Flexible MoS2 Anchored on Ge-Containing Carbon Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
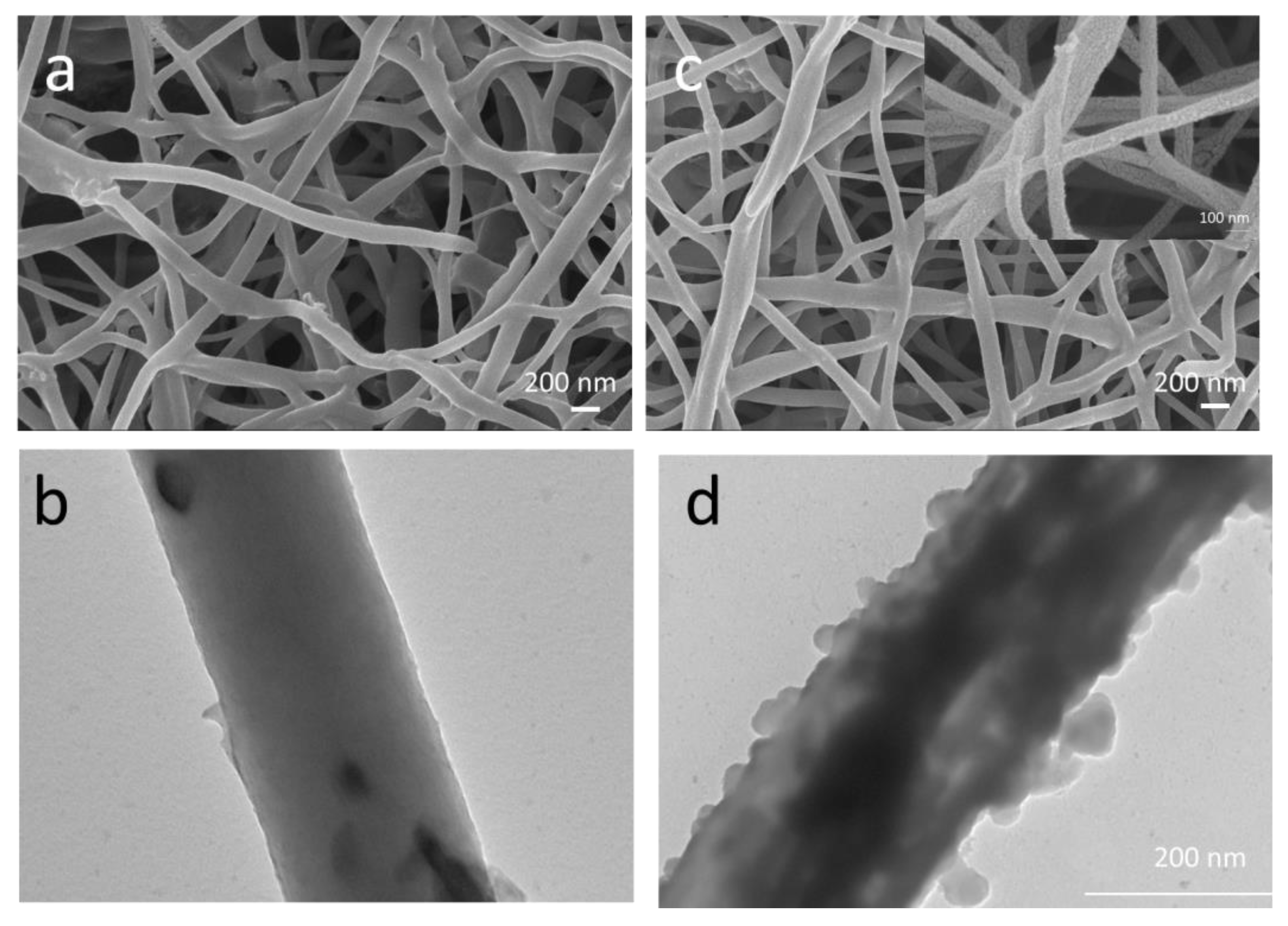
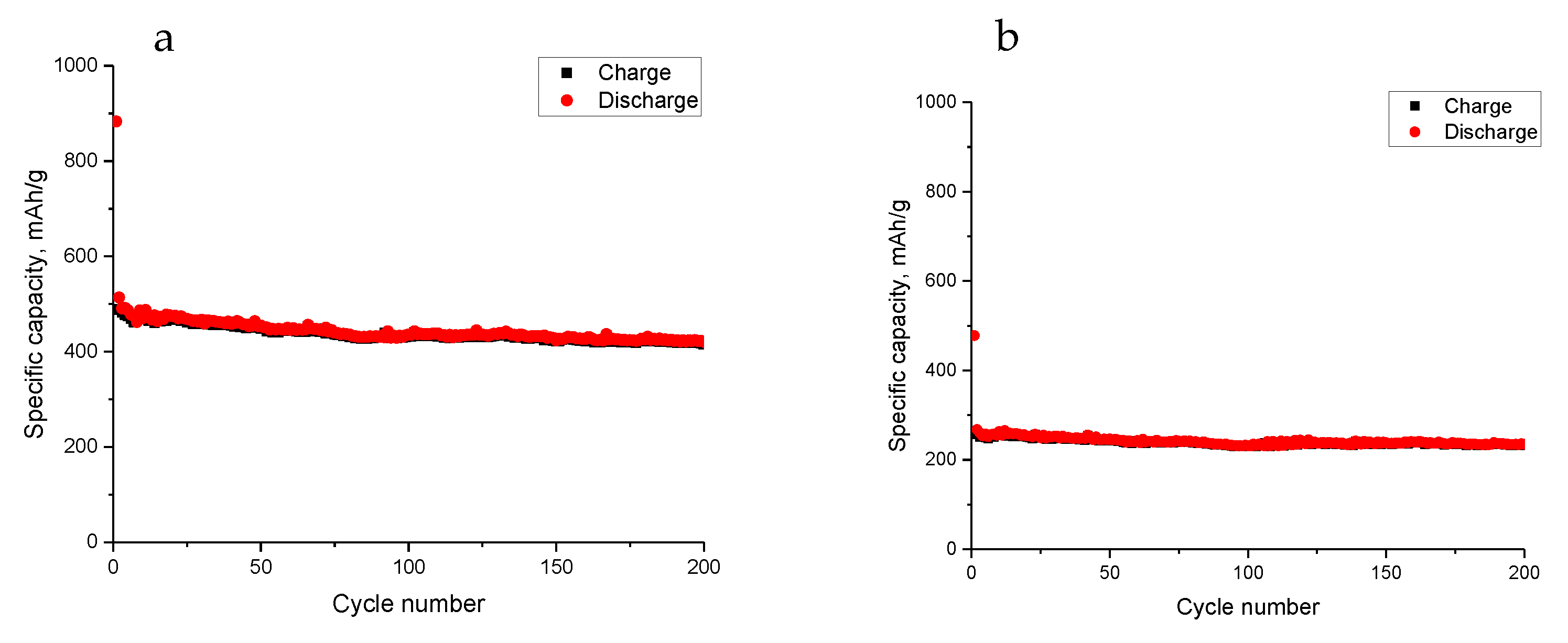
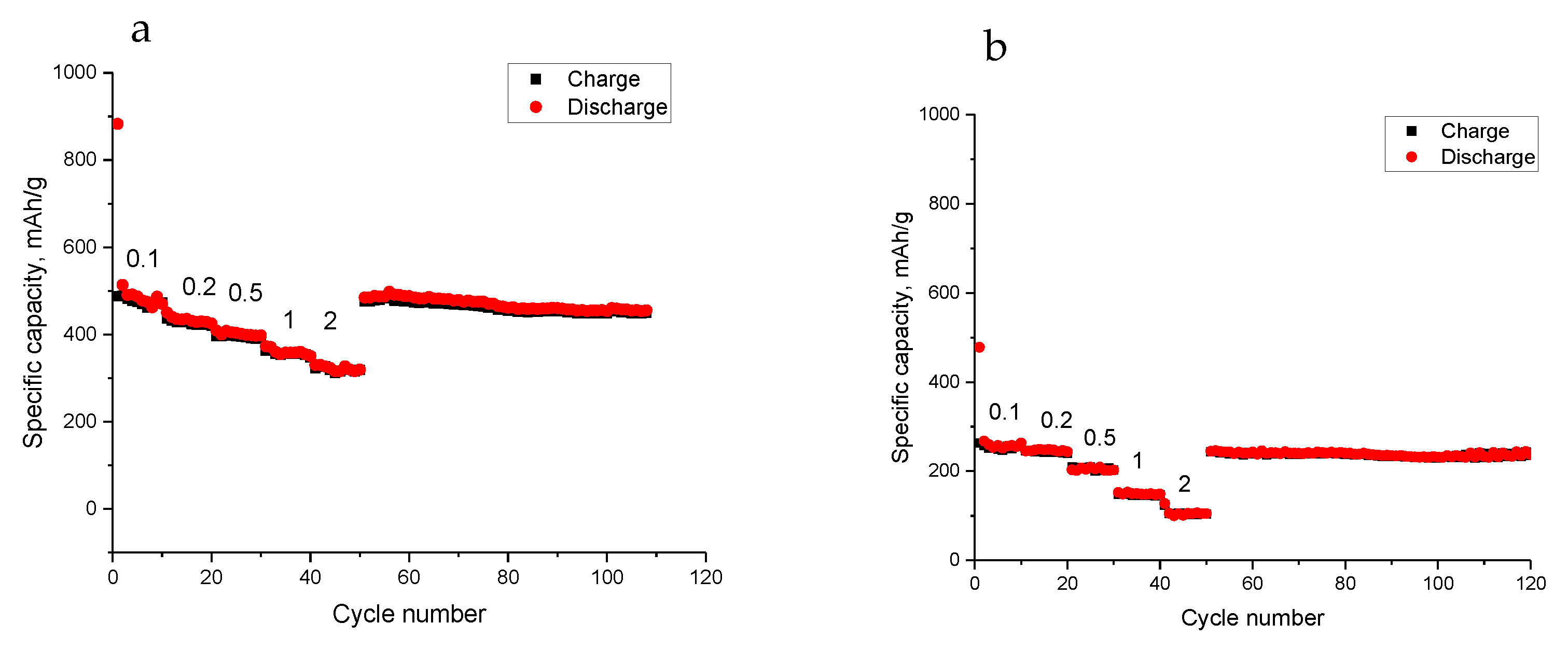
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, X.; Chen, L.; Yanilmaz, M.; Lu, X.; Yang, K.; Hu, K.; Liu, Y.; Zhang, X. From nature, requite to nature: Bio-based cellulose and its derivatives for construction of green zinc batteries. Chem. Eng. J. 2023, 454, 140311. [Google Scholar] [CrossRef]
- Lu, Y.; Yanilmaz, M.; Chen, C.; Ge, Y.; Dirican, M.; Zhu, J.; Li, Y.; Zhang, X. Lithium-substituted sodium layered transition metal oxide fibers as cathodes for sodium-ion batteries. Energy Storage Mater. 2015, 1, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Abdolrazzaghian, E.; Zhu, J.; Kim, J.; Yanilmaz, M. MoS2-Decorated Graphene@porous Carbon nanofiber Anodes via Centrifugal Spinning. Nanomaterials 2022, 12, 2505. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yanilmaz, M.; Chen, C.; Dirican, M.; Ge, Y.; Zhu, J.; Zhang, X. Centrifugally spun SnO2 microfibers composed of interconnected nanoparticles as the anode in sodium-ion batteries. ChemElectroChem 2015, 2, 1947–1956. [Google Scholar] [CrossRef]
- Al Rai, A.; Yanilmaz, M. High-performance nanostructured bio-based carbon electrodes for energy storage applications. Cellulose 2021, 28, 5169–5218. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Muhammad, S.; Wei, Z.; Zhu, J.; Duan, X. Hierarchical N-doping germanium/carbon nanofibers as anode for high-performance lithium-ion and sodium-ion batteries. Nanotechnology 2020, 31, 015402. [Google Scholar] [CrossRef]
- Kohandehghan, A.; Cui, K.; Kupsta, M.; Ding, J.; Memarzadeh Lotfabad, E.; Kalisvaart, W.P.; Mitlin, D. Activation with Li enables facile sodium storage in germanium. Nano Lett. 2014, 14, 5873–5882. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.-Y.; Sun, J. Recent progress in the carbon-based frameworks for high specific capacity anodes/cathode in lithium/sodium ion batteries. New Carbon Mater. 2021, 36, 106–116. [Google Scholar] [CrossRef]
- Youn, D.H.; Patterson, N.A.; Park, H.; Heller, A.; Mullins, C.B. Facile synthesis of Ge/N-doped carbon spheres with varying nitrogen content for lithium ion battery anodes. ACS Appl. Mater. Interfaces 2016, 8, 27788–27794. [Google Scholar] [CrossRef]
- Liu, J.; Song, K.; Zhu, C.; Chen, C.-C.; van Aken, P.A.; Maier, J.; Yu, Y. Ge/C nanowires as high-capacity and long-life anode materials for Li-ion batteries. ACS Nano 2014, 8, 7051–7059. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; He, W.; Xu, X.; Liu, Z.; Shen, J.; Hu, Y.; Chen, Z.; Liu, J. Scalable synthesis of Li2GeO3/expanded graphite as a high-performance anode for Li-ion batteries. J. Alloys Compd. 2022, 898, 162893. [Google Scholar] [CrossRef]
- Xu, X.; Li, F.; Zhang, D.; Liu, Z.; Zuo, S.; Zeng, Z.; Liu, J. Self-Sacrifice Template Construction of Uniform Yolk–Shell ZnS@ C for Superior Alkali-Ion Storage. Adv. Sci. 2022, 9, 2200247. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, X.; Liu, J.; Liu, Z.; Shen, J.; Li, F.; Hu, R.; Yang, L.; Ouyang, L.; Zhu, M. Facile Synthesis of Peapod-Like Cu3Ge/Ge@ C as a High-Capacity and Long-Life Anode for Li-Ion Batteries. Chem.-A Eur. J. 2019, 25, 11486–11493. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Wang, Y.; Xiao, P.; Zhang, W.; Rao, S.; Wang, Z. Construction of N-doped C@MoS2 heteroshell with the yolk of Sn nanoparticles as high-performance anodes for sodium-ion batteries. J. Alloys Compd. 2022, 892, 162157. [Google Scholar] [CrossRef]
- Yu, X.; Chen, C.; Li, R.; Yang, T.; Wang, W.; Dai, Y. Construction of SnS2@ MoS2@rGO heterojunction anode and their half/full sodium ion storage performances. J. Alloys Compd. 2022, 896, 162784. [Google Scholar] [CrossRef]
- Petrovičovà, B.; Ferrara, C.; Brugnetti, G.; Ritter, C.; Fracchia, M.; Ghigna, P.; Pollastri, S.; Triolo, C.; Spadaro, L.; Ruffo, R.; et al. Effect of germanium incorporation on the electrochemical performance of electrospun Fe2O3 nanofibers-based anodes in sodium-ion batteries. Appl. Sci. 2021, 11, 1483. [Google Scholar] [CrossRef]
- Wang, X.; Fan, L.; Gong, D.; Zhu, J.; Zhang, Q.; Lu, B. Core–shell Ge@ Graphene@TiO2 nanofibers as a high-capacity and cycle-stable anode for lithium and sodium ion battery. Adv. Funct. Mater. 2016, 26, 1104–1111. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Asiri, A.M.; Zhang, X. Centrifugally spun porous carbon microfibers as interlayer for Li–S batteries. J. Mater. Sci. 2020, 55, 3538–3548. [Google Scholar] [CrossRef]
- Consales, M.; Cutolo, A.; Penza, M.; Aversa, P.; Giordano, M.; Cusano, A. Fiber optic chemical nanosensors based on engineered single-walled carbon nanotubes. J. Sens. 2008, 2008, 936074. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhu, G.; Kang, Q.; Huang, Z.-D.; Feng, X.; Li, Y.; Liu, R.; Ma, Y. Towards free-standing MoS 2 nanosheet electrocatalysts supported and enhanced by N-doped CNT–graphene foam for hydrogen evolution reaction. RSC Adv. 2015, 5, 55396–55400. [Google Scholar] [CrossRef]
- Yan, Y.; Ge, X.; Liu, Z.; Wang, J.-Y.; Lee, J.-M.; Wang, X. Facile synthesis of low crystalline MoS 2 nanosheet-coated CNTs for enhanced hydrogen evolution reaction. Nanoscale 2013, 5, 7768–7771. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yin, D.; Zhang, M. Sandwich-like MoS2@ SnO2@C with high capacity and stability for sodium/potassium ion batteries. Small 2018, 14, e1703818. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Chen, T.; Hu, B.; Sun, Z.; Pan, L. GeO2 decorated reduced graphene oxide as anode material of sodium ion battery. Electrochim. Acta 2015, 173, 193–199. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Zhang, X.; Zhang, X.; Chen, S. Ionic liquid electrodeposition of Ge nano-film on Cu wire mesh as stable anodes for lithium-ion batteries. Ionics 2020, 26, 2225–2231. [Google Scholar] [CrossRef]
- Gao, C.; Kim, N.D.; Salvatierra, R.V.; Lee, S.-K.; Li, L.; Li, Y.; Sha, J.; Silva, G.A.L.; Fei, H.; Xie, E. Germanium on seamless graphene carbon nanotube hybrids for lithium ion anodes. Carbon 2017, 123, 433–439. [Google Scholar] [CrossRef]
- Liu, R.; Luo, F.; Zeng, L.; Liu, J.; Xu, L.; He, X.; Xu, Q.; Huang, B.; Qian, Q.; Wei, M.; et al. Dual carbon decorated germanium-carbon composite as a stable anode for sodium/potassium-ion batteries. J. Colloid Interface Sci. 2021, 584, 372–381. [Google Scholar] [CrossRef]
- Chen, C.; Li, G.; Lu, Y.; Zhu, J.; Jiang, M.; Hu, Y.; Cao, L.; Zhang, X. Chemical vapor deposited MoS2/electrospun carbon nanofiber composite as anode material for high-performance sodium-ion batteries. Electrochim. Acta 2016, 222, 1751–1760. [Google Scholar] [CrossRef]
- Zeng, L.; Luo, F.; Xia, X.; Yang, M.-Q.; Xu, L.; Wang, J.; Feng, X.; Qian, Q.; Wei, M.; Chen, Q. An Sn doped 1T–2H MoS 2 few-layer structure embedded in N/P co-doped bio-carbon for high performance sodium-ion batteries. Chem. Commun. 2019, 55, 3614–3617. [Google Scholar] [CrossRef]
- Zheng, F.; Pan, Q.; Yang, C.; Xiong, X.; Ou, X.; Hu, R.; Chen, Y.; Liu, M. Sn-MoS2-C@ C Microspheres as a Sodium-Ion Battery Anode Material with High Capacity and Long Cycle Life. Chem.-A Eur. J. 2017, 23, 5051–5058. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanilmaz, M.; Kim, J.J. Flexible MoS2 Anchored on Ge-Containing Carbon Nanofibers. Nanomaterials 2023, 13, 75. https://doi.org/10.3390/nano13010075
Yanilmaz M, Kim JJ. Flexible MoS2 Anchored on Ge-Containing Carbon Nanofibers. Nanomaterials. 2023; 13(1):75. https://doi.org/10.3390/nano13010075
Chicago/Turabian StyleYanilmaz, Meltem, and Jung Joong Kim. 2023. "Flexible MoS2 Anchored on Ge-Containing Carbon Nanofibers" Nanomaterials 13, no. 1: 75. https://doi.org/10.3390/nano13010075




