Review of Electrochemically Synthesized Resistive Switching Devices: Memory Storage, Neuromorphic Computing, and Sensing Applications
Abstract
:1. Introduction
2. Resistive Switching Effects
2.1. Unipolar and Bipolar Resistive Switching
2.2. Resistive Switching Mechanisms
2.2.1. Electrochemical Metallization Memory
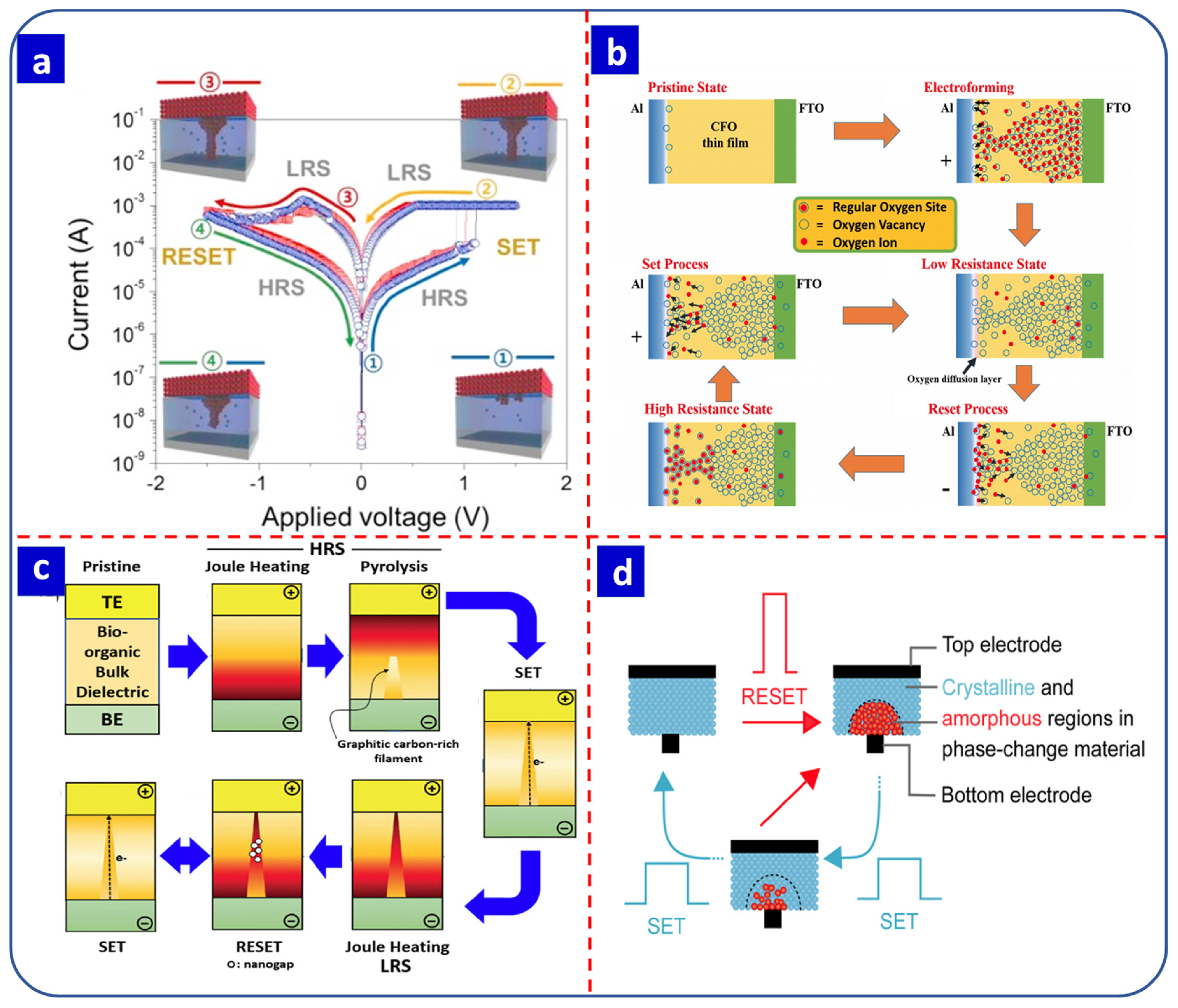
2.2.2. Valance-Change Memory
2.2.3. Thermochemical Memory
2.2.4. Phase-Change Memory
3. Resistive Switching Devices and Their Applications
3.1. Memory Storage
3.2. Neuromorphic Computing
| Requirements | Refs. | ||
|---|---|---|---|
| Memory storage | Operating voltage | <1 V | [3,76,77,78,79] |
| Type of switching | Digital, analog | ||
| Switching layer thickness | 50 nm | ||
| Power consumption | ~10 pJ/transition | ||
| Switching time | <10 ns/transition | ||
| Endurance | >109 cycles | ||
| Data retention | >10 years (85 °C) | ||
| MIM cell size | ~500 nm2 | ||
| 3D stacking | Yes | ||
| Neuromorphic computing | Operating voltage | <1 V | [1,2,91] |
| Type of switching | Analog | ||
| Switching layer thickness | 50 nm | ||
| Power consumption | ~10 pJ/transition | ||
| Switching time | <10 ns/transition | ||
| Endurance | >109 cycles | ||
| Data retention | >10 years | ||
| MIM cell size | ~500 nm2 | ||
| Potentiation and depression (P&D) | Yes | ||
| Spike-timing-dependent plasticity (STDP) | Yes | ||
| Spike-rate-dependent plasticity (SRDP) | Yes | ||
| Sensor | Operating voltage | <1 V | [3,75] |
| Type of switching | Digital, analog | ||
| Switching layer thickness | 50 nm | ||
| Power consumption | ~10 pJ/transition | ||
| Switching time | <10 ns/transition | ||
| Endurance | >109 cycles | ||
| Data retention | >10 years (85 °C) | ||
| MIM cell size | ~500 nm2 | ||
| Response time | Low as possible | ||
3.3. Sensor Applications
3.4. Other Applications

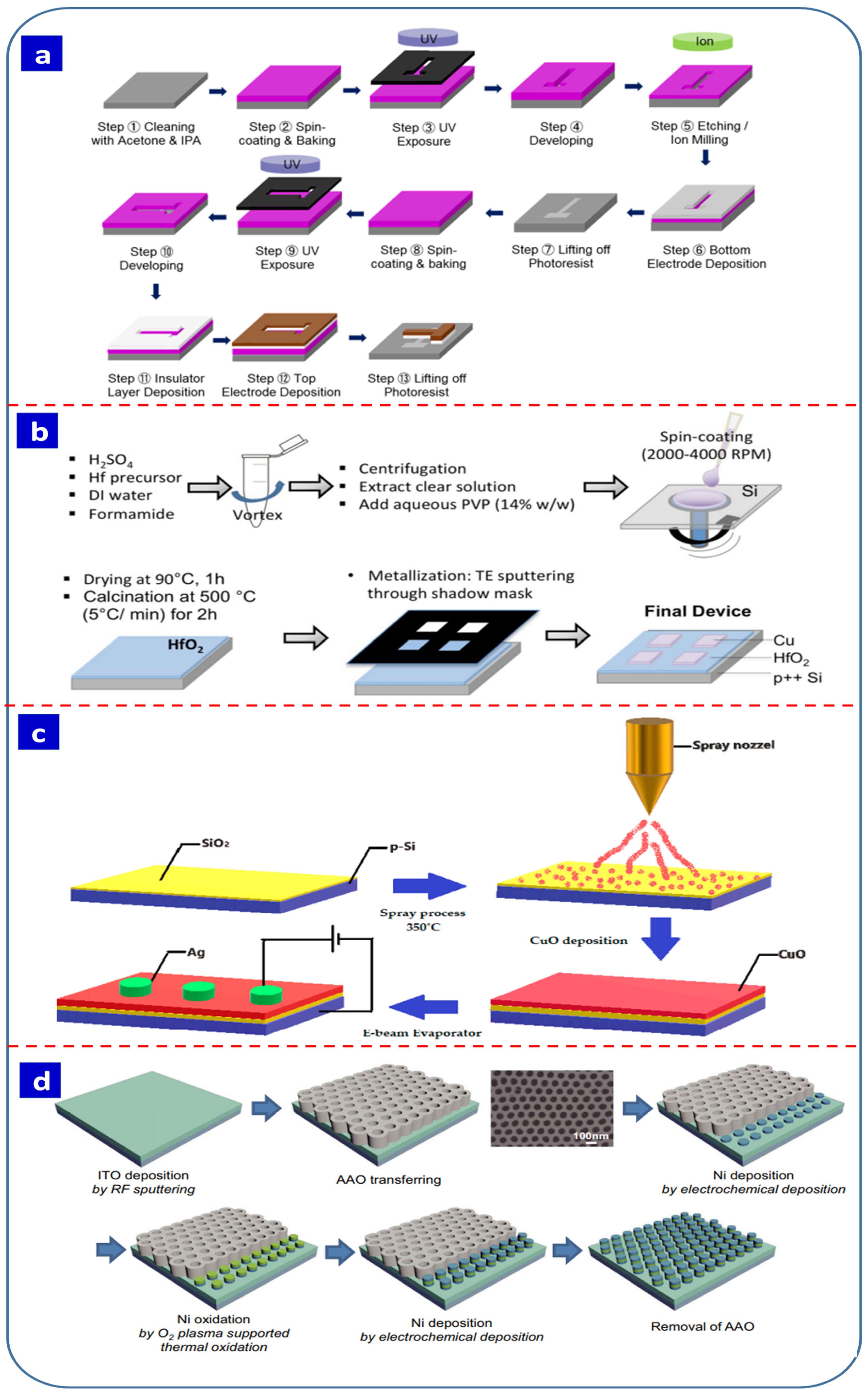
4. Fabrication Techniques for Resistive Switching Devices
5. Electrochemical Methods for Resistive Switching Devices
5.1. Electrodeposition

5.2. Anodization
6. Electrodeposited Resistive Switching Materials for Memory Storage Applications
6.1. Carbon-Based Resistive Switching Devices
6.2. ZnO-Based Resistive Switching Devices
6.3. TiO2-Based Resistive Switching Devices
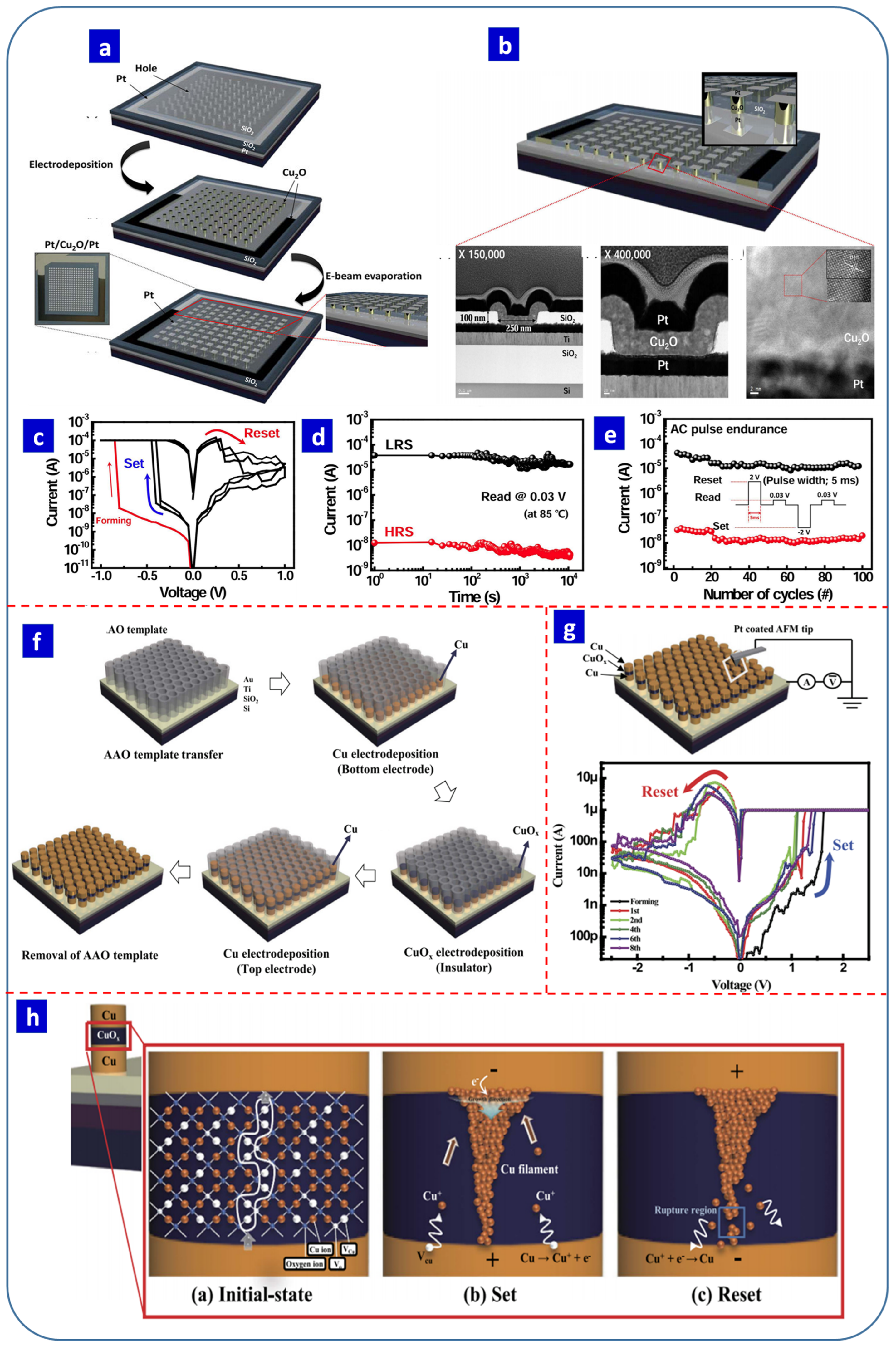
6.4. Cu2O-Based Resistive Switching Devices
6.5. Other Metal-Oxide- and Composite-Based Resistive Switching Devices
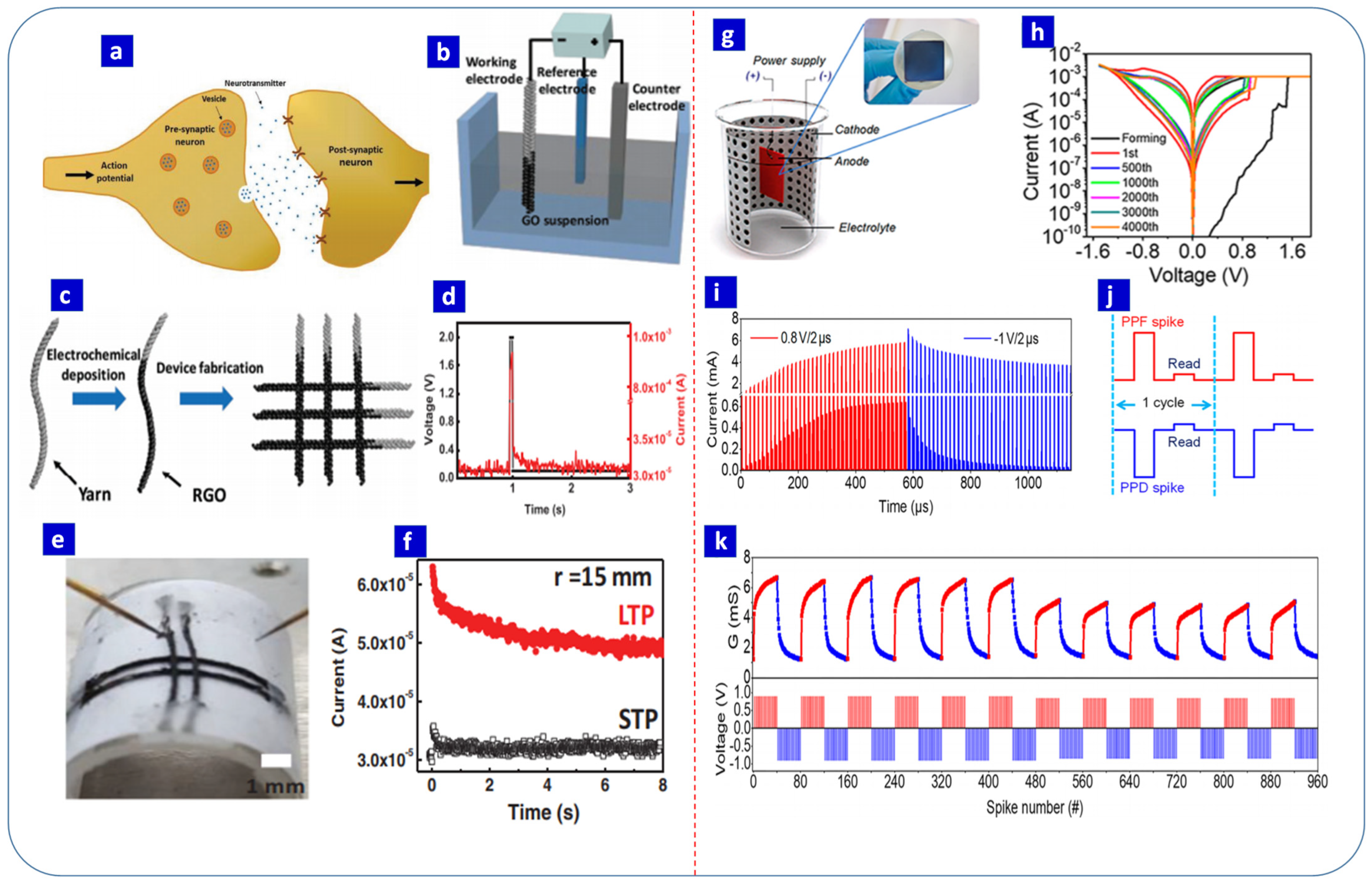
7. Electrochemically Synthesized Resistive Switching Materials for Neuromorphic Computing Applications
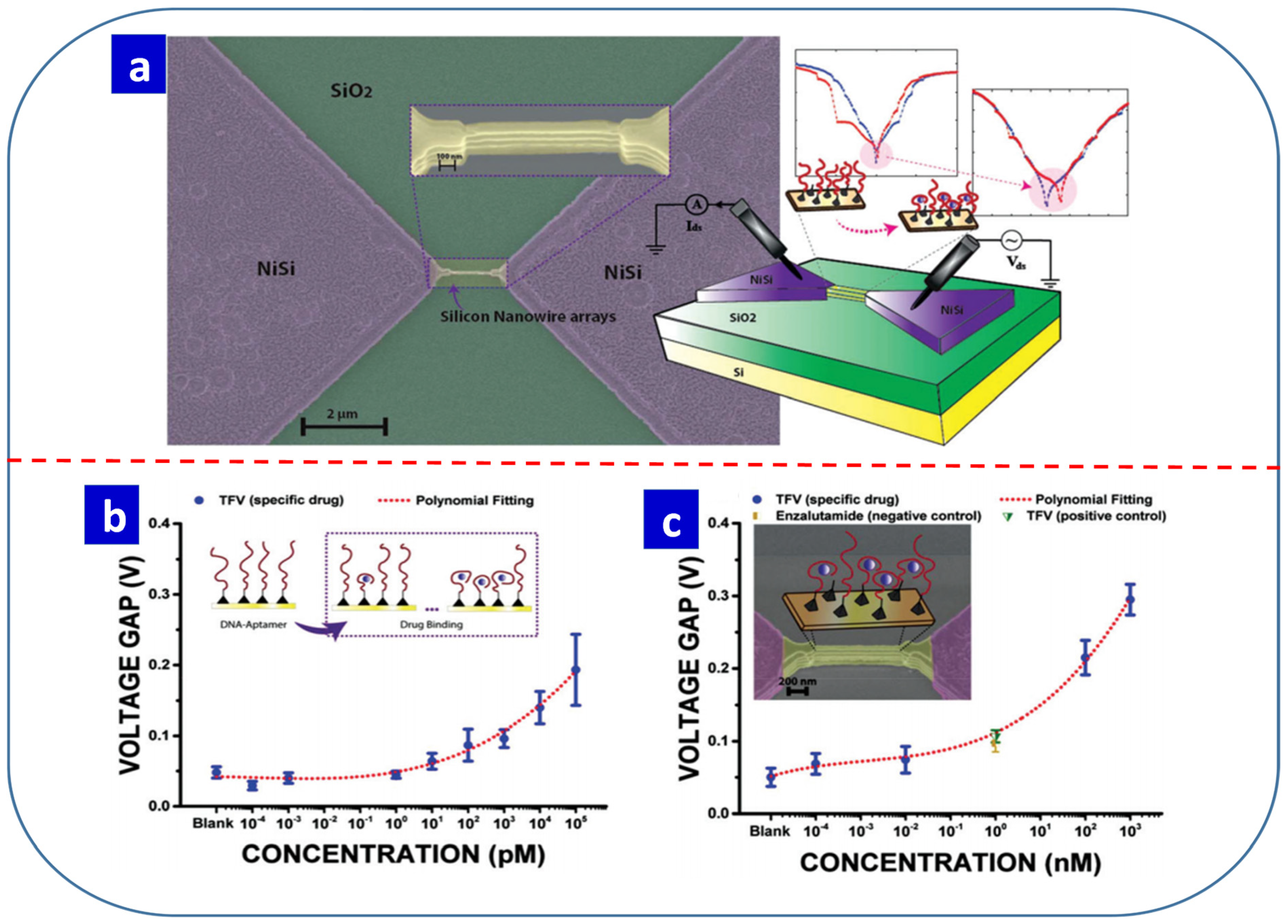
8. Electrochemically Synthesized Resistive Switching Materials for Sensing Applications
9. Future Outlook
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, S.G.; Han, J.S.; Kim, H.; Kim, S.Y.; Jang, H.W. Recent advances in memristive materials for artificial synapses. Adv. Electron. Mater. 2018, 3, 1800457. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Wang, L.; Chen, F.; Liang, D. Scientific big data and digital earth. Chin. Sci. Bull. 2014, 59, 5066–5073. [Google Scholar] [CrossRef]
- Lanza, M.; Wong, H.S.P.; Pop, E.; Ielmini, D.; Strukov, D.; Regan, B.C.; Larcher, L.; Villena, M.A.; Yang, J.J.; Gouxand Belmonte, L. A Recommended Methods to Study Resistive Switching Devices. Adv. Electron. Mater. 2019, 5, 1800143. [Google Scholar] [CrossRef] [Green Version]
- Chua, L. Memristor-the missing circuit element. IEEE Trans. Circuit Theory 1971, 18, 507–519. [Google Scholar] [CrossRef]
- Strukov, D.B.; Snider, G.S.; Stewart, D.R.; Williams, R.S. The missing memristor found. Nature 2008, 453, 80–83. [Google Scholar] [CrossRef]
- Dongale, T.D.; Khot, K.V.; Mohite, S.V.; Desai, N.D.; Shinde, S.S.; Patil, V.L.; Vanalkar, S.A.; Moholkar, A.V.; Rajpure, K.Y.; Bhosale, P.N.; et al. Effect of write voltage and frequency on the reliability aspects of memristor-based RRAM. Int. Nano Lett. 2017, 7, 209–216. [Google Scholar] [CrossRef]
- Le Gallo, M.; Sebastian, A. An overview of phase-change memory device physics. J. Phys. D Appl. Phys. 2020, 53, 213002. [Google Scholar] [CrossRef]
- Louis, J.; Hoffer, B.; Kvatinsky, S. Performing Memristor-Aided Logic (MAGIC) using STT-MRAM. In Proceedings of the 2019 26th IEEE International Conference on Electronics, Circuits and Systems (ICECS), Genoa, Italy, 27–29 November 2019; pp. 787–790. [Google Scholar]
- Kund, M.; Beitel, G.; Pinnow, C.U.; Rohr, T.; Schumann, J.; Symanczyk, R.; Ufert, K.; Muller, G. Conductive bridging RAM (CBRAM): An emerging non-volatile memory technology scalable to sub 20 nm. In Proceedings of the IEEE InternationalElectron Devices Meeting, 2005, IEDM Technical Digest, Washington, DC, USA, 5 December 2005; pp. 754–757. [Google Scholar]
- Shen, Z.; Zhao, C.; Qi, Y.; Mitrovic, I.Z.; Yang, L.; Wen, J.; Huang, Y.; Li, P.; Zhao, C. Memristive non-volatile memory based on graphene materials. Micromachines 2020, 11, 341. [Google Scholar] [CrossRef] [Green Version]
- Maan, A.K.; James, A.P. Voltage controlled memristor threshold logic gates. In Proceedings of the 2016 IEEE Asia Pacific Conference on Circuits and Systems, Jeju, Republic of Korea, 25–28 October 2016; pp. 376–379. [Google Scholar]
- Xia, Q.; Yang, J.J. Memristive crossbar arrays for brain-inspired computing. Nat. Mater. 2019, 18, 309–323. [Google Scholar] [CrossRef]
- Dongale, T.D.; Patil, P.J.; Desai, N.K.; Chougule, P.P.; Kumbhar, S.M.; Waifalkar, P.P.; Patil, P.B.; Vhatkar, R.S.; Takale, M.V.; Gaikwad, P.K.; et al. TiO2 based nanostructured memristor for RRAM and neuromorphic applications: A simulation approach. Nano Converg. 2016, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Gallas, J.A. Stability diagrams for a memristor oscillator. Eur. Phys. J. Spec. Top. 2019, 228, 2081–2091. [Google Scholar] [CrossRef] [Green Version]
- Ibrayev, T.; Fedorova, I.; Maanand, A.K.; James, A.P. Memristive operational amplifiers. Procedia Comput. Sci. 2014, 41, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Vishnu, S.; Saji, S.A.; Rohit, R.; Ramakrishnan, V.N. Application of memristors in active filters. In Proceedings of the 2016 3rd International Conference on Devices, Circuits and Systems (ICDCS), Coimbatore, India, 3–5 March 2016; pp. 84–88. [Google Scholar]
- Yuan, F.; Li, Y. A chaotic circuit constructed by a memristor, a memcapacitor and a meminductor. Chaos 2019, 29, 101101. [Google Scholar] [CrossRef] [PubMed]
- Vahl, A.; Carstensen, J.; Kaps, S.; Lupan, O.; Strunskus, T.; Adelung, R.; Faupel, F. Concept and modelling of memsensors as two terminal devices with enhanced capabilities in neuromorphic engineering. Sci. Rep. 2019, 9, 4361. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; Kim, K.H.; Lee, B.R.; Park, J.H.; Schubert, E.F.; Kim, T.G. Glass-based transparent conductive electrode: Its application to visible-to-ultraviolet light-emitting diodes. ACS Appl. Mater. Interfaces 2016, 8, 35668–35677. [Google Scholar] [CrossRef]
- Kim, H.D.; An, H.M.; Kim, K.H.; Kim, S.J.; Kim, C.S.; Cho, J.; Schubert, E.F.; Kim, T.G. A universal method of producing transparent electrodes using wide-bandgap materials. Adv. Funct. Mater. 2014, 24, 1575–1581. [Google Scholar] [CrossRef]
- Park, N.W.; Kang, D.Y.; Lee, W.Y.; Yoon, Y.S.; Kim, G.S.; Saitoh, E.; Kim, T.G.; Lee, S.K. Controllable seebeck coefficients of a metal-diffused aluminum oxide layer via conducting filament density and energy filtering. ACS Appl. Mater. Interfaces 2019, 11, 23303–23312. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Lee, W.Y.; Park, N.W.; Yoon, Y.S.; Kim, G.S.; Kim, T.G.; Lee, S.K. Tunable thermal conductivity in aluminum oxide resistive based switching structures by conducting filament diffusion. J. Alloys Compd. 2019, 790, 992–1000. [Google Scholar] [CrossRef]
- Kamble, G.U.; Shetake, N.P.; Yadav, S.D.; Teli, A.M.; Patil, D.S.; Pawar, S.A.; Karanjkar, M.M.; Patil, P.S.; Shin, J.C.; Orlowskiand, M.K.; et al. Coexistence of filamentary and homogeneous resistive switching with memristive and meminductive memory effects in Al/MnO2/SS thin film metal–insulator–metal device. Int. Nano Lett. 2018, 8, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Kamble, G.U.; Takaloo, A.V.; Teli, A.M.; Kim, Y.J.; Sonar, P.; Dongale, T.D.; Kim, D.K.; Kim, T.W. Highly-stable memristive devices with synaptic characteristics based on hydrothermally synthesized MnO2 active layers. J. Alloys Compd. 2021, 872, 159653. [Google Scholar] [CrossRef]
- Dongale, T.D.; Bagade, A.A.; Mohite, S.V.; Rananavare, A.D.; Orlowski, M.K.; Kamat, R.K.; Rajpure, K.Y. Bipolar resistive switching with coexistence of mem-elements in the spray deposited CoFe2O4 thin film. J. Mater. Sci. Mater. Electron. 2018, 29, 3231–3238. [Google Scholar] [CrossRef]
- Dongle, V.S.; Dongare, A.A.; Mullani, N.B.; Pawar, P.S.; Patil, P.B.; Heo, J.; Park, T.J.; Dongale, T.D. Development of self-rectifying ZnO thin film resistive switching memory device using successive ionic layer adsorption and reaction method. J. Mater. Sci. Mater. Electron. 2018, 29, 18733–18741. [Google Scholar] [CrossRef]
- Hadi, S.A.; Humood, K.M.; Abi Jaoude, M.; Abu Nahla, H.; FAlShehhi, H.; Mohammad, B. Bipolar Cu/HfO2/p++ Si memristors by sol-gel spin coating method and their application to environmental sensing. Sci. Rep. 2019, 9, 9983. [Google Scholar] [CrossRef] [Green Version]
- Khot, A.C.; Dongale, T.D.; Nirmal, K.A.; Deepthi, J.K.; Sutar, S.S.; Kim, T.G. 2D Ti3C2Tx MXene-derived self-assembled 3D TiO2 nanoflowers for nonvolatile memory and synaptic learning applications. J. Mater. Sci. Technol. 2023, 150, 1–10. [Google Scholar] [CrossRef]
- Desai, T.R.; Dongale, T.D.; Patil, S.R.; Tiwari, A.P.; Pawar, P.K.; Kamat, R.K.; Kim, T.G. Synaptic learning functionalities of inverse biomemristive device based on trypsin for artificial intelligence application. J. Mater. Res. Technol. 2021, 11, 1100–1110. [Google Scholar] [CrossRef]
- Verrelli, E.; Gray, R.J.; O’Neill, M.; Kelly, S.M.; Kemp, N.T. Microwave oven fabricated hybrid memristor devices for non-volatile memory storage. Mater. Res. Express 2014, 1, 46305. [Google Scholar] [CrossRef] [Green Version]
- Rafique, A.F.; Zaini, J.H.; Esa, M.Z.B.; Nauman, M.M. Printed memory devices using electrohydrodynamic deposition technique. Appl. Phys. A 2020, 126, 134. [Google Scholar] [CrossRef]
- Patil, A.P.; Nirmal, K.A.; Mali, S.S.; Hong, C.K.; Kim, T.G.; Patil, P.S.; Dongale, T.D. Tuning the analog and digital resistive switching properties of TiO2 by nanocompositing Al-doped ZnO. Mater. Sci. Semicond. Process 2020, 115, 105110. [Google Scholar] [CrossRef]
- Pragnya, P.; Pinkowitz, A.; Hull, R.; Gall, D. Electrochemical memristive devices based on submonolayer metal deposition. APL Mater. 2019, 7, 101121. [Google Scholar] [CrossRef]
- Gurrappa, I.; Binder, L. Electrodeposition of nanostructured coatings and their characterization—A review. Sci. Technol. Adv. Mater. 2008, 9, 043001. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Xiao, M.; Zhou, N.Y. Carbon nanowalls: A new material for resistive switching memory devices. Carbon 2017, 120, 54–62. [Google Scholar] [CrossRef]
- Jing, S.; Younis, A.; Chu, D.; Li, S. Resistive switching characteristics in electrochemically synthesized ZnO films. AIMS Mater. Sci. 2015, 2, 28–36. [Google Scholar] [CrossRef]
- Miller, K.; Nalwa, K.S.; Bergerud, A.; Neihart, N.M.; Chaudhary, S. Memristive behavior in thin anodic titania. IEEE Electron Device Lett. 2010, 31, 737–739. [Google Scholar] [CrossRef]
- Dulal, S.M.S.I.; Yun, H.J.; Shin, C.B.; Kim, C.K. Electrodeposition of CoWP film: III. Effect of pH and temperature. Electrochim. Acta 2007, 53, 934–943. [Google Scholar] [CrossRef]
- Pan, F.; Gao, S.; Chen, C.; Song, C.; Zeng, F. Recent progress in resistive random access memories: Materials, switching mechanisms, and performance. Mater. Sci. Eng. R Rep. 2014, 83, 1–59. [Google Scholar] [CrossRef]
- Mohammad, B.; Abi Jaoude, M.; Kumar, V.; Al Homouz, D.M.; Nahla, H.A.; Al-Qutayri, M.; Christoforou, N. State of the art of metal oxide memristor devices. Nanotechnol. Rev. 2016, 5, 311–329. [Google Scholar] [CrossRef]
- Lim, E.W.; Ismail, R. Conduction mechanism of valence change resistive switching memory: A survey. Electronics 2015, 4, 586–613. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Feng, Y.; Huang, P.; Liu, L.; Kang, J. Low-power resistive switching characteristic in HfO2/TiOx bi-layer resistive random-access memory. Nanoscale Res. Lett. 2019, 14, 157. [Google Scholar] [CrossRef] [Green Version]
- Koza, J.A.; Schroen, I.P.; Willmering, M.M.; Switzer, J.A. Electrochemical synthesis and nonvolatile resistance switching of Mn3O4 thin films. Chem. Mater. 2014, 26, 4425–4432. [Google Scholar] [CrossRef]
- Lelmini, D.; Waser, R. Wiley-VCH Book; Wiley: Weinheim, Germany, 2016; ISBN 978-527-33417-9. [Google Scholar]
- Fowler, B.W.; Chang, Y.F.; Zhou, F.; Wang, Y.; Chen, P.Y.; Xue, F.; Chen, Y.T.; Bringhurst, B.; Pozder, S.; Lee, J.C. Electroforming and resistive switching in silicon dioxide resistive memory devices. RSC Adv. 2015, 5, 21215–21236. [Google Scholar] [CrossRef]
- Hu, W.; Peng, Y.; She, Y.; Tang, B.; Qiu, J.; Tang, X.; Bao, D. Insights to the influences of electroforming process on resistive switching types in Pt/InGaZnO/W memory device. Ceram. Int. 2018, 44, S88–S92. [Google Scholar] [CrossRef]
- Patil, A.P.; Revadekar, C.C.; Kamble, G.U.; Kundale, S.S.; Kadam, S.J.; Sutar, S.S.; Dongale, T.D. Investigations on resistive switching effect and time series statistical analysis of solution combustion synthesized ZnTiO3 memristive device. J. Mater. Sci. Mater. Electron. 2022, 33, 23390–23403. [Google Scholar] [CrossRef]
- Ahir, N.A.; Takaloo, A.V.; Nirmal, K.A.; Kundale, S.S.; Chougale, M.Y.; Bae, J.; Dongale, T.D. Capacitive coupled non-zero I–V and type-II memristive properties of the NiFe2O4–TiO2 nanocomposite. Mater. Sci. Semicond. Process 2021, 125, 105646. [Google Scholar] [CrossRef]
- Tzouvadaki, I.; Aliakbarinodehi, N.; De Micheli, G.; Carrara, S. The memristive effect as a novelty in drug monitoring. Nanoscale 2017, 9, 9676–9684. [Google Scholar] [CrossRef]
- Asif, M.; Kumar, A. Existence of bipolar and unipolar resistive switching in CaZrO3 thin film device. J. Alloys Compd. 2021, 859, 158373. [Google Scholar] [CrossRef]
- Tsai, T.L.; Ho, T.H.; Tseng, T.Y. Unipolar resistive switching behaviors and mechanisms in an annealed Ni/ZrO2/TaN memory device. J. Phys. D Appl. Phys. 2015, 48, 035108. [Google Scholar] [CrossRef]
- Tang, H.H.; Whang, T.J.; Su, Y.K. Interpretations for coexistence of unipolar and bipolar resistive switching behaviors in Pt/NiO/ITO structure. Jpn. J. Appl. Phys. 2019, 58, SDDE14. [Google Scholar] [CrossRef]
- Hao, A.; Ismail, M.; He, S.; Huang, W.; Qin, N.; Bao, D. Coexistence of unipolar and bipolar resistive switching behaviors in NiFe2O4 thin film devices by doping Ag nanoparticles. J. Appl. Phys. 2018, 123, 085108. [Google Scholar] [CrossRef]
- Akinaga, H.; Shima, H. Resistive random access memory (ReRAM) based on metal oxides. Proc. IEEE 2010, 98, 2237–2251. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, L.; Xu, X.; Miao, J.; Jiang, Y. Effect of oxide/oxide interface on polarity dependent resistive switching behavior in ZnO/ZrO2 heterostructures. Appl. Phys. Lett. 2014, 104, 192903. [Google Scholar] [CrossRef]
- Chakraborty, I.; Bodurtha, K.J.; Heeder, N.J.; Godfrin, M.P.; Tripathi, A.; Hurt, R.H.; Shukla, A.; Bose, A. Massive electrical conductivity enhancement of multilayer graphene/polystyrene composites using a nonconductive filler. ACS Appl. Mater. Interfaces 2014, 6, 16472–16475. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, C.B.; Lee, D.; Lee, S.R.; Chang, M.; Hur, J.H.; Kim, Y.B.; Kim, C.J.; Seo, D.H.; Seo, S.; et al. A fast, high-endurance and scalable non-volatile memory device made from asymmetric Ta2O5−x/TaO2−x bilayer structures. Nat. Mater. 2011, 10, 625–630. [Google Scholar] [CrossRef]
- Valov, I. Redox-based resistive switching memories (ReRAMs): Electrochemical systems at the atomic scale. ChemElectroChem 2014, 1, 26–36. [Google Scholar] [CrossRef]
- Munjal, S.; Khare, N. Valence change bipolar resistive switching accompanied with magnetization switching in CoFe2O4 thin film. Sci. Rep. 2017, 7, 12427. [Google Scholar] [CrossRef] [Green Version]
- Russo, U.; Ielmini, D.; Cagli, C.; Lacaita, A.L. Self-accelerated thermal dissolution model for reset programming in unipolar resistive-switching memory (RRAM) devices. IEEE Trans. Electron Devices 2009, 56, 193–200. [Google Scholar] [CrossRef]
- Nandakumar, S.R.; Boybat, I.; Le Gallo, M.; Eleftheriou, E.; Sebastian, A.; Rajendran, B. Experimental demonstration of supervised learning in spiking neural networks with phase-change memory synapses. Sci. Rep. 2020, 10, 8080. [Google Scholar] [CrossRef] [PubMed]
- Valov, I. Interfacial interactions and their impact on redox-based resistive switching memories (ReRAMs). Semicond. Sci. Technol. 2017, 32, 093006. [Google Scholar] [CrossRef]
- Cheong, K.Y.; Tayeb, I.A.; Zhao, F.; Abdullah, J.M. Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application. Nanotechnol. Rev. 2021, 10, 680–709. [Google Scholar] [CrossRef]
- Raoux, S.; Xiong, F.; Wuttig, M.; Pop, E. Phase change materials and phase change memory. MRS Bull. 2014, 39, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Jeon, D.S.; Dongale, T.D.; Kim, T.G. Low power Ti-doped NbO2-based selector device with high selectivity and low OFF current. J. Alloys Compd. 2021, 884, 161041. [Google Scholar] [CrossRef]
- Burr, G.W.; Breitwisch, M.J.; Franceschini, M.; Garetto, D.; Gopalakrishna, K.; Jackson, B.; Shenoy, R.S. Phase change memory technology. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process Meas. Phenom. 2010, 28, 223–262. [Google Scholar] [CrossRef] [Green Version]
- Fong, S.W.; Neumann, C.M.; Wong, H.S.P. Phase-change memory-Towards a storage-class memory. IEEE Trans. Electron Devices 2017, 64, 4374–4385. [Google Scholar] [CrossRef]
- Kim, S.; Burr, G.W.; Kim, W.; Nam, S.W. Phase-change memory cycling endurance. MRS Bull. 2019, 44, 710–714. [Google Scholar] [CrossRef]
- Huang, R.; Kissling, G.P.; Kashtiban, R.; Noori, Y.J.; Cicvarić, K.; Zhang, W.; de Groot, C.K. Towards a 3D GeSbTe phase-change memory with integrated selector by non-aqueous electrodeposition. Faraday Discuss. 2019, 213, 339–355. [Google Scholar] [CrossRef] [Green Version]
- Bricalli, A.; Ambrosi, E.; Laudato, M.; Maestro, M.; Rodriguez, R.; Ielmini, D. Resistive switching device technology based on silicon oxide for improved ON–OFF ratio Part II: Select devices. IEEE Trans. Electron Devices 2017, 65, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Pang, W.; Feng, Z.; Chen, X.; Chen, Y.; Zhang, Q.; Zhang, H.; Sun, C.; Yang, J.J.; Zhang, D. Enabling selectivity and fast recovery of ZnO nanowire gas sensors through resistive switching. Sens. Actuators B Chem. 2017, 238, 357–363. [Google Scholar] [CrossRef]
- Zhao, W.; Moreau, M.; Deng, E.; Zhang, Y.; Portal, J.M.; Klein, J.O.; Bocquet, M.; Aziza, H.; Deleruyelle, D.; Muller, C.; et al. Synchronous non-volatile logic gate design based on resistive switching memories. IEEE Trans. Circuits Syst. I Regul. Pap. 2013, 61, 443–454. [Google Scholar] [CrossRef]
- Simanjuntak, F.M.; Panda, D.; Wei, K.H.; Tseng, T.Y. Status and prospects of ZnO-based resistive switching memory devices. Nanoscale Res. Lett. 2016, 11, 368. [Google Scholar] [CrossRef] [Green Version]
- Rani, A.; Khot, A.C.; Jang, I.G.; Kim, T.G. Heterostructure carbon packed MoSSe nanospheres for flexible ReRAM and synapse devices. Carbon 2022, 189, 104–112. [Google Scholar] [CrossRef]
- Qiu, J.T.; Samanta, S.; Dutta, M.; Ginnaram, S.; Maikap, S. Controlling resistive switching by using an optimized MoS2 interfacial layer and the role of top electrodes on ascorbic acid sensing in TaOx based RRAM. Langmuir 2019, 35, 3897–3906. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhu, S.; Mao, S.; Zheng, P.; Xia, Y.; Yang, F.; Lei, M.; Zhao, Y. From dead leaves to sustainable organic resistive switching memory. J. Colloid Interface Sci. 2018, 513, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Govoreanu, B.; Kar, G.S.; Chen, Y.Y.; Paraschiv, V.; Kubicek, S.; Fantini, A.; Jurczak, M. 10 × 10 nm2 Hf/HfOx crossbar resistive RAM with excellent performance, reliability and low-energy operation. In Proceedings of the 2011 International Electron Devices Meeting, Washington, DC, USA, 5–7 December 2011; pp. 31–36. [Google Scholar]
- Lanza, M.; Waser, R.; Ielmini, D.; Yang, J.J.; Goux, L.; Suñe, J.; Kenyon, A.J.; Mehonic, A.; Spiga, S.; Rana, V.; et al. Standards for the characterization of endurance in resistive switching devices. ACS Nano 2021, 15, 17214–17231. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chen, Y.S.; Chen, P.S.; Gu, P.Y.; Hsu, Y.Y.; Wang, S.M.; Liu, W.H.; Tsai, C.H.; Sheu, S.S.; Chiang, P.C.; et al. Evidence and solution of over-RESET problem for HfOx based resistive memory with sub-ns switching speed and high endurance. In Proceedings of the 2010 International Electron Devices Meeting, San Francisco, CA, USA, 6–8 December 2010; pp. 17–19. [Google Scholar]
- Sahu, D.P.; Jammalamadaka, S.N. Remote control of resistive switching in TiO2 based resistive random access memory device. Sci. Rep. 2017, 7, 17224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, D.S.; Park, J.H.; Kang, D.Y.; Dongale, T.D.; Kim, T.G. Forming-ready resistance random access memory using randomly pre-grown conducting filaments via pre-forming. Mater. Sci. Semicond. Process 2020, 110, 104951. [Google Scholar] [CrossRef]
- Khot, A.C.; Dongale, T.D.; Nirmal, K.A.; Sung, J.H.; Lee, H.J.; Nikam, R.D.; Kim, T.G. Amorphous boron nitride memristive device for high-density memory and neuromorphic computing applications. ACS Appl. Mater. Interfaces 2022, 14, 10546–10557. [Google Scholar] [CrossRef]
- Raeber, T.J.; Zhao, Z.C.; Murdoch, B.J.; McKenzie, D.R.; McCulloch, D.G.; Partridge, J.G. Resistive switching and transport characteristics of an all-carbon memristor. Carbon 2018, 136, 280–285. [Google Scholar] [CrossRef]
- Ouyang, J. Two-terminal resistive switching memory devices with a polymer film embedded with nanoparticles. J. Mater. Chem. 2015, C3, 7243–7261. [Google Scholar] [CrossRef]
- Pradel, A.; Frolet, N.; Ramonda, M.; Piarristeguy, A.; Ribes, M. Bipolar resistance switching in chalcogenide materials. Phys. Status Solidi 2011, 208, 2303–2308. [Google Scholar] [CrossRef]
- Yu, M.J.; Son, K.R.; Khot, A.C.; Kang, D.Y.; Sung, J.H.; Jang, I.G.; Dange, Y.D.; Dongale, T.D.; Kim, T.G. Three Musketeers: Demonstration of multilevel memory, selector and synaptic behaviors from an Ag-GeTe based chalcogenide material. J. Mater. Res. Technol. 2021, 15, 1984–1995. [Google Scholar] [CrossRef]
- Gupta, V.; Lucarelli, G.; Castro, S.; Brown, T.; Ottavi, M. Perovskite based low power synaptic memristor device for neuromorphic application. In Proceedings of the 14th International Conference on Design & Technology of Integrated Systems in Nanoscale Era (DTIS), Mykonos, Greece, 16–18 April 2019; pp. 1–6. [Google Scholar]
- Kim, M.K.; Lee, J.S. Short-term plasticity and long-term potentiation in artificial biosynapses with diffusive dynamics. ACS Nano 2018, 12, 1680–1687. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yu, H.C.; Huang, C.Y.; Chung, W.L.; Wu, S.L.; Su, Y.K. Nonvolatile bio-memristor fabricated with egg albumen film. Sci. Rep. 2015, 5, 10022. [Google Scholar] [CrossRef] [Green Version]
- Khot, A.C.; Dongale, T.D.; Park, J.H.; Kesavan, A.V.; Kim, T.G. Ti3C2-based MXene oxide nanosheets for resistive memory and synaptic learning applications. ACS Appl. Mater. Interfaces 2021, 13, 5216–5227. [Google Scholar] [CrossRef]
- Majumdar, S.; Tan, H.; Qin, Q.H.; van Dijken, S. Energy-efficient organic ferroelectric tunnel junction memristors for neuromorphic computing. Adv. Electron. Mater. 2019, 5, 1800795. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.R.; Zhou, L.; Mao, J.Y.; Ren, Y.; Yang, J.Q.; Yang, G.H.; Zhu, X.; Han, S.T.; Roy, V.A.; Zhou, Y. Artificial synapse emulated by charge trapping-based resistive switching device. Adv. Mater. Technol. 2019, 4, 1800342. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Zhao, Y.; Wang, Y.; Zhang, J.; Fang, L.; Jin, S.; Shao, Y.; Huang, J. Recent advances in transistor-based artificial synapses. Adv. Funct. Mater. 2019, 29, 1903700. [Google Scholar] [CrossRef]
- Stoliar, P.; Yamada, H.; Toyosaki, Y.; Sawa, A. Spike-shape dependence of the spike-timing dependent synaptic plasticity in ferroelectric-tunnel-junction synapses. Sci. Rep. 2019, 9, 17740. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Yun, M.J.; Kim, K.H.; Kim, S.; Kim, H.D. Advanced recovery and high-sensitive properties of memristor-based gas sensor devices operated at room temperature. ACS Sens. 2021, 6, 4217–4224. [Google Scholar] [CrossRef]
- Fang, X.; Chen, X.; Liu, Y.; Li, Q.; Zeng, Z.; Maiyalagan, T.; Mao, S. Nanocomposites of Zr(IV)-based metal–organic frameworks and reduced graphene oxide for electrochemically sensing ciprofloxacin in water. ACS Appl. Nano Mater. 2019, 2, 2367–2376. [Google Scholar] [CrossRef]
- Kang, D.Y.; Kim, B.H.; Lee, T.H.; Shim, J.W.; Kim, S.; Sung, H.J.; Chang, K.J.; Kim, T.G. Dopant-tunable ultrathin transparent conductive oxides for efficient energy conversion devices. Nano-Micro Lett. 2021, 13, 211. [Google Scholar] [CrossRef]
- Park, T.H.; Son, K.R.; Hirayama, H.; Kim, T.G. High-efficiency and high-reliability deep-UV light-emitting diodes using transparent Ni-implanted AlN ohmic electrodes. IEEE Access 2021, 9, 166617–166623. [Google Scholar] [CrossRef]
- Son, K.R.; Hong, S.H.; Yu, M.J.; Kim, T.G. Thermally stable and conductive nickel-incorporated gallium oxide thin-film electrode for efficient GaN microscale light-emitting diode arrays. Appl. Surf. Sci. 2022, 604, 154560. [Google Scholar] [CrossRef]
- Loy, D.J.; Dananjaya, P.A.; Chakrabarti, S.; Tan, K.H.; Chow, S.C.; Toh, E.H.; Lew, W.S. Oxygen vacancy density dependence with a hopping conduction mechanism in multilevel switching behavior of HfO2-based resistive random access memory devices. ACS Appl. Electron. Mater. 2020, 2, 3160–3170. [Google Scholar] [CrossRef]
- Walke, P.D.; Yuldashev, S.U.; Magotra, V.K.; Lee, D.J.; Abdullaev, S.; Kang, T.W.; Jeon, H.C. Memristive devices from CuO nanoparticles. Nanomaterials 2020, 10, 1677. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Lee, J.S. Self-assembled nanostructured resistive switching memory devices fabricated by templated bottom-up growth. Sci. Rep. 2016, 6, 18967. [Google Scholar] [CrossRef] [Green Version]
- Shaari, N.A.A.; Sauki, N.S.M.; Herman, S.H. Effect of annealing time on memristive behavior of sol-gel spincoated ZnO-based memristive device. AIP Conf. Proc. 2016, 1774, 050018. [Google Scholar]
- Huang, J.S.; Lee, C.Y.; Chin, T.S. Forming-free bipolar memristive switching of ZnO films deposited by cyclic-voltammetry. Electrochim. Acta 2013, 91, 62–68. [Google Scholar] [CrossRef]
- Jiang, L.; Lv, F.C.; Yang, R.; Hu, D.C.; Guo, X. Forming-free artificial synapses with Ag point contacts at interface. J. Materiomics 2019, 5, 296–302. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lin, Y.S.; Cheng, C.W.; Jhang, W.C. Annealing effect on the performance of copper oxide resistive memory devices. IEEE Trans. Electron. Devices 2020, 67, 976–983. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, D.; Kang, D.Y.; Jeon, D.S.; Kim, T.G. Nanoscale 3D stackable Ag-doped HfOx-based selector devices fabricated through low-temperature hydrogen annealing. ACS Appl. Mater. Interfaces 2019, 11, 29408–29415. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, M.; Li, Y.; Long, S.; Lv, H.; Liu, Q.; Liu, M. Highly scalable resistive switching memory in metal nanowire crossbar arrays fabricated by electron beam lithography. J. Vac. Sci. Technol. B 2016, 34, G105. [Google Scholar] [CrossRef]
- Sun, B.; Wei, L.; Li, H.; Jia, X.; Wu, J.; Chen, P. The DNA strand assisted conductive filament mechanism for improved resistive switching memory. J. Mater. Chem. C 2015, 3, 12149–12155. [Google Scholar] [CrossRef]
- Moazzeni, A.; Riyahi Madvar, H.; Hamedi, S.; Kordrostami, Z. Fabrication of Graphene Oxide-Based Resistive Switching Memory by the Spray Pyrolysis Technique for Neuromorphic Computing. ACS Appl. Nano Mater. 2023, 6, 2236–2248. [Google Scholar] [CrossRef]
- Dongale, T.D.; Pawar, P.S.; Tikke, R.S.; Mullani, N.B.; Patil, V.B.; Teli, A.M.; Patil, P.S. Mimicking the synaptic weights and human forgetting curve using hydrothermally grown nanostructured CuO memristor device. J. Nanosci. Nanotechnol. 2018, 18, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Tripathy, N.; Pradhan, D.; Sahu, P.K.; Kar, J.P. Electrical characteristics of dip coated TiO2 thin films with various withdrawal speeds for resistive switching applications. Appl. Surf. Sci. 2018, 449, 181–185. [Google Scholar] [CrossRef]
- Chandane, P.T.; Dongale, T.D.; Patil, P.B.; Tiwari, A.P. Organic resistive switching device based on cellulose-gelatine microcomposite fibers. J. Mater. Sci. Mater. Electron. 2019, 30, 21288–21296. [Google Scholar] [CrossRef]
- Dongale, T.D.; Khot, A.C.; Takaloo, A.V.; Kim, T.G. Facile synthesis of nickel cobaltite quasi-hexagonal nanosheets for multilevel resistive switching and synaptic learning applications. NPG Asia Mater. 2021, 13, 16. [Google Scholar] [CrossRef]
- Dongale, T.D.; Khot, S.S.; Patil, A.A.; Wagh, S.V.; Patil, P.B.; Dubal, D.P.; Kim, T.G. Bifunctional nanoparticulated nickel ferrite thin films: Resistive memory and aqueous battery applications. Mater. Des. 2021, 201, 109493. [Google Scholar] [CrossRef]
- Varshney, G.; Kanel, S.R.; Kempisty, D.M.; Varshney, V.; Agrawal, A.; Sahle-Demessie, E.; Varma, R.S.; Nadagouda, M.N. Nanoscale TiO2 films and their application in remediation of organic pollutants. Coord. Chem. Rev. 2016, 306, 43–64. [Google Scholar] [CrossRef]
- Kwon, S.; Seo, H.; Lee, H.; Jeon, K.J.; Young Park, J. Reversible bistability of conductance on graphene/CuOx/Cu nanojunction. Appl. Phys. Lett. 2012, 100, 123101. [Google Scholar] [CrossRef] [Green Version]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene–gold nanoparticles hybrid—Synthesis, functionalization, and application in a electrochemical and surface-enhanced raman scattering biosensor. Materials 2016, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Pham, N.P.; Boellard, E.; Sarro, P.M.; Burghartz, J.N. Spin, spray coating and electrodeposition of photoresist for MEMS structures-A comparison. Eurosensors 2002, 81, 81–86. [Google Scholar]
- Lokhande, P.E.; Chavan, U.S.; Pandey, A. Materials and fabrication methods for electrochemical supercapacitors: Overview. Electrochem. Energy Rev. 2020, 3, 155–186. [Google Scholar] [CrossRef]
- Endres, F.; El Abedin, S.Z. Nanoscale electrodeposition of germanium on Au (111) from an ionic liquid: An in situ STM study of phase formation Part I. Ge from GeBr4. Phys. Chem. Chem. Phys. 2002, 4, 1640–1648. [Google Scholar] [CrossRef]
- Lincot, D. Electrodeposition of semiconductors. Thin Solid Film. 2005, 487, 40–48. [Google Scholar] [CrossRef]
- Karatutlu, A.; Barhoum, A.; Sapelkin, A. Liquid-phase synthesis of nanoparticles and nanostructured materials. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–28. [Google Scholar]
- Wu, M.S.; Huang, C.Y.; Lin, K.H. Electrophoretic deposition of nickel oxide electrode for high-rate electrochemical capacitors. J. Power Sources 2009, 186, 557–564. [Google Scholar] [CrossRef]
- Khurana, G.; Misra, P.; Kumar, N.; Kooriyattil, S.; Scott, J.F.; Katiyar, R.S. Enhanced resistive switching in forming-free graphene oxide films embedded with gold nanoparticles deposited by electrophoresis. Nanotechnology 2015, 27, 15702. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M.; Ghorbani, M. Electrophoretic deposition of titania nanoparticles in different alcohols: Kinetics of deposition. J. Am. Ceram. Soc. 2011, 94, 2354–2361. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Lahiri, A.; Pulletikurthi, G.; Endres, F. A review on the electroless deposition of functional materials in ionic liquids for batteries and catalysis. Front. Chem. 2019, 7, 85. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Wallace, G.G. Tin nanoparticles decorated copper oxide nanowires for selective electrochemical reduction of aqueous CO2 to CO. J. Mater. Chem. A 2016, 4, 10710–10718. [Google Scholar] [CrossRef]
- Wu, Z.; Ge, S.; Zhang, M.; Li, W.; Tao, K. Synthesis of nickel nanoparticles supported on metal oxides using electroless plating: Controlling the dispersion and size of nickel nanoparticles. J. Colloid Interface Sci. 2009, 330, 359–366. [Google Scholar] [CrossRef]
- Quintero, D.; Galvis, O.; Calderón, J.A.; Castaño, J.G.; Echeverrí, F. Effect of electrochemical parameters on the formation of anodic films on commercially pure titanium by plasma electrolytic oxidation. Surf. Coat. Technol. 2014, 258, 1223–1231. [Google Scholar] [CrossRef]
- Zaffora, A.; Di Quarto, F.; Habazaki, H.; Valov, I.; Santamaria, M. Electrochemically prepared oxides for resistive switching memories. Faraday Discuss. 2019, 213, 165–181. [Google Scholar] [CrossRef] [PubMed]
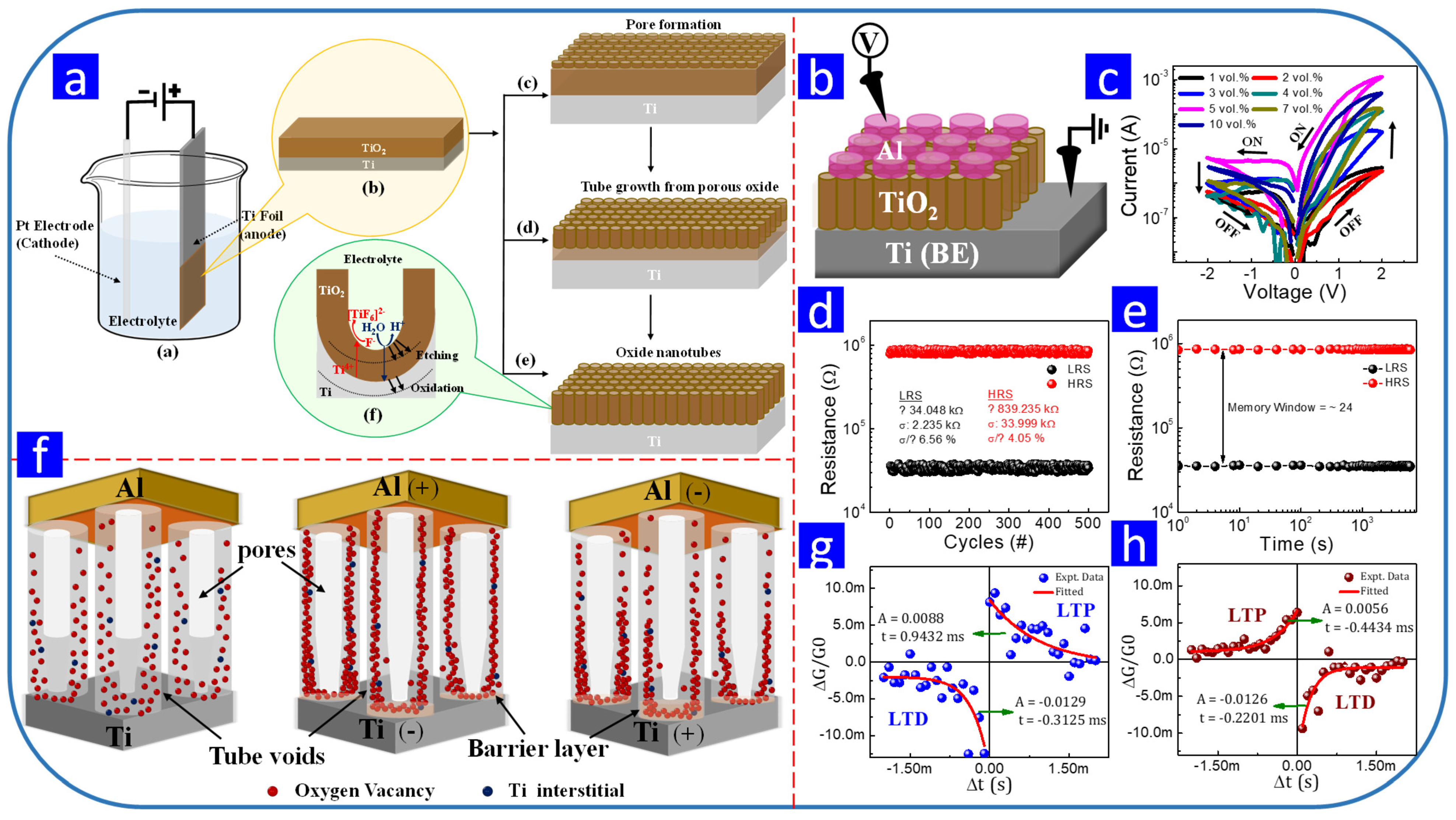
- Nirmal, K.A.; Nhivekar, G.S.; Khot, A.C.; Dongale, T.D.; Kim, T.G. Unraveling the effect of the water content in the electrolyte on the resistive switching properties of self-assembled one-dimensional anodized TiO2 nanotubes. J. Phys. Chem. Lett. 2022, 13, 7870–7880. [Google Scholar] [CrossRef] [PubMed]
- Verwey, E. Electrolytic conduction of a solid insulator at high fields. The formation of the anodic oxide film on aluminium. Physica 1935, 2, 1059–1063. [Google Scholar] [CrossRef]
- Cabrera, N.; Mott., N.F. Theory of the oxidation of metals. Rep. Prog. Phys. 1949, 12, 163–184. [Google Scholar] [CrossRef]
- Dewald, J.F. A theory of the kinetics of formation of anode films at high fields. J. Electrochem. Soc. 1955, 102, 1. [Google Scholar] [CrossRef]
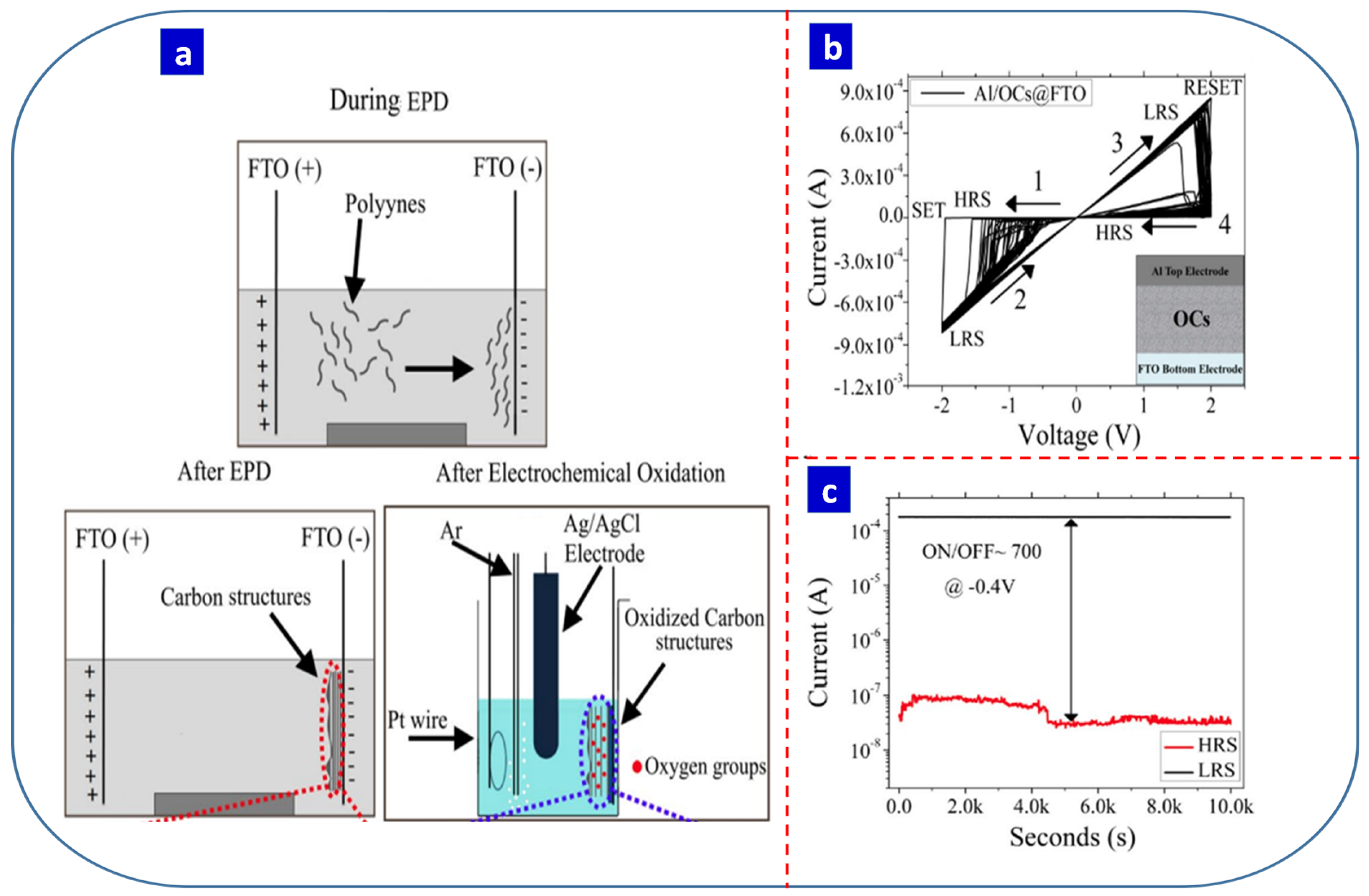
- Russo, P.; Xiao, M.; Zhou, N.Y. Electrochemical oxidation induced multi-level memory in carbon-based resistive switching devices. Sci. Rep. 2019, 9, 1564. [Google Scholar] [CrossRef] [Green Version]
- Pal, P.; Wang, Y.H. Interconversion of complementary resistive switching from graphene oxide based bipolar multilevel resistive switching device. Appl. Phys. Lett. 2020, 117, 054101. [Google Scholar] [CrossRef]
- Kim, T.; Kim, D.K.; Kim, J.; Pak, J.J. Resistive switching behaviour of multi-stacked PVA/graphene oxide+ PVA composite/PVA insulating layer-based RRAM devices. Semicond. Sci. Technol. 2019, 34, 065006. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chang, K.C.; Chang, T.C.; Chen, H.L.; Young, T.F.; Tsai, T.M.; Zhang, R.; Chu, T.J.; Ciou, J.F.; Lou, J.C.; et al. Resistance switching induced by hydrogen and oxygen in diamond-like carbon memristor. IEEE Electron Device Lett. 2014, 35, 1016–1018. [Google Scholar] [CrossRef]
- Pei, Y.; Zhou, Z.; Chen, A.P.; Chen, J.; Yan, X. A carbon-based memristor design for associative learning activities and neuromorphic computing. Nanoscale 2020, 12, 13531–13539. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.; Hwang, J.H.; Kim, T.W.; Lee, S.S.; Jeon, W. Sustainable resistance switching performance from composite-type ReRAM device based on carbon Nanotube@Titania core–shell wires. Sci. Rep. 2020, 10, 18830. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, R.; Kumar, A.; Kumar, D.; Kumar, M. Enhanced resistive switching in graphene oxide based composite thin film for nonvolatile memory applications. Mater. Res. Express 2019, 6, 105621. [Google Scholar] [CrossRef]
- Zhou, G.; Ren, Z.; Wang, L.; Wu, J.; Sun, B.; Zhou, A.; Zhang, G.; Zheng, S.; Duan, S.; Song, Q. Resistive switching memory integrated with amorphous carbon-based nanogenerators for self-powered device. Nano Energy 2019, 63, 103793. [Google Scholar] [CrossRef]
- Dugu, S.; Pavunny, S.P.; Limbu, T.B.; Weiner, B.R.; Morell, G.; Katiyar, R.S. A graphene integrated highly transparent resistive switching memory device. APL Mater. 2018, 6, 058503. [Google Scholar] [CrossRef]
- Tsai, C.L.; Xiong, F.; Pop, E.; Shim, M. Resistive random access memory enabled by carbon nanotube crossbar electrodes. ACS Nano 2013, 7, 5360–5366. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Chen, S.; Cao, J.; Wei, B.; Liu, J.; Zhang, Y. Graphene oxide for nonvolatile memory application by using electrophoretic technique. Mater. Today Commun. 2020, 25, 101537. [Google Scholar] [CrossRef]
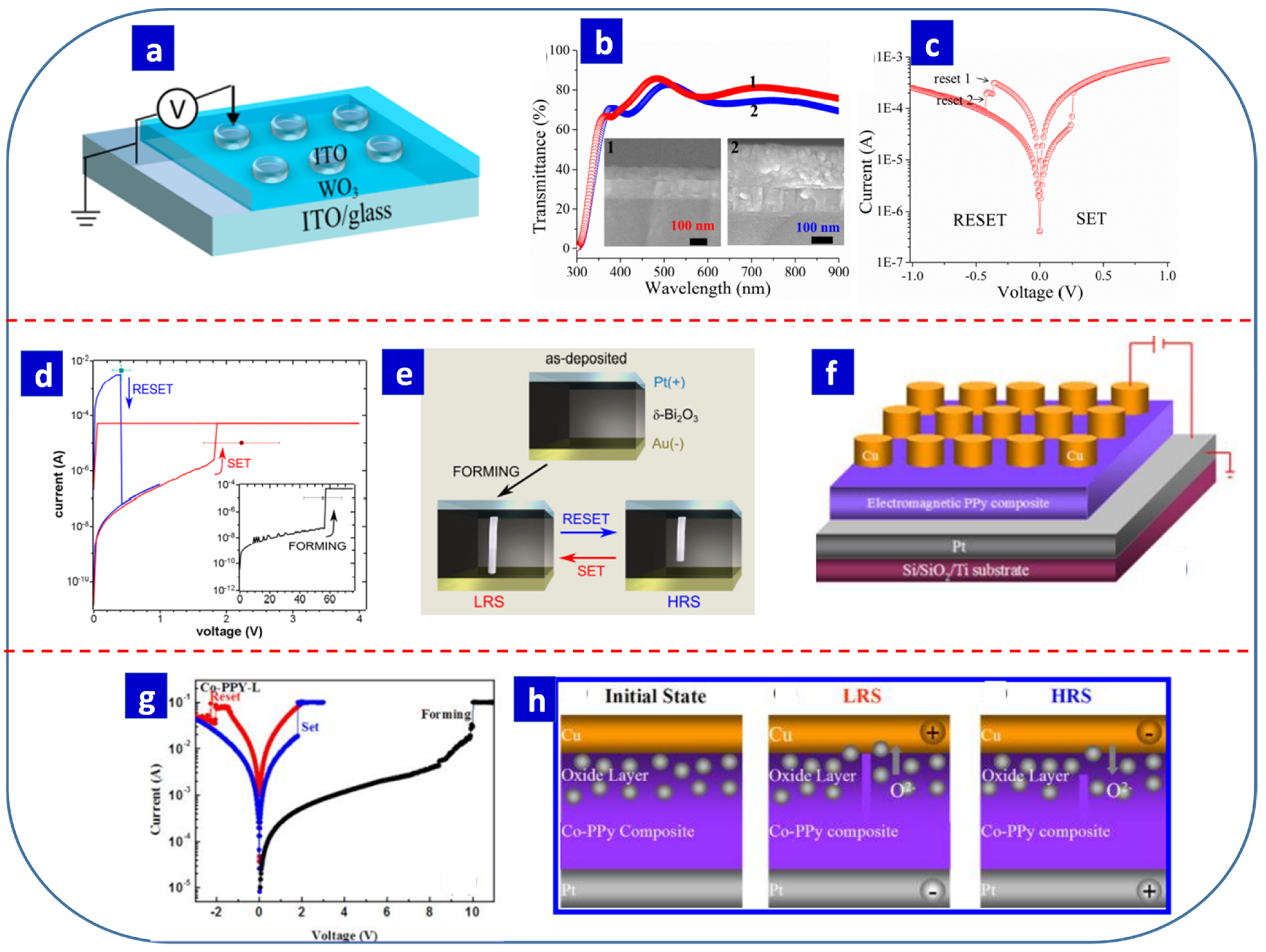
- Fauzi, F.B.; Ani, M.H.; Othman, R.; Azhar, A.Z.A.; Mohamed, M.A.; Herman, S.H. Fabrication of flexible Au/ZnO/ITO/PET memristor using dilute electrodeposition method. IOP Conf. Ser. Mater. Sci. Eng. 2015, 99, 012002. [Google Scholar] [CrossRef]
- Yusoff, M.M.; Ani, M.H. Synthesis and characterization of ZnO thin film memristor. Adv. Mat. Res. 2013, 701, 172–175. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, X.; Zheng, X.; Liu, Y.; Shen, Y.; Zhang, Y. Influence of carrier concentration on the resistive switching characteristics of a ZnO-based memristor. Nano Res. 2016, 9, 1116–1124. [Google Scholar] [CrossRef]
- Quintana, A.; Gómez, A.; Baró, M.D.; Suriñach, S.; Pellicer, E.; Sort, J. Self-templating faceted and spongy single-crystal ZnO nanorods: Resistive switching and enhanced piezoresponse. Mater. Des. 2017, 133, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Younis, A.; Chu, D.; Li, S. Bi-stable resistive switching characteristics in Ti-doped ZnO thin films. Nanoscale Res. Lett. 2013, 8, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauzi, F.B.; Othman, R.; Mohamed, M.A.; Herman, S.H.; Azhar, A.Z.A.; Ani, M.H. Transition metal oxide (TMO) thin film memristor on Cu substrate using dilute electrodeposition method. Mater. Trans. 2015, 56, 1302–1306. [Google Scholar] [CrossRef] [Green Version]
- Fauzi, F.B.; Ani, M.H.; Herman, S.H.; Mohamed, M.A. Dilute electrodeposition of TiO2 and ZnO thin film memristors on Cu substrate. IOP Conf. Ser. Mater. Sci. Eng. 2018, 340, 012006. [Google Scholar] [CrossRef]
- Younis, A.; Chu, D.; Lin, X.; Yi, J.; Dang, F.; Li, S. High-performance nanocomposite based memristor with controlled quantum dots as charge traps. ACS Appl. Mater. Interfaces 2013, 5, 2249–2254. [Google Scholar] [CrossRef]
- Ocampo, O.; Antúnez, E.E.; Agarwal, V. Memristive devices from porous silicon–ZnO/VO2 nanocomposites. Superlattices Microstruct. 2015, 88, 198–203. [Google Scholar] [CrossRef]
- Chu, D.; Younis, A.; Li, S. Direct growth of TiO2 nanotubes on transparent substrates and their resistive switching characteristics. J. Phys. D Appl. Phys. 2012, 45, 355306. [Google Scholar] [CrossRef]
- Hazra, A.; Acharyya, D.; Bhattacharyya, P. Electrochemically grown nono-structured TiO2 based low power resistive random access memory. In Proceedings of the IEEE International Conference ON Emerging Trends in Computing. Communication and Nanotechnology (ICECCN), Tirunelveli, India, 25–26 March 2013; pp. 558–563. [Google Scholar]
- Yoo, J.; Lee, K.; Tighineanu, A.; Schmuki, P. Highly ordered TiO2 nanotube-stumps with memristive response. Electrochem. Commun. 2013, 34, 177–180. [Google Scholar] [CrossRef]
- Yin, X.B.; Tian, K.; Tan, Z.H.; Yang, R.; Guo, X. Polarity reversal in the bipolar switching of anodic TiO2 film. J. Electrochem. Soc. 2015, 162, E271. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, F.; Mao, S.; Zhu, S.; Jia, Y.; Yuan, L.; Salmen, M.; Sun, B. Effect of anodic oxidation time on resistive switching memory behavior based on amorphous TiO2 thin films device. Chem. Phys. Lett. 2018, 706, 477–482. [Google Scholar] [CrossRef]
- Tao, D.W.; Jiang, Z.J.; Chen, J.B.; Qi, B.J.; Zhang, K.; Wang, C.W. Stable resistive switching characteristics from highly ordered Cu/TiO2/Ti nanopore array membrane memristors. Appl. Surf. Sci. 2021, 539, 148161. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Y.; Zhu, K.; Sun, F.; Guo, C.; Wu, X.; Cheng, G.; Zheng, R. Core-shell copper nanowire-TiO2 nanotube arrays with excellent bipolar resistive switching properties. Electrochim. Acta 2019, 316, 133–142. [Google Scholar] [CrossRef]
- Gul, E.; Gokcen, D. Active memristive layer deposition via Mn (II)-assisted anodic oxidation of titanium. ECS J. Solid State Sci. Technol. 2020, 9, 054004. [Google Scholar] [CrossRef]
- Aglieri, V.; Zaffora, A.; Lullo, G.; Santamaria, M.; Di Franco, F.; Cicero, U.L.; Mosca, M.; Macaluso, R. Resistive switching in microscale anodic titanium dioxide-based memristors. Superlattices Microstruct. 2018, 113, 135–142. [Google Scholar] [CrossRef]
- Conti, D.; Lamberti, A.; Porro, S.; Rivolo, P.; Chiolerio, A.; Pirri, C.F.; Ricciardi, C. Memristive behaviour in poly-acrylic acid coated TiO2 nanotube arrays. Nanotechnology 2016, 27, 485208. [Google Scholar] [CrossRef]
- Han, U.B.; Lee, J.S. Integration scheme of nanoscale resistive switching memory using bottom-up processes at room temperature for high-density memory applications. Sci. Rep. 2016, 6, 28966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, U.B.; Lee, J.S. Bottom-up synthesis of ordered metal/oxide/metal nanodots on substrates for nanoscale resistive switching memory. Sci. Rep. 2016, 6, 25537. [Google Scholar] [CrossRef] [Green Version]
- Kundale, S.S.; Patil, A.P.; Patil, S.L.; Patil, P.B.; Kamat, R.K.; Kim, D.K.; Kim, T.G.; Dongale, T.D. Effects of switching layer morphology on resistive switching behavior: A case study of electrochemically synthesized mixed-phase copper oxide memristive devices. Appl. Mater. Today 2022, 27, 101460. [Google Scholar] [CrossRef]
- Katwal, R.; Kaur, H.; Sharma, G.; Naushad, M.; Pathania, D. Electrochemical synthesized copper oxide nanoparticles for enhanced photocatalytic and antimicrobial activity. J. Ind. Eng. Chem 2015, 31, 173–184. [Google Scholar] [CrossRef]
- Bhaumik, A.; Haque, A.; Karnati, P.; Taufique, M.F.N.; Patel, R.; Ghosh, K. Copper oxide based nanostructures for improved solar cell efficiency. Thin Solid Film. 2014, 572, 126–133. [Google Scholar] [CrossRef]
- Dubal, D.P.; Dhawale, D.S.; Salunkhe, R.R.; Jamdade, V.S.; Lokhande, C.D. Fabrication of copper oxide multilayer nanosheets for supercapacitor application. J. Alloys Compd. 2010, 492, 26–30. [Google Scholar] [CrossRef]
- Rydosz, A. The use of copper oxide thin films in gas-sensing applications. Coatings 2018, 8, 425. [Google Scholar] [CrossRef] [Green Version]
- Yazdanparast, S.; Koza, J.A.; Switzer, J.A. Copper nanofilament formation during unipolar resistance switching of electrodeposited cuprous oxide. Chem. Mater. 2015, 27, 5974–5981. [Google Scholar] [CrossRef]
- Yazdanparast, S. Parameters controlling microstructures and resistance switching of electrodeposited cuprous oxide thin films. Appl. Surf. Sci. 2016, 389, 632–638. [Google Scholar] [CrossRef]
- Patil, R.R.; Patil, S.V.; Sabnis, A.M.; Khot, K.V.; Kamat, R.K.; Dongale, T.D.; Kim, D.K. Resistive switching memory properties of electrodeposited Cu2O thin films. J. Nano-Elect. Phy. 2020, 12, 2035. [Google Scholar] [CrossRef]
- Park, K.; Lee, J.S. Flexible resistive switching memory with a Ni/CuOx/Ni structure using an electrochemical deposition process. Nanotechnology 2016, 27, 125203. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, J.S. Design of electrodeposited bilayer structures for reliable resistive switching with self-compliance. ACS Appl. Mater. Interfaces 2016, 8, 32918–32924. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, C.P.; Bärner, K.; Marchenkov, V.V.; Zeng, Y. Resistance switching properties of Cu2S film by electrochemical deposition. Appl. Surf. Sci. 2016, 360, 875–879. [Google Scholar] [CrossRef]
- Qian, K.; Cai, G.; Nguyen, V.C.; Chen, T.; Lee, P. Direct observation of conducting filaments in tungsten oxide based transparent resistive switching memory. ACS Appl. Mater. Interfaces 2016, 8, 27885–27891. [Google Scholar] [CrossRef]
- Koza, J.A.; Bohannan, E.W.; Switzer, J.A. Superconducting filaments formed during nonvolatile resistance switching in electrodeposited δ-Bi2O3. ACS Nano 2013, 7, 9940–9946. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gao, M.; Yu, L.; Lu, L.; Xu, X.; Jiang, Y. Co nanoparticles induced resistive switching and magnetism for the electrochemically deposited polypyrrole composite films. ACS Appl. Mater. Interfaces 2014, 6, 17823–17830. [Google Scholar] [CrossRef]
- Dongale, T.D.; Khot, A.C.; Takaloo, A.V.; Son, K.R.; Kim, T.G. Multilevel resistive switching and synaptic plasticity of nanoparticulated cobaltite oxide memristive device. J. Mater. Sci. Technol. 2021, 78, 81–91. [Google Scholar] [CrossRef]
- Kang, D.Y.; Rani, A.; Yoo, K.J.; Kim, T.G. Improved threshold switching characteristics of vanadium oxide/oxynitride-based multilayer selector in a cross-point array. J. Alloys Compd. 2022, 922, 166192. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.H.; Dongale, T.D.; Kim, T.G. Vacancy-modulated self-rectifying characteristics of NiOx/Al2O3-based nanoscale ReRAM devices. J. Alloys Compd. 2020, 821, 153247. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, J.H.; Chang, Y.W.; Hwang, S.S.; Yoo, K.H. Reversible resistive switching behaviors in NiO nanowires. Appl. Phys. Lett. 2008, 93, 033503. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.; Eom, H.; Kim, W.B.; Yoo, B. Resistive switching characteristics of Au/P-doped NiO/Au segmented nanowires synthesized by electrochemical deposition. Jpn. J. Appl. Phys. 2014, 53, 024202. [Google Scholar] [CrossRef]
- Sophocleous, M.; Mohammadian, N.; Majewski, L.A.; Georgiou, J. Solution-processed low voltage tantalum-based memristive switches. Mater. Lett. 2020, 269, 127676. [Google Scholar] [CrossRef]
- Zaffora, A.; Cho, D.Y.; Lee, K.S.; Di Quarto, F.; Waser, R.; Santamaria, M.; Valov, I. Electrochemical tantalum oxide for resistive switching memories. Adv. Mater. 2017, 29, 1703357. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, Y.; Lee, S.K.; Ruan, G.; Kim, T.W.; Fei, H.; Lee, S.H.; Kim, D.Y.; Yoon, J.; Tour, J.M. Flexible nanoporous WO3–x nonvolatile memory device. ACS Nano 2016, 10, 7598–7603. [Google Scholar] [CrossRef]
- Koza, J.A.; He, Z.; Miller, A.S.; Switzer, J.A. Resistance switching in electrodeposited VO2 thin films. Chem. Mater. 2011, 23, 4105–4108. [Google Scholar] [CrossRef]
- Kim, D.; Park, J.H.; Jeon, D.S.; Dongale, T.D.; Kim, T.G. Ta2O5-y-based ReRAM device with annealing-free Ag: ZrNx-based bilayer selector device. J. Alloys Compd. 2021, 854, 157261. [Google Scholar] [CrossRef]
- Younis, A.; Chu, D.; Lin, X.; Lee, J.; Li, S. Bipolar resistive switching in p-type Co3O4 nanosheets prepared by electrochemical deposition. Nanoscale Res. Lett. 2013, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Jamilpanah, L.; Khademi, I.; Gharehbagh, J.S.; Mohseni, S.A.; Mohseni, S.M. Promising memristive behavior in MoS2–MoO2–MoO3 scalable composite thin films. J. Alloys Compd. 2020, 835, 155291. [Google Scholar] [CrossRef]
- Han, J.H.; Muralidhar, R.; Waser, R.; Bazant, M.Z. Resistive switching in aqueous nanopores by shock electrodeposition. Electrochim. Acta 2016, 222, 370–375. [Google Scholar] [CrossRef] [Green Version]
- Avila, L.B.; Müller, C.K.; Hildebrand, D.; Faita, F.L.; Baggio, B.F.; Cid, C.C.P.; Pasa, A. Resistive switching in electrodeposited prussian blue layers. Materials 2020, 13, 5618. [Google Scholar] [CrossRef]
- Han, N.; Park, M.U.; Yoo, K.H. Memristive switching in Bi1–xSbx nanowires. ACS Appl. Mater. Interfaces 2016, 8, 9224–9230. [Google Scholar] [CrossRef]
- Müller, R.; Rouault, O.; Katzenmeyer, A.; Goux, L.; Wouters, D.J.; Genoe, J.; Heremans, P. Electrodeposition of copper tetracyanoquinodimethane for bipolar resistive switching non-volatile memories. Philos. Trans. R. Soc. A 2009, 367, 4191–4201. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.L.; Redekar, R.S.; Pawar, O.Y.; Kundale, S.S.; Sutar, S.S.; More, K.V.; Tarwal, N.L. Precursor-dependent resistive switching properties of nanostructured g-C3N4: Statistical and experimental investigations. J. Mater. Sci. Mater. Electron. 2023, 34, 155. [Google Scholar] [CrossRef]
- Mullani, N.B.; Kumbhar, D.D.; Lee, D.H.; Kwon, M.J.; Cho, S.Y.; Oh, N.; Park, J.H. Surface Modification of a Titanium Carbide MXene Memristor to Enhance Memory Window and Low-Power Operation. Adv. Funct. Mater. 2023, 2300343. [Google Scholar] [CrossRef]
- Jagadhane, K.S.; Dongale, T.D.; Nikam, A.S.; Tadavalekar, N.B.; Kamat, R.K.; Kolekar, G.B.; Anbhule, P.V. Tetraphenylethene Carbothioamide-Based Organic Stimuli-Responsive Mechanochromic Memristive Devices with Non-Volatile Memory and Synaptic Learning Functionalities. ChemistrySelect 2023, 8, e202300026. [Google Scholar] [CrossRef]
- Patil, A.R.; Dongale, T.D.; Kamat, R.K.; Rajpure, K.Y. Spray pyrolysis deposited iron tungstate memristive device for artificial synapse application. Mater. Today Commun. 2021, 29, 102900. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, B.; Tang, J.; Li, X.; Wu, W.; Qian, H.; Wu, H. Analog-type resistive switching devices for neuromorphic computing. Phys. Status Solidi Rapid Res. Lett. 2019, 13, 1900204. [Google Scholar] [CrossRef]
- More, S.S.; Patil, P.A.; Kadam, K.D.; Patil, H.S.; Patil, S.L.; Pawar, A.V.; Dongale, T.D. Resistive switching and synaptic properties modifications in gallium-doped zinc oxide memristive devices. Results Phys. 2019, 12, 1946–1955. [Google Scholar] [CrossRef]
- Park, Y.; Park, M.J.; Lee, J.S. Reduced graphene oxide-based artificial synapse yarns for wearable textile device applications. Adv. Funct. Mater. 2018, 281, 804123. [Google Scholar] [CrossRef]
- Chen, S.; Noori, S.; Villena, M.A.; Shi, Y.; Han, T.; Zuo, Y.; Pedeferri, M.; Strukov, D.; Lanza, M.; Diamanti, M.V. Memristive electronic synapses made by anodic oxidation. Chem. Mater. 2019, 31, 8394–8401. [Google Scholar] [CrossRef]
- Choi, S.; Jang, S.; Moon, J.H.; Kim, J.C.; Jeong, H.Y.; Jang, P.; Lee, K.J.; Wang, G. A self-rectifying TaOy/nanoporous TaOx memristor synaptic array for learning and energy-efficient neuromorphic systems. NPG Asia Mater. 2018, 10, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Ginnaram, S.; Qiu, J.T.; Maikap, S. Controlling Cu migration on resistive switching. artificial synapse, and glucose/saliva detection by using an optimized AlOx interfacial layer in a-COx-based conductive bridge random access memory. ACS Omega 2020, 5, 7032–7043. [Google Scholar] [CrossRef] [Green Version]
- Pawar, A.V.; Kanapally, S.S.; Kadam, K.D.; Patil, S.L.; Dongle, V.S.; Jadhav, S.A.; Kim, S.; Dongale, T.D. MemSens: A new detection method for heavy metals based on silver nanoparticle assisted memristive switching principle. J. Mater. Sci. Mater. Electron. 2019, 30, 11383–11394. [Google Scholar] [CrossRef]
- Sangaonkar, G.M.; Desai, M.P.; Dongale, T.D.; Pawar, K.D. Selective interaction between phytomediated anionic silver nanoparticles and mercury leading to amalgam formation enables highly sensitive. colorimetric and memristor-based detection of mercury. Sci. Rep. 2020, 10, 2037. [Google Scholar] [CrossRef] [Green Version]
- Vidiš, M.; Pleceni, T.; Moško, M.; Tomašec, S.; Roch, T.; Satrapinskyy, L.; Grančič, B.; Peceni, A. Gasistor: A memristor based gas-triggered switch and gas sensor with memory. Appl. Phys. Lett. 2019, 115, 093504. [Google Scholar] [CrossRef]
- Puppo, F.; Doucey, M.A.; Di Ventra, M.G.; Micheli, D.; Carrara, S. Memristor-based devices for sensing. In Proceedings of the 2014 IEEE International Symposium on Circuits and Systems (ISCAS), Melbourne, Australia, 1–5 June 2014; pp. 2257–2260. [Google Scholar]
- Abunahla, H.; Mohammad, B.; Mahmoud, L.; Darweesh, M.; Alhawari, M.; Jaoud, A.; Hitt, G.W. Memsens: Memristor-based radiation sensor. IEEE Sens. J. 2018, 18, 3198–3205. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Chen, X.; Wu, W. Electrolyte-oxide-semiconductor structures as pH sensors based on resistive-switching characteristic. In Proceedings of the IEEE 29th International Conference on Micro Electro Mechanical Systems, Shanghai, China, 24–28 January 2016; pp. 897–900. [Google Scholar]
- Hilder, M.; Winther-Jensen, B.; Li, D.; Forsyth, M.; MacFarlane, D.R. Direct electro-deposition of graphene from aqueous suspensions. Phys. Chem. Chem. Phys. 2011, 13, 9187–9193. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Yan, F.; Liu, L.; Li, M.; Zhang, M.; Shang, L.; Xiao, L.; Ao, Y. One-step electrodeposition of Cu/CNT/CF multiscale reinforcement with substantially improved thermal/electrical conductivity and interfacial properties of epoxy composites. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105530. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, W.; Yu, C.; Zheng, Z.; Chen, Y.; Wang, J.; Liu, J.; Ma, K.; Ren, W. Electrochemical synthesis of Al/CuO thermite films on copper substrates. Ind. Eng. Chem. Res. 2019, 58, 7131–7138. [Google Scholar] [CrossRef]
- Rezaei, M.; Tabaian, S.; Haghshenas, D.F. Electrochemical nucleation and growth of Pd/PdCo core–shell nanoparticles with enhanced activity and durability as fuel cell catalyst. J. Mater. Chem. A 2014, 2, 4588–4597. [Google Scholar] [CrossRef]
- Gorelikov, I.; Kumacheva, E. Electrodeposition of polymer-semiconductor nanocomposite films. Chem. Mater. 2004, 16, 4122–4127. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, K.; Subramanian, P.; Xu, M.; Luo, J.; Fransaer, J. Electrochemical deposition of metal–organic framework films and their applications. J. Mater. Chem. A 2020, 8, 7569–7587. [Google Scholar] [CrossRef]



| Sr. No. | Method | Advantages | Disadvantages | Copper Oxide-Based Device Performance | Refs. |
|---|---|---|---|---|---|
| 1 | Sputtering |
|
| VSET: 0.7 V VRESET: −0.5 V Endurance: 103 cycles Retention: 104 s Memory Window: 105 | [105,106,116] |
| 2 | Chemical vapor deposition |
|
| VSET: −2 V VRESET: 1.5 V Endurance: 10 cycles Retention: 50 s Memory Window: 7 | [117,118] |
| 3 | Spray pyrolysis |
|
| VSET: 1.5 V VRESET: −0.65 V Endurance: 50 cycles Retention: 103 s Memory Window: 103 | [110,116] |
| 4 | Spin coating |
|
| VSET: 2.25 V VRESET: −2.25 V Endurance: 102 cycles Retention: 800 s Memory Window: 50 | [109,119] |
| 5 | Hydrothermal |
|
| VSET: 1 V VRESET: −1 V Endurance:10 cycles Retention: 600 s Memory Window: 102 | [111,116,120] |
| 6 | Sol–gel |
|
| - | [116,118,120] |
| 7 | Electrochemical |
|
| VSET: 0.7 V VRESET: −0.7 V Endurance: 5 × 103 cycles Retention: 103 s Memory Window: 102 | [104,116,118,120] |
| RS Material | Synthesis Method | Electrolyte | Applied Voltage | Time | VSET (V) | VRESET (V) | ON/OFF Ratio | Endurance Cycles | Data Retention (s) | Mode | Device Structure | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbon nanowalls | Electrophoretic | Polyyne solution by arc discharge | 30 V | 1 h | −2 | 2 | - | 150 | 5 × 104 | Bipolar | Al/CNWs/FTO | Memory Storage | [35] |
| Carbon structures | Electrophoretic | Polyyne solution by arc discharge | 30 V | 2 h | −2 | 2 | 700 | 50 | 1 × 104 | Bipolar | Al/OCs@FTO | Memory Storage | [137] |
| Graphene oxide | Electrophoretic | 0.2 mol/L GO solution | 10 V/cm | 1 min | 1.7 V | −1.7 V | 10 | - | 102 | Bipolar | Al/GO/ITO | Memory Storage | [147] |
| RS Material | Synthesis Method | Electrolyte | Applied Voltage/Current | Temp. °C | Time | VSET (V) | VRESET (V) | ON/OFF Ratio | Endurance Cycles | Data Retention (s) | Mode | Device Structure | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZnO | Electrodeposition | 0.01 M Zn(NO3)2·6H2O | 1 mA | 70 | 20 min | 3 | −3 | - | 2000 | 1800 | Bipolar | Au/ZnO rod/FTO | Memory storage | [36] |
| ZnO | Electrodeposition | 0.01 M ZnCl2 | 1.5 V | - | 20 min | 1.9 | −2 | - | - | - | Bipolar | - | Memory storage | [149] |
| ZnO | Cyclic voltammetry deposition | 0.01 M Zn(NO3)2·6H2O, 0.1 M KNO3 | ±0.9 V | 65 ± 2 | 300 s | 1 | −1 | 50 | 200 | 104 | Bipolar | Pt/ZnO/ITO | Memory storage | [104] |
| ZnO | Electrodeposition | 5 mM Zn(NO3)2·6H2O and HNO3 | −0.9 V | 80 | 1000 s | 2.7 | −2.5 | - | 100 | 1000 | Bipolar | Au/ZnO/AZO | Memory storage | [150] |
| ZnO | Electrodeposition | 0.005M ZnCl2 | 2.0 V | RT | - | 4 | −4 | - | 100 | - | Bipolar | Au/ZnO/ITO/PET | Memory storage | [148] |
| ZnO | Electrodeposition and hydrothermal | ZnSO4. 7H2O Na2SO4 H3BO3, H₂SO₄ | −1.5 V | 25 | 400 s | −4 | 5 | - | - | 10 | Bipolar | Pt (AFM tip)/ZnO/Zn | Memory storage | [151] |
| Ti-ZnO | Electrodeposition | 0.1 M Zn (NO3)2·6H2O with 2% (NH4)2TiF6 | 1 mA | 75 | 30 min | 2.7 | −2.9 | 14 | 200 | 2000 | Bipolar | Au/Ti-ZnO/ITO | Memory storage | [152] |
| ZnO-Cu2O-CuO | Electrodeposition | 0.005 M ZnCl2 | 2.0 V | RT | 120 s | 2 | −2 | - | - | - | Bipolar | Au/ZnO-Cu2O-CuO/Cu | Memory storage | [153] |
| ZnO-Cu2O-CuO | Electrodeposition | 0.005 M ZnCl2 | 1 V | - | - | 2 | −2 | 1.08 | - | - | Bipolar | Au/ZnO-Cu2O-CuO/Cu | Memory storage | [154] |
| CeO2−ZnO | Electrodeposition and dip coating | 0.01 M Zn(NO3)2∙6H2O | 2 mA | 75 | 30 min | −1.9 | 2.08 | - | 200 | 10,000 | Bipolar | Au/CeO2−ZnO/FTO | Memory storage | [155] |
| ZnO-Si | Anodization and sol–gel | - | - | - | - | 10 | −10 | ~2.4 | 100 | - | Bipolar | Al/ZnO/Si/Al | Memory storage | [156] |
| RS Material | Synthesis Method | Electrolyte | Applied Voltage | Temp. °C | Time | VSET (V) | VRESET (V) | ON/OFF Ratio | Endurance Cycles | Data Retention (s) | Mode | Device Structure | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TiO2 | Anodization | 0.27 M NH4F,glycerol | 30 V | - | 10 s | 1 | −1 | - | 3 | - | Bipolar | Ag/TiO2/Ti | Memory Storage | [37] |
| TiO2 | Electrodeposition | (NH4)2TiF6 and HBO3 | −0.8 V | 90 | 30 min | 2.5 | −1.3 | - | 7000 | - | Bipolar | Au/TiO2/FTO | Memory Storage | [157] |
| TiO2 | Anodization | 1 M H2SO4 | 10 V | - | 15 min | 0.24 | −0.24 | 25 | 100 | - | Bipolar | Au/TiO2/Ti | Memory Storage | [158] |
| TiO2 | Anodization | HF and H3PO4 | - | - | 2 h | −1 | 1 | - | 20 | - | Bipolar | Pt/TiO2/Ti | Memory Storage | [159] |
| TiO2 | Anodization | 0.1 mol/L H2SO4 | 20 V | - | 30 min | 2 | −2 | - | 50 | 103 | Bipolar | Pt/TiO2/Ti | Memory Storage | [160] |
| TiOx | CV Anodization | 100 mM CH3SO3H, Mn(CH 3SO3)2 | −0.5 to 10 V | - | - | 1 | −1 | 600 | - | Bipolar | Ag/TiOx/Ti/Au | Memory Storage | [164] | |
| TiO2 | Anodization | 1 M H3PO4 | 10 V | - | 20 min | −2 | 2 | - | 20 | Bipolar | Cu/TiO2/Ti | Memory Storage | [165] | |
| TiO2 | Anodization | 0.5 wt% NH4F,ethylene glycol, water | 60 V | RT | 30 s | 2 | −2 | - | 20 | - | Bipolar | Ti/TiO2/PAA/Pt | Memory Storage | [166] |
| TiO2 | Anodization | 0.25 wt% NH4F and ethylene glycol | 60 V | - | 5 min | 0.37 | −0.4 | 25 | 16 | - | Bipolar | Ag/TiO2/Ti | Memory Storage | [161] |
| Core–shell copper nanowire-TiO2 nanotube | Anodization | 0.5 wt% NH4F, 3 vol% H3PO4 3 vol% DI water in ethylene glycol | 80 V | RT | 2 h | 1.8 | −2.5 | 40 | 10,000 | 104 | Bipolar | Au/TiO2-Cu/Ti | Memory Storage | [163] |
| RS Material | Synthesis Method | Electrolyte | Applied Voltage/Current Density | pH | Time | VSET (V) | VRESET (V) | Forming Voltage | ON/ OFF Ratio | Endurance Cycles | Data Retention (s) | Mode | Device Structure | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu2O | Electrodeposition | 0.2 M CuSO4.5H2O, 0.2 M L(+)-tartaric acid, 3 M NaOH | 1 mA/cm2 | - | - | 4 | 1 | 34 ± 6.7 V | 10,000 | 170 | 8 × 104 | Unipolar | Au−Pd/Cu2O/Au | Memory Storage | [175] |
| Cu2O | Electrodeposition | 0.6 M CuSO4·5H2O, 3 M lactic acid, 2M NaOH | 1 mA/cm2 | 9 | - | −0.36 | 0.31 | ~0.8 | >1000 | 100 | 104 | Bipolar | Pt/Cu2O/Pt | Memory Storage | [167] |
| Cu2O | Electrodeposition | 0.6 M CuSO4·5H2O, 3 M lactic acid, 2 M NaOH | 1 mA/cm2 | 9 | 30 s | 1.3 | −0.75 | 1.5 | ~1000 | 8 | - | Bipolar | Cu/CuOx/Cu | Memory Storage | [168] |
| Cu2O | Electrodeposition | 1 M CuSO4.5H2O, 6 M sulfuric acid, NaOH | 1–4 V | - | - | ~−2 | 2 | 5 | - | - | - | Bipolar | Al/Cu2O/FTO | Memory Storage | [176] |
| CuOx | Electrodeposition | 0.6 M CuSO4.5H2O, 3 M lactic acid, NaOH | 4 mA | - | 30 s | 0.5 | −0.3 | 0.75 | - | 100 | 104 | Bipolar | Ni/CuOx/Ni | Memory Storage | [177] |
| CuOx | Electrodeposition | 0.6 M CuSO4.5H2O, 3 M lactic acid, NaOH | −0.4 V vs. Ag/AgCl | 9 and 11.5 | 100 s | 2.5 | −3 | 4 | - | 100 | 104 | Bipolar | Pt/CuOx/CuOx/Pt | Memory Storage | [178] |
| RS Material | Synthesis Method | Electrolyte | Applied Voltage/Current Density | pH | Time | VSET (V) | VRESET (V) | ON/OFF Ratio | Endurance Cycles | Data Retention (s) | Mode | Device Structure | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NiO | Electrodeposition and thermal oxidation | Ni2SO4 ·6H2O (0.5M) H3PO3 (0.67 M) | 3 mA/cm2 | - | - | 1.2 | 0.52 | - | 100 | - | Unipolar | W/NiO/Au | Memory Storage | [186] |
| NiO | Electrodeposition and thermal oxidation | Ni(H2NSO3)2, NiCl2·6H2O, H3BO3, CH3COONa, H2SO4 | 4 mA/cm2 | 3.4 | 1 min | 9 | 6 | ~100 | 10 | - | Unipolar | Ni/NiO/Ni | Memory Storage | [102] |
| P-doped NiO | Electrodeposition and thermal oxidation | 1 M Ni2SO4 ·6H2O, 0.2 M NiCl₂, and 0.5 M H3BO3 | 5 mA/cm2 | - | 5 min | 0.8 | −0.7 | - | - | - | Bipolar | Au/P-doped NiO/Au | Memory Storage | [187] |
| TaOx | Anodization | 1 mM citric acid | - | - | - | 4.5 | −5 | 80 | - | Bipolar | Ta/TaOx/Pt | Memory Storage | [188] | |
| Ta2O5 | Anodization | H3BO3, Na2B4O7 | - | 8 | - | 0.45–0.65 V | 0.45 0.65 V | >10 | 106 | 104 | Bipolar | Ta/Ta2O5/Pt | Memory Storage | [189] |
| Ta2O5 | Anodization | 0.42 M H3BO3, 0.08 M Na2B4O7 | 10 mV s−1 | 8 | - | 0.45 | −0.9 | - | 1000 | 104 | Bipolar | Ta/anodic Ta2O5/Pt | Memory Storage | [132] |
| WO3−x | Anodization | NH4F, ethylene glycol | 20 V | - | 20 s | 1 | −1 | 105 | 1000 | 105 | Bipolar | Cu/WO3−x/ITO/PET | Memory Storage | [190] |
| WO3 | Electrodeposition | Na2WO4·H2O, H2O2, HClO4 | −0.7 V | 1.2 | 60 s | 0.25 | −0.42 | - | 320 | >104 | Bipolar | ITO/WO3/ITO | Memory Storage | [180] |
| Mn3O4 | Electrodeposition | Mn(II) acetate, sodium acetate | 0.3 V | 6 | - | 0.72 | 2.5 | - | 30 | - | Unipolar | AuPd/Mn3O4/Au | Memory Storage | [43] |
| δ-Bi2O3 | Electrodeposition | 0.1 M Bi(NO3)3, 0.25 M L-tartaric acid and 2.5 M KOH | 5 mA/cm2 | - | - | 2.2 | 0.4 | 106 | 100 | 5 × 104 | Unipolar | Pt/δ-Bi2O3/Au | Memory Storage | [181] |
| VO2 | Electrodeposition | - | - | - | - | 0.5 | −0.5 | - | - | - | Bipolar | Ag/VO2/Pt | Memory Storage | [191] |
| CO3O4 | Electrodeposition | 0.1 M Co(NO3)2·6H2O | −0.8 V | - | 20 min | 1.5 | −1.93 | - | 200 | >104 | Bipolar | Au/CO3O4/ITO | Memory Storage | [193] |
| MoS2-MoO2-MoO3 | Electrodeposition | 0.39 M Na2MoO4·2H2O and 0.88 M Na2S·5H2O | - | 7 | - | 1.15 0.2 | −0.5 −0.1 | - | - | - | Bipolar | Al/MoSO/FTO Ag/MoSO/FTO | Memory Storage | [194] |
| Prussian blue | Electrodeposition | 0.25 mM K3Fe(CN)6, 0.25 mM FeCl3, 1.0 M KCl and 5.0 mM HCl | 0.3 V | 2 | - | −0.6 V | ~+0.6 V | - | 200 | - | Bipolar | Au/Prussian blue/Ag | Memory Storage | [196] |
| Bi1−xSbx | Electrodeposition | 0.05 M SbCl2 0.05 M Bi(NO3)3·5H2O dimethyl sulfoxide | −0.8 V | - | - | 1.35 | −1.2 V | 104 | 300 | - | Bipolar | Pt/Bi1-xSbx/Pt | Memory Storage | [197] |
| Co−PPy composite | Electrodeposition (CV) | 0.1 mol/L Na2SO4, 0.001 mol/L sodium dodecylbenzenseulfonate, and 0.1 mol/L pyrrole Co nanoparticles | 0.5–1.0 V | 3 | - | 1.07 to 2.3 | −1.29 to −2.3 | - | - | 104 | Bipolar | Cu/Co−PPy composite/Pt | Memory Storage | [182] |
| RS Material | Synthesis Method | Electrolyte | Deposition Potential/ Current Density | Temp. | VSET (V) | VRESET (V) | Device Structure | Cross Point Array | Synaptic Properties | Advantages | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TiO2 Nanotubes | Anodization | Ethylene glycol + NH4F + distilled water | 30 V | - | 2 | −2 | Al/TiO2 nanotubes/Ti | - | Potentiation–depression, STDP-based Hebbian learning rules | Low-cost artificial synaptic device that can mimic the basic and advanced synaptic learning properties | [133] |
| CuXO | Anodization | 3 M KOH | 10 mA/cm2 for 150 s | - | 8 | −8 | Pt/CuXO/Cu | - | Potentiation–depression, STDP-based Hebbian learning rules | Low-cost artificial synaptic device that can mimic the basic and advanced synaptic learning properties | [169] |
| Reduced Graphene Oxide | Electrophoretic | Aqueous suspension of graphene oxide | −1.1 V | 60 °C | - | - | Conductive yarns/RGO/conductive yarns | 2 × 2 | EPSC, PPF, and a transition from short-term plasticity to long-term plasticity | Operated stably without degradation during mechanical bending; artificial synapses for wearable neuromorphic systems | [205] |
| Anodic TiO2−x | Anodization | 0.5 M NaOH | 2.5 V | - | 0.8 | −1.6 | Pt/anodic TiO2−x/Ti | - | Potentiation and depression | A cheap and effective route to fabricate competitive electronic synapses | [206] |
| TaOy/nano-porous TaOx | Anodization | Aqueous H2SO4 and HF | 50 V for 10 s | - | 4 | −4 | Pt/TaOy/nanoporous TaOx/Ta | 4 × 4 | LTP, LTD, and STDP | 89.08% recognition accuracy for MNIST handwritten digit images | [207] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundale, S.S.; Kamble, G.U.; Patil, P.P.; Patil, S.L.; Rokade, K.A.; Khot, A.C.; Nirmal, K.A.; Kamat, R.K.; Kim, K.H.; An, H.-M.; et al. Review of Electrochemically Synthesized Resistive Switching Devices: Memory Storage, Neuromorphic Computing, and Sensing Applications. Nanomaterials 2023, 13, 1879. https://doi.org/10.3390/nano13121879
Kundale SS, Kamble GU, Patil PP, Patil SL, Rokade KA, Khot AC, Nirmal KA, Kamat RK, Kim KH, An H-M, et al. Review of Electrochemically Synthesized Resistive Switching Devices: Memory Storage, Neuromorphic Computing, and Sensing Applications. Nanomaterials. 2023; 13(12):1879. https://doi.org/10.3390/nano13121879
Chicago/Turabian StyleKundale, Somnath S., Girish U. Kamble, Pradnya P. Patil, Snehal L. Patil, Kasturi A. Rokade, Atul C. Khot, Kiran A. Nirmal, Rajanish K. Kamat, Kyeong Heon Kim, Ho-Myoung An, and et al. 2023. "Review of Electrochemically Synthesized Resistive Switching Devices: Memory Storage, Neuromorphic Computing, and Sensing Applications" Nanomaterials 13, no. 12: 1879. https://doi.org/10.3390/nano13121879







