Optical and Structural Analysis of TiO2–SiO2 Nanocomposite Thin Films Fabricated via Pulsed Laser Deposition Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Target Preparation
2.2. Substrate Preparation
2.3. Deposition Parameters
2.4. Thin Film Characterizations
3. Results and Discussion
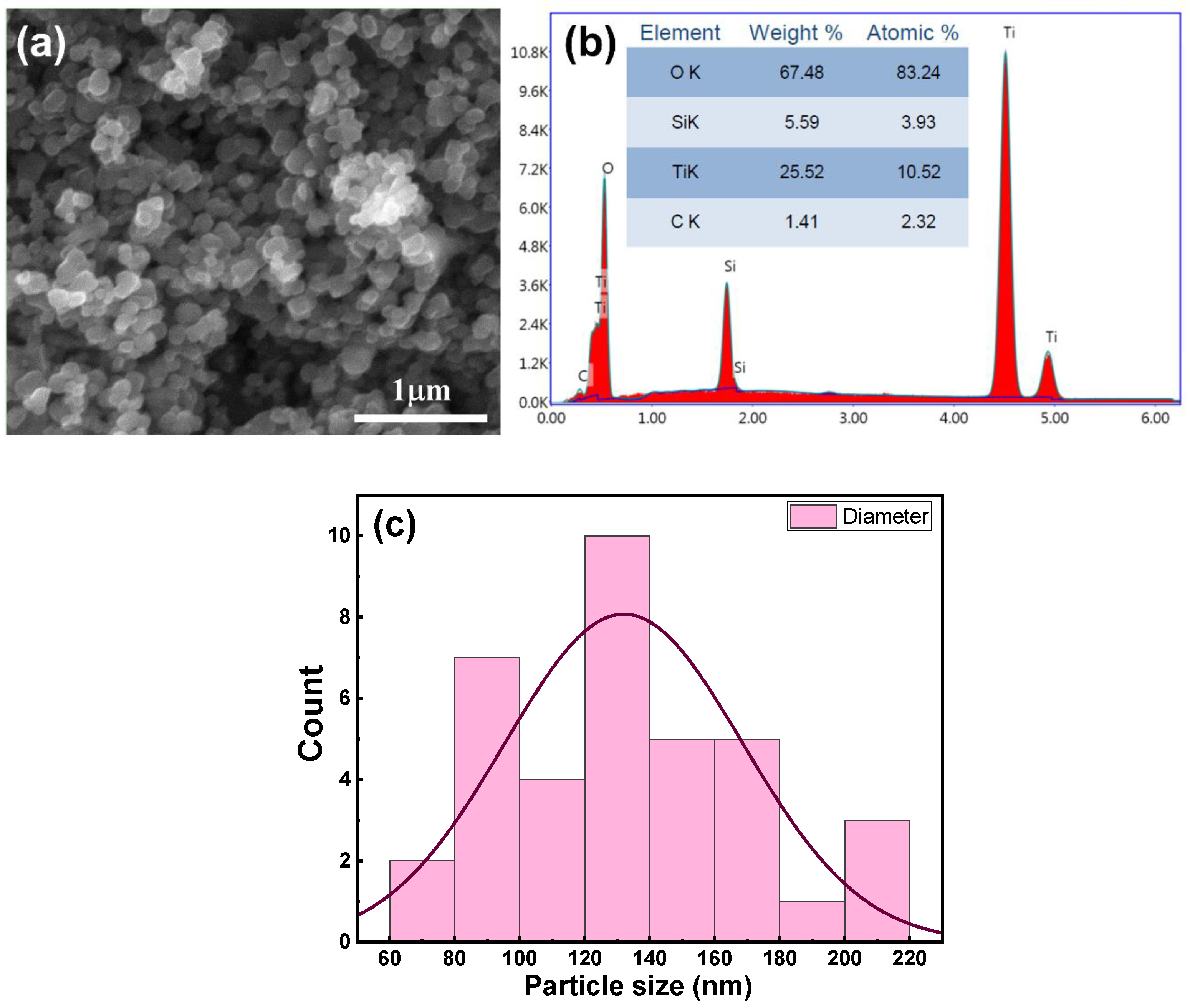
3.1. Nanocomposite Target Properties
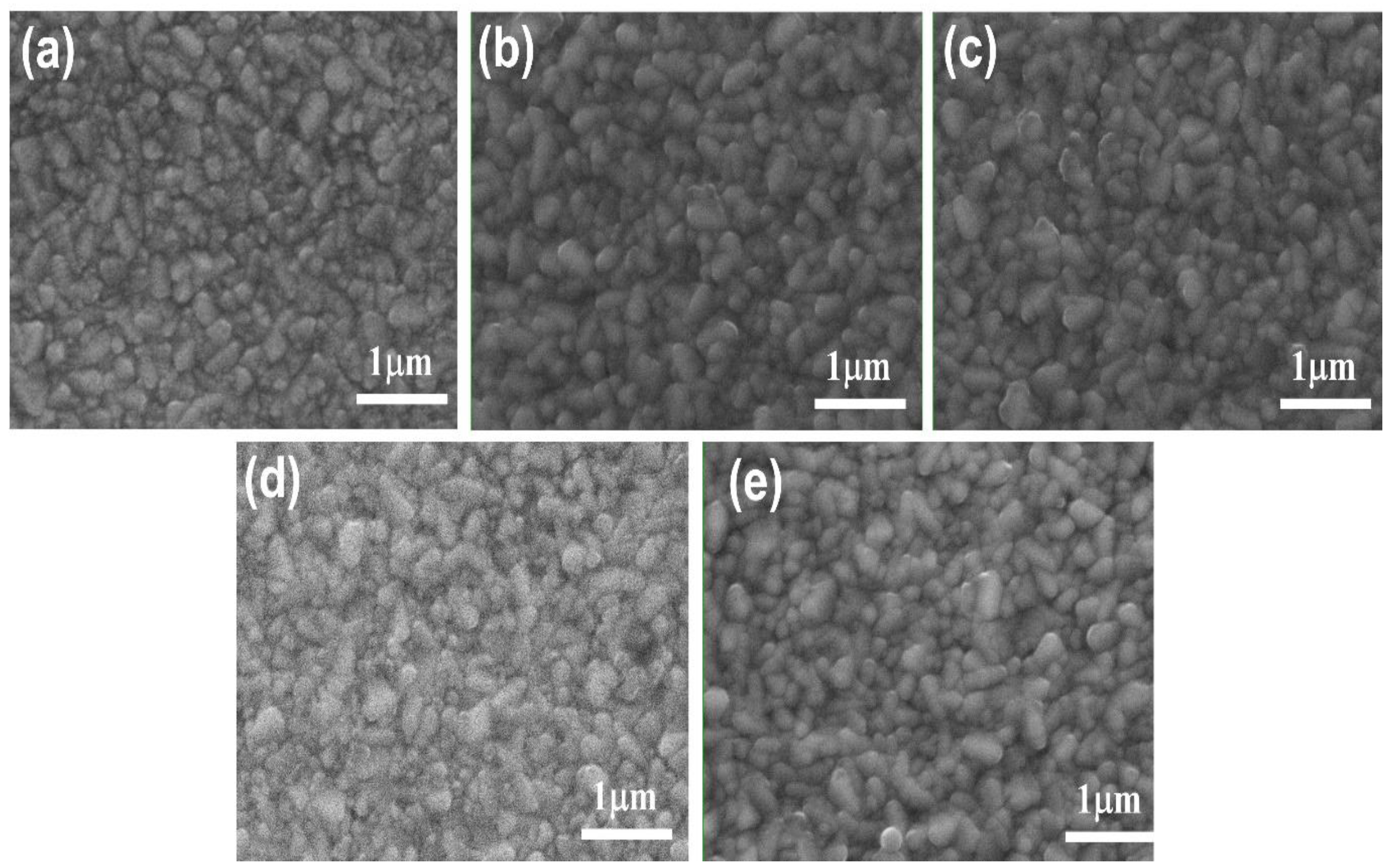
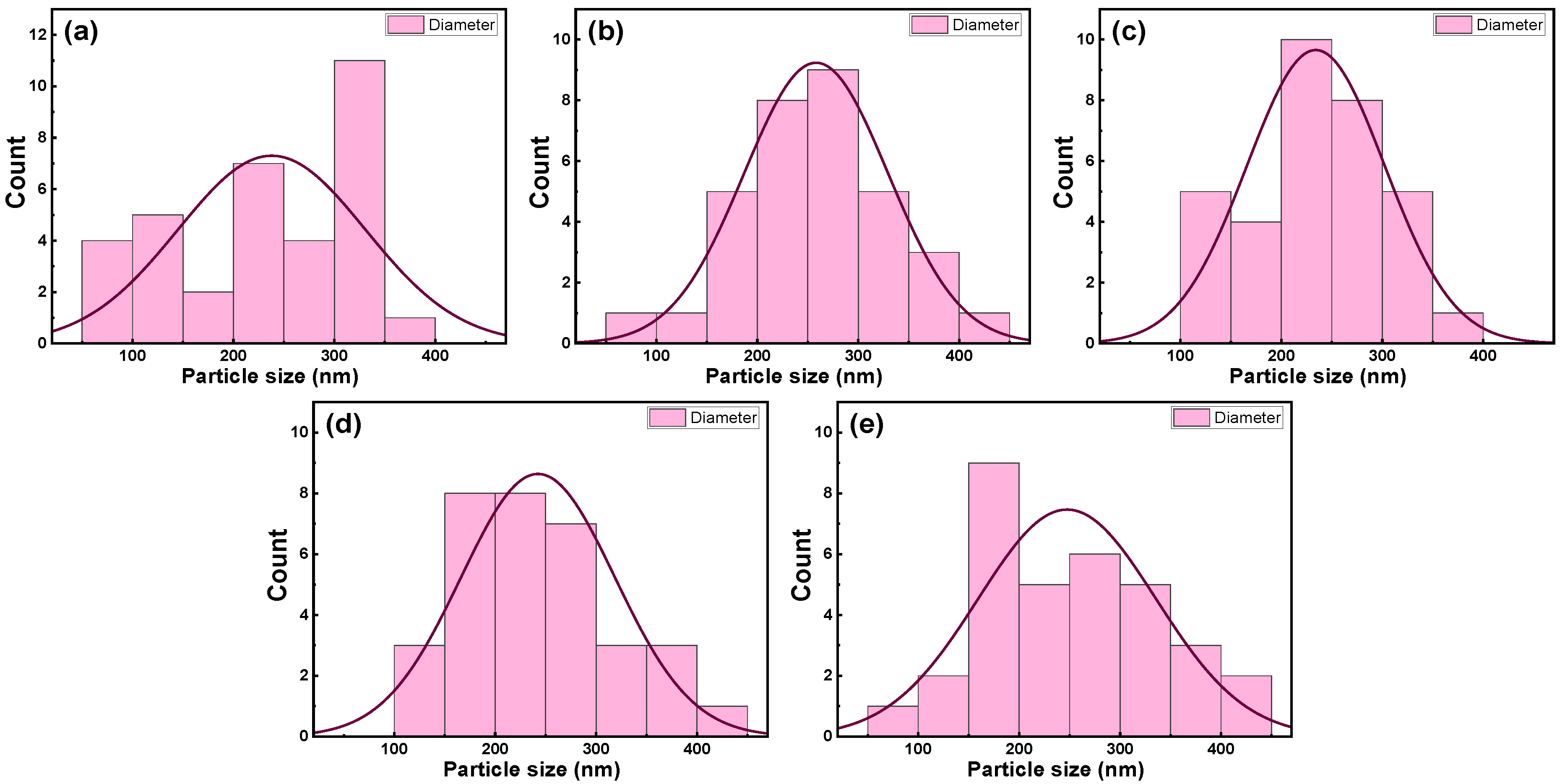
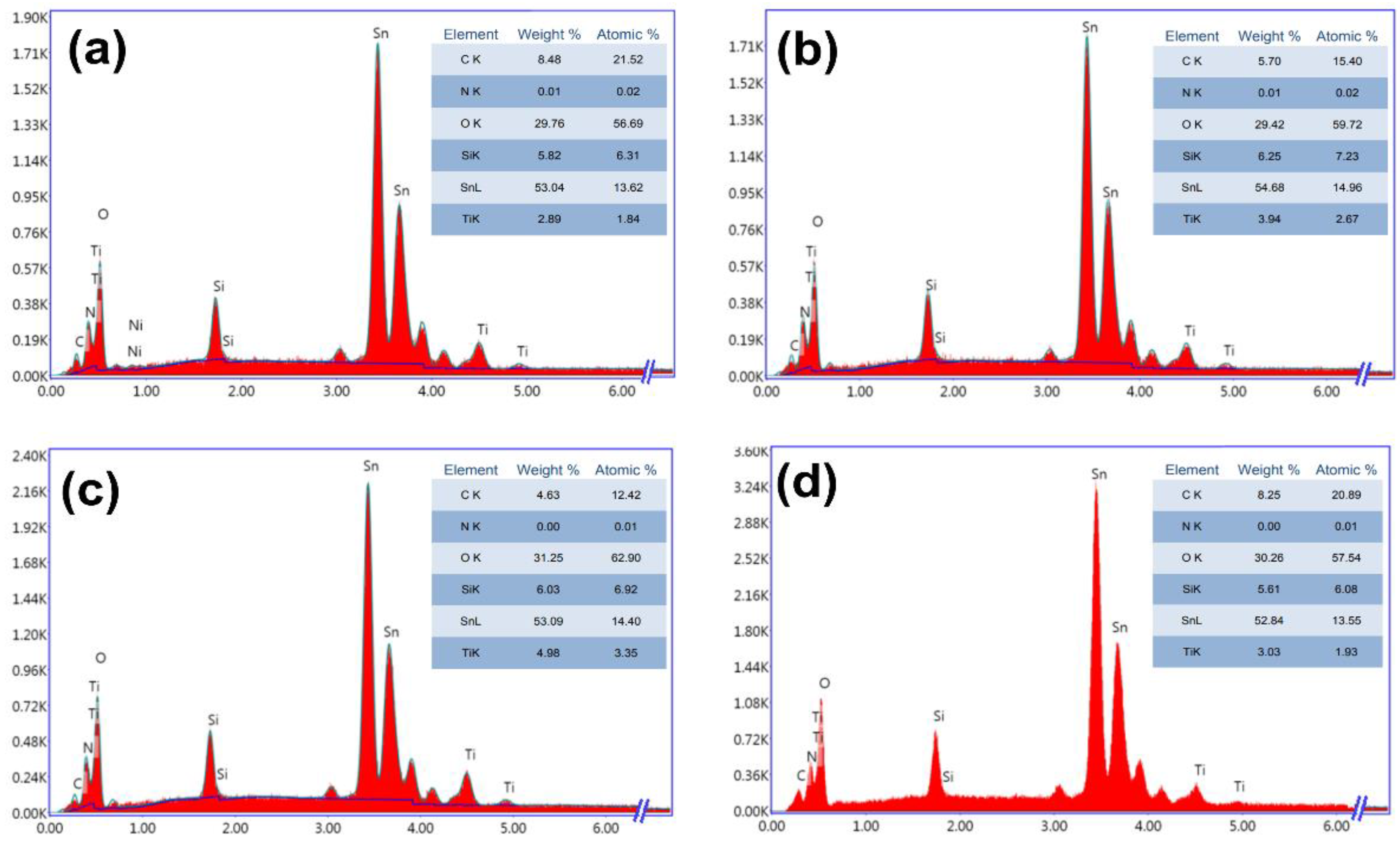
3.2. Nanocomposite Thin Films Characterizations
3.2.1. SEM Morphological Analysis
3.2.2. Energy Dispersive X-ray Analysis
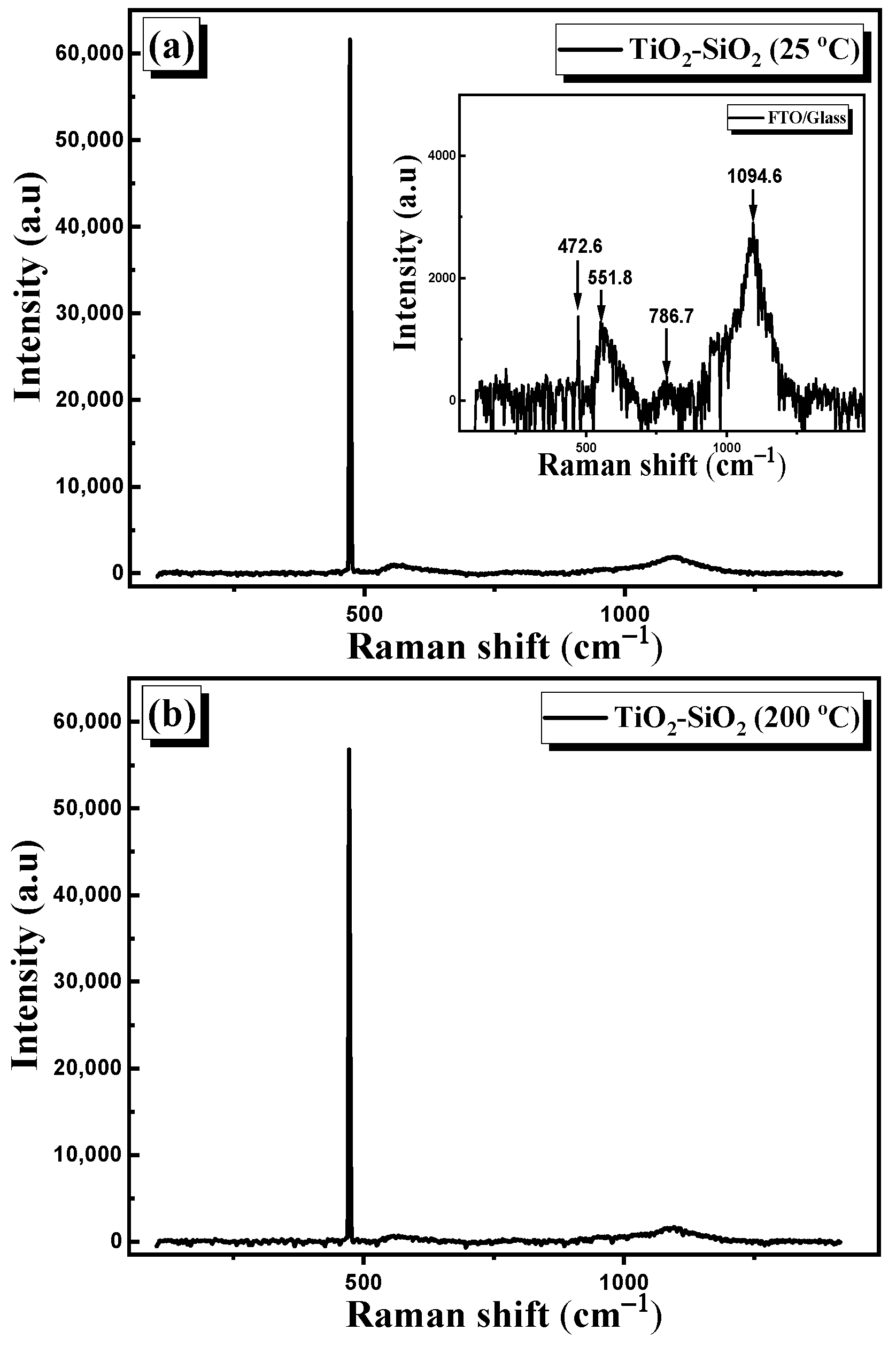
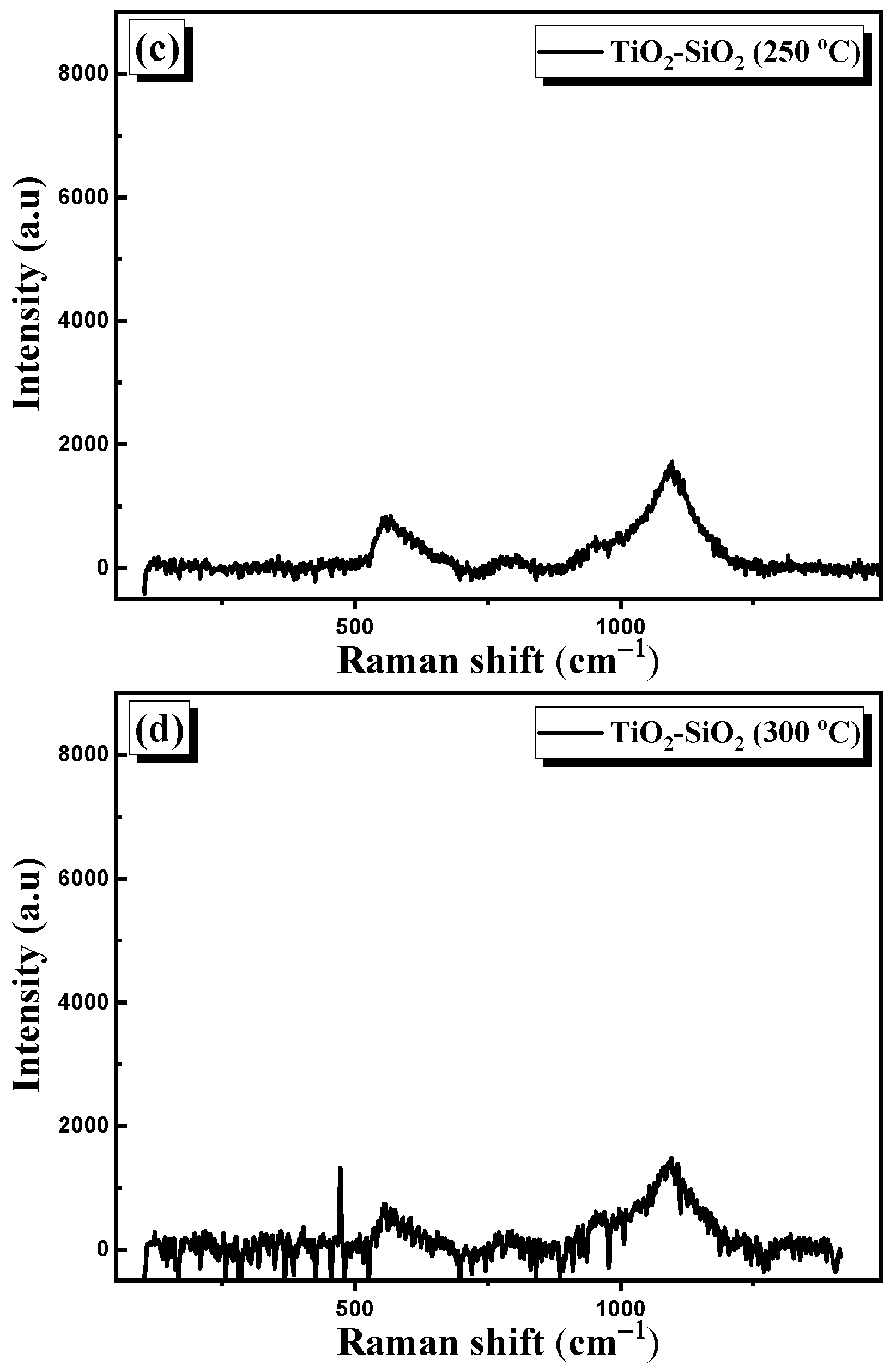
3.2.3. Raman Spectroscopy
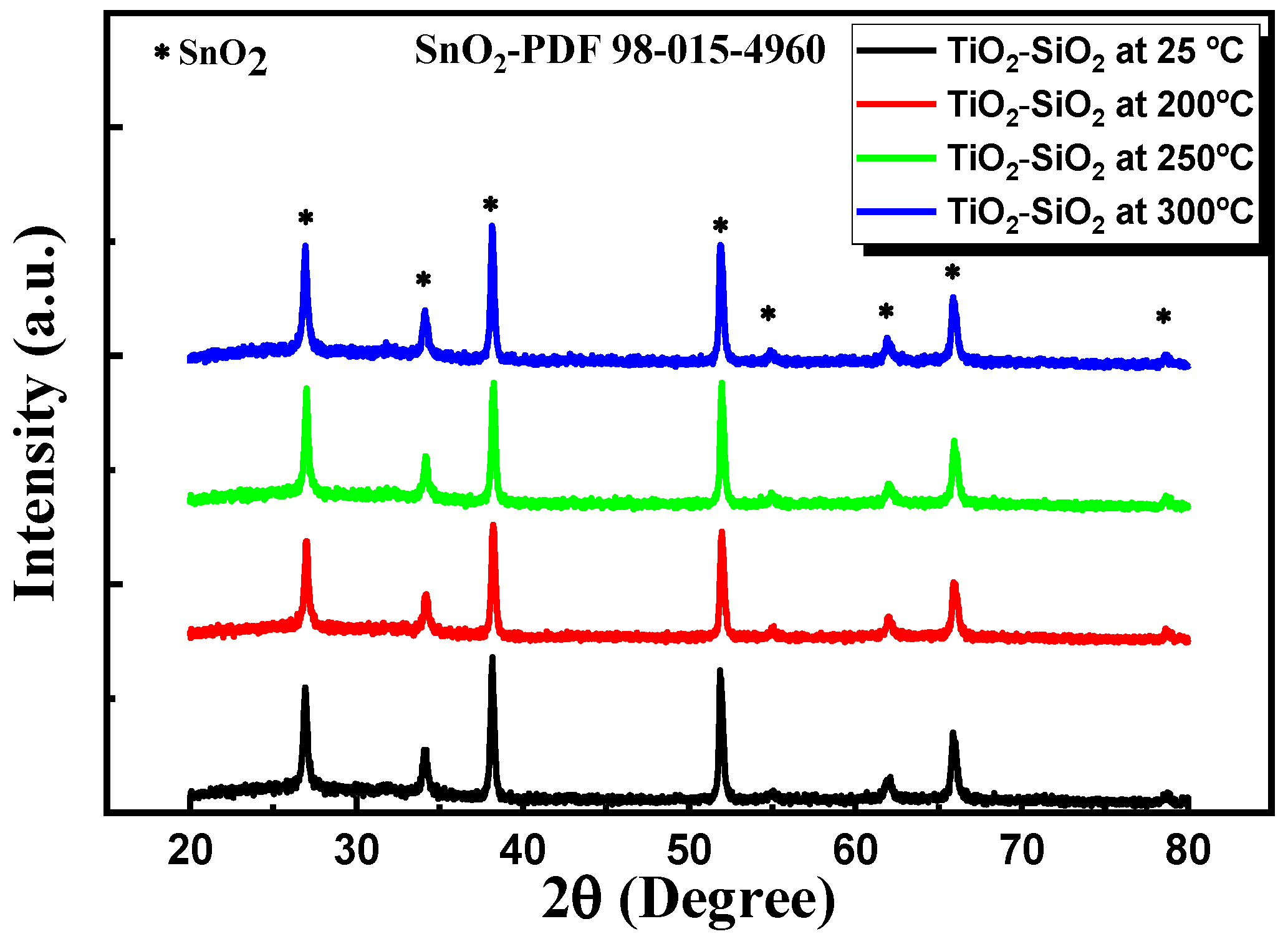
3.2.4. XRD Characterization
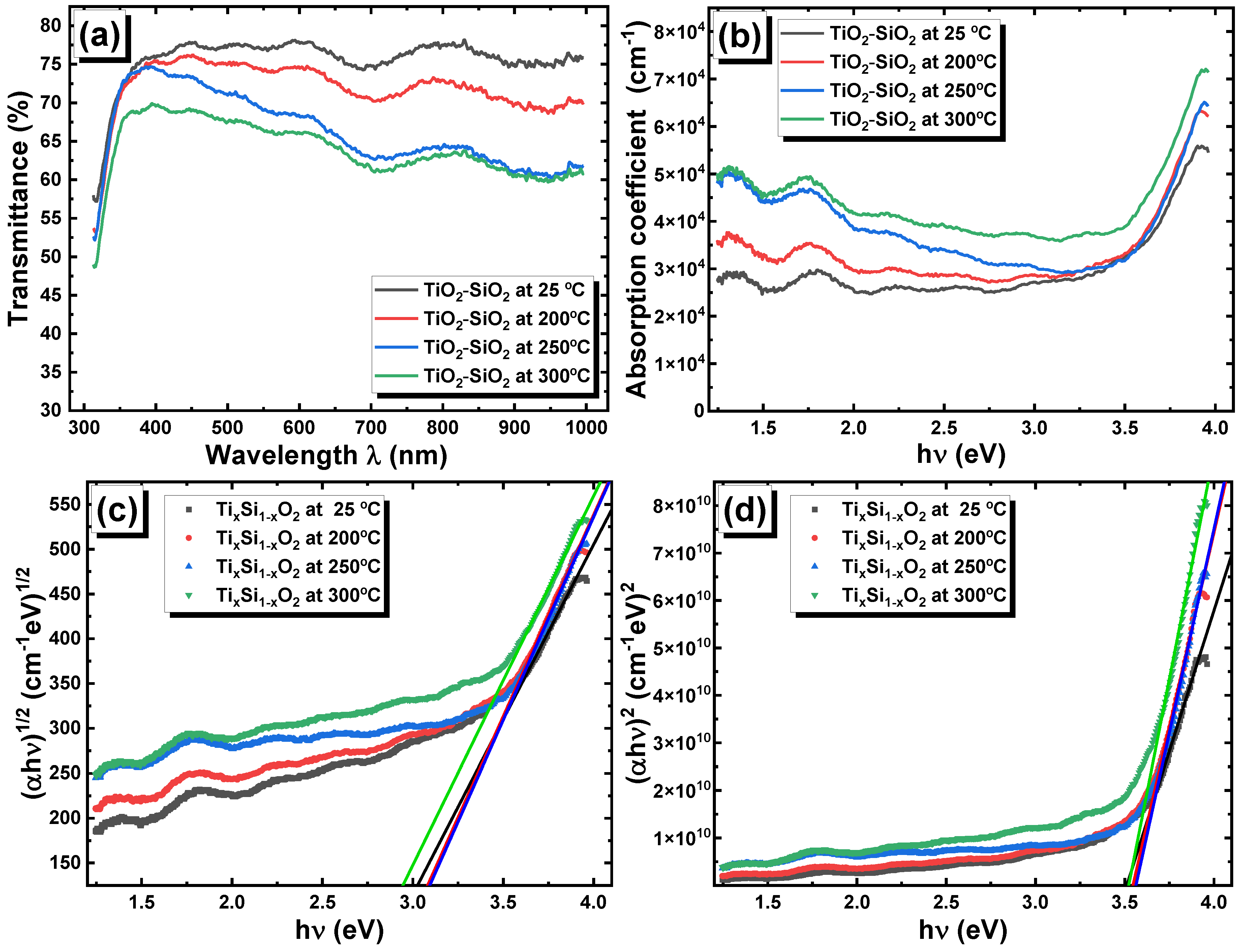
3.2.5. Optical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef]
- Sun, S.; Song, P.; Cui, J.; Liang, S. Amorphous TiO2 nanostructures: Synthesis, fundamental properties and photocata-lytic applications. Catal. Sci. Technol. 2019, 9, 4198–4215. [Google Scholar] [CrossRef]
- Möls, K.; Aarik, L.; Mändar, H.; Kasikov, A.; Niilisk, A.; Rammula, R.; Aarik, J. Influence of phase composition on optical properties of TiO2: Dependence of refractive index and band gap on formation of TiO2-II phase in thin films. Opt. Mater. 2019, 96, 109335. [Google Scholar] [CrossRef]
- O’regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Zhu, W.; Zheng, G.; Cao, S.; He, H. Thermal conductivity of amorphous SiO2 thin film: A molecular dynamics study. Sci. Rep. 2018, 8, 10537. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Zhao, S.; Dong, W. Hydrophobic and optical properties of silica antireflective coating prepared via sol-gel method. Mater. Res. Express. 2021, 8, 046403. [Google Scholar] [CrossRef]
- Masuda, Y.; Jinbo, Y.; Koumoto, K. Room temperature CVD of TiO2 thin films and their electronic properties. Sci. Adv. Mater. 2009, 1, 138–143. [Google Scholar] [CrossRef]
- Baek, G.; Baek, J.H.; Kim, H.M.; Lee, S.; Jin, Y.; Park, H.S.; Kil, D.S.; Kim, S.; Park, Y.; Park, J.S. Atomic layer chemical vapor deposition of SiO2 thin films using a chlorine-free silicon precursor for 3D NAND applications. Ceram. Int. 2021, 47, 9036–9042. [Google Scholar] [CrossRef]
- Weng, X.; Fisher, P.; Skowronski, M.; Salvador, P.A.; Maksimov, O. Structural characterization of TiO2 films grown on LaAlO3 and SrTiO3 substrates using reactive molecular beam epitaxy. J. Cryst. Growth 2008, 310, 545–550. [Google Scholar] [CrossRef]
- Shibata, H.; Kimura, S.; Takatoh, H. Deposition of SiO2 thin films by combined low-energy ion-beam and molecular-beam epitaxial method. Jpn. J. Appl. Phys. 2000, 39, 1327. [Google Scholar] [CrossRef]
- Xu, J.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. TiO2 coatings via atmospheric-pressure plasma-enhanced chemical vapor deposition for enhancing the UV-resistant properties of transparent plastics. ACS Omega 2021, 6, 1370–1377. [Google Scholar] [CrossRef]
- Ryu, C.I.; Choa, S.H. Evaluation of Argon as a Carrier Gas of Liquid Material Vaporization During the Plasma-Enhanced Chemical Vapor Deposition (PECVD) Silicon Oxide Process. Sci. Adv. Mater. 2020, 12, 583–588. [Google Scholar] [CrossRef]
- Devi, K.P.; Goswami, P.; Chaturvedi, H. Fabrication of nanocrystalline TiO2 thin films using Sol-Gel spin coating technology and investigation of its structural, morphology and optical characteristics. Appl. Surf. Sci. 2022, 591, 153226. [Google Scholar] [CrossRef]
- Wang, J.; Ran, Q.; Xu, X.; Zhu, B.; Zhang, W. August. Preparation and Optical Properties of TiO2–SiO2 thin films by Sol-gel Dipping Method. IOP Conf. Ser. Earth Environ. Sci. 2019, 310, 042029. [Google Scholar] [CrossRef]
- Kadri, L.; Bulai, G.; Carlescu, A.; George, S.; Gurlui, S.; Leontie, L.; Doroftei, C.; Adnane, M. Effect of Target Sintering Temperature on the Morphological and Optical Properties of Pulsed Laser Deposited TiO2 Thin Films. Coatings 2021, 11, 561. [Google Scholar] [CrossRef]
- Koike, R.; Suzuki, R.; Katayama, K.; Higashihata, M.; Ikenoue, H.; Nakamura, D. Formation dynamics of SiO2 nanoparticles produced by laser ablation in ambient gases. Appl. Phys. A 2022, 128, 971. [Google Scholar] [CrossRef]
- Subedi, B.; Puli, V.S.; Boyd, I.W.; Chrisey, D.B. Pulsed Laser Deposition of Thin Films. In Handbook of Laser Technology and Applications, 2nd ed.; Guo, C., Sing, S.C., Eds.; CRC Press: Boca Raton, FL, USA, 2021; Volume 3, p. 111124. [Google Scholar]
- Haider, A.J.; Alawsi, T.; Haider, M.J.; Taha, B.A.; Marhoon, H.A. A comprehensive review on pulsed laser deposition technique to effective nanostructure production: Trends and challenges. Opt. Quantum Electron. 2022, 54, 488. [Google Scholar] [CrossRef]
- Axente, E.; Socol, G. Pulsed Laser Deposition of Thin Films: Recent Advances and Challenge. Coatings 2022, 12, 368. [Google Scholar] [CrossRef]
- Rosales, A.; Ortiz-Frade, L.; Medina-Ramirez, I.E.; Godínez, L.A.; Esquivel, K. Self-cleaning of SiO2–TiO2 coating: Effect of sonochemical synthetic parameters on the morphological, mechanical, and photocatalytic properties of the films. Ultrason. Sonochem. 2021, 73, 105483. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Tang, Q.; He, B. Toward elevated light harvesting: Efficient dye-sensitized solar cells with titanium dioxide/silica photoanodes. RSC Adv. 2015, 5, 46260–46266. [Google Scholar] [CrossRef]
- Zhang, L.; Hörantner, M.T.; Zhang, W.; Yan, Q.; Snaith, H.J. Near-neutral-colored semitransparent perovskite films using a combination of colloidal self-assembly and plasma etching. Sol. Energy Mater Sol. Cells 2017, 160, 193–202. [Google Scholar] [CrossRef]
- Hodroj, A.; Chaix-Pluchery, O.; Audier, M.; Gottlieb, U.; Deschanvres, J.L. Thermal annealing of amorphous Ti–Si–O thin films. J. Mater. Res. 2008, 23, 755–759. [Google Scholar] [CrossRef]
- Zhao, W.; Jia, H.; Qu, J.; Yang, C.; Wang, Y.; Zhu, J.; Wu, H.; Liu, G. Sol-gel synthesis of TiO2–SiO2 hybrid films with tunable refractive index for broadband antireflective coatings covering the visible range. J. Solgel Sci. Technol. 2022, 1–17. [Google Scholar] [CrossRef]
- Mitronika, M.; Villeneuve-Faure, C.; Massol, F.; Boudou, L.; Ravisy, W.; Besland, M.P.; Goullet, A.; Richard-Plouet, M. TiO2–SiO2 mixed oxide deposited by low pressure PECVD: Insights on optical and nanoscale electrical properties. Appl. Surf. Sci. 2021, 541, 148510. [Google Scholar] [CrossRef]
- Wang, J.; Bai, L.; Xiao, Y.; Luo, Y.; Zhu, B.; Zhang, W. Synthesis, wettability and optical properties of TixO2– Si(1-x)O2 composite thin films. IOP Conf. Ser. Mater. Sci. Eng. 2020, 738, 012032. [Google Scholar] [CrossRef]
- Kunti, A.K.; Chowdhury, M.; Sharma, S.K.; Gupta, M.; Chaudhary, R.J. Influence of O2 pressure on structural, morphological and optical properties of TiO2–SiO2 composite thin films prepared by pulsed laser deposition. Thin Solid Film. 2017, 629, 79–89. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Budiman, W.J.; Yudasari, N.; Isnaeni, K.T.; Wibowo, A. Facile and green fabrication of microwave-assisted reduced graphene oxide/titanium dioxide nanocomposites as photocatalysts for rhodamine 6G degradation. ACS Omega 2021, 6, 32166–32177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, L.; Shao, M.; Huang, J.; Ding, M.; Deng, X.; Wei, X.; Xu, X. Anodic oxidation synthesis of one-dimensional TiO2 nanostructures for photocatalytic and field emission properties. J. Nanomater. 2014, 2014, 831752. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, D.; Wang, N.; Zhang, F.; Zhao, B.; Tan, S.; Zhang, J. Inverted polymer solar cells with TiO2 electron extraction layers prepared by magnetron sputtering. Sci. China Chem. 2013, 56, 1573–1577. [Google Scholar] [CrossRef]
- Su, T.S.; Hsieh, T.Y.; Hong, C.Y.; Wei, T.C. Electrodeposited ultrathin TiO2 blocking layers for efficient perovskite so-lar cells. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.D. Pulsed-Laser Deposition of Silicon Dioxide Thin-films Using the Molecular Fluorine Laser. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 1997. [Google Scholar]
- Wang, X.; Wang, X.; Di, Q.; Zhao, H.; Liang, B.; Yang, J. Mutual effects of fluorine dopant and oxygen vacancies on structural and luminescence characteristics of F doped SnO2 nanoparticles. Materials 2017, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Li, Y.; Ren, X.; Zhao, Y.; Gao, F.; Zhao, H. 2D SnO2 nanosheets: Synthesis, characterization, structures, and excellent sensing performance to ethylene glycol. Nanomaterials 2018, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Shcheblanov, N.S.; Mantisi, B.; Umari, P.; Tanguy, A. Detailed analysis of plastic shear in the Raman spectra of SiO2 glass. J. Non-Cryst. Solids 2015, 428, 6–19. [Google Scholar] [CrossRef]
- Asl, H.Z.; Rozati, S.M. High-quality spray-deposited fluorine-doped tin oxide: Effect of film thickness on structural, morphological, electrical, and optical properties. Appl. Phys. A 2019, 125, 689. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin, H.S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef]
- Huong, H.T.; Nhu, T.T.Q.; Nang, H.X.; Tuan, P.A.; Huy, P.T. Super-hydrophilic Ce3+-doped TiO2-SiO2 nanocomposite thin films with high optical transmittance by a simple spin-coating method. Thin Solid Film. 2023, 768, 139730. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadri, L.; Abderrahmane, A.; Bulai, G.; Carlescu, A.; Doroftei, C.; Motrescu, I.; Gurlui, S.; Leontie, L.; Adnane, M. Optical and Structural Analysis of TiO2–SiO2 Nanocomposite Thin Films Fabricated via Pulsed Laser Deposition Technique. Nanomaterials 2023, 13, 1632. https://doi.org/10.3390/nano13101632
Kadri L, Abderrahmane A, Bulai G, Carlescu A, Doroftei C, Motrescu I, Gurlui S, Leontie L, Adnane M. Optical and Structural Analysis of TiO2–SiO2 Nanocomposite Thin Films Fabricated via Pulsed Laser Deposition Technique. Nanomaterials. 2023; 13(10):1632. https://doi.org/10.3390/nano13101632
Chicago/Turabian StyleKadri, Laid, Abdelkader Abderrahmane, Georgiana Bulai, Aurelian Carlescu, Corneliu Doroftei, Iuliana Motrescu, Silviu Gurlui, Liviu Leontie, and Mohamed Adnane. 2023. "Optical and Structural Analysis of TiO2–SiO2 Nanocomposite Thin Films Fabricated via Pulsed Laser Deposition Technique" Nanomaterials 13, no. 10: 1632. https://doi.org/10.3390/nano13101632







