Enhancement of Perovskite Solar Cells by TiO2-Carbon Dot Electron Transport Film Layers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Fabrication of TiO2 Blocking Layer, C-Dot, and Perovskite Solar Cells
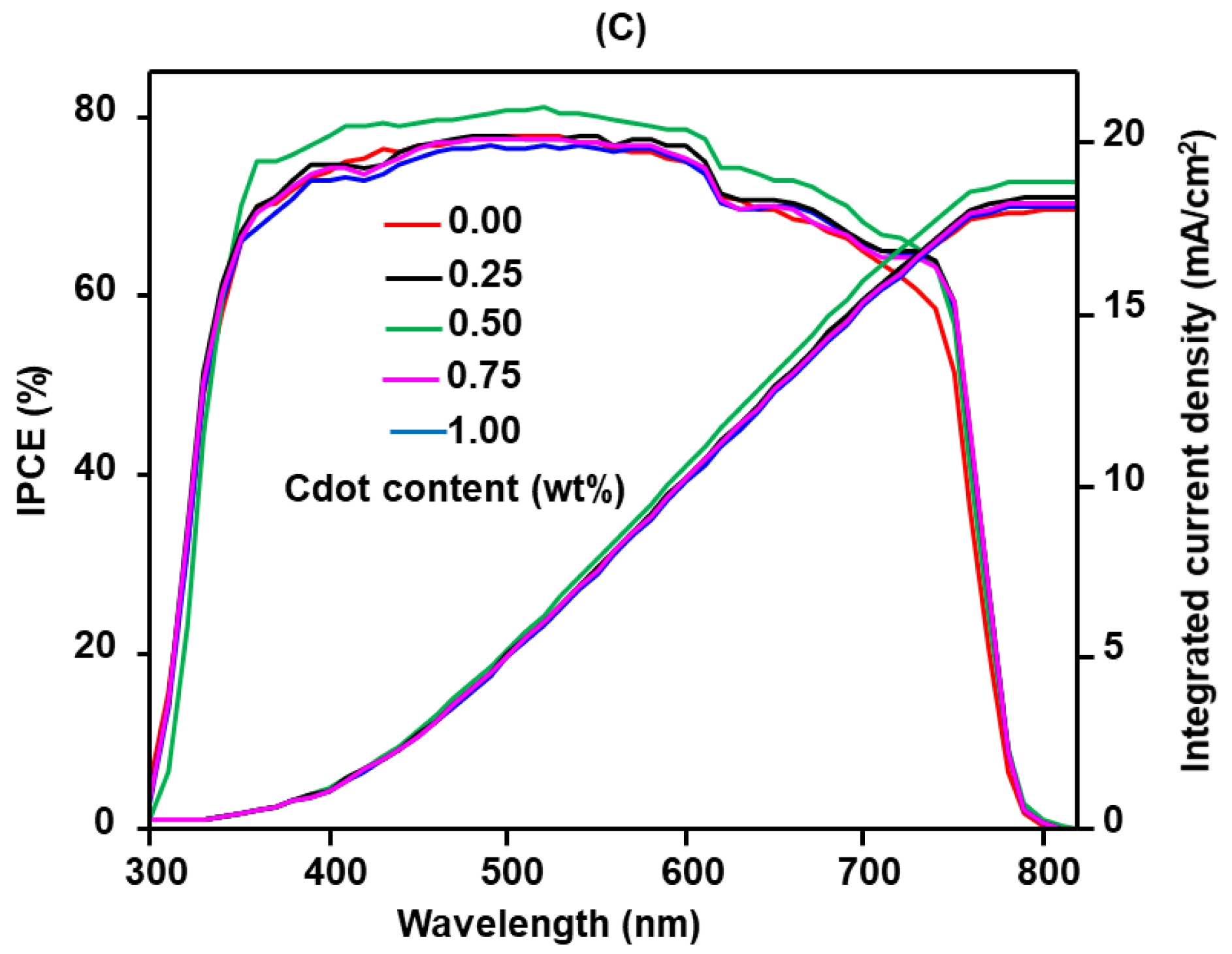
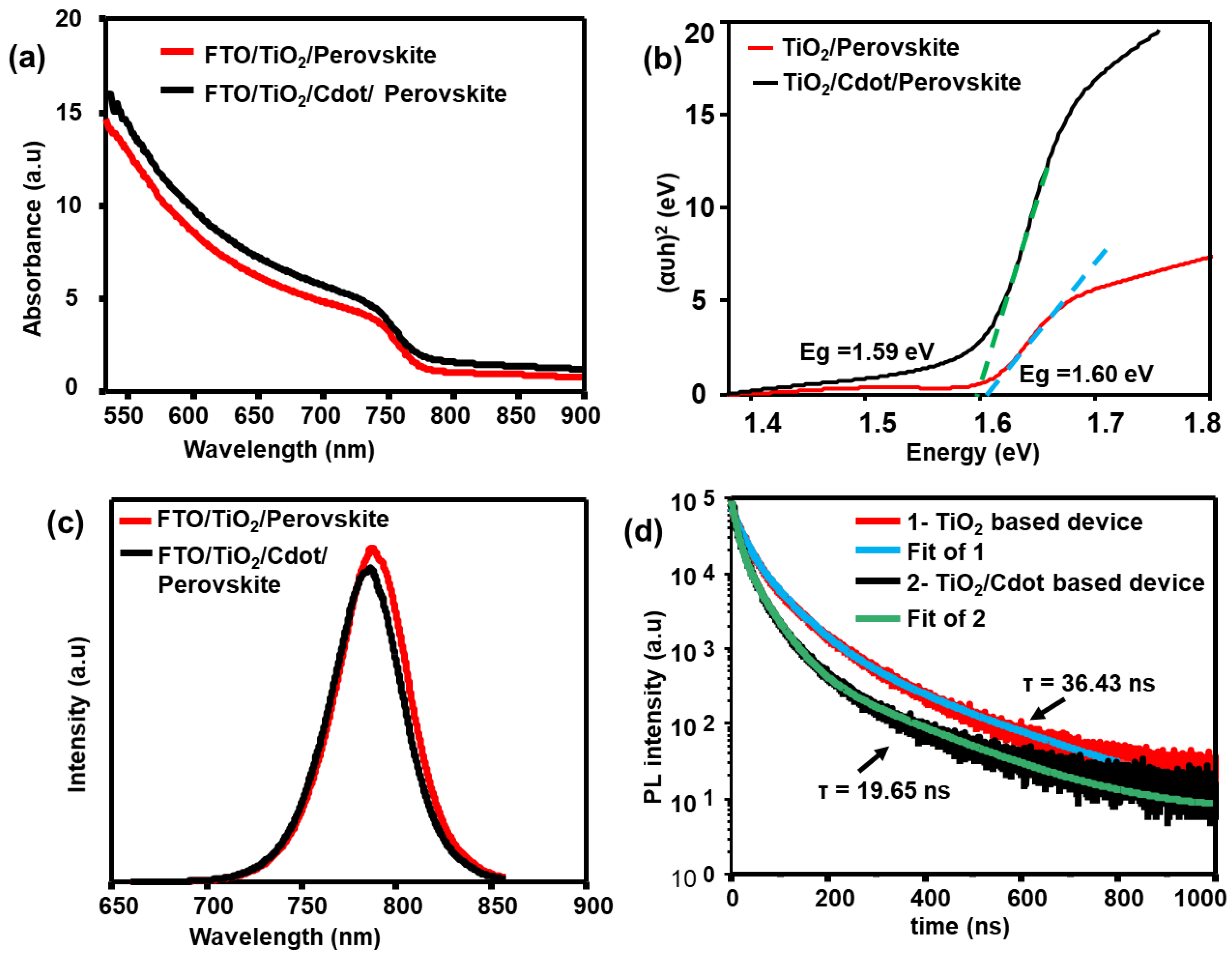
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lim, K.-G.; Lee, T.-W. Planar heterojunction organometal halide perovskite solar cells: Roles of interfacial layers. Energy Environ. Sci. 2016, 9, 12–30. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef]
- Mazzarella, L.; Lin, Y.H.; Kirner, S.; Morales-Vilches, A.B.; Korte, L.; Albrecht, S.; Crossland, E.; Stannowski, B.; Case, C.; Snaith, H.J. Infrared light management using a nanocrystalline silicon oxide interlayer in monolithic perovskite/silicon heterojunction tandem solar cells with efficiency above 25%. Adv. Energy Mater. 2019, 9, 1803241. [Google Scholar] [CrossRef]
- Yang, Z.; Chueh, C.-C.; Liang, P.-W.; Crump, M.; Lin, F.; Zhu, Z.; Jen, A.K.-Y. Effects of formamidinium and bromide ion substitution in methylammonium lead triiodide toward high-performance perovskite solar cells. Nano Energy 2016, 22, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Kelly, T.L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat. Photonics 2014, 8, 133–138. [Google Scholar] [CrossRef]
- You, J.; Hong, Z.; Yang, Y.; Chen, Q.; Cai, M.; Song, T.-B.; Chen, C.-C.; Lu, S.; Liu, Y.; Zhou, H. Low-temperature solution-processed perovskite solar cells with high efficiency and flexibility. ACS Nano. 2014, 8, 1674–1680. [Google Scholar] [CrossRef]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.; Kanatzidis, M.G. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photonics 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Nagaoka, H.; Ma, F.; Dequilettes, D.W.; Vorpahl, S.M.; Glaz, M.S.; Colbert, A.E.; Ziffer, M.E.; Ginger, D.S. Zr incorporation into TiO2 electrodes reduces hysteresis and improves performance in hybrid perovskite solar cells while increasing carrier lifetimes. J. Phys. Chem. Lett. 2015, 6, 669–675. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, J.; Ding, J.; Hu, J.S.; Wang, D. A rutile TiO2 electron transport layer for the enhancement of charge collection for efficient perovskite solar cells. Angew. Chem. Int. Ed. 2019, 58, 9414–9418. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J. Low-temperature solution-processed tin oxide as an alternative electron transporting layer for efficient perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tian, W.; Gu, B.; Zhu, Y.; Li, L. TiO2 electron transport bilayer for highly efficient planar perovskite solar cell. Small 2017, 13, 1701535. [Google Scholar] [CrossRef]
- Cao, J.; Wu, B.; Chen, R.; Wu, Y.; Hui, Y.; Mao, B.W.; Zheng, N. Efficient, hysteresis-free, and stable perovskite solar cells with ZnO as electron-transport layer: Effect of surface passivation. Adv. Mater. Technol. 2018, 30, 1705596. [Google Scholar] [CrossRef] [PubMed]
- Zhen, C.; Wu, T.; Chen, R.; Wang, L.; Liu, G.; Cheng, H.-M. Strategies. Sustain. Chem. Eng. 2019, 7, 4586–4618. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, K.; Sun, H.; Gu, B.; Lu, H.; Cao, F.; Xiong, J.; Li, L. TiO2 phase junction electron transport layer boosts efficiency of planar perovskite solar cells. Adv. Sci. 2018, 5, 1700614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parida, B.; Singh, A.; Oh, M.; Jeon, M.; Kang, J.-W.; Kim, H. Effect of compact TiO2 layer on structural, optical, and performance characteristics of mesoporous perovskite solar cells. Mater. Today 2019, 18, 176–183. [Google Scholar] [CrossRef]
- Prochowicz, D.; Tavakoli, M.M.; Wolska-Pietkiewicz, M.; Jędrzejewska, M.; Trivedi, S.; Kumar, M.; Zakeeruddin, S.M.; Lewiński, J.; Graetzel, M.; Yadav, P. Suppressing recombination in perovskite solar cells via surface engineering of TiO2 ETL. Solar Energy 2020, 197, 50–57. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. C 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Efa, M.T.; Imae, T. Effects of carbon dots on ZnO nanoparticle-based dye-sensitized solar cells. Electrochim. Acta 2019, 303, 204–210. [Google Scholar] [CrossRef]
- Etefa, H.F.; Imae, T.; Yanagida, M. Enhanced photosensitization by carbon dots Co-adsorbing with dye on p-type semiconductor (Nickel Oxide) solar cells. ACS Appl. Mater. Interfaces 2020, 12, 18596–18608. [Google Scholar] [CrossRef] [PubMed]
- Geleta, T.A.; Imae, T. Nanocomposite photoanodes consisting of p-NiO/n-ZnO heterojunction and carbon quantum dot additive for dye-sensitized solar cells. ACS Appl. Nano Mater. 2021, 4, 236–249. [Google Scholar] [CrossRef]
- Chang, C.C.; Geleta, T.A.; Imae, T. Effect of carbon dots on supercapacitor performance of carbon nanohorn/conducting polymer composites. Bull. Chem. Soc. Jpn. 2021, 94, 454–462. [Google Scholar] [CrossRef]
- Do, T.T.A.; Grijalvo, S.; Imae, T.; Garcia-Celma, M.J.; Rodríguez-Abreu, C. A nanocellulose-based platform towards targeted chemo-photodynamic/photothermal cancer therapy. Carbohydr. Polym. 2021, 270, 118366. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-W.; Chen, Y.-H.; Li, M.-H.; Wu, Y.-S.; Chiu, P.-T.; Lin, Y.-P.; Tung, Y.-L.; Tsai, S.-Y.; Chen, P. Low-temperature growth of uniform ultrathin TiO2 blocking layer for efficient perovskite solar cell. Org. Electron. 2019, 75, 105379. [Google Scholar] [CrossRef]
- Ren, H.; Zou, X.; Cheng, J.; Ling, T.; Bai, X.; Chen, D. Facile solution spin-coating SnO2 thin film covering cracks of TiO2 hole blocking layer for perovskite solar cells. Coatings 2018, 8, 314. [Google Scholar] [CrossRef] [Green Version]
- Pang, S.; Zhang, C.; Zhang, H.; Dong, H.; Chen, D.; Zhu, W.; Xi, H.; Chang, J.; Lin, Z.; Zhang, J. Boosting performance of perovskite solar cells with Graphene quantum dots decorated SnO2 electron transport layers. Appl. Surf. Sci. 2020, 507, 145099. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M. Pseudo-halide anion engineering for α-FAPbI 3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Feng, Y.; Zhang, X.; Liu, J.; Hou, W.; Jia, J.; Zhang, B. XPS study on changes of lead on the channel surface of microchannel plate reduced by hydrogen. IOP Conf. Ser. Mater. Sci. Eng. C 2019, 490, 022067. [Google Scholar] [CrossRef]
- McGettrick, J.D.; Hooper, K.; Pockett, A.; Baker, J.; Troughton, J.; Carnie, M.; Watson, T. Sources of Pb (0) artefacts during XPS analysis of lead halide perovskites. Mater. Lett. 2019, 251, 98–101. [Google Scholar] [CrossRef]
- Ma, J.; Qin, M.; Li, Y.; Zhang, T.; Xu, J.; Fang, G.; Lu, X. Guanidinium doping enabled low-temperature fabrication of high-efficiency all-inorganic CsPbI2 Br perovskite solar cells. J. Mater. Chem. 2019, 7, 27640–27647. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Hong, C.K. A-Site Rubidium Cation-Incorporated CsPbI2Br All- Inorganic Perovskite Solar Cells Exceeding 17% Efficiency. Solar RRL 2020, 4, 2000164. [Google Scholar] [CrossRef]
- Chen, P.; Bai, Y.; Wang, S.; Lyu, M.; Yun, J.H.; Wang, L. In situ growth of 2D perovskite capping layer for stable and efficient perovskite solar cells. Adv. Funct. Mater. 2018, 28, 1706923. [Google Scholar] [CrossRef]
- Ashraf, A.; Dastgheib, S.A.; Mensing, G.; Shannon, M.A. Surface characteristics of selected carbon materials exposed to supercritical water. J. Supercrit. Fluids 2013, 76, 32–40. [Google Scholar] [CrossRef]
- Pham, N.D.; Singh, A.; Chen, W.; Hoang, M.T.; Yang, Y.; Wang, X.; Wolff, A.; Wen, X.; Jia, B.; Sonar, P. Self-assembled carbon dot-wrapped perovskites enable light trapping and defect passivation for efficient and stable perovskite solar cells. J. Mater. Chem. A 2021, 9, 7508–7521. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Bi, D.; Gao, P.; Scopelliti, R.; Oveisi, E.; Luo, J.; Grätzel, M.; Hagfeldt, A.; Nazeeruddin, M.K. High-performance perovskite solar cells with enhanced environmental stability based on amphiphile-modified CH3NH3PbI3. Adv. Mater. 2016, 28, 2910–2915. [Google Scholar] [CrossRef]
- Gao, N.; Huang, L.; Li, T.; Song, J.; Hu, H.; Liu, Y.; Ramakrishna, S. Application of carbon dots in dye-sensitized solar cells: A review. J. Appl. Polym. Sci. 2020, 137, 48443. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Bao, Z.; Tang, W.; Wu, H.; Pan, J.; Hu, J.; Zeng, H. Surface states engineering carbon dots as multi-band light active sensitizers for ZnO nanowire array photoanode to boost solar water splitting. Carbon 2017, 121, 201–208. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, J.; Wang, Z.; Mu, C.; Fan, Z.; Du, L.; Bai, Y.; Fan, L.; Yan, H.; Phillips, D.L. Efficiency enhancement of perovskite solar cells through fast electron extraction: The role of graphene quantum dots. J. Am. Chem. Soc. 2014, 136, 3760–3763. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, E.; Vallés-Pelarda, M.; Hames, B.C.; Sanchez, R.S.; Barea, E.M.; Maghraoui-Meherzi, H.; Mora-Seró, I. Transformation of PbI2, PbBr2 and PbCl2 salts into MAPbBr3 perovskite by halide exchange as an effective method for recombination reduction. Phys. Chem. Chem. Phys. 2017, 19, 10913–10921. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zheng, E.; Bian, J.; Wang, X.-F.; Tian, W.; Sanehira, Y.; Miyasaka, T. Low-temperature SnO2-based electron selective contact for efficient and stable perovskite solar cells. J. Mater. Chem. A 2015, 3, 10837–10844. [Google Scholar] [CrossRef]
- Li, C.; Wang, F.; Xu, J.; Yao, J.; Zhang, B.; Zhang, C.; Xiao, M.; Dai, S.; Li, Y.; Tan, Z.A. Efficient perovskite/fullerene planar heterojunction solar cells with enhanced charge extraction and suppressed charge recombination. Nanoscale 2015, 7, 9771–9778. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Kapil, G.; Sakamoto, K.; Hirotani, D.; Kamrudin, M.A.; Wang, Z.; Hamada, K.; Nomura, D.; Kang, H.-G.; Nagayoshi, H. Efficient, hysteresis free, inverted planar flexible perovskite solar cells via perovskite engineering and stability in cylindrical encapsulation. Sustain. Energy Fuels 2019, 3, 1739–1748. [Google Scholar] [CrossRef]
- Liang, P.W.; Liao, C.Y.; Chueh, C.C.; Zuo, F.; Williams, S.T.; Xin, X.K.; Lin, J.; Jen, A.K.Y. Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells. Adv. Mater. 2014, 26, 3748–3754. [Google Scholar] [CrossRef]
- Wu, R.; Yang, J.; Xiong, J.; Liu, P.; Zhou, C.; Huang, H.; Gao, Y.; Yang, B. Efficient electron-blocking layer-free planar heterojunction perovskite solar cells with a high open-circuit voltage. Org. Electron. 2015, 26, 265–272. [Google Scholar] [CrossRef]
- Wang, J.-F.; Zhu, L.; Zhao, B.-G.; Zhao, Y.-L.; Song, J.; Gu, X.-Q.; Qiang, Y.-H. Surface engineering of perovskite films for efficient solar cells. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Huang, X.; Chee, M.; Hao, F.; Dong, P. Carbon-based perovskite solar cells: From single-junction to modules. Carbon Energy 2019, 1, 109–123. [Google Scholar] [CrossRef]

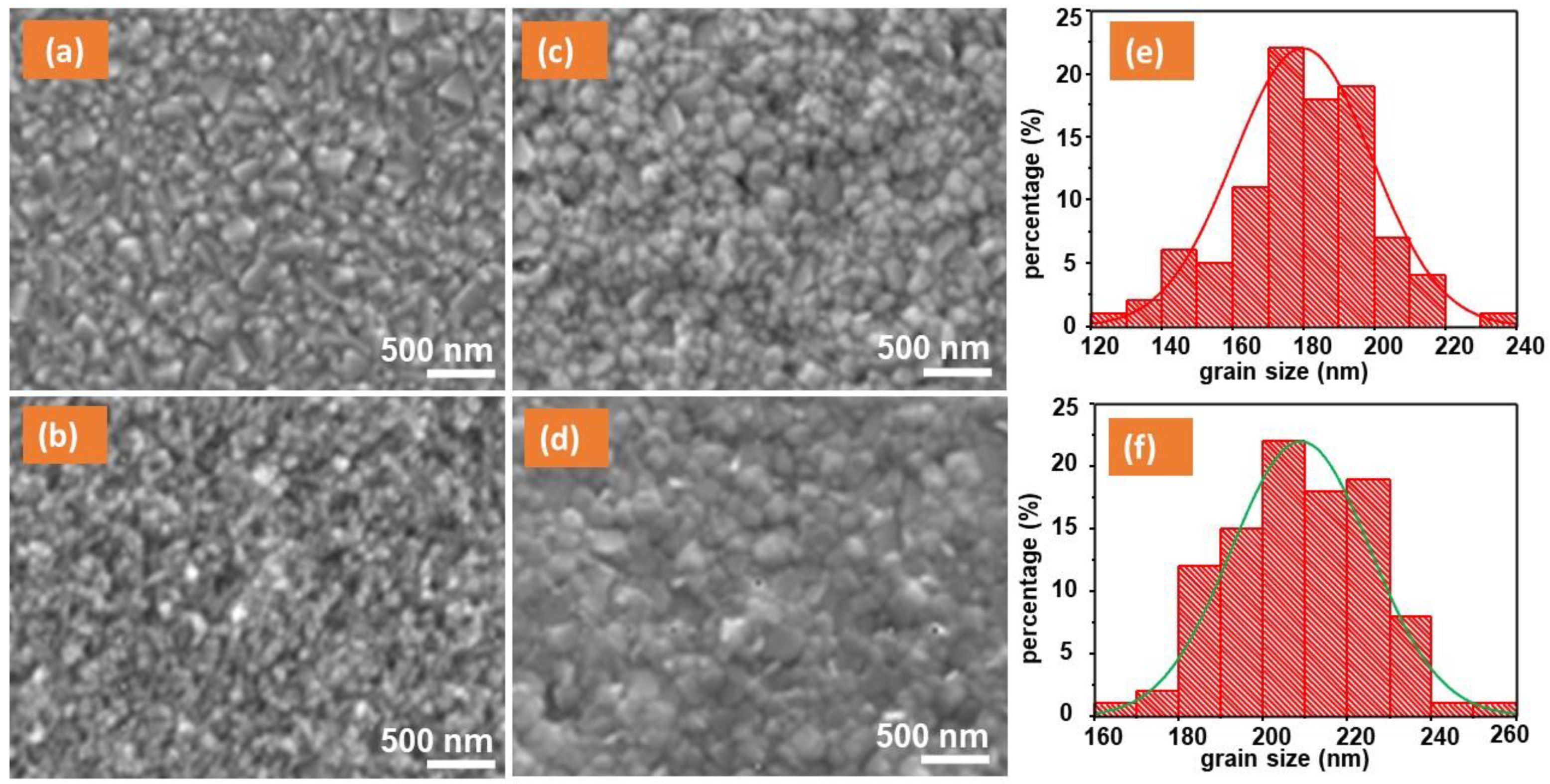
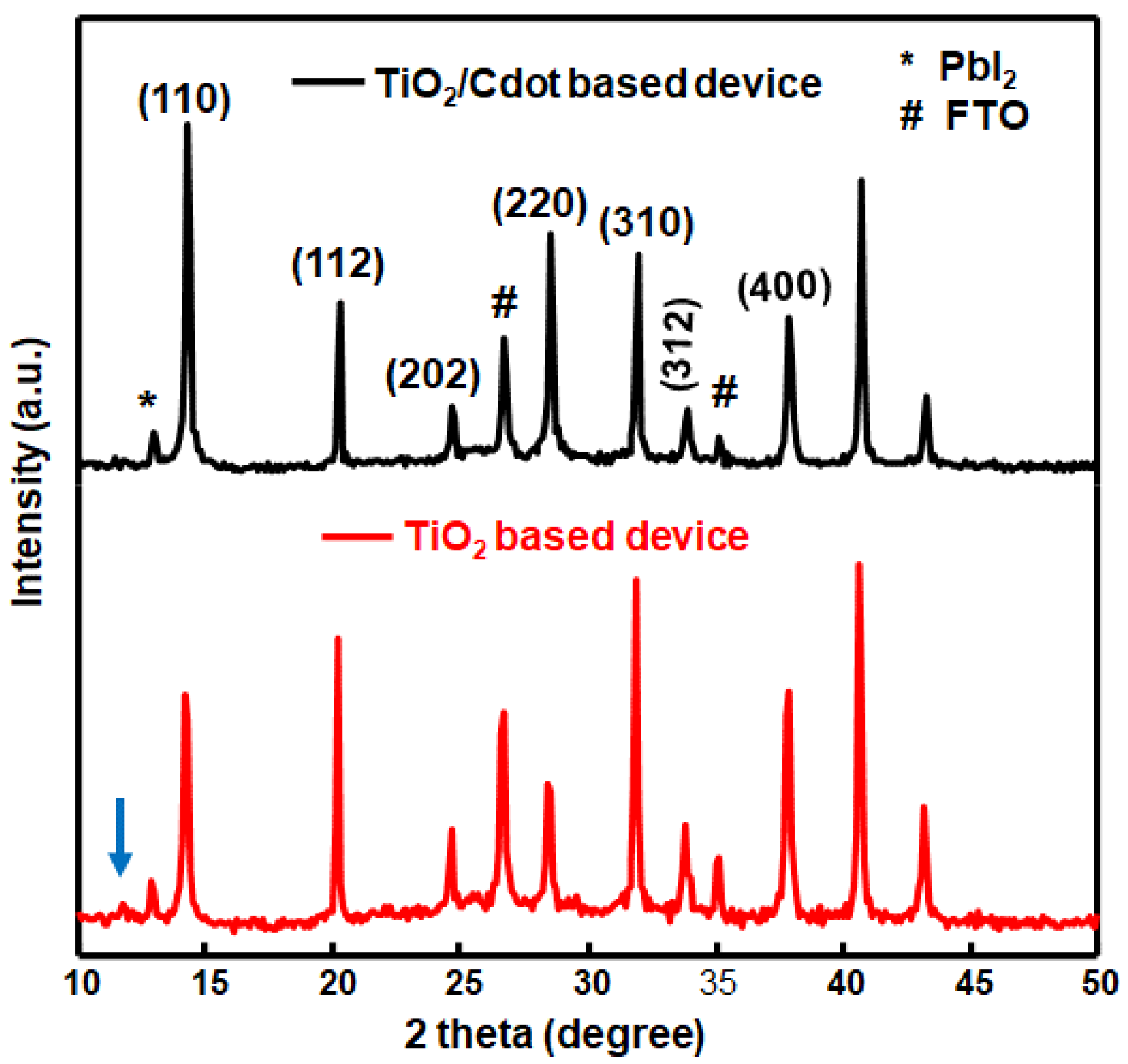
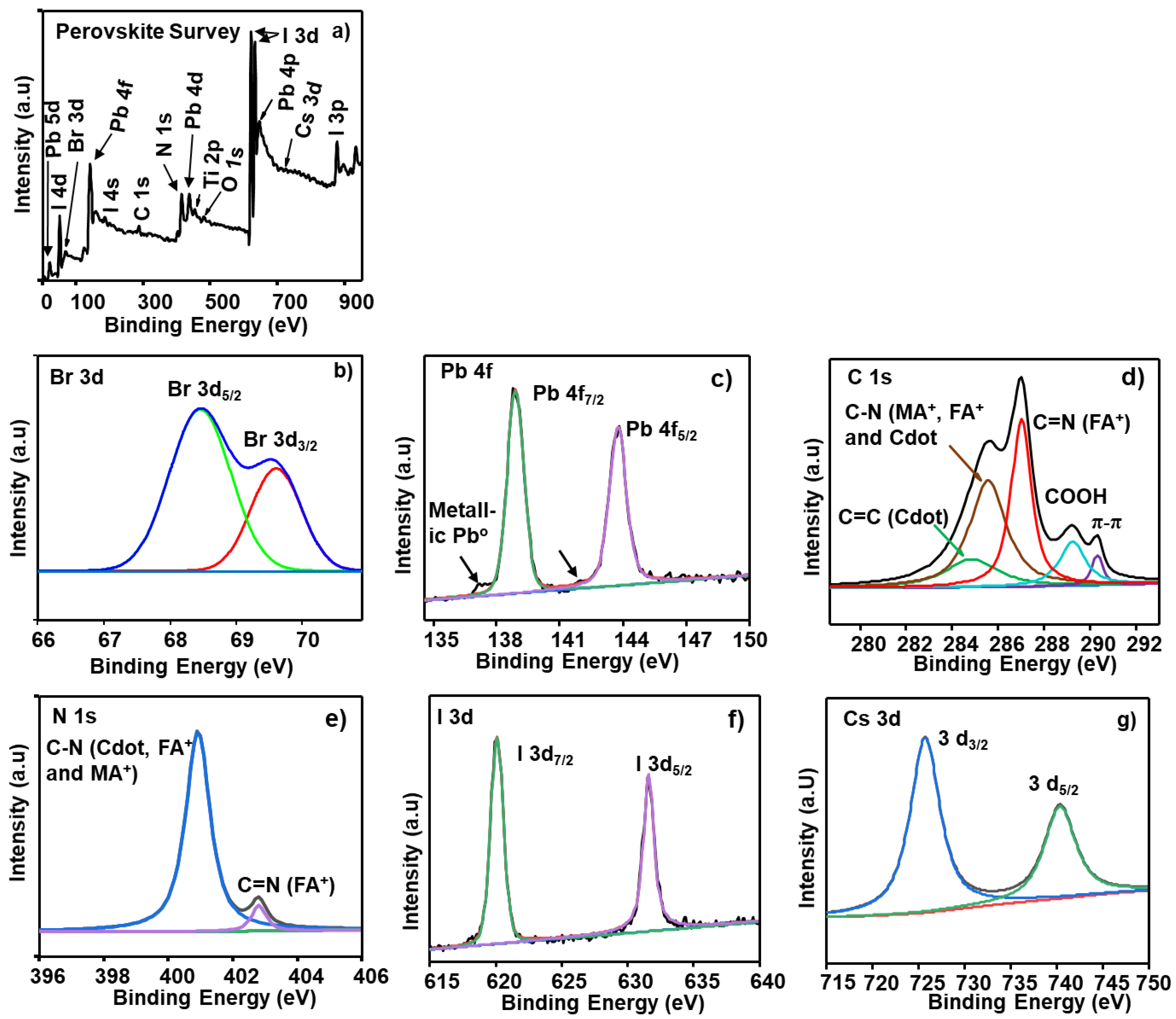
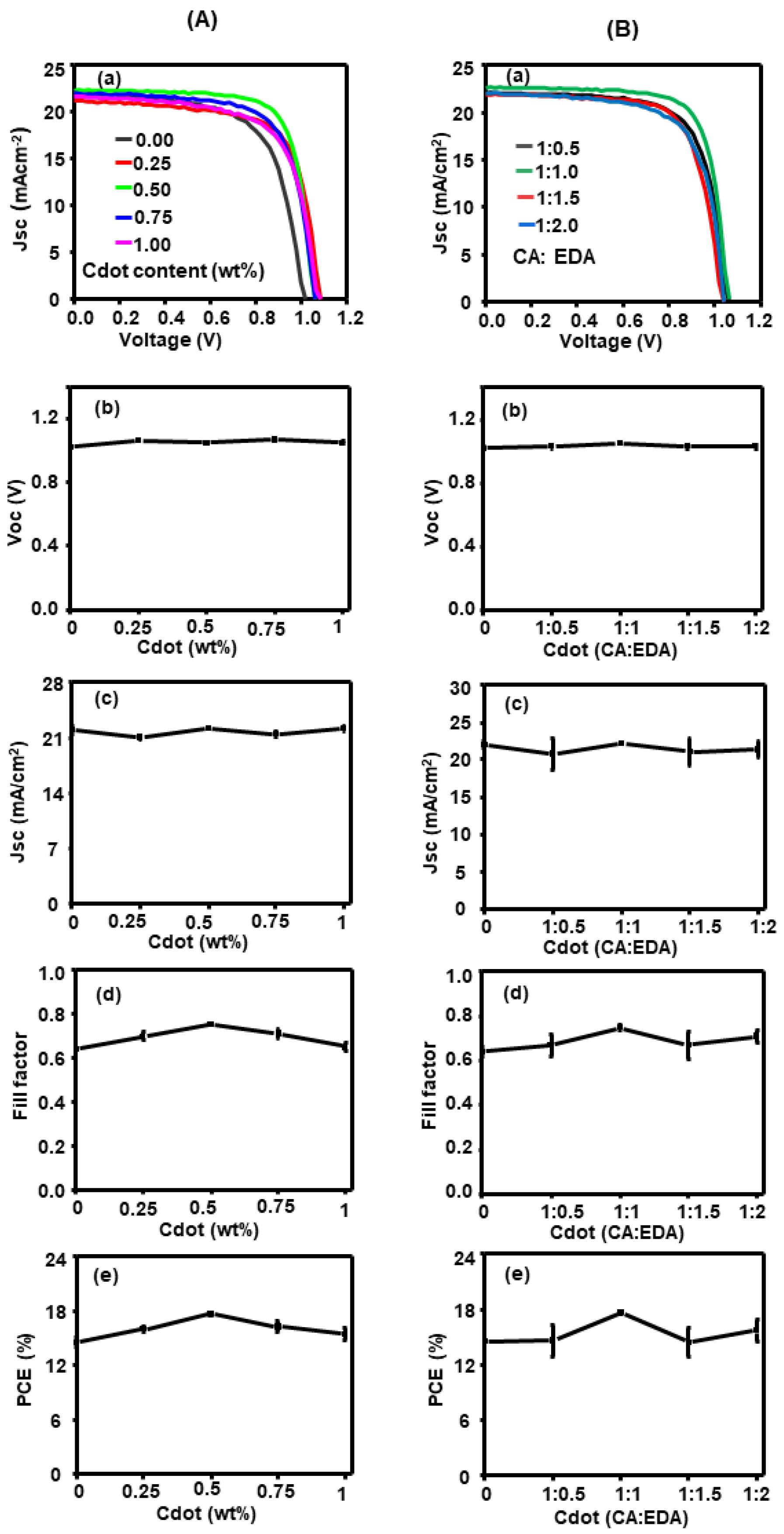


| Element | Binding Energy (eV) | Area Intensity (a.u) | Bond Assignment |
|---|---|---|---|
| Br 3d | 68.5 | 42.9 | Br− 3d5/2 |
| 69.6 | 14.9 | Br− 3d3/2 | |
| Pb 4f | 138.9 | 955.2 | Pb2+ 4f7/2 |
| 143.8 | 959.1 | Pb2+ 4f5/2 | |
| C 1s | 284.7 | 47.8 | C=C (Cdot) |
| 285.6 | 126.5 | C–N (FA+, MA+ and Cdot) | |
| 287.1 | 107.6 | C=N (FA) | |
| 289.2 | 37.6 | COOH (Cdot) | |
| 290.3 | 9.8 | Graphitic π–π* satellite (Cdot) | |
| N 1s | 400.9 | 123.5 | C–N (FA+, MA+ and Cdot) |
| 402.8 | 9.4 | C=N (FA+) | |
| I 3d | 620.1 | 3440.8 | I− 3d7/2 |
| 631.6 | 3515.3 | I− 3d5/2 | |
| Cs 3d | 725.7 | 130.5 | Cs+ 3d3/2 |
| 740.3 | 72.0 | Cs+ 3d5/2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadeta, T.F.; Huang, K.-W.; Imae, T.; Tung, Y.-L. Enhancement of Perovskite Solar Cells by TiO2-Carbon Dot Electron Transport Film Layers. Nanomaterials 2023, 13, 186. https://doi.org/10.3390/nano13010186
Yadeta TF, Huang K-W, Imae T, Tung Y-L. Enhancement of Perovskite Solar Cells by TiO2-Carbon Dot Electron Transport Film Layers. Nanomaterials. 2023; 13(1):186. https://doi.org/10.3390/nano13010186
Chicago/Turabian StyleYadeta, Tamasgen Fikadu, Kuo-Wei Huang, Toyoko Imae, and Yung-Liang Tung. 2023. "Enhancement of Perovskite Solar Cells by TiO2-Carbon Dot Electron Transport Film Layers" Nanomaterials 13, no. 1: 186. https://doi.org/10.3390/nano13010186




