Engendering High Energy Density LiFePO4 Electrodes with Morphological and Compositional Tuning
Abstract
:1. Introduction
2. Materials and Methods
3. Results
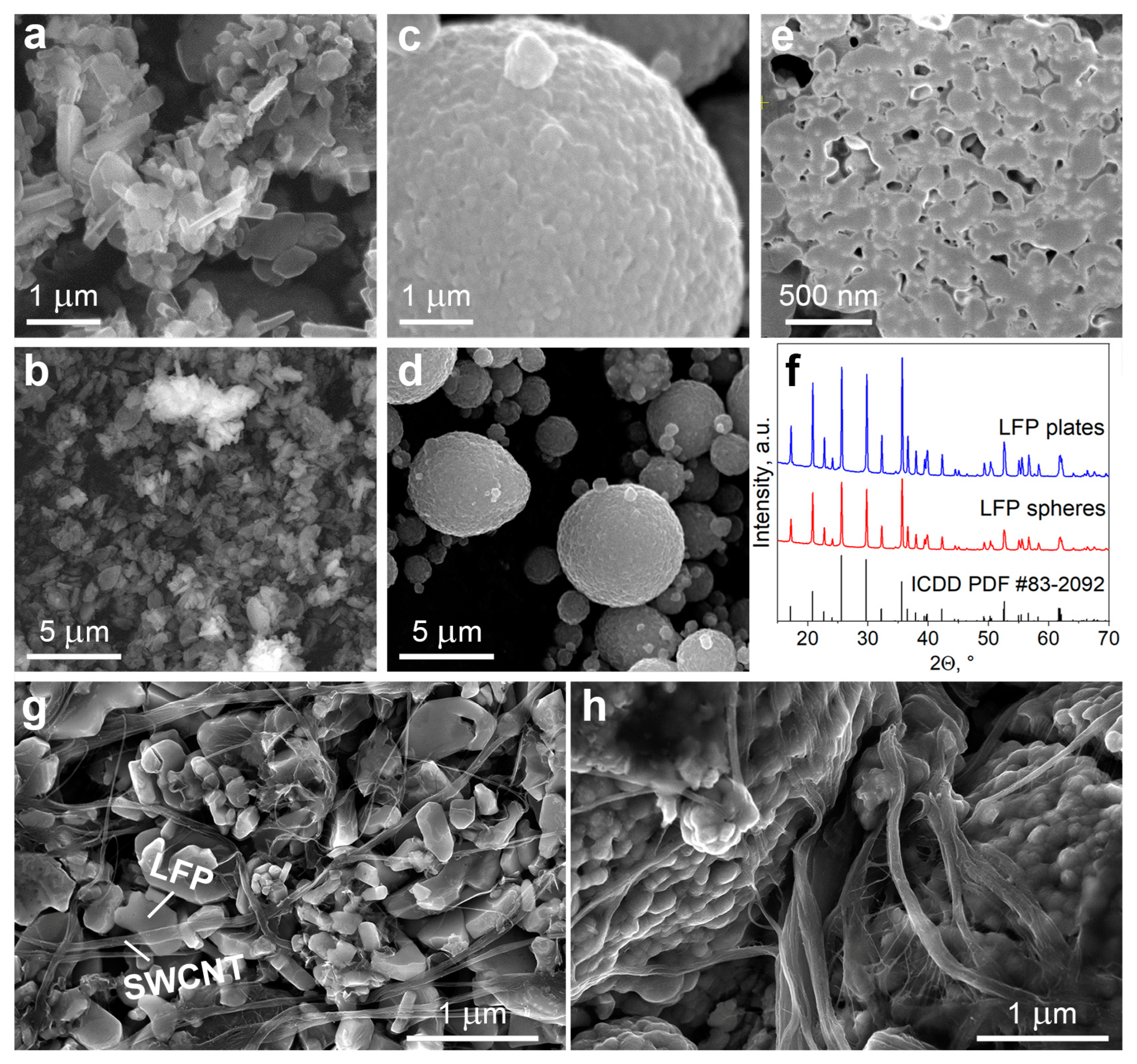
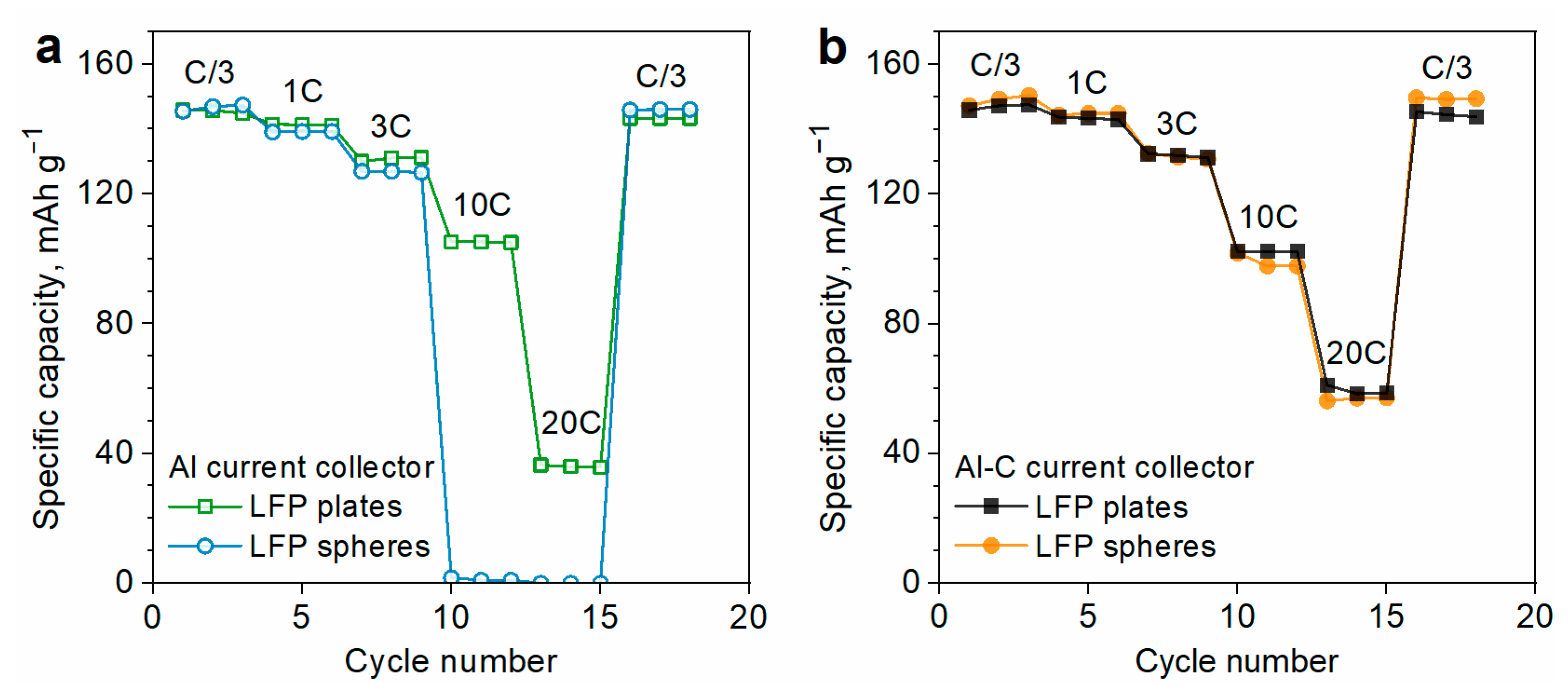
3.1. Effect of LFP Morphology
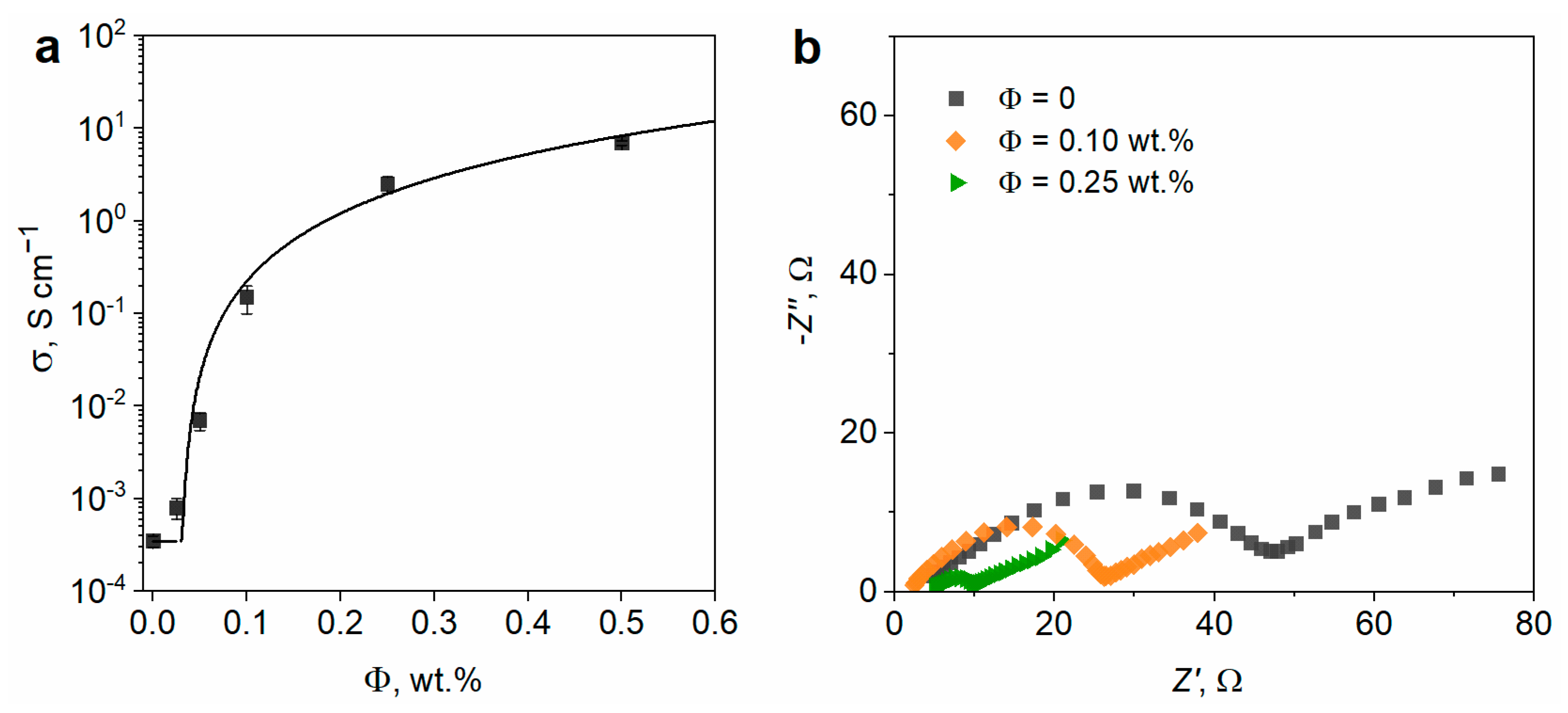
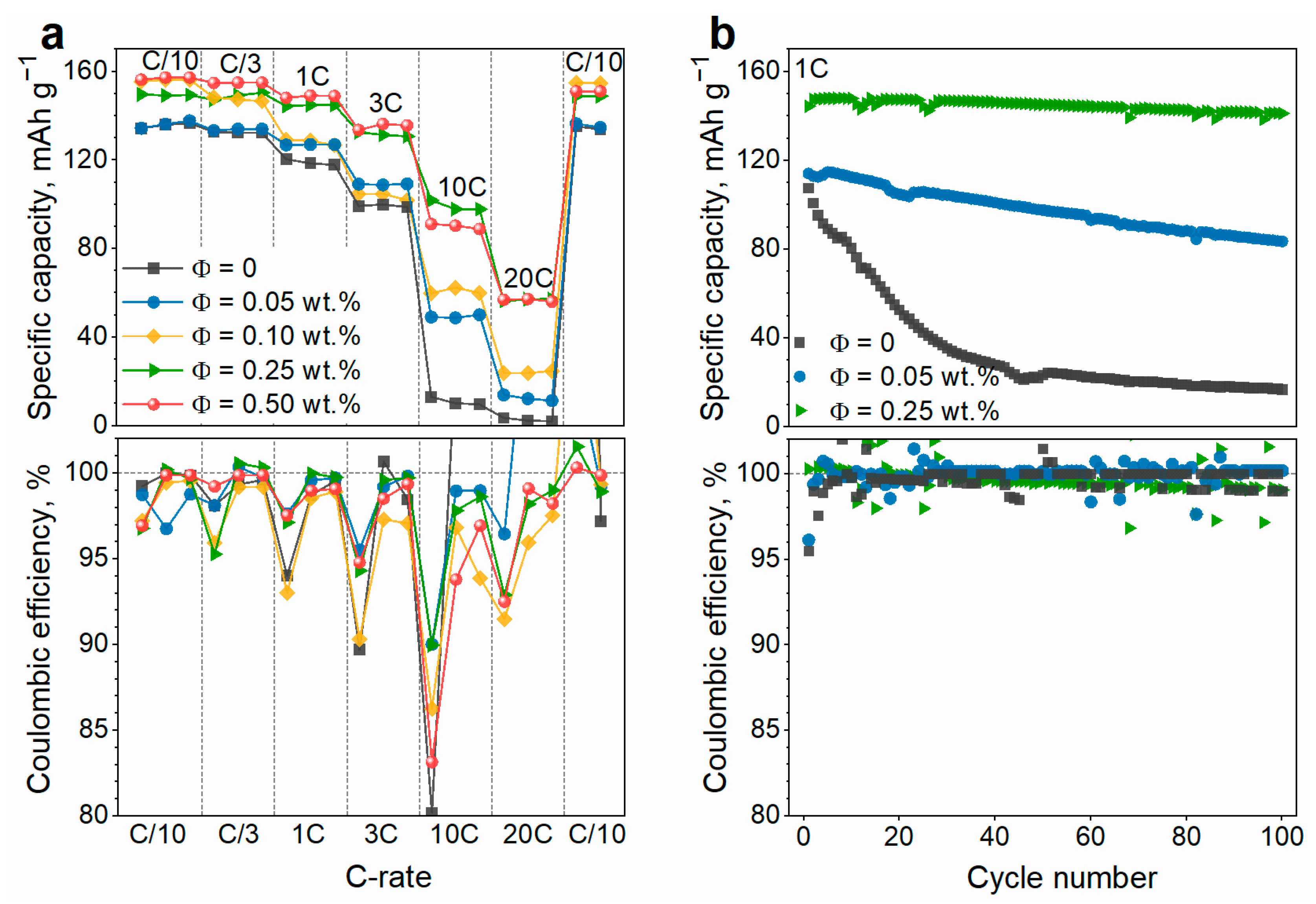
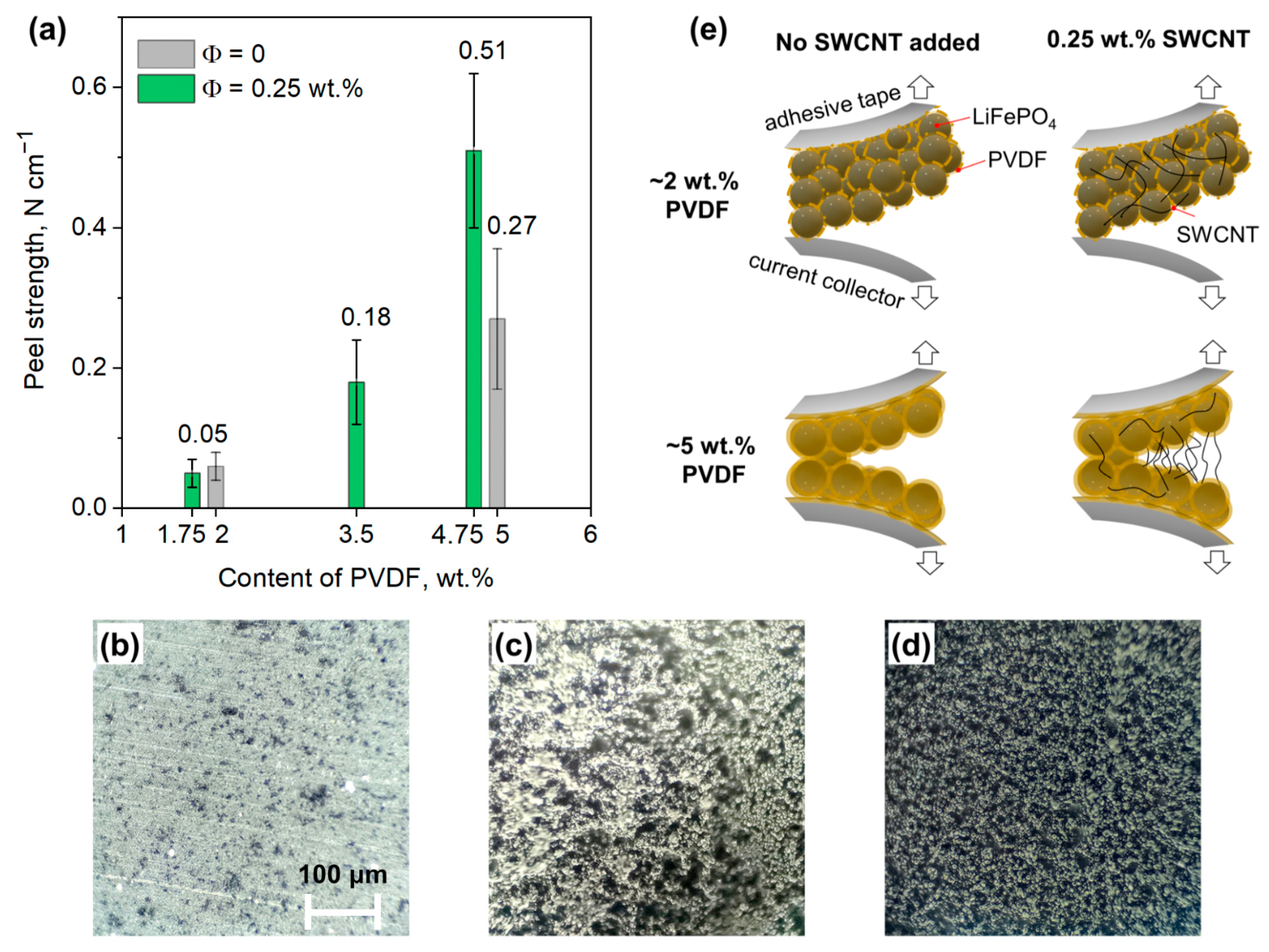
3.2. Effect of the SWCNT Content
3.3. Effect of the Binder Amount
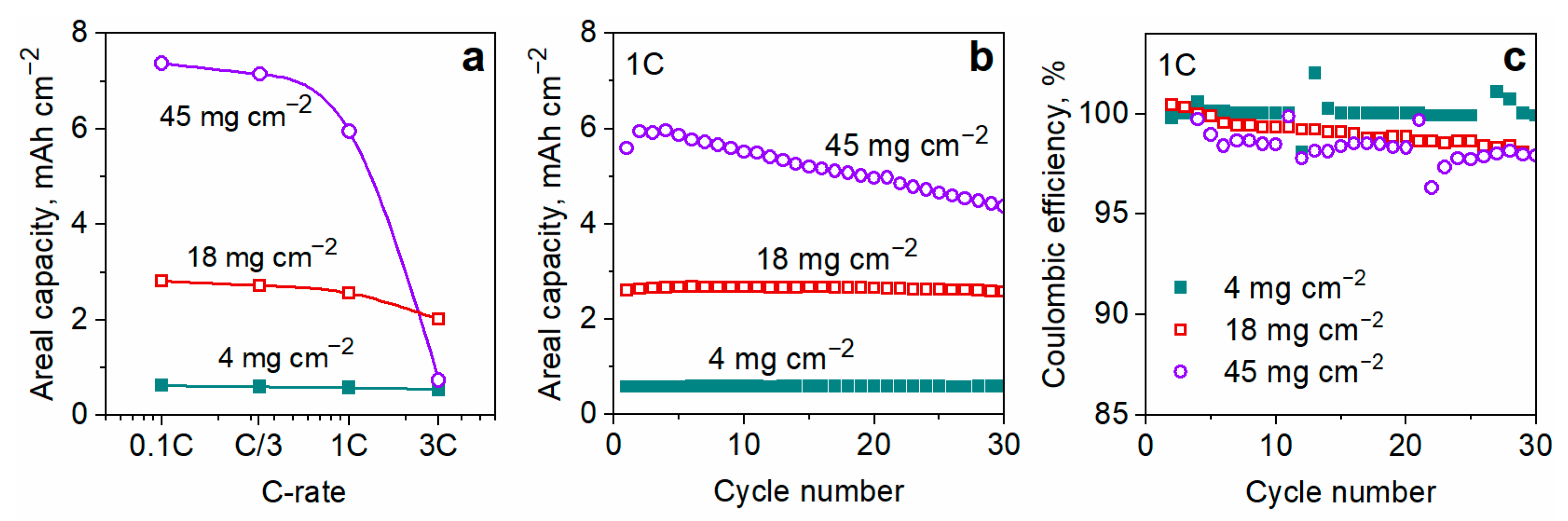
3.4. Effect of the Electrode Thickness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review. Electrochem. Energ. Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef]
- Choi, D.; Shamim, N.; Crawford, A.; Huang, Q.; Vartanian, C.K.; Viswanathan, V.V.; Paiss, M.D.; Alam, M.J.E.; Reed, D.M.; Sprenkle, V.L. Li-Ion Battery Technology for Grid Application. J. Power Sources 2021, 511, 230419. [Google Scholar] [CrossRef]
- Chen, S.-P.; Lv, D.; Chen, J.; Zhang, Y.-H.; Shi, F.-N. Review on Defects and Modification Methods of LiFePO4 Cathode Material for Lithium-Ion Batteries. Energy Fuels 2022, 36, 1232–1251. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Sundarrajan, S.; Chellappan, V.; Reddy, M.V.; Ramakrishna, S.; Zaghib, K. Recent Development in Carbon-LiFePO4 Cathodes for Lithium-Ion Batteries: A Mini Review. Batteries 2022, 8, 133. [Google Scholar] [CrossRef]
- Xiuqin, O.; Lin, P.; Haichen, G.; Yichen, W.; Jianwei, L. Temperature-Dependent Crystallinity and Morphology of LiFePO4 Prepared by Hydrothermal Synthesis. J. Mater. Chem. 2012, 22, 9064–9068. [Google Scholar] [CrossRef]
- Wang, X.; Wen, L.; Zheng, Y.; Liu, H.; Liang, G. Facile Synthesis and Electrochemical Properties of High Tap Density LiFePO4/C. Ionics 2019, 25, 4589–4596. [Google Scholar] [CrossRef]
- Sun, S.; An, Q.; Tian, Z.; Zhao, X.; Shen, X. Low-Temperature Synthesis of LiFePO4 Nanoplates/C Composite for Lithium Ion Batteries. Energy Fuels 2020, 34, 11597–11605. [Google Scholar] [CrossRef]
- Vu, A.; Stein, A. Lithium Iron Phosphate Spheres as Cathode Materials for High Power Lithium Ion Batteries. J. Power Sources 2014, 245, 48–58. [Google Scholar] [CrossRef]
- Fisher, C.A.J.; Saiful Islam, M. Surface Structures and Crystal Morphologies of LiFePO4: Relevance to Electrochemical Behaviour. J. Mater. Chem. 2008, 18, 1209–1215. [Google Scholar] [CrossRef]
- Nan, C.; Lu, J.; Li, L.; Li, L.; Peng, Q.; Li, Y. Size and Shape Control of LiFePO4 Nanocrystals for Better Lithium Ion Battery Cathode Materials. Nano Res. 2013, 6, 469–477. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Xue, J.; Ke, X.; Liang, G. LiFePO4/C Composites with High Compaction Density as Cathode Materials for Lithium-Ion Batteries with High Volumetric Energy Density. Ionics 2021, 27, 4687–4694. [Google Scholar] [CrossRef]
- Sun, J.; Li, Z.; Ren, X.; Wang, L.; Liang, G. High Volumetric Energy Density of LiFePO4/C Microspheres Based on Xylitol-Polyvinyl Alcohol Complex Carbon Sources. J. Alloys Compd. 2019, 773, 788–795. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, G.-S.; Choi, Y.-M.; Park, W.I.; Rogers, J.A.; Paik, U. Comparison of Multiwalled Carbon Nanotubes and Carbon Black as Percolative Paths in Aqueous-Based Natural Graphite Negative Electrodes with High-Rate Capability for Lithium-Ion Batteries. J. Power Sources 2008, 184, 308–311. [Google Scholar] [CrossRef]
- Sheem, K.; Lee, Y.H.; Lim, H.S. High-Density Positive Electrodes Containing Carbon Nanotubes for Use in Li-Ion Cells. J. Power Sources 2006, 158, 1425–1430. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Peng, H.-J.; Zhang, Q.; Huang, J.-Q.; Liu, X.-F.; Wang, L.; He, X.; Zhu, W.; Wei, F. Hierarchical Carbon Nanotube/Carbon Black Scaffolds as Short- and Long-Range Electron Pathways with Superior Li-Ion Storage Performance. ACS Sustain. Chem. Eng. 2014, 2, 200–206. [Google Scholar] [CrossRef]
- Tian, R.; Alcala, N.; O’Neill, S.J.K.; Horvath, D.V.; Coelho, J.; Griffin, A.J.; Zhang, Y.; Nicolosi, V.; O’Dwyer, C.; Coleman, J.N. Quantifying the Effect of Electronic Conductivity on the Rate Performance of Nanocomposite Battery Electrodes. ACS Appl. Energy Mater. 2020, 3, 2966–2974. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Guo, H.; Wang, Z.; Peng, W.; Yang, Y.; Liang, R. Effect of Carbon Nanotube on the Electrochemical Performance of C-LiFePO4/Graphite Battery. J. Power Sources 2008, 184, 522–526. [Google Scholar] [CrossRef]
- Napolskiy, F.; Avdeev, M.; Yerdauletov, M.; Ivankov, O.; Bocharova, S.; Ryzhenkova, S.; Kaparova, B.; Mironovich, K.; Burlyaev, D.; Krivchenko, V. On the Use of Carbon Nanotubes in Prototyping the High Energy Density Li-ion Batteries. Energy Technol. 2020, 8, 2000146. [Google Scholar] [CrossRef]
- Lin, X.; Shu, J.; Wu, K.; Shao, L.; Li, P.; Shui, M.; Wang, D.; Long, N.; Ren, Y. Improved Electrochemical Property of Pb(NO3)2 by Carbon Black, Graphene and Carbon Nanotube. Electrochim. Acta 2014, 137, 767–773. [Google Scholar] [CrossRef]
- Ning, G.; Zhang, S.; Xiao, Z.; Wang, H.; Ma, X. Efficient Conducting Networks Constructed from Ultra-Low Concentration Carbon Nanotube Suspension for Li Ion Battery Cathodes. Carbon 2018, 132, 323–328. [Google Scholar] [CrossRef]
- Jessl, S.; Beesley, D.; Engelke, S.; Valentine, C.J.; Stallard, J.C.; Fleck, N.; Ahmad, S.; Cole, M.T.; De Volder, M. Carbon Nanotube Conductive Additives for Improved Electrical and Mechanical Properties of Flexible Battery Electrodes. Mater. Sci. Eng. A 2018, 735, 269–274. [Google Scholar] [CrossRef]
- Zhu, P.; Gastol, D.; Marshall, J.; Sommerville, R.; Goodship, V.; Kendrick, E. A Review of Current Collectors for Lithium-Ion Batteries. J. Power Sources 2021, 485, 229321. [Google Scholar] [CrossRef]
- Striebel, K.; Shim, J.; Sierra, A.; Yang, H.; Song, X.; Kostecki, R.; McCarthy, K. The Development of Low Cost LiFePO4-Based High Power Lithium-Ion Batteries. J. Power Sources 2005, 146, 33–38. [Google Scholar] [CrossRef]
- Wu, H.-C.; Wu, H.-C.; Lee, E.; Wu, N.-L. High-Temperature Carbon-Coated Aluminum Current Collector for Enhanced Power Performance of LiFePO4 Electrode of Li-Ion Batteries. Electrochem. Commun. 2010, 12, 488–491. [Google Scholar] [CrossRef]
- Kubarkov, A.V.; Asharchuk, A.A.; Drozhzhin, O.A.; Karpushkin, E.A.; Stevenson, K.J.; Antipov, E.V.; Sergeyev, V.G. Effect of Polymer Binders with Single-Walled Carbon Nanotubes on the Electrochemical and Physicochemical Properties of the LiFePO4 Cathode. ACS Appl. Energy Mater. 2021, 4, 12310–12318. [Google Scholar] [CrossRef]
- Belharouak, I.; Johnson, C.; Amine, K. Synthesis and Electrochemical Analysis of Vapor-Deposited Carbon-Coated LiFePO4. Electrochem. Commun. 2005, 7, 983–988. [Google Scholar] [CrossRef]
- Gurova, O.A.; Arhipov, V.E.; Koroteev, V.O.; Guselnikova, T.Y.; Asanov, I.P.; Sedelnikova, O.V.; Okotrub, A.V. Purification of Single-Walled Carbon Nanotubes Using Acid Treatment and Magnetic Separation. Phys. Status Solidi B 2019, 256, 1800742. [Google Scholar] [CrossRef]
- Reynolds, C.D.; Slater, P.R.; Hare, S.D.; Simmons, M.J.H.; Kendrick, E. A Review of Metrology in Lithium-Ion Electrode Coating Processes. Mater. Des. 2021, 209, 109971. [Google Scholar] [CrossRef]
- ASTM D1876-01; Standard Test Method for Peel Resistance of Adhesives (T-Peel Test). ASTM International: West Conshohocken, PA, USA, 2001. [CrossRef]
- Wen, L.; Sun, J.; An, L.; Wang, X.; Ren, X.; Liang, G. Effect of Conductive Material Morphology on Spherical Lithium Iron Phosphate. Nanomaterials 2018, 8, 904. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; An, L.; Zhao, X.; Liang, G. Blended Spherical Lithium Iron Phosphate Cathodes for High Energy Density Lithium–Ion Batteries. Ionics 2019, 25, 61–69. [Google Scholar] [CrossRef]
- Ying, J.; Lei, M.; Jiang, C.; Wan, C.; He, X.; Li, J.; Wang, L.; Ren, J. Preparation and Characterization of High-Density Spherical Li0.97Cr0.01FePO4/C Cathode Material for Lithium Ion Batteries. J. Power Sources 2006, 158, 543–549. [Google Scholar] [CrossRef]
- Loghavi, M.M.; Askari, M.; Babaiee, M.; Ghasemi, A. Improvement of the Cyclability of Li-Ion Battery Cathode Using a Chemical-Modified Current Collector. J. Electroanal. Chem. 2019, 841, 107–110. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Zhao, X.; Wang, L.; Liang, G. Effect of Spherical Particle Size on the Electrochemical Properties of Lithium Iron Phosphate. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2019, 34, 549–557. [Google Scholar] [CrossRef]
- Choi, W.; Shin, H.-C.; Kim, J.M.; Choi, J.-Y.; Yoon, W.-S. Modeling and Applications of Electrochemical Impedance Spectroscopy (EIS) for Lithium-Ion Batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Dreyer, W.; Jamnik, J.; Guhlke, C.; Huth, R.; Moškon, J.; Gaberšček, M. The Thermodynamic Origin of Hysteresis in Insertion Batteries. Nature Mater. 2010, 9, 448–453. [Google Scholar] [CrossRef]
- Wei, W.; Guo, L.; Qiu, X.; Qu, P.; Xu, M.; Guo, L. Porous Micro-Spherical LiFePO4/CNT Nanocomposite for High-Performance Li-Ion Battery Cathode Material. RSC Adv. 2015, 5, 37830–37836. [Google Scholar] [CrossRef]
- Hwang, J.; Kong, K.C.; Chang, W.; Jo, E.; Nam, K.; Kim, J. New Liquid Carbon Dioxide Based Strategy for High Energy/Power Density LiFePO4. Nano Energy 2017, 36, 398–410. [Google Scholar] [CrossRef]
- Wang, B.; Liu, T.; Liu, A.; Liu, G.; Wang, L.; Gao, T.; Wang, D.; Zhao, X.S. A Hierarchical Porous C@LiFePO4/Carbon Nanotubes Microsphere Composite for High-Rate Lithium-Ion Batteries: Combined Experimental and Theoretical Study. Adv. Energy Mater. 2016, 6, 1600426. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A Review and Analysis of Electrical Percolation in Carbon Nanotube Polymer Composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Ezquerra, T.A.; Canalda, J.C.; Sanz, A.; Linares, A. On the Electrical Conductivity of PVDF Composites with Different Carbon-Based Nanoadditives. Colloid Polym. Sci. 2014, 292, 1989–1998. [Google Scholar] [CrossRef]
- Krause, B.; Barbier, C.; Kunz, K.; Pötschke, P. Comparative Study of Singlewalled, Multiwalled, and Branched Carbon Nanotubes Melt Mixed in Different Thermoplastic Matrices. Polymer 2018, 159, 75–85. [Google Scholar] [CrossRef]
- Pang, H.; Xu, L.; Yan, D.-X.; Li, Z.-M. Conductive Polymer Composites with Segregated Structures. Prog. Polym. Sci. 2014, 39, 1908–1933. [Google Scholar] [CrossRef]
- Predtechenskiy, M.R.; Khasin, A.A.; Smirnov, S.N.; Bezrodny, A.E.; Bobrenok, O.F.; Dubov, D.Y.; Kosolapov, A.G.; Lyamysheva, E.G.; Muradyan, V.E.; Saik, V.O.; et al. New Perspectives in SWCNT Applications: Tuball SWCNTs. Part 2. New Composite Materials through Augmentation with Tuball. Carbon Trends 2022, 8, 100176. [Google Scholar] [CrossRef]
- Gong, C.; Xue, Z.; Wen, S.; Ye, Y.; Xie, X. Advanced Carbon Materials/Olivine LiFePO4 Composites Cathode for Lithium Ion Batteries. J. Power Sources 2016, 318, 93–112. [Google Scholar] [CrossRef]
- Yoo, J.-K.; Oh, Y.; Park, T.; Lee, K.E.; Um, M.-K.; Yi, J.-W. Optimization of Carbon Nanotubes as Conductive Additives for High-Energy-Density Electrodes for Lithium-Ion Batteries. Energy Technol. 2019, 7, 1800845. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, R.; Liu, G.; Song, X.; Battaglia, V.S. Cooperation between Active Material, Polymeric Binder and Conductive Carbon Additive in Lithium Ion Battery Cathode. J. Phys. Chem. C 2012, 116, 4875–4882. [Google Scholar] [CrossRef]
- Lee, Y.K. The Effect of Active Material, Conductive Additives, and Binder in a Cathode Composite Electrode on Battery Performance. Energies 2019, 12, 658. [Google Scholar] [CrossRef]
- Haselrieder, W.; Westphal, B.; Bockholt, H.; Diener, A.; Höft, S.; Kwade, A. Measuring the Coating Adhesion Strength of Electrodes for Lithium-Ion Batteries. Int. J. Adhes. Adhes. 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Billot, N.; Günther, T.; Schreiner, D.; Stahl, R.; Kranner, J.; Beyer, M.; Reinhart, G. Investigation of the Adhesion Strength along the Electrode Manufacturing Process for Improved Lithium-Ion Anodes. Energy Technol. 2020, 8, 1801136. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V.S. A Comprehensive Understanding of Electrode Thickness Effects on the Electrochemical Performances of Li-Ion Battery Cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- de la Torre-Gamarra, C.; Sotomayor, M.E.; Sanchez, J.-Y.; Levenfeld, B.; Várez, A.; Laïk, B.; Pereira-Ramos, J.-P. High Mass Loading Additive-Free LiFePO4 Cathodes with 500 μm Thickness for High Areal Capacity Li-Ion Batteries. J. Power Sources 2020, 458, 228033. [Google Scholar] [CrossRef]
- Yu, D.Y.W.; Donoue, K.; Inoue, T.; Fujimoto, M.; Fujitani, S. Effect of Electrode Parameters on LiFePO4 Cathodes. J. Electrochem. Soc. 2006, 153, A835. [Google Scholar] [CrossRef]
- Rousselot, S.; Antitomaso, P.; Savignac, L.; Généreux, S.; Taylor, L.W.; Bibienne, T.; Pasquali, M.; Schougaard, S.B.; Dollé, M. PEDOT Assisted CNT Self-Supported Electrodes for High Energy and Power Density. Electrochim. Acta 2020, 349, 136418. [Google Scholar] [CrossRef]
- Raj, H.; Sil, A. Energy and Power Densities of Novel Composite Electrode Driven by Synergy of Poly(3,4-Ethylene Dioxythiophene):Poly(Styrene Sulfonate) and Single Walled Carbon Nanotubes for Lithium-Ion Battery. J. Power Sources 2020, 458, 228052. [Google Scholar] [CrossRef]

| Morphology of LFP Particles | Tap Density of LFP Particles, g cm−3 | Electrode Density, g cm−3 | Volumetric Capacity of Electrode 2, mAh cm−3 |
|---|---|---|---|
| plates | 0.8 | 0.81 ± 0.2 | 116.1 |
| spheres | 1.7 | 1.76 ± 0.2 | 255.0 |
| Content of LFP, wt.% | Loading of LFP, mg cm−2 | Content of CNT, wt,% | Density of Electrode, g cm−3 | Specific Capacity (10C-Rate), mAh g−1 | Areal Capacity (1C-Rate), mAh cm−2 | Free-Standing Electrode | Reference |
|---|---|---|---|---|---|---|---|
| 95 | 4.0 | 0.25 | 1.8 | 100 | 0.39 | – | This study |
| 95 | 4.8 | 0.25 | 1.4 | 105 | 0.50 | – | [25] |
| 95 | 7.0 | 5 | n/a | 105 | 0.96 | + | [54] |
| 81 | 6.0 | 4 | n/a | 118 | 0.79 | + | [54] |
| 86 | n/a | 4.6 | n/a | 100 | n/a | – | [55] |
| 98 | 45 | 0.25 | 1.8 | n/a | 5.95 | + | This study |
| 90 | 40 | 1 | 1.3 | n/a | 5.1 | – | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubarkov, A.V.; Babkin, A.V.; Drozhzhin, O.A.; Stevenson, K.J.; Antipov, E.V.; Sergeyev, V.G. Engendering High Energy Density LiFePO4 Electrodes with Morphological and Compositional Tuning. Nanomaterials 2023, 13, 1771. https://doi.org/10.3390/nano13111771
Kubarkov AV, Babkin AV, Drozhzhin OA, Stevenson KJ, Antipov EV, Sergeyev VG. Engendering High Energy Density LiFePO4 Electrodes with Morphological and Compositional Tuning. Nanomaterials. 2023; 13(11):1771. https://doi.org/10.3390/nano13111771
Chicago/Turabian StyleKubarkov, Aleksei V., Alexander V. Babkin, Oleg A. Drozhzhin, Keith J. Stevenson, Evgeny V. Antipov, and Vladimir G. Sergeyev. 2023. "Engendering High Energy Density LiFePO4 Electrodes with Morphological and Compositional Tuning" Nanomaterials 13, no. 11: 1771. https://doi.org/10.3390/nano13111771







