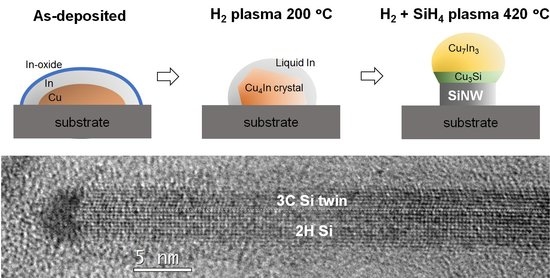
Evolution of Cu-In Catalyst Nanoparticles under Hydrogen Plasma Treatment and Silicon Nanowire Growth Conditions
Abstract
:1. Introduction
2. Materials and Methods
- -
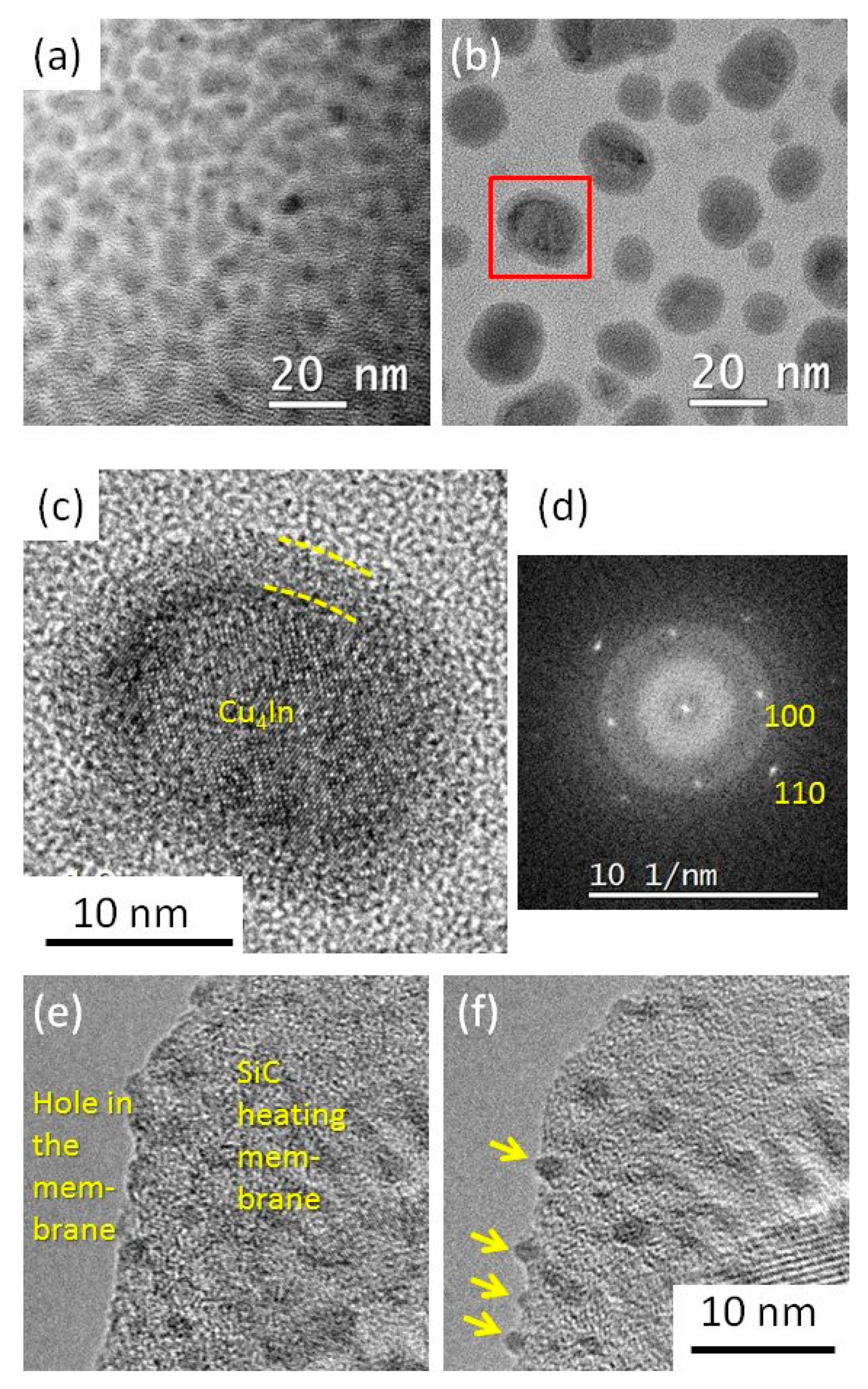
- Step I—hydrogen plasma pre-treatment at 200 °C. For this step, the H2 flow rate, the gas pressure P, the RF plasma power density and the duration time t were 100 sccm, 0.8 mbar, 56.7 mW/cm2, and 2 min, respectively. To evaluate the effect of plasma, we compared the Cu-In NPs after the plasma treatment with the NPs having experienced the same temperature annealing but without plasma. To follow the effect of plasma in real time, we made the same experiment in situ, in the TEM (with smaller catalysts deposited on the Protochips™ heating membranes);
- -
- Step II—SiNW growth at 420 °C. For this step, a second plasma treatment was carried out in the same run in the PECVD reactor by adding a SiH4 flow of 5 sccm to 100 sccm H2 and changing the process parameters to the following values: P of 1.42 mbar, RF power density of 17 mW/cm2 for t = 3 min. To understand the effect of the plasma and that of adding silane, we compare the Cu-In catalyst NPs at the head of the obtained SiNWs with (i) those NPs that have undergone exactly the same plasma pre-treatment at 200 °C as mentioned above and the 420 °C anneal treatment but the latter in vacuum, and (ii) the NPs that have undergone the 420 °C plasma treatment, but with only hydrogen and no SiH4 in the plasma (the other experimental parameters remain unchanged as in step I).
3. Results and Discussion
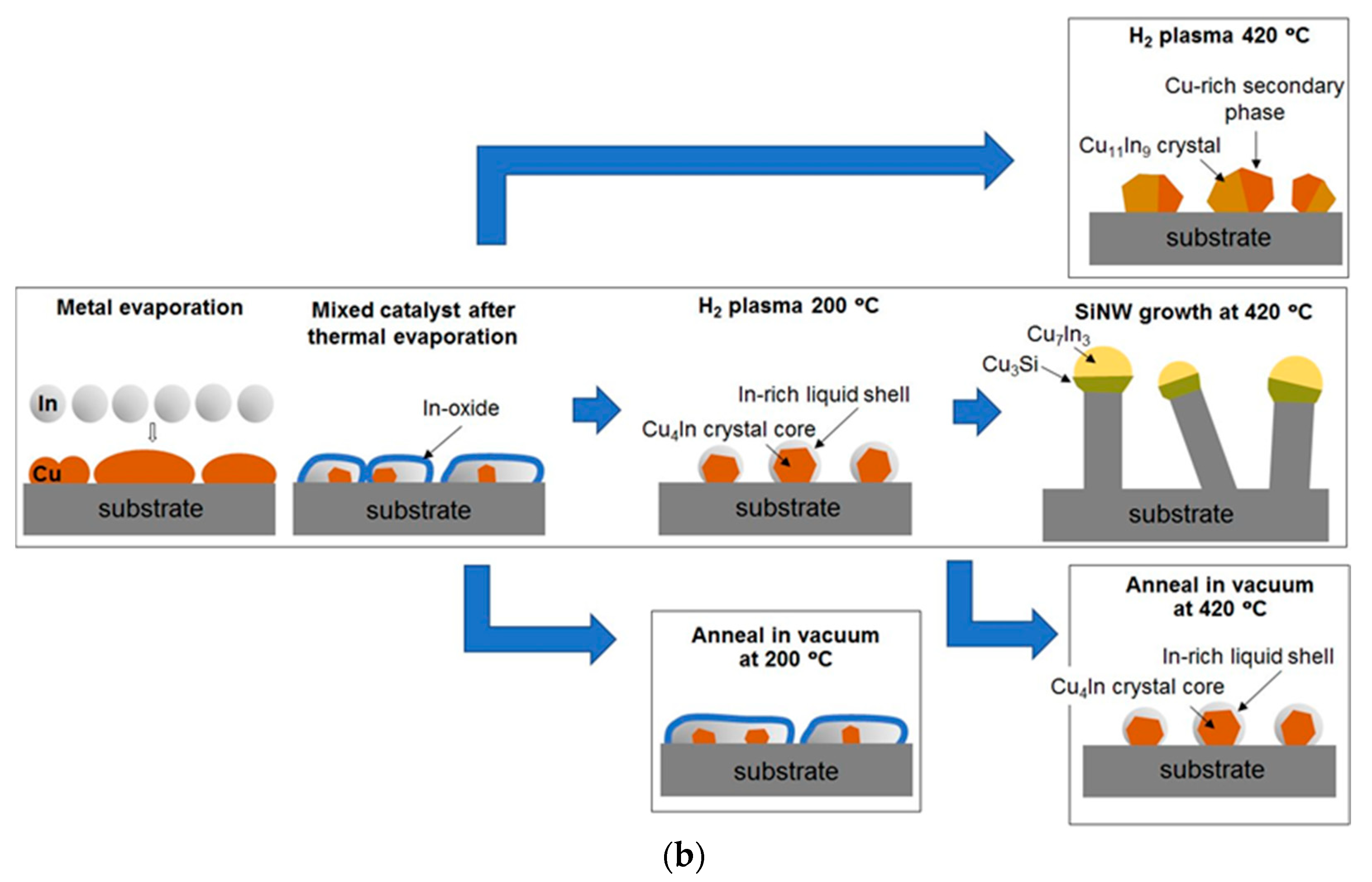
3.1. Evolution of Bimetallic Cu-In Catalyst NPs throughout the Process
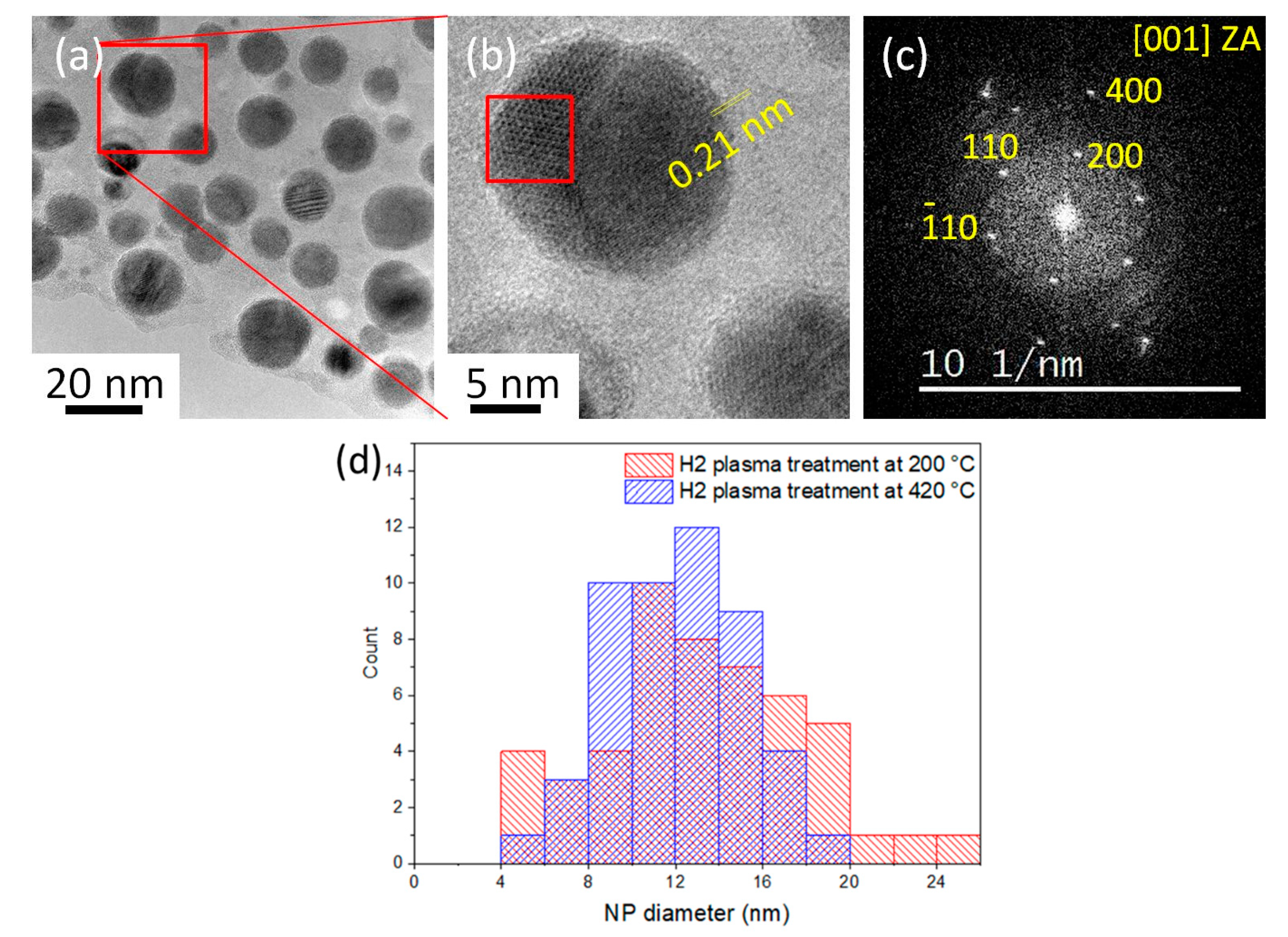
3.1.1. 200 °C H2 Plasma Treatment
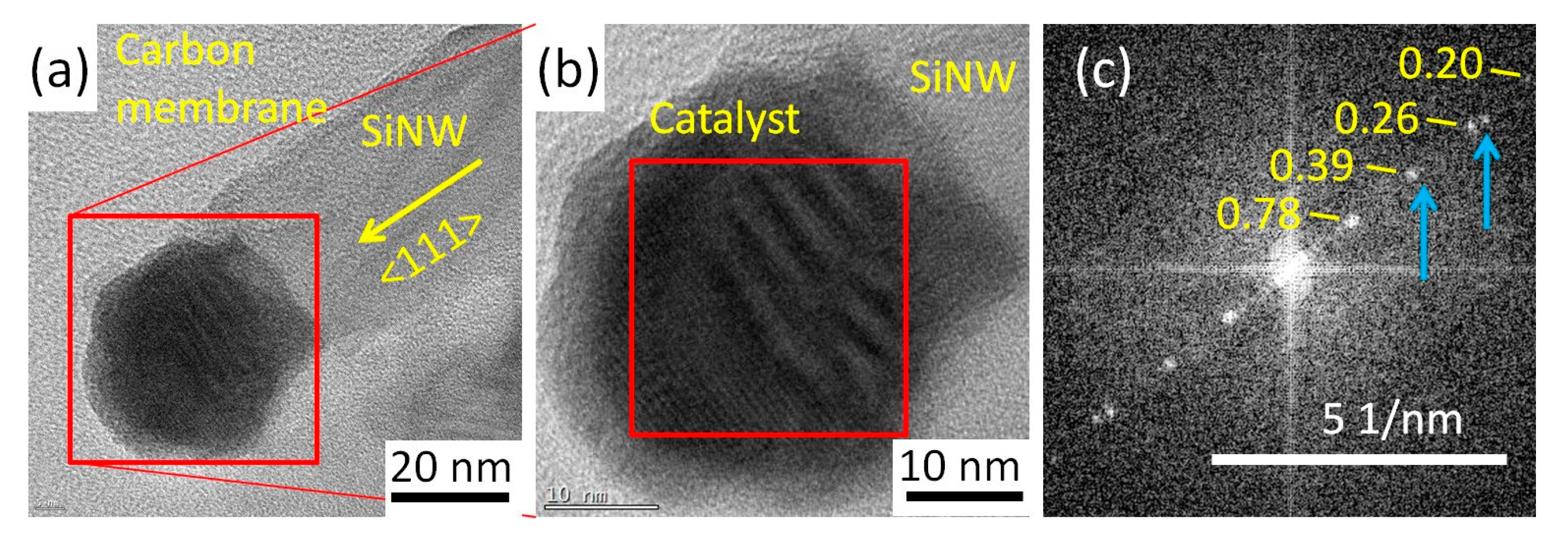
3.1.2. 420 °C Plasma Treatment and Si Nanowire Growth
3.1.3. Catalyst Evolution: Summary
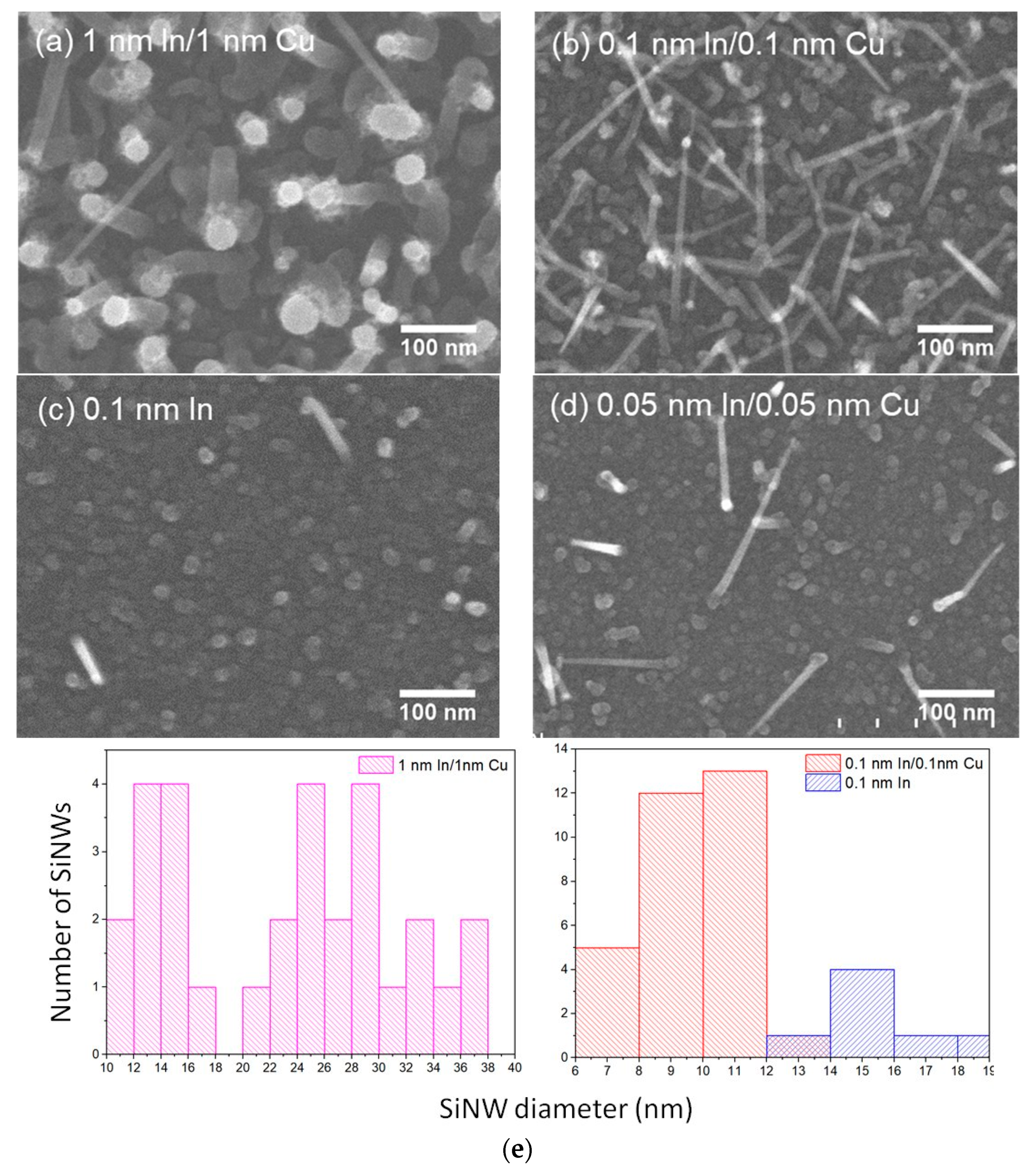
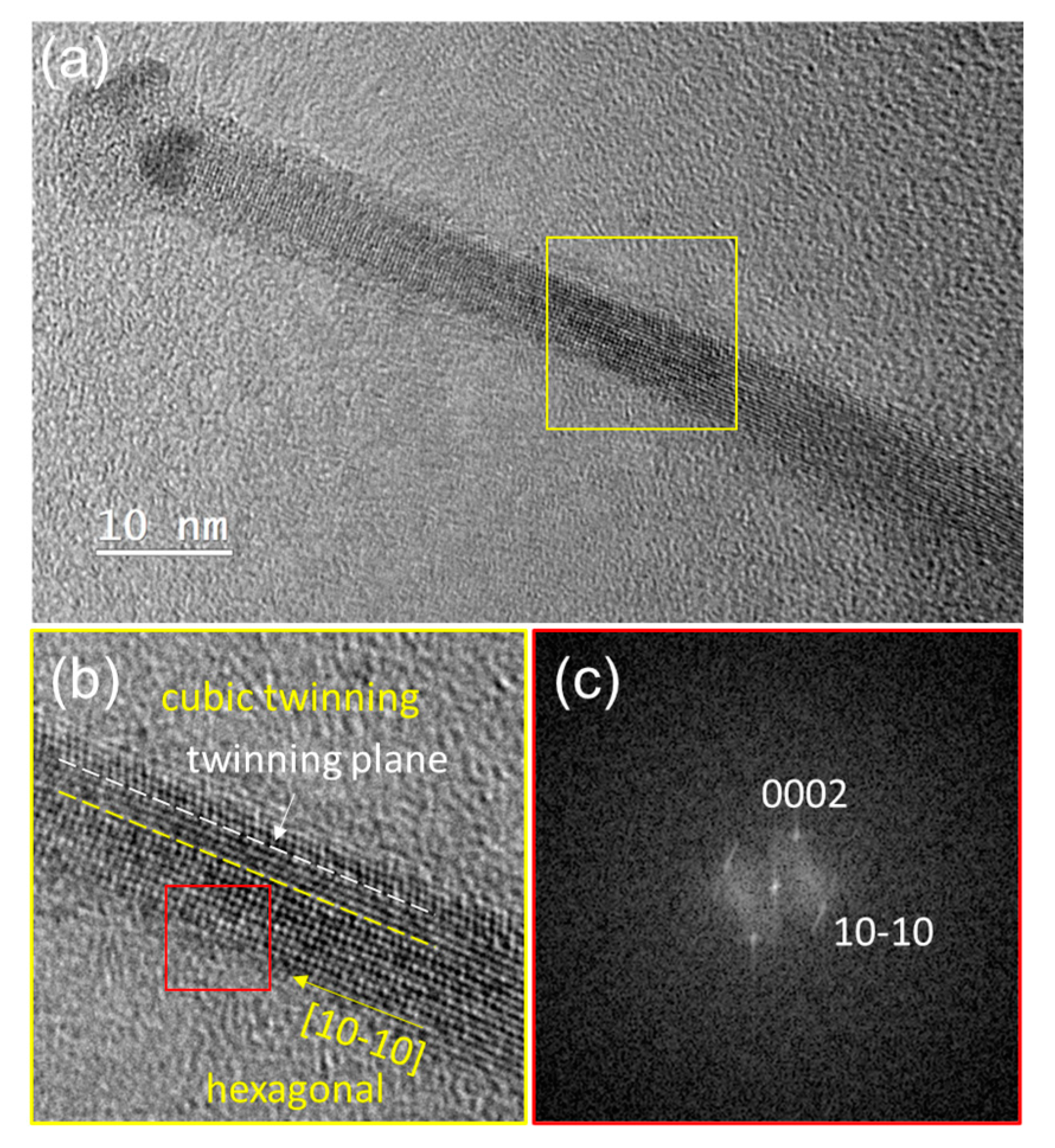
3.2. Influence of Catalyst Composition on the Distribution and Structure of SiNWs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, J.; Odom, T.W.; Lieber, C.M. Chemistry and physics in one dimension: Synthesis and properties of nanowires and nanotubes. Acc. Chem. Res. 1999, 32, 435–445. [Google Scholar] [CrossRef]
- Lieber, C.M.; Wang, Z.L. Functional Nanowires. MRS Bull. 2011, 32, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Zheng, G.; Lieber, C.M. (Eds.) Emergence of Nanowires. In Nanowires: Building Blocks for Nanoscience and Nanotechnology; Springer: Cham, Switzerland, 2016; pp. 1–13. [Google Scholar]
- Lysov, A.; Vinaji, S.; Offer, M.; Gutsche, C.; Regolin, I.; Mertin, W.; Geller, M.; Prost, W.; Bacher, G.; Tegude, F.-J. Spatially resolved photoelectric performance of axial GaAs nanowire pn-diodes. Nano Res. 2011, 4, 987–995. [Google Scholar] [CrossRef]
- Chen, K.-I.; Li, B.-R.; Chen, Y.-T. Silicon nanowire field-effect transistor-based biosensors for biomedical diagnosis and cellular recording investigation. Nano Today 2011, 6, 131–154. [Google Scholar] [CrossRef]
- Könenkamp, R.; Word, R.C.; Schlegel, C. Vertical nanowire light-emitting diode. Appl. Phys. Lett. 2004, 85, 6004–6006. [Google Scholar] [CrossRef] [Green Version]
- Tong, C.; Delamarre, A.; De Lépinau, R.; Scaccabarozzi, A.; Oehler, F.; Harmand, J.-C.; Collin, S.; Cattoni, A. GaAs/GaInP nanowire solar cell on Si with state-of-the-art Voc and quasi-Fermi level splitting. Nanoscale 2022, 14, 12722–12735. [Google Scholar] [CrossRef]
- Wagner, R.S.; Ellis, W.C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Cui, Y.; Lieber, C.M. Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science 2001, 291, 851–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puglisi, R.A.; Bongiorno, C.; Caccamo, S.; Fazio, E.; Mannino, G.; Neri, F.; Scalese, S.; Spucches, D.; La Magna, A. Chemical Vapor Deposition Growth of Silicon Nanowires with Diameter Smaller than 5 nm. ACS Omega 2019, 4, 17967–17971. [Google Scholar] [CrossRef] [Green Version]
- Holmes, J.D.; Johnston, K.P.; Doty, R.C.; Korgel, B.A. Control of thickness and orientation of solution-grown silicon nanowires. Science 2000, 287, 1471–1473. [Google Scholar] [CrossRef] [PubMed]
- Kuykendall, T.R.; Altoe, M.V.P.; Ogletree, D.F.; Aloni, S. Catalyst-directed crystallographic orientation control of GaN nanowire growth. Nano Lett. 2014, 14, 6767–6773. [Google Scholar] [CrossRef] [PubMed]
- Lugstein, A.; Steinmair, M.; Hyun, Y.J.; Bertagnolli, E.; Pongratz, P. Ga∕Au alloy catalyst for single crystal silicon-nanowire epitaxy. Appl. Phys. Lett. 2007, 90, 023109. [Google Scholar] [CrossRef]
- Maksimova, K.Y.; Kozlov, A.A.; Shvets, P.V.; Koneva, U.Y.; Yurkevich, O.V.; Lebedev, O.I.; Vyvenko, O.F.; Mikhailovskii, V.Y.; Goikhman, A.Y. Copper-stabilized Si/Au nanowhiskers for advanced nanoelectronic applications. ACS Omega 2018, 3, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lu, J.; Qian, S.; Misra, S.; Yu, L.; Xu, J.; Xu, L.; Wang, J.; Shi, Y.; Chen, K.; et al. Bi-Sn alloy catalyst for simultaneous morphology and doping control of silicon nanowires in radial junction solar cells. Appl. Phys. Lett. 2015, 107, 163105. [Google Scholar] [CrossRef]
- Behroudj, A.; Geiger, D.; Strehle, S. Epitaxial bottom-up growth of silicon nanowires on oxidized silicon by alloy-catalyzed gas-phase synthesis. Nano Lett. 2019, 19, 7895–7900. [Google Scholar] [CrossRef]
- Chou, Y.C.; Wen, C.Y.; Reuter, M.C.; Su, D.; Stach, E.A.; Ross, F.M. Controlling the growth of Si/Ge nanowires and heterojunctions using silver-gold alloy catalysts. ACS Nano 2012, 6, 6407–6415. [Google Scholar] [CrossRef]
- Ngo, É.; Wang, W.; Bulkin, P.; Florea, I.; Foldyna, M.; Roca i Cabarrocas, P.; Maurice, J.-L. Liquid-assisted vapor–solid–solid silicon nanowire growth mechanism revealed by in situ TEM when using Cu–Sn bimetallic catalysts. J. Phys. Chem. C 2021, 125, 19773–19779. [Google Scholar] [CrossRef]
- Wang, W.; Ngo, É.; Florea, I.; Foldyna, M.; Roca i Cabarrocas, P.; Maurice, J.-L. High density of quantum-sized silicon nanowires with different polytypes grown with bimetallic catalysts. ACS Omega 2021, 6, 26381–26390. [Google Scholar] [CrossRef]
- Hu, T.; Seifner, M.S.; Snellman, M.; Jacobsson, D.; Sedrpooshan, M.; Ternero, P.; Messing, M.E.; Dick, K.A. Direct observation of liquid–solid two-phase seed particle-assisted kinking in GaP nanowire growth. Small Struct. 2023, 2300011. [Google Scholar] [CrossRef]
- Amato, M.; Kaewmaraya, T.; Zobelli, A.; Palummo, M.; Rurali, R. Crystal phase effects in Si nanowire polytypes and their homojunctions. Nano Lett. 2016, 16, 5694–5700. [Google Scholar] [CrossRef] [Green Version]
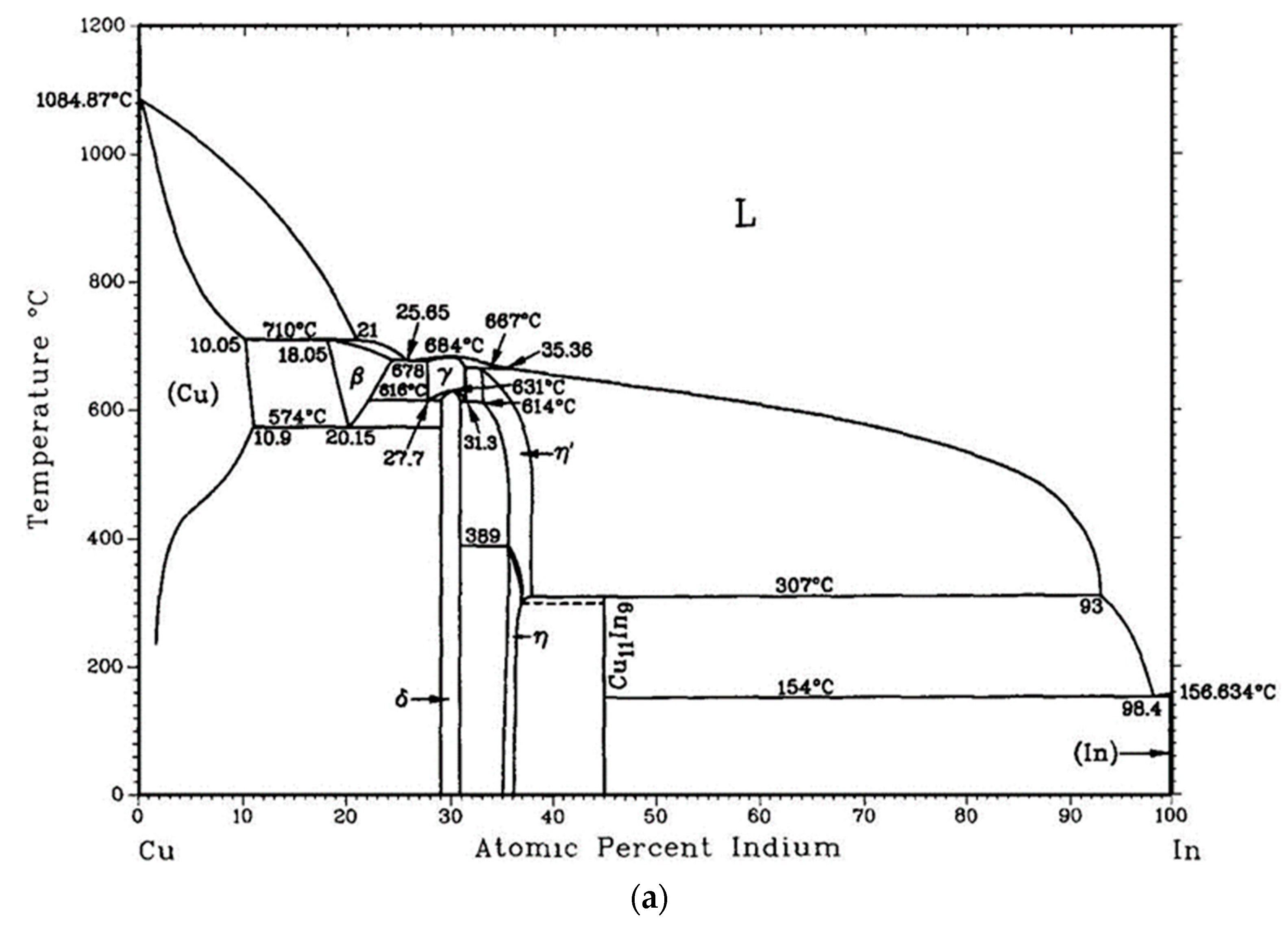
- Okamoto, H. Comment on Cu-In (Copper-Indium). J. Phase Equilib. 1994, 15, 226–227. [Google Scholar] [CrossRef]
- Olesinski, R.; Kanani, N.; Abbaschian, G. The In Si (Indium-Silicon) system. Bull. Alloy Phase Diagr. 1985, 6, 128–130. [Google Scholar] [CrossRef]
- Yu, L.; Chen, W.; O’Donnell, B.; Patriarche, G.; Bouchoule, S.; Pareige, P.; Rogel, R.; Claire Salaun, A.; Pichon, L.; Roca i Cabarrocas, P. Growth-in-place deployment of in-plane silicon nanowires. Appl. Phys. Lett. 2011, 99, 203104. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-G.; Lee, C.-Y.; Jung, S.-B. Interfacial reactions and intermetallic compound growth between indium and copper. J. Mater. Sci. Mater. Electron. 2004, 15, 95–98. [Google Scholar] [CrossRef]
- Orbey, N.; Birkmire, R.W.; Russell, T.W.F.; Jones, G.A. Copper-Indium Alloy Transformations. J. Phase Equilib. 2000, 21, 509. [Google Scholar] [CrossRef]
- Park, J.-S.; Jeong, J.K.; Chung, H.-J.; Mo, Y.-G.; Kim, H.D. Electronic transport properties of amorphous indium-gallium-zinc oxide semiconductor upon exposure to water. Appl. Phys. Lett. 2008, 92, 072104. [Google Scholar] [CrossRef] [Green Version]
- Ramanujam, J.; Singh, U.P. Copper indium gallium selenide based solar cells—A review. Energy Environ. Sci. 2017, 10, 1306–1319. [Google Scholar] [CrossRef]
- Scofield, J.H.; Duda, A.; Albin, D.; Ballard, B.L.; Predecki, P.K. Sputtered molybdenum bilayer back contact for copper indium diselenide-based polycrystalline thin-film solar cells. Thin Solid Film. 1995, 260, 26–31. [Google Scholar] [CrossRef]
- Yang, J.; Kim, J.-Y.; Yu, J.H.; Ahn, T.-Y.; Lee, H.; Choi, T.-S.; Kim, Y.-W.; Joo, J.; Ko, M.J.; Hyeon, T. Copper–indium–selenide quantum dot-sensitized solar cells. Phys. Chem. Chem. Phys. 2013, 15, 20517–20525. [Google Scholar] [CrossRef]
- Yu, C.L.; Wang, S.S.; Chuang, T.H. Intermetallic compounds formed at the interface between liquid indium and copper substrates. J. Electron. Mater. 2002, 31, 488–493. [Google Scholar] [CrossRef]
- Castilla-Amorós, L.; Schouwink, P.; Oveisi, E.; Okatenko, V.; Buonsanti, R. Tailoring morphology and elemental distribution of Cu-In nanocrystals via galvanic replacement. J. Am. Chem. Soc. 2022, 144, 18286–18295. [Google Scholar] [CrossRef]
- Rasul, S.; Anjum, D.H.; Jedidi, A.; Minenkov, Y.; Cavallo, L.; Takanabe, K. A Highly Selective Copper–Indium Bimetallic Electrocatalyst for the Electrochemical Reduction of Aqueous CO2 to CO. Angew. Chem. Int. Ed. 2015, 54, 2146–2150. [Google Scholar] [CrossRef]
- Luo, W.; Xie, W.; Mutschler, R.; Oveisi, E.; De Gregorio, G.L.; Buonsanti, R.; Züttel, A. Selective and Stable Electroreduction of CO2 to CO at the Copper/Indium Interface. ACS Catal. 2018, 8, 6571–6581. [Google Scholar] [CrossRef]
- Ahn, S.; Choi, Y.J.; Kim, K.; Eo, Y.-J.; Cho, A.; Gwak, J.; Yun, J.H.; Shin, K.; Ahn, S.K.; Yoon, K. Amorphous Cu–In–S Nanoparticles as Precursors for CuInSe2 Thin-Film Solar Cells with a High Efficiency. ChemSusChem 2013, 6, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kim, C.; Yun, J.H.; Gwak, J.; Jeong, S.; Ryu, B.-H.; Yoon, K. CuInSe2 (CIS) Thin Film Solar Cells by Direct Coating and Selenization of Solution Precursors. J. Phys. Chem. C 2010, 114, 8108–8113. [Google Scholar] [CrossRef]
- Stolt, L.; Hedström, J.; Kessler, J.; Ruckh, M.; Velthaus, K.O.; Schock, H.W. ZnO/CdS/CuInSe2 thin-film solar cells with improved performance. Appl. Phys. Lett. 1993, 62, 597–599. [Google Scholar] [CrossRef]
- Sommadossi, S.; Gust, W.; Mittemeijer, E.J. Phase characterisation and kinetic behaviour of diffusion soldered Cu/In/Cu interconnections. Mater. Sci. Technol. 2003, 19, 528–534. [Google Scholar] [CrossRef]
- Sommadossi, S.; Litynska, L.; Zieba, P.; Gust, W.; Mittemeijer, E.J. Transmission electron microscopy investigation of the microstructure and chemistry of Si/Cu/In/Cu/Si interconnections. Mater. Chem. Phys. 2003, 81, 566–568. [Google Scholar] [CrossRef]
- Wojewoda, J.; Zieba, P.; Faryna, M.; Gust, W.; Mittemeijer, E.J.; Litynska, L. Characterization of Interfacial Reactions in Cu/In/Cu Joints. Microchim. Acta 2004, 145, 107–110. [Google Scholar] [CrossRef]
- Wang, W. Plasma-Enhanced CVD Growth of Cubic and Hexagonal Diamond Silicon Nanowires with Liquid-Solid Mixed Catalysts for Photovoltaic Applications. Ph.D. Thesis, Institut Polytechnique de Paris, Palaiseau, France, 2021. Available online: https://theses.hal.science/tel-03297142 (accessed on 30 June 2023).
- Maurice, J.-L.; Bulkin, P.; Ngo, É.; Wang, W.; Foldyna, M.; Florea, I.; Roca i Cabarrocas, P.; Béjaud, R.; Hardouin Duparc, O. Visualizing the effects of plasma-generated H atoms in situ in a transmission electron microscope. Eur. Phys. J. Appl. Phys. 2022, 97, 7. [Google Scholar] [CrossRef]
- Liu, X.; Davis, R.W.; Hughes, L.C.; Rasmussen, M.H.; Bhat, R.; Zah, C.-E.; Stradling, J. A study on the reliability of indium solder die bonding of high power semiconductor lasers. J. Appl. Phys. 2006, 100, 013104. [Google Scholar] [CrossRef]
- Tang, J.; Maurice, J.-L.; Fossard, F.; Florea, I.; Chen, W.; Johnson, E.V.; Foldyna, M.; Yu, L.; Roca i Cabarrocas, P. Natural occurrence of the diamond hexagonal structure in silicon nanowires grown by a plasma-assisted vapour-liquid-solid method. Nanoscale 2017, 9, 8113–8118. [Google Scholar] [CrossRef]
- Stadelmann, P.A. EMS—A software package for electron diffraction analysis and HREM image simulation in materials science. Ultramicroscopy 1987, 21, 131–145. [Google Scholar] [CrossRef]
- Stadelmann, P. JEMS-Swiss. Available online: https://www.jems-swiss.ch/ (accessed on 15 January 2022).
- Zoubian, F.; Renaut, N.; Latrasse, L. Distributed elementary ECR microwave plasma sources supplied by solid state generators for production of large area plasmas without scale limitation: Plasma density measurements and comparison with simulation. Plasma Res. Express 2021, 3, 025010. [Google Scholar] [CrossRef]
- Sigeneger, F.; Ellis, J.; Harhausen, J.; Lang, N.; van Helden, J.H. Verified modeling of a low pressure hydrogen plasma generated by electron cyclotron resonance. Plasma Sources Sci. Technol. 2022, 31, 105011. [Google Scholar] [CrossRef]
- Che, G.C.; Ellner, M. Powder crystal data for the high-temperature phases Cu4In, Cu9In4(h) and Cu2In(h). Powder Diffr. 2013, 7, 107–108. [Google Scholar] [CrossRef]
- Bolcavage, A.; Chen, S.W.; Kao, C.R.; Chang, Y.A.; Romig, A.D. Phase equilibria of the Cu-In system I: Experimental investigation. J. Phase Equilib. 1993, 14, 14–21. [Google Scholar] [CrossRef]
- Subramanian, P.R.; Laughlin, D.E. The Cu−In (Copper-Indium) system. Bull. Alloy Phase Diagr. 1989, 10, 554–568. [Google Scholar] [CrossRef]
- Bahari, Z.; Dichi, E.; Legendre, B.; Dugué, J. The equilibrium phase diagram of the copper–indium system: A new investigation. Thermochim. Acta 2003, 401, 131–138. [Google Scholar] [CrossRef]
- Koster, A.S.; Wolff, L.R.; Visser, G.J. Structure of copper–indium Cu7In3. Acta Crystallogr. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 3094–3096. [Google Scholar] [CrossRef]
- Yao, Y.; Fan, S. Si nanowires synthesized with Cu catalyst. Mater. Lett. 2007, 61, 177–181. [Google Scholar] [CrossRef]
- Rajasekharan, T.P.; Schubert, K. Kristallstruktur von Cu11In9. Int. J. Mater. Res. 1981, 72, 275–278. [Google Scholar] [CrossRef]
- Drevillon, B.; Kumar, S.; Roca i Cabarrocas, P.; Siefert, J. I nsitu investigation of the optoelectronic properties of transparent conducting oxide/amorphous silicon interfaces. Appl. Phys. Lett. 1989, 54, 2088–2090. [Google Scholar] [CrossRef]
- Campbell, C.T. Ultrathin metal films and particles on oxide surfaces: Structural, electronic and chemisorptive properties. Surf. Sci. Rep. 1997, 27, 1–111. [Google Scholar] [CrossRef]
- Wang, W.; Ngo, É.; Florea, I.; Foldyna, M.; Roca i Cabarrocas, P.; Maurice, J.-L. Room temperature growth of silica nanowires on top of ultrathin Si nanowires synthesized with Sn-Cu bimetallic seeds. Phys. Status Solidi A 2021, 218, 2100409. [Google Scholar] [CrossRef]

| Step I: 200 °C | Step II: 420 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Catalyst | Substrate | T (°C) | H2 Flow Rate (sccm) | P (mbar) | Power Density (mW/cm2) | T (°C) | H2 + SiH4 Flow Rate (sccm) | P (mbar) | Power Density (mW/cm2) |
| 1 | 1 nm In/1 nm Cu | carbon-coated gold TEM grid | 200 | 100 | 0.8 | 56.7 | ||||
| 2 | 200 | 100 | 0.8 | 0 | ||||||
| 3 | 200 | 100 | 0.8 | 56.7 | 420 | 100 + 5 | 1.42 | 17 | ||
| 4 | 200 | 100 | 0.8 | 56.7 | 420 | 0 | 1.42 | 0 | ||
| 5 | 420 | 100 | 0.8 | 56.7 | ||||||
| 6 | 0.6 nm In/0.2 nm Cu | SiC membrane | 250 | 30 | Power of 50 W | 370 | 30 + 1.5 | 3 × 10−2 | Power of 50 W | |
| 7 | 1 nm In/1 nm Cu | Si (001) | Growth parameters: same as group 3 | |||||||
| 8 | 0.1 nm In/0.1 nm Cu | |||||||||
| 9 | 0.1 nm In | |||||||||
| 10 | 0.05 nm In/0.05 nm Cu | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Ngo, É.; Bulkin, P.; Zhang, Z.; Foldyna, M.; Roca i Cabarrocas, P.; Johnson, E.V.; Maurice, J.-L. Evolution of Cu-In Catalyst Nanoparticles under Hydrogen Plasma Treatment and Silicon Nanowire Growth Conditions. Nanomaterials 2023, 13, 2061. https://doi.org/10.3390/nano13142061
Wang W, Ngo É, Bulkin P, Zhang Z, Foldyna M, Roca i Cabarrocas P, Johnson EV, Maurice J-L. Evolution of Cu-In Catalyst Nanoparticles under Hydrogen Plasma Treatment and Silicon Nanowire Growth Conditions. Nanomaterials. 2023; 13(14):2061. https://doi.org/10.3390/nano13142061
Chicago/Turabian StyleWang, Weixi, Éric Ngo, Pavel Bulkin, Zhengyu Zhang, Martin Foldyna, Pere Roca i Cabarrocas, Erik V. Johnson, and Jean-Luc Maurice. 2023. "Evolution of Cu-In Catalyst Nanoparticles under Hydrogen Plasma Treatment and Silicon Nanowire Growth Conditions" Nanomaterials 13, no. 14: 2061. https://doi.org/10.3390/nano13142061