrossRef]
- Xiong, T.; Yu, Z.G.; Wu, H.; Du, Y.; Xie, Q.; Chen, J.; Zhang, Y.W.; Pennycook, S.J.; Lee, W.S.V.; Xue, J. Defect engineering of oxygen-deficient manganese oxide to achieve high-performing aqueous zinc ion battery. Adv. Energy Mater. 2019, 9, 1803815. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Liu, X.; Li, L. Biomass derived interconnected hierarchical micro-meso-macro-porous carbon with ultrahigh capacitance for supercapacitor. Carbon 2019, 147, 540–549. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Wang, S.; Liu, X.; Li, L. Microwave/freeze casting assisted fabrication of carbon frameworks derived from embedded upholder in tremella for superior performance supercapacitors. Energy Storage Mater. 2019, 18, 447–455. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, S.; Li, L.; Dou, S. Bio-nanotechnology in high-performance supercapacitors. Adv. Energy Mater. 2017, 7, 1700592. [Google Scholar] [CrossRef]
- Beguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Miller, J.R.; Burke, A.F. Electrochemical capacitors: Challenges and opportunities for real-world applications. Electrochem. Soc. Interface 2008, 17, 53–57. [Google Scholar] [CrossRef]
- Park, S.; Jayaraman, S. Smart textiles: Wearable electronic systems. MRS Bull. 2003, 28, 585–591. [Google Scholar] [CrossRef]
- Shim, B.S.; Chen, W.; Doty, C.; Xu, C.; Kotov, N.A. Smart electronic yarns and wearable fabrics for human biomonitoring made by carbon nanotube coating with polyelectrolytes. Nano Lett. 2008, 8, 4151–4157. [Google Scholar] [CrossRef]
- Selvaraj, A.R.; Muthusamy, A.; Kim, H.-J.; Senthil, K.; Prabakar, K. Ultrahigh surface area biomass derived 3D hierarchical porous carbon nanosheet electrodes for high energy density supercapacitors. Carbon 2021, 174, 463–474. [Google Scholar] [CrossRef]
- Chmiola, J.; Largeot, C.; Taberna, P.-L.; Simon, P.; Gogotsi, Y. Monolithic Carbide-Derived Carbon Films for Micro-Supercapacitors. Science 2010, 328, 480–483. [Google Scholar] [CrossRef] [Green Version]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.-L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.W.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H.M. 3D Aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. Int. Ed. 2008, 47, 373–376. [Google Scholar] [CrossRef]
- Wang, Y.; Foo, C.Y.; Hoo, T.K.; Ng, M.; Lin, J. Designed smart system of the sandwiched and concentric architecture of RuO2/C/RuO2 for high performance in electrochemical energy storage. Chem. Eur. J. 2010, 16, 3598–3603. [Google Scholar] [CrossRef]
- Ramya, R.; Sivasubramanian, R.; Sangaranarayanan, M. Conducting polymers-based electrochemical supercapacitors—Progress and prospects. Electrochim. Acta 2013, 101, 109–129. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Dubal, D.P.; Holze, R. Self-assembly of stacked layers of Mn3O4 nanosheets using a scalable chemical strategy for enhanced, flexible, electrochemical energy storage. J. Power Sources 2013, 238, 274–282. [Google Scholar] [CrossRef]
- Dubal, D.P.; Kim, J.G.; Kim, Y.; Holze, R.; Kim, W.B. Demonstrating the highest supercapacitive performance of branched MnO2 nanorods grown directly on flexible substrates using controlled chemistry at ambient temperature. Energy Technol. 2013, 1, 125–130. [Google Scholar] [CrossRef]
- Gund, G.S.; Dubal, D.P.; Patil, B.H.; Shinde, S.S.; Lokhande, C.D. Enhanced activity of chemically synthesized hybrid graphene oxide/Mn3O4 composite for high performance supercapacitors. Electrochim. Acta 2013, 92, 205–215. [Google Scholar] [CrossRef]
- Kiamahalleh, M.V.; Zein, S.H.S.; Najafpour, G.; SATA, S.A.; Buniran, S. Multiwalled carbon nanotubes based nanocomposites for supercapacitors: A review of electrode materials. Nano 2012, 7, 1230002. [Google Scholar] [CrossRef]
- Halper, M.S.; Ellenbogen, J.C. Supercapacitors: A Brief Overview; The MITRE Corporation: McLean, VA, USA, 2006; pp. 1–34. [Google Scholar]
- Choi, H.; Yoon, H. Nanostructured electrode materials for electrochemical capacitor applications. Nanomaterials 2015, 5, 906–936. [Google Scholar] [CrossRef] [Green Version]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nanosci. Technol. 2009, 320–329. [Google Scholar]
- Kulandaivalu, S.; Sulaiman, Y. Recent advances in layer-by-layer assembled conducting polymer based composites for supercapacitors. Energies 2019, 12, 2107. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.I.; Sunarso, J.; Wong, B.T.; Lin, H.; Yu, A.; Jia, B. Towards enhanced energy density of graphene-based supercapacitors: Current status, approaches, and future directions. J. Power Sources 2018, 396, 182–206. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.H.; Tasfiah Azad, A.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Wu, Y.; Ran, F. Vanadium nitride quantum dot/nitrogen-doped microporous carbon nanofibres electrode for high-performance supercapacitors. J. Power Sources 2017, 344, 1–10. [Google Scholar] [CrossRef]
- Xianwen, M.; Hatton, T.A.; Gregory, C.R. A reviewof electrospun carbon fibres as electrode materials for energy storage. Curr. Org. Chem. 2013, 17, 1390–1401. [Google Scholar]
- Tong, L.; Liu, J.; Boyer, S.M.; Sonnenberg, L.A.; Fox, M.T.; Ji, D.; Feng, J.; Bernier, W.E.; Jones Jr, W.E. Vapor-phase polymerized poly(3,4-ethylenedioxythiophene) (PEDOT)/TiO2 composite fibres as electrode materials for supercapacitors. Electrochim. Acta 2017, 224, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.M.; Ramachandran, R.; Mani, V.; Saraswathi, R. Recent advancements in electrode materials for the high-performance electrochemical supercapacitors: A review. Int. J. Electrochem. Sci. 2014, 9, 4072–4085. [Google Scholar]
- Gan, J.K.; Lim, Y.S.; Pandikumar, A.; Huang, N.M.; Lim, H.N. Graphene/polypyrrole coated carbon nanofibre core–shell architecture electrode for electrochemical capacitors. RSC Adv. 2015, 5, 12692–12699. [Google Scholar] [CrossRef]
- Chang, W.-M.; Wang, C.-C.; Chen, C.-Y. Plasma-induced polyaniline grafted on carbon nanotube-embedded carbon nanofibres for high-performance supercapacitors. Electrochim. Acta 2016, 212, 130–140. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, C.; Song, X.; Liu, J.; Zhao, L.; Zhang, P.; Gao, L. Engineering the volumetric effect of Polypyrrole for auto-deformable supercapacitor. Chem. Eng. J. 2019, 374, 59–67. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Chen, Z.; Zhong, L. Cellulose carbon aerogel/PPy composites for highperformance supercapacitor. Carbohydr. Polym. 2019, 215, 322–329. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, J.; Bai, B.; Qiu, A.; Losic, D.; Shi, D.; Chen, M. Free-standing PEDOT/polyaniline conductive polymer hydrogel for flexible solid-state supercapacitors. Electrochim. Acta 2019, 322, 134769. [Google Scholar] [CrossRef]
- Wang, H.; Gao, M.; Zhu, Y.; Zhou, H.; Liu, H.; Gao, L.; Wu, M. A flexible 3-D structured carbon molecular sieve@PEDOT composite electrode for supercapacitor. J. Electroanal. Chem. 2018, 826, 191–197. [Google Scholar] [CrossRef]
- Ravit, R.; Abdullah, J.; Ahmad, I.; Sulaiman, Y. Electrochemical performance of poly(3, 4-ethylenedioxythipohene)/nanocrystalline cellulose (PEDOT/NCC) film for supercapacitor. Carbohydr. Polym. 2019, 203, 128–138. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, V. Current technology of supercapacitors: A review. J. Electron. Mater. 2020, 49, 3520–3532. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.C.; Yu, F.; Zhao, X.S.; Wang, H. Boosting the cycling stability of transition metal compounds-based supercapacitors. Energy Storage Mater. 2019, 16, 545–573. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Xu, C.; Chen, H. A review on the synthesis of CuCo2O4-based electrode materials and their applications in supercapacitors. J. Mater. 2021, 7, 98–126. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, F.; Yan, Q.; Wu, X. Investigation on electrochemical behaviors of NiCo2O4 battery-type supercapacitor electrodes: The role of an aqueous electrolyte. Inorg. Chem. Front. 2017, 4, 1642–1648. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Sun, J.; Zhang, Y.; Chen, H.; Xu, C. Simple solvothermal synthesis of magnesium cobaltite microflowers as a battery grade material with high electrochemical performances. Ceram. Int. 2019, 45, 14642–14651. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Ryu, K.S. CuCo2O4 flowers/Ni-foam architecture as a battery type positive electrode for high performance hybrid supercapacitor applications. Electrochim. Acta 2017, 238, 99–106. [Google Scholar] [CrossRef]
- Sun, J.; Li, S.; Han, X.; Liao, F.; Zhang, Y.; Gao, L.; Chen, H.; Xu, C. Rapid hydrothermal synthesis of snowflake-like ZnCo2O4/ZnO mesoporous microstructures with excellent electrochemical performances. Ceram. Int. 2019, 45, 12243–12250. [Google Scholar] [CrossRef]
- Guo, X.; Chen, C.; Zhang, Y.; Xu, Y.; Pang, H. The application of transition metal cobaltites in electrochemistry. Energy Storage Mater. 2019, 23, 439–465. [Google Scholar] [CrossRef]
- Ryu, K.S.; Kim, K.M.; Park, N.G.; Park, Y.J.; Chang, S.H. Symmetric redox supercapacitor with conducting polyaniline electrodes. J. Power Sources 2002, 103, 305–309. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.X.; Kaner, R.B. Polyaniline nanofibers: A unique polymer nanostructure for versatile applications. Acc. Chem. Res. 2008, 42, 135–145. [Google Scholar] [CrossRef]
- Huang, J.X.; Kaner, R.B. Nanofiber formation in the chemical polymerization of aniline: A mechanistic study. Angew. Chem. 2004, 116, 5941–5945. [Google Scholar] [CrossRef]
- Chiou, N.R.; Epstein, A.J. Polyaniline nanofibers prepared by dilute polymerization. Adv. Mater. 2005, 17, 1679–1683. [Google Scholar] [CrossRef]
- Huang, J.X.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.X.; Kaner, R.B. A general chemical route to polyaniline nanofibers. J. Am. Chem. Soc. 2004, 126, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Sivakkumar, S.R.; Kim, W.J.; Choi, J.A.; Macfarlane, D.R.; Forsyth, M.; Kim, D.W. Electrochemical performance of polyaniline nanofibres and polyaniline/multi-walled carbon nanotube composite as an electrode material for aqueous redox supercapacitors. J. Power Sources 2007, 171, 1062–1068. [Google Scholar] [CrossRef]
- Li, H.L.; Wang, J.X.; Chu, Q.X.; Wang, Z.; Zhang, F.B.; Wang, S.C. Theoretical and experimental specific capacitance of polyaniline in sulfuric acid. J. Power Sources 2009, 190, 578–586. [Google Scholar] [CrossRef]
- Xu, J.J.; Wang, K.; Zu, S.Z.; Han, B.H.; Wei, Z.X. Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef]
- Meng, C.Z.; Liu, C.H.; Chen, L.Z.; Hu, C.H.; Fan, S.S. Highly flexible and all-solid-state paperlike polymer supercapacitors. Nano Lett. 2010, 10, 4025–4031. [Google Scholar] [CrossRef]
- Srinivasan, R.; Elaiyappillai, E.; Nixon, E.J.; Lydia, I.S.; Johnson, P.M. Enhanced electrochemical behaviour of Co-MOF/PANI composite electrode for supercapacitors. Inorg. Chim. Acta 2020, 502, 119393. [Google Scholar] [CrossRef]
- Maier, M.A.; Babu, R.S.; Sampaio, D.M.; de Barros, A.L.F. Binder-free polyaniline interconnected metal hexacyanoferrates nanocomposites (Metal = Ni, Co) on carbon fibers for flexible supercapacitors. J. Mater. Sci. Mater. Electron. 2017, 28, 17405–17413. [Google Scholar] [CrossRef]
- Babu, R.S.; de Barros, A.L.F.; Maier, M.A.; Sampaio, D.M.; Balamurugan, J.; Lee, J.H. Novel polyaniline/manganese hexacyanoferrate nanoparticles on carbon fiber as binder-free electrode for flexible supercapacitors. Compos. B Eng. 2018, 143, 141–147. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.-C.J.; Kitagawa, S. Metal-organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.-R.; Suslick, K.S. Chapter nine—Mechanochemical reactions of metal-organic frameworks. Adv. Inorg Chem. 2018, 71, 403–434. [Google Scholar]
- Chul, H.D.; Vinodh, R.; Gopi, C.V.V.M.; Deviprasath, C.; Kim, H.-J.; Yi, M. Effect of the cobalt and zinc ratio on the preparation of zeolitic imidazole frameworks (ZIFs): Synthesis, characterization and supercapacitor applications. Dalton Trans. 2019, 48, 14808–14819. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, P.; Bharadwaj, L.M.; Paul, A.; Deep, A. Luminescent nanocrystal metal organic framework based biosensor for molecular recognition. Inorg. Chem. Commun. 2014, 43, 114–117. [Google Scholar] [CrossRef]
- Shown, I.; Ganguly, A.; Chen, L.C.; Chen, K.H. Conducting polymer-based flexible supercapacitor. Energy Sci. Eng. 2015, 3, 2–26. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; You, J.; Park, M.-S.; Al Hossain, M.S.; Yamauchi, Y.; Kim, J.H. Conductive polymers for next-generation energy storage systems: Recent progress and new functions. Mater. Horiz. 2016, 3, 517–535. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Wu, X.; Luo, C.; Wang, Q.; Li, J.; Hu, L. Conducting polymer coated metal-organic framework nanoparticles: Facile synthesis and enhanced electromagnetic absorption properties. Synth. Met. 2017, 228, 18–24. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Ren, L.; Piao, Q.; Zhong, J.; Wang, Y.; Li, H.; Chen, Y.; Wang, B. Flexible solid-state supercapacitor based on a metal-organic framework interwoven by electrochemically-deposited PANI. J. Am. Chem. Soc. 2015, 137, 4920–4923. [Google Scholar] [CrossRef]
- Xu, M.; Guo, H.; Zhang, T.; Zhang, J.; Wang, X.; Yang, W. High-performance zeolitic imidazolate frameworks derived three-dimensional Co3S4/polyaniline nanocomposite for supercapacitors. J. Energy Storage 2021, 35, 102303. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Ali, S.R.; Farid, S.; Afzal, A.M. Co-MOF/polyaniline-based electrode material for high performance supercapattery devices. Electrochim. Acta 2020, 346, 136039. [Google Scholar] [CrossRef]
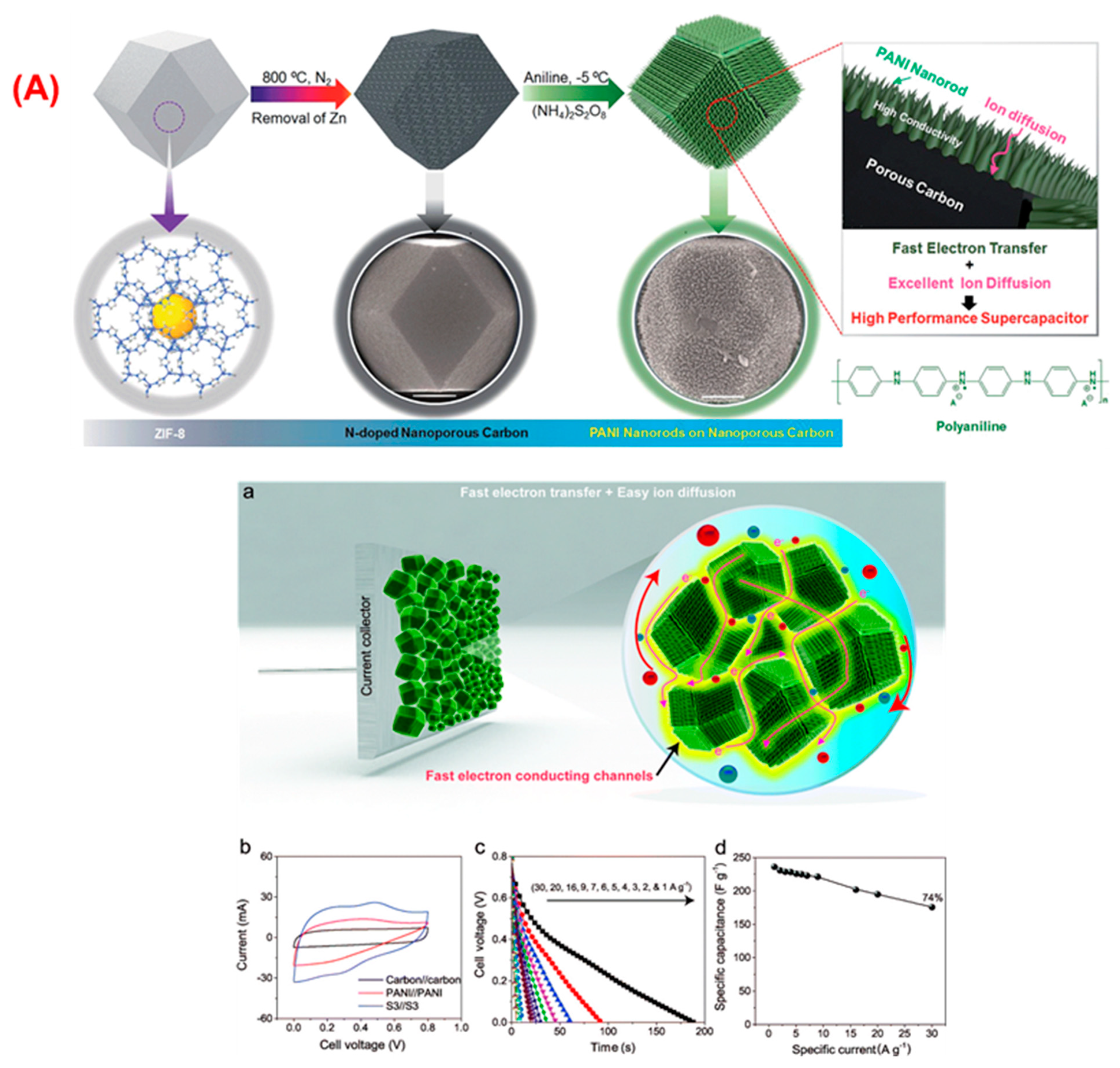
- Yao, M.; Zhao, X.; Zhang, Q.; Zhang, Y.; Wang, Y. Polyaniline nanowires aligned on MOFs-derived nanoporous carbon as high-performance electrodes for supercapacitor. Electrochim. Acta 2021, 390, 138804. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Tang, J.; Kobayashi, N.; Kim, J.; Ide, Y.; Tominaka, S.; Kim, J.H.; Yamauchi, Y. Ultrahigh performance supercapacitors utilizing core–shell nanoarchitectures from a metal–organic framework-derived nanoporous carbon and a conducting polymer. Chem. Sci. 2016, 7, 5704–5713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantin, A.M.; Gavrilov, N.; Pasti, I.A.; Moravkova, Z.; Acharya, U.; Unterweger, C.; Breitenbach, S.; Zhigunov, A.; Bober, P. Polyaniline-metal organic framework (Fe-BTC) composite for electrochemical applications. Polymer 2020, 208, 122945. [Google Scholar]
- Wang, M.; Ma, Y.; Ye, J. Controllable layer-by-layer assembly of metal-organic frameworks/polyaniline membranes for flexible solid-state microsupercapacitors. J. Power Sources 2020, 474, 228681. [Google Scholar] [CrossRef]
- Guo, S.N.; Zhu, Y.; Yan, Y.Y.; Min, Y.L.; Fan, J.C.; Xu, Q.J.; Yun, H. (Metal-Organic Framework)-Polyaniline sandwich structure composites as novel hybrid electrode materials for high-performance supercapacitor. J. Power Sources 2016, 316, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Wang, Q.; Ma, Z.; Ji, Z.; Wang, X.; Song, D.; Liu, Y.; Wang, N. A high-capacitance flexible solid-state supercapacitor based on polyaniline and metal-organic framework (UiO-66) composites. J. Power Sources 2018, 379, 350–361. [Google Scholar] [CrossRef]
- Liu, P.-Y.; Zhao, J.-J.; Dong, Z.-P.; Liu, Z.-L.; Wang, Y.-Q. Interwoving polyaniline and a metal-organic framework grown in situ for enhanced supercapacitor behavior. J. Alloys Compd. 2021, 854, 157181. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Ouyang, K.; Xu, Z. Two-Dimensional Metal-organic framework nanosheets grown on carbon fiber paper interwoven with polyaniline as an electrode for supercapacitors. Energy Fuels 2021, 35, 19818–19826. [Google Scholar] [CrossRef]
- Udayan, A.P.M.; Sadak, O.; Gunasekaran, S. Metal-organic framework/polyaniline nanocomposites for lightweight energy storage. ACS Appl. Energy Mater. 2020, 3, 12368–12377. [Google Scholar] [CrossRef]
- Neisi, Z.; Ansari-Asl, Z.; Dezfuli, A.S. Polyaniline/Cu(II) metal-organic frameworks composite for high performance supercapacitor electrode. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1838–1847. [Google Scholar] [CrossRef]
- Tian, D.; Wang, C.; Lu, X. Metal—Organic frameworks and their derived functional materials for supercapacitor electrode application. Adv. Energy Sustain. Res. 2021, 2, 2100024. [Google Scholar] [CrossRef]
- Gong, J.; Xu, Z.; Tang, Z.; Zhong, J.; Zhang, L. Highly compressible 3-D hierarchical porous carbon nanotube/metal organic framework/polyaniline hybrid sponges supercapacitors. AIP Adv. 2019, 9, 055032. [Google Scholar] [CrossRef]
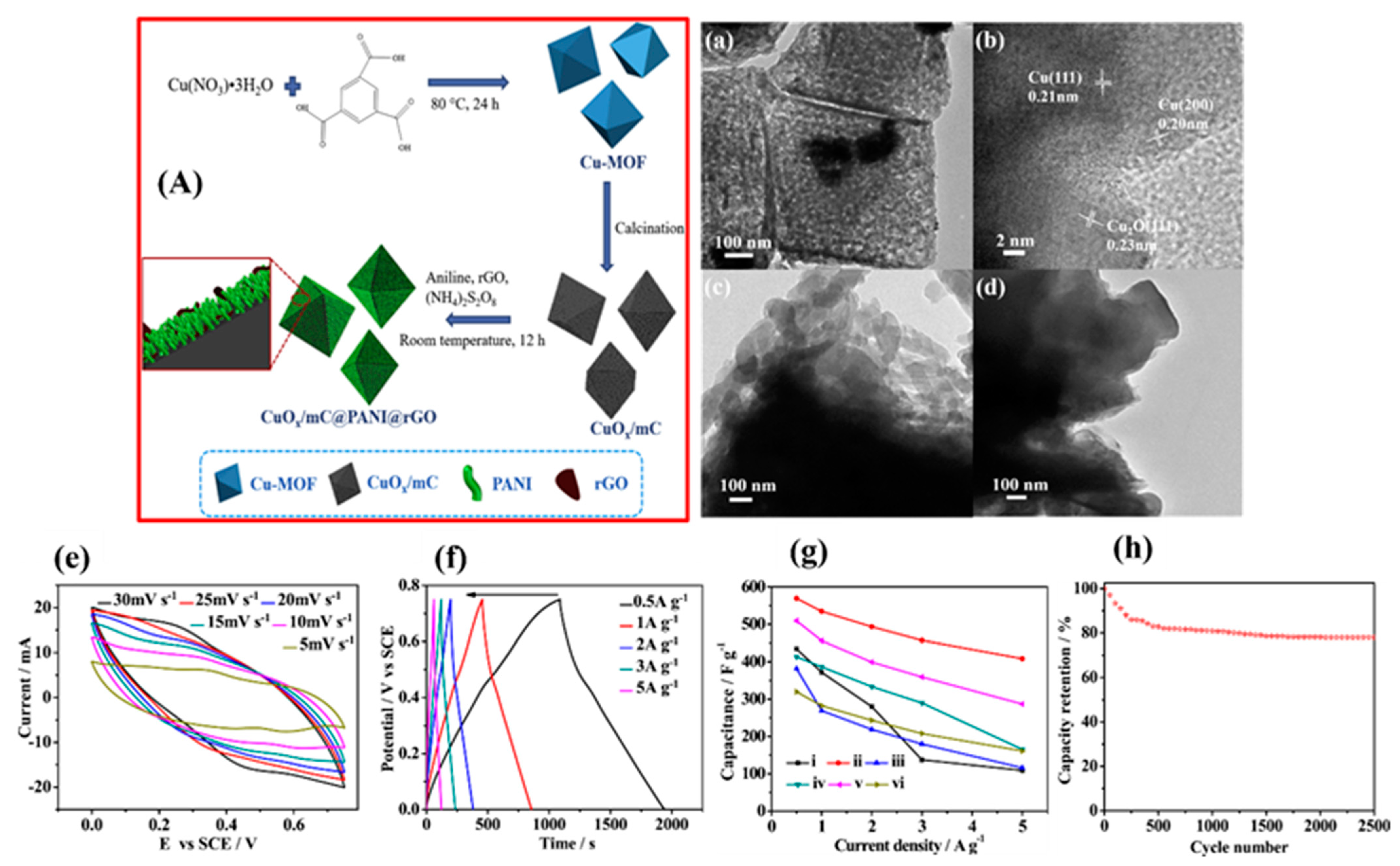
- He, L.; Liu, J.; Yang, L.; Song, Y.; Wang, M.; Peng, D.; Zhang, Z.; Fang, S. Copper metal-organic framework-derived CuOx-coated three-dimensional reduced graphene oxide and polyaniline composite: Excellent candidate free-standing electrodes for high-performance supercapacitors. Electrochim. Acta 2018, 275, 133–144. [Google Scholar] [CrossRef]
- Wang, Y.G.; Li, H.Q.; Xia, Y.Y. Ordered whisker like polyaniline grown on the surface of mesoporous carbon and its electrochemical capacitance performance. Adv. Mater. 2006, 18, 2619–2623. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Jin, L.-N.; Wang, H.-T.; Kang, X.-H.; Bian, S.-W. Fabrication of three-dimensional composite textile electrodes by metal-organic framework, zinc oxide, graphene and polyaniline for all-solid-state supercapacitors. J. Colloid Interface Sci. 2018, 530, 29–36. [Google Scholar] [CrossRef]
- Sundriyal, S.; Kaur, H.; Bhardwaj, S.K.; Mishra, S.; Kim, K.-H.; Deep, A. Metal-organic frameworks and their composites as efficient electrodes for supercapacitor applications. Coord. Chem. Rev. 2018, 369, 15–38. [Google Scholar] [CrossRef]
- Fu, D.; Li, H.; Zhang, X.-M.; Han, G.; Zhou, H.; Chang, Y. Flexible solid-state supercapacitor fabricated by metal-organic framework/graphene oxide hybrid interconnected with PEDOT. Mater. Chem. Phys. 2016, 179, 166–173. [Google Scholar] [CrossRef]
- Mulzer, C.R.; Shen, L.; Bisbey, R.P.; McKone, J.R.; Zhang, N.; Abruña, H.D.; Dichtel, W.R. Superior Charge storage and power density of a conducting polymer-modified covalent organic framework. ACS Cent. Sci. 2016, 2, 667–673. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, H.; Li, Z.; Sun, P.; Liu, F.; Dong, C.; Wang, J.; Li, Z.; Wu, M.; Zhang, C.; et al. Synthesis of delaminated layered double hydroxides and their assembly with graphene oxide for supercapacitor application. J. Alloys Compd. 2017, 711, 31–41. [Google Scholar] [CrossRef]
- Yu, L.; Shi, N.; Liu, Q.; Wang, J.; Yang, B.; Wang, B.; Yan, H.; Sun, Y.; Jing, X. Facile synthesis of exfoliated Co–Al LDH–carbon nanotube composites with high performance as supercapacitor electrodes. Phys. Chem. Chem. Phys. 2014, 16, 17936–17942. [Google Scholar] [CrossRef] [PubMed]
- Vinodh, R.; Babu, R.S.; Atchudan, R.; Kim, H.-J.; Yi, M.; Samyn, L.M.; de Barros, A.L.F. Fabrication of High-Performance Asymmetric Supercapacitor Consists of Nickel Oxide and Activated Carbon (NiO//AC). Catalysts 2022, 12, 375. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Thirukumaran, P.; Vinodh, R.; Lee, Y.R. Green synthesis of nitrogen-doped carbon nanograss for supercapacitors. J. Taiwan Inst. Chem. Eng. 2019, 102, 475–486. [Google Scholar] [CrossRef]
- Xing, T.; Ouyang, Y.; Chen, Y.; Zheng, L.; Wu, C.; Wang, X. P-doped ternary transition metal oxide as electrode material of asymmetric supercapacitor. J. Energy Storage 2020, 28, 101248. [Google Scholar] [CrossRef]
- Masikhwa, T.M.; Barzegar, F.; Dangbegnon, J.K.; Bello, A.; Madito, M.J.; Momodu, D.; Manyala, N. Asymmetric supercapacitor based on VS2 nanosheets and activated carbon materials. RSC Adv. 2016, 6, 38990–39000. [Google Scholar] [CrossRef] [Green Version]
- Neeraj, N.S.; Mordina, B.; Srivastava, A.K.; Mukhopadhyay, K.; Prasad, N.E. Impact of process condi-tions on the electrochemical performances of NiMoO4 nanorods and activated carbon based asymmetric supercapacitor. Appl. Surf. Sci. 2019, 473, 807–819. [Google Scholar] [CrossRef]
- Gao, L.; Xiong, L.; Xu, D.; Cai, J.; Huang, L.; Zhou, J.; Zhang, L. Distinctive Construction of Chitin-Derived Hierarchically Porous Carbon Microspheres/Polyaniline for High-Rate Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 28918–28927. [Google Scholar] [CrossRef]
- Salleh, N.A.; Kheawhom, S.; Mohamad, A.A. Chitosan as biopolymer binder for graphene in superca-pacitor electrode. Results Phys. 2021, 25, 104244. [Google Scholar] [CrossRef]


| S. No. | Electrode Materials | Specific Capacitance | Electrolyte | Specific Energy | Specific Power | Cyclability/ Capacitance Retention | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 3-ES | 2-ES | |||||||
| 1 | PANI-ZIF-67/CC | 2146 mF cm−2 @ 10 mV s−1 | SSC: 35 mF cm−2 | 3-ES: 3 M KCl; SSC: H2SO4/PVA | 0.0161 mWh cm−3 | 0.833 W cm−3 | 2000 GCD cycles; 80% | [93] |
| 2 | Co3S4/PANI | 106 F g−1 @ 1 A g−1 | ASC: 114.6 F g−1 @ 1 A g−1 | 3-ES and ASC: 6 M KOH | 40.75 Wh kg−1 | 800 W kg−1 | 20,000 GCD cycles; 88% | [94] |
| 3 | MOF/PANI | 162.5 C g−1 @ 0.4 A g−1 | ASC: 104.5 C g−1 @ 1 A g−1 | 3-ES and ASC: 1 M KOH | 23.2 Wh kg−1 | 1600 W kg−1 | 3000 GCD cycles; 146% | [95] |
| 4 | PC-MOFs/PANI | 534.16 F g−1 @ 0.2 A g−1 | SSC: 140 F g−1 @ 0.2 A g−1 | SSC: H2SO4/PVA | 9.72 μWh cm−2 | 199.99 μW cm−2 | 10,000 cycles; 94.4% | [96] |
| 5 | MOF/PANI | 1100 F g−1@ 1 mV s−1 | SSC: 236 140 F g−1 @ 1 A g−1 | 1 M H2SO4 | 21 Wh kg−1 | 400 W kg−1 | 20,000 GCD cycles; 86% | [97] |
| 6 | PANI/Fe-BTC | 346 F g−1 @ 20 mV s−1 | --- | 0.5 M H2SO4 | --- | --- | --- | [98] |
| 7 | MOF/PANI | 719.2 mF cm−2 @ 0.5 mA cm−2 | MSCs: 528.5 mF cm−2 @ 10 mA cm−2 | MSCs: H2SO4/PVA | 443.7 mW cm−3 | 3218.4 μW cm−2 | 6000 GCD cycles; 87.6% | [99] |
| 8 | Zn-MOF/PANI | 477 F g−1 @ 1 A g−1 | --- | 1 M H2SO4 | --- | --- | --- | [100] |
| 9 | PANI/UiO-66 | 1015 F g−1 @ 1 A g−1 | SSC: 647 F g−1 @ 1 A g−1 | H2SO4/PVA | 78.8 Wh kg−1 | 200 W kg−1 | 5000 GCD cycles; 91% | [101] |
| 10 | ZIF-67@PANI | 2497 F g−1 @ 1 A g−1 | SSC: 512 F g−1 @ 1 A g−1 | KOH | 71.1 Wh kg−1 | 504.72 W kg−1 | 9000 GCD cycles; 92.3% | [102] |
| 11 | CFP/ZIF-L/PANI | 681 mF cm−2 @ 1 mA cm−2 | --- | 3 M KCl | --- | --- | 3000 GCD cycles; 82.6% | [103] |
| 12 | ZIF-8/PANI | 395.4 F g−1 @ 0.2 A g−1 | ASC: 28.1 mF cm−2 @ 0.1 mA cm−2 | 1 M H2SO4 | 3.2 μWh cm−2 | 1.1 mW cm−2 | 1000 GCD cycles; 78.4% | [104] |
| 13 | PANI/Cu-MOF | 734 F g−1 @ 5 mV s−1 | --- | 6 M KOH | --- | --- | 4000 GCD cycles; 98% | [105] |
| 14 | CNT/MOF/PANI | 342.5 F g−1 @ 1 A g−1 | --- | --- | 28.9 Wh kg−1 | ~800 W kg−1 | --- | [107] |
| 15 | CuOx@mC@PANI@rGO | 534.5 F g−1@ 1 A g−1 | --- | 1 M H2SO4 | --- | --- | 2500 GCD cycles; 80% | [108] |
| 16 | PANI/ZnO/ZIF-8/G/PC | 1.378 F cm−2 @ 1 mA cm−1 | SSC: --- | SSC: H2SO4/PVA | 235 μWh cm−3 | 1542 μW cm−3 | --- | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinodh, R.; Babu, R.S.; Sambasivam, S.; Gopi, C.V.V.M.; Alzahmi, S.; Kim, H.-J.; de Barros, A.L.F.; Obaidat, I.M. Recent Advancements of Polyaniline/Metal Organic Framework (PANI/MOF) Composite Electrodes for Supercapacitor Applications: A Critical Review. Nanomaterials 2022, 12, 1511. https://doi.org/10.3390/nano12091511
Vinodh R, Babu RS, Sambasivam S, Gopi CVVM, Alzahmi S, Kim H-J, de Barros ALF, Obaidat IM. Recent Advancements of Polyaniline/Metal Organic Framework (PANI/MOF) Composite Electrodes for Supercapacitor Applications: A Critical Review. Nanomaterials. 2022; 12(9):1511. https://doi.org/10.3390/nano12091511
Chicago/Turabian StyleVinodh, Rajangam, Rajendran Suresh Babu, Sangaraju Sambasivam, Chandu V. V. Muralee Gopi, Salem Alzahmi, Hee-Je Kim, Ana Lucia Ferreira de Barros, and Ihab M. Obaidat. 2022. "Recent Advancements of Polyaniline/Metal Organic Framework (PANI/MOF) Composite Electrodes for Supercapacitor Applications: A Critical Review" Nanomaterials 12, no. 9: 1511. https://doi.org/10.3390/nano12091511