A Strategy for Tuning the Structure, Morphology, and Magnetic Properties of MnFe2O4/SiO2 Ceramic Nanocomposites via Mono-, Di-, and Trivalent Metal Ion Doping and Annealing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis
2.3. Characterization
3. Results and Discussion
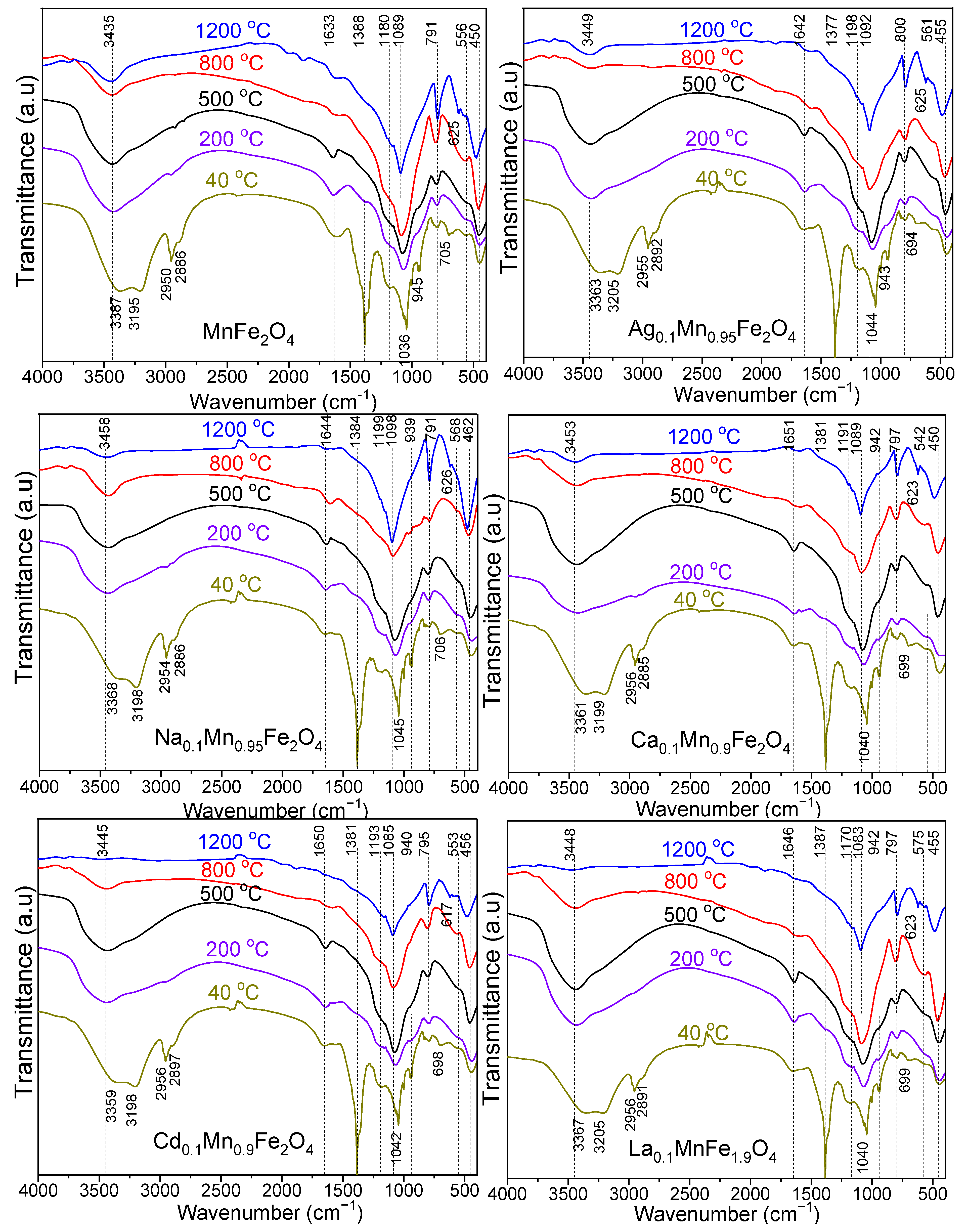
3.1. FT–IR Analysis
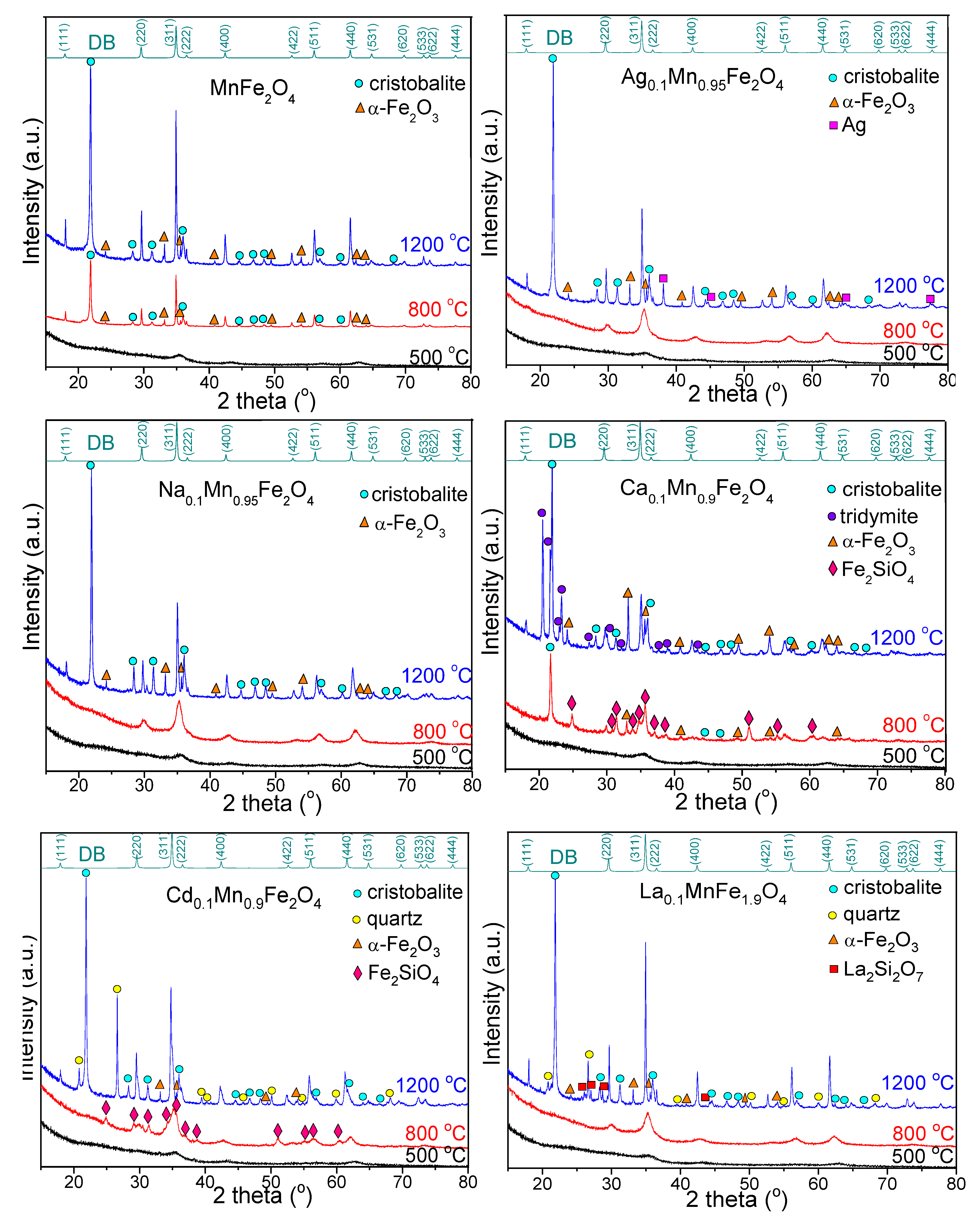
3.2. Structural Analysis
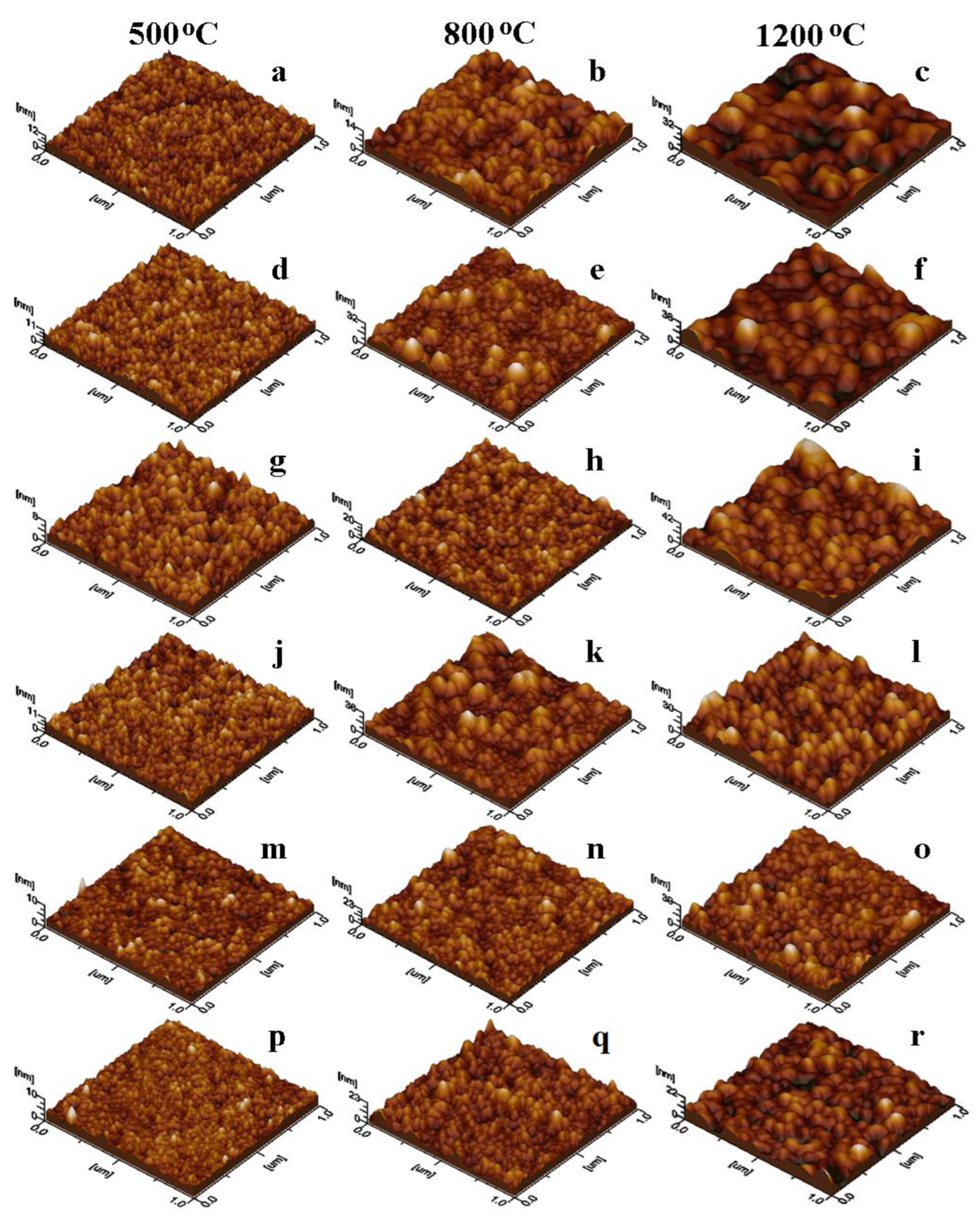
3.3. Morphological Analysis
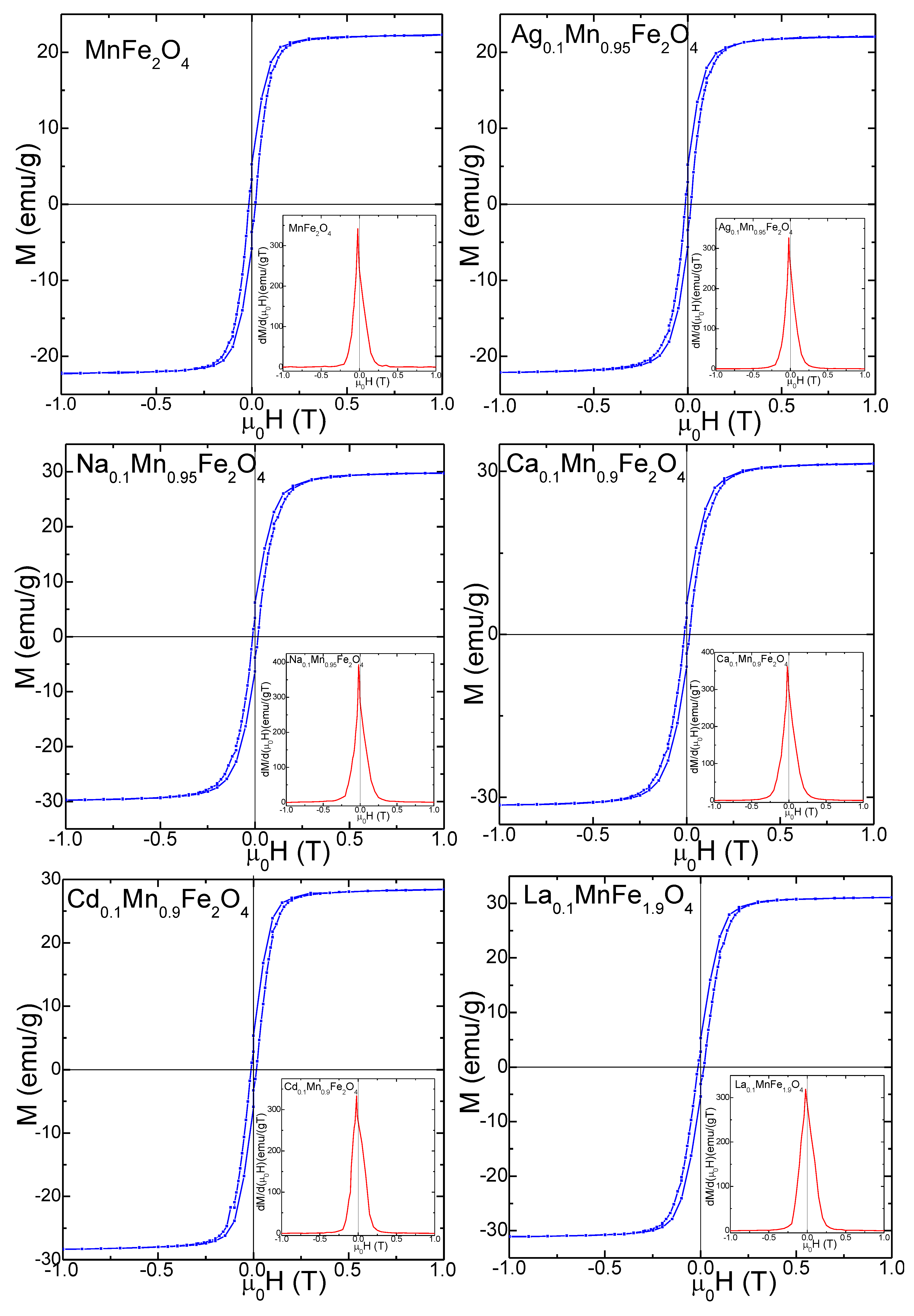
3.4. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhlaghi, N.; Najafpour-Darzi, G. Manganese ferrite (MnFe2O4) nanoparticles: From synthesis to application—A review. J. Ind. Eng. Chem. 2021, 103, 292–304. [Google Scholar] [CrossRef]
- Dippong, T.; Deac, I.G.; Cadar, O.; Levei, E.A. Effect of silica embedding on the structure, morphology and magnetic behavior of (Zn0.6Mn0.4Fe2O4)δ/(SiO2)(100−δ) nanoparticles. Nanomaterials 2021, 11, 2232. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Deac, I.G.; Petean, I.; Cadar, O. Dependence of structural, morphological and magnetic properties of manganese ferrite on Ni-Mn substitution. Int. J. Mol. Sci. 2022, 23, 3097. [Google Scholar] [CrossRef] [PubMed]
- Dippong, T.; Levei, E.A.; Cadar, O.; Deac, I.G.; Lazar, M.; Borodi, G.; Petean, I. Effect of amorphous SiO2 matrix on structural and magnetic properties of Cu0.6Co0.4Fe2O4/SiO2 nanocomposites. J. Alloys Compd. 2020, 849, 156695. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O. Investigation of structural, morphological and magnetic properties of MFe2O4 (M = Co, Ni, Zn, Cu, Mn) obtained by thermal decomposition. Int. J. Mol. Sci. 2022, 23, 8483. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, H.; Zhang, C.; Feng, J.; Pu, S.; Ren, Y.; Wang, Y. Enhanced catalytic ozonation treatment of dibutyl phthalate enabled by porous magnetic Ag-doped ferrospinel MnFe2O4 materials: Performance and mechanism. Chem. Eng. J. 2018, 354, 42–52. [Google Scholar] [CrossRef]
- Baig, M.M.; Zulfiqar, S.; Yousuf, M.A.; Touqeer, M.; Ullah, S.; Agboola, P.O.; Warsi, M.F.; Shakir, I. Structural and photocatalytic properties of new rare earth La3+ substituted MnFe2O4 ferrite nanoparticles. Ceram. Int. 2020, 46, 23208–23217. [Google Scholar] [CrossRef]
- Kalaiselvan, C.R.; Laha, S.S.; Somvanshi, S.B.; Tabish, T.A.; Thorat, N.D.; Sahu, N.K. Manganese ferrite (MnFe2O4) nanostructures for cancer theranostics. Coord. Chem. Rev. 2022, 473, 214809. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Liu, D.; Li, X.; Liang, H. Enhanced catalytic performance of Cu-doped MnFe2O4 magnetic ferrites: Tetracycline hydrochloride attacked by superoxide radicals efficiently in a strong alkaline environment. Chemosphere 2022, 297, 134154. [Google Scholar] [CrossRef]
- Shayestefar, M.; Mashreghi, A.; Hasani, S.; Rezvan, M.T. Optimization of the structural and magnetic properties of MnFe2O4 doped by Zn and Dy using Taguchi method. J. Magn. Magn. Mater. 2022, 541, 168390. [Google Scholar] [CrossRef]
- Angadi, J.V.; Shigihalli, N.B.; Batoo, K.M.; Hussain, S.; Sekhar, E.V.; Wang, S.S.; Kubrin, P. Synthesis and study of transition metal doped ferrites useful for permanent magnet and humidity sensor applications. J. Magn. Magn. Mater. 2022, 564, 170088. [Google Scholar] [CrossRef]
- Debnath, S.; Das, R. Study of the optical properties of Zn doped Mn spinel ferrite nanocrystals shows multiple emission peaks in the visible range—A promising soft ferrite nanomaterial for deep blue LED. J. Mol. Struct. 2020, 1199, 127044. [Google Scholar] [CrossRef]
- Mallesh, S.; Srinivas, V. A comprehensive study on thermal stability and magnetic properties of MnZn-ferrite nanoparticles. J. Magn. Magn. Mater. 2019, 475, 290–303. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liang, J.; Huang, C.; Li, W. Synthesis and antibacterial activity of magnetic MnFe2O4/Ag composite particles. Nanosci. Nanotechnol. Lett. 2014, 6, 385–391. [Google Scholar] [CrossRef]
- Andrade, R.G.D.; Ferreira, D.; Veloso, S.R.S.; Santos-Pereira, C.; Castanheira, E.M.S.; Côrte-Real, M.; Rodrigues, L.R. Synthesis and cytotoxicity assessment of citrate-coated calcium and manganese ferrite nanoparticles for magnetic hyperthermia. Pharmaceutics 2022, 14, 2694. [Google Scholar] [CrossRef] [PubMed]
- Dippong, T.; Levei, E.A.; Leostean, C.; Cadar, O. Impact of annealing temperature and ferrite content embedded in SiO2 matrix on the structure, morphology and magnetic characteristics of (Co0.4Mn0.6Fe2O4)δ (SiO2)100−δ nanocomposites. J. Alloys Compd. 2021, 868, 159203. [Google Scholar] [CrossRef]
- Kozerozhets, I.V.; Panasyuk, G.P.; Semenov, E.A.; Avdeeva, V.V.; Danchevskaya, M.N.; Simonenko, N.P.; Vasiliev, M.G.; Kozlova, L.O.; Ivakin, I.D. Recrystallization of nanosized boehmite in an aqueous medium. Powder Technol. 2023, 416, 118030. [Google Scholar] [CrossRef]
- Kang, S.; Wang, C.; Chen, J.; Meng, T.; Jiaqiang, E. Progress on solvo/hydrothermal synthesis and optimization of the cathode materials of lithium-ion battery. J. Energy Storage 2023, 67, 107515. [Google Scholar] [CrossRef]
- Ravindra, A.V.; Ju, S. Mesoporous CoFe2O4 nanocrystals: Rapid microwave-hydrothermal synthesis and effect of synthesis temperature on properties. Mater. Chem. Phys. 2023, 303, 127818. [Google Scholar] [CrossRef]
- Baublytė, M.; Vailionis, A.; Sokol, D.; Skaudžius, R. Enhanced functionality of Scots pine sapwood by in situ hydrothermal synthesis of GdPO4·H2O:Eu3+ Composites in woods matrix. Ceram. Int. 2023, in press. [CrossRef]
- Marques de Gois, M.; de Alencar Souza, L.W.; Nascimento Cordeiro, C.H.; Tavares da Silva, I.B.; Soares, J.M. Study of morphology and magnetism of MnFe2O4–SiO2 composites. Ceram. Int. 2023, 49, 11552–11562. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O. Formation, structure and magnetic properties of MFe2O4@SiO2 (M = Co, Mn, Zn, Ni, Cu) nanocomposites. Materials 2021, 14, 1139. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Zhang, L.; Wang, J.; Feng, X.; Zhao, L.; Rao, H.; Wang, Y.; Dai, J. Preparation of SiO2-MnFe2O4 composites via one-pot hydrothermal synthesis method and microwave absorption investigation in S-band. Molecules 2019, 24, 2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Ma, S.; Sun, J.; Xia, C.; Liu, C.; Wang, Z.; Zhao, X.; Gao, F.; Gong, Q.; Song, B.; et al. Manganese ferrite nanoparticle micellar nanocomposites as MRI contrast agent for liver imaging. Biomaterials 2009, 30, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Kurtan, U.; Admir, M.; Yildiz, A.; Baykal, A. Synthesis and magnetically recyclable MnFe2O4@SiO2@Ag nanocatalyst: Its high catalytic performances for azo dyes and nitro compunds reduction. Appl. Surf. Sci. 2016, 376, 16–25. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Zhang, X.J.; Wang, S.W.; Wang, G.S.; Yin, P.G. Enhanced microwave absorption material of ternary nanocomposites based on MnFe2O4@SiO2, polyaniline and polyvinylidene fluoride. RSC Adv. 2006, 6, 88104–88109. [Google Scholar] [CrossRef]
- Shirzadi-Ahodashti, M.; Ebrahimzadeh, M.A.; Ghoreishi, S.M.; Naghizadeh, A.; Mortazavi-Derazkola, S. Facile and eco-benign synthesis of a novel MnFe2O4@SiO2@Au magnetic nanocomposite with antibacterial properties and enhanced photocatalytic activity under UV and visible-light irradiations. Appl. Organomet. Chem. 2020, 34, e5614. [Google Scholar]
- Kavkhani, R.; Hajalilou, A.; Abouzari-Lotf, E.; Ferreira, L.P.; Cruz, M.M.; Yusefi, M.; Arvini, E.; Ogholbey, A.B.; Ismail, U.N. CTAB assisted synthesis of MnFe2O4@ SiO2 nanoparticles for magnetic hyperthermia and MRI application. Mater. Today 2023, 31, 103412. [Google Scholar] [CrossRef]
- Asghar, K.; Qasim, M.; Das, D. Preparation and characterization of mesoporous magnetic MnFe2O4@mSiO2 nanocomposite for drug delivery application. Mater. Today Proc. 2020, 26, 87–93. [Google Scholar] [CrossRef]
- Desai, H.B.; Hathiya, L.J.; Joshi, H.H.; Tanna, A.R. Synthesis and characterization of photocatalytic MnFe2O4 nanoparticles. Mater. Today Proc. 2020, 21, 1905–1910. [Google Scholar] [CrossRef]
- Kozerozhets, V.; Semenov, E.A.; Avdeeva, V.V.; Ivakin, Y.D.; Kupreenko, S.Y.; Egorov, A.V.; Kholodkova, A.A.; Vasilev, M.G.; Kozlova, L.O.; Panasyuk, G.P. State and forms of water in dispersed aluminum oxides and hydroxides. Ceram. Int. 2023, in press. [CrossRef]
- Calvin, J.J.; Rosen, P.F.; Ross, N.L.; Navrotsky, A.; Woodfield, B.F. Review of surface water interactions with metal oxide nanoparticles. J. Mater. Res. 2019, 34, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Balarabe, B.Y.; Bowmik, S.; Ghosh, A.; Maity, P. Photocatalytic dye degradation by magnetic XFe2O3 (X: Co, Zn, Cr, Sr, Ni, Cu, Ba, Bi, and Mn) nanocomposites under visible light: A cost efficiency comparison. J. Magn. Magn. Mater. 2022, 562, 169823. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Toloman, D.; Barbu-Tudoran, L.; Cadar, O. Investigation on the formation, structural and photocatalytic properties of mixed Mn-Zn ferrites nanoparticles embedded in SiO2 matrix. J. Alloys Compd. 2021, 158, 105281. [Google Scholar] [CrossRef]
- Ajeesha, T.L.; Manikandan, A.; Anantharaman, A.; Jansi, S.; Durka, M.; Almessiere, M.A.; Slimani, Y.; Baykal, A.; Asiri, A.M.; Kasmery, H.A.; et al. Structural investigation of Cu doped calcium ferrite (Ca1−xCuxFe2O4; x = 0, 0.2, 0.4, 0.6, 0.8, 1) nanomaterials prepared by co-precipitation method. J. Mater. Res. Technol. 2022, 18, 705–719. [Google Scholar] [CrossRef]
- Liandi, A.R.; Cahyana, A.H.; Kusumah, A.J.F.; Lupitasari, A.; Alfariza, D.N.; Nuraini, R.; Sari, R.W.; Kusumasari, F.C. Recent trends of spinel ferrites (MFe2O4: Mn, Co, Ni, Cu, Zn) applications as an environmentally friendly catalyst in multicomponent reactions: A review. Case Stud. Chem. Environ. Eng. 2023, 7, 100303. [Google Scholar] [CrossRef]
- Rout, J.; Choudhary, R.N.P.; Sharma, H.B.; Shannigrahi, S.R. Effect of co-substitutions (Ca–Mn) on structural, electrical and magnetic characteristics of bismuth ferrite. Ceram. Int. 2015, 41, 9078–9087. [Google Scholar] [CrossRef]
- Torre, F.; Sanchez, T.A.; Doppiu, S.; Bengoechea, M.O.; Ergueta, P.L.A.; Palomo del Barrio, E. Effect of atomic substitution on the sodium manganese ferrite thermochemical cycle for hydrogen production. Mater. Today Energy 2022, 29, 101094. [Google Scholar] [CrossRef]
- Yadav, B.S.; Vishwakarma, A.K.; Singh, A.K.; Kumar, N. Oxygen vacancies induced ferromagnetism in RF-sputtered and hydrothermally annealed zinc ferrite (ZnFe2O4) thin films. Vacuum 2023, 207, 111617. [Google Scholar] [CrossRef]
- Sharma, A.D.; Sharma, H.B. Structural, optical, and dispersive parameters of (Gd, Mn) co-doped BiFeO3 thin film. Mater. Today Proc. 2022, 65, 2837–2843. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Şakar, B.C.; Kundakci, M.; Gürbulak, B.; Yıldırım, M. Analysis of magnesium ferrite and nickel doped magnesium ferrite thin films grown by spray pyrolysis. Mater. Today Proc. 2021, 46, 6920–6923. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C.; Morales, M.P. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef]
- Chavarría-Rubio, J.A.; Cortés-Hernández, D.A.; Garay-Tapia, A.M.; Hurtado-López, G.F. The role of lanthanum in the structural, magnetic and electronic properties of nanosized mixed manganese ferrites. J. Magn. Magn. Mater. 2022, 553, 169253. [Google Scholar] [CrossRef]
- Caruntu, D.; Caruntu, G.; O’Connor, C.J. Magnetic properties of variable-sized Fe3O4 nanoparticles synthesized from nonaqueous homogeneous solutions of polyols. J. Phys. D Appl. Phys. 2007, 40, 5801–5809. [Google Scholar] [CrossRef] [Green Version]


| Parameter | Temp (°C) | MnFe2O4 | Ag0.1Mn0.95Fe2O4 | Na0.1Mn0.95Fe2O4 | Ca0.1Mn0.9Fe2O4 | Cd0.1Mn0.9Fe2O4 | La0.1MnFe1.9O4 | Error |
|---|---|---|---|---|---|---|---|---|
| DXRD (nm) | 500 | 14.2 | 10.2 | 14.0 | 11.8 | 12.0 | 11.1 | ±1.3 |
| 800 | 16.7 | 14.7 | 15.9 | 15.3 | 16.3 | 16.2 | ±1.6 | |
| 1200 | 66.3 | 55.4 | 40.1 | 58.0 | 56.5 | 50.0 | ±5.5 | |
| DC (%) | 500 | 61.5 | 48.5 | 48.9 | 61.2 | 60.8 | 59.9 | ±5.0 |
| 800 | 70.2 | 62.0 | 69.4 | 66.9 | 63.9 | 68.0 | ±6.6 | |
| 1200 | 90.1 | 88.8 | 86.3 | 85.5 | 89.5 | 88.6 | ±8.7 | |
| a (Å) | 500 | 8.445 | 8.414 | 8.427 | 8.409 | 8.418 | 8.441 | ±0.01 |
| 800 | 8.485 | 8.457 | 8.462 | 8.443 | 8.467 | 8.478 | ±0.01 | |
| 1200 | 8.544 | 8.504 | 8.510 | 8.491 | 8.533 | 8.517 | ±0.01 | |
| V (Å3) | 500 | 602.3 | 595.7 | 598.4 | 594.6 | 596.5 | 601.4 | ±0.01 |
| 800 | 610.9 | 604.6 | 605.9 | 601.9 | 607.0 | 609.4 | ±0.01 | |
| 1200 | 623.7 | 615.0 | 616.3 | 612.2 | 621.3 | 617.8 | ±0.01 | |
| dA (Å) | 500 | 3.657 | 3.643 | 3.649 | 3.641 | 3.645 | 3.655 | ±0.01 |
| 800 | 3.674 | 3.662 | 3.664 | 3.656 | 3.666 | 3.671 | ±0.01 | |
| 1200 | 3.700 | 3.682 | 3.685 | 3.677 | 3.695 | 3.688 | ±0.01 | |
| dB (Å) | 500 | 2.986 | 2.975 | 2.979 | 2.973 | 2.976 | 2.984 | ±0.01 |
| 800 | 2.999 | 2.990 | 2.992 | 2.985 | 2.994 | 2.997 | ±0.01 | |
| 1200 | 3.021 | 3.007 | 3.009 | 3.002 | 3.017 | 3.011 | ±0.01 | |
| dp (g/cm3) | 500 | 4.133 | 4.375 | 4.187 | 4.298 | 4.340 | 4.388 | ±0.01 |
| 800 | 4.255 | 4.554 | 4.299 | 4.420 | 4.471 | 4.472 | ±0.01 | |
| 1200 | 4.334 | 4.633 | 4.474 | 4.549 | 4.555 | 4.577 | ±0.01 | |
| dXRD (g/cm3) | 500 | 5.087 | 5.322 | 5.110 | 5.119 | 5.264 | 5.278 | ±0.01 |
| 800 | 5.015 | 5.244 | 5.047 | 5.057 | 5.173 | 5.209 | ±0.01 | |
| 1200 | 4.912 | 5.155 | 4.962 | 4.972 | 5.054 | 5.138 | ±0.01 | |
| P (%) | 500 | 18.7 | 17.8 | 18.0 | 16.0 | 17.5 | 16.9 | ± 1.6 |
| 800 | 15.1 | 13.2 | 14.8 | 12.6 | 13.6 | 14.1 | ± 1.2 | |
| 1200 | 11.8 | 10.1 | 9.83 | 8.51 | 9.87 | 10.9 | ± 1.0 |
| Gel | Temperature (°C) | H (nm) | Rq (nm) | DAFM (nm) | Surface (nm2) |
|---|---|---|---|---|---|
| MnFe2O4 | 500 | 12 | 1.34 | 15 | 1012 |
| 800 | 14 | 1.60 | 18 | 1017 | |
| 1200 | 32 | 3.73 | 70 | 1022 | |
| Ag0.1Mn0.95Fe2O4 | 500 | 11 | 1.28 | 13 | 1019 |
| 800 | 32 | 3.08 | 16 | 1023 | |
| 1200 | 38 | 4.78 | 57 | 1035 | |
| Na0.1Mn0.95Fe2O4 | 500 | 8 | 0.92 | 17 | 1014 |
| 800 | 20 | 2.29 | 20 | 1031 | |
| 1200 | 42 | 5.25 | 45 | 1044 | |
| Ca0.1Mn0.9Fe2O4 | 500 | 11 | 1.29 | 12 | 1017 |
| 800 | 36 | 3.81 | 18 | 1027 | |
| 1200 | 30 | 4.17 | 60 | 1039 | |
| Cd0.1Mn0.9Fe2O4 | 500 | 10 | 0.50 | 14 | 1009 |
| 800 | 23 | 2.41 | 18 | 1037 | |
| 1200 | 39 | 3.87 | 58 | 1054 | |
| La0.1MnFe1.9O4 | 500 | 10 | 0.54 | 13 | 1014 |
| 800 | 23 | 2.40 | 19 | 1030 | |
| 1200 | 22 | 2.28 | 55 | 1022 | |
| Error | – | ± 1.0 | ± 0.20 | ± 5.0 | ±5.0 |
| Parameter | Temp (°C) | MnFe2O4 | Ag0.1Mn0.95Fe2O4 | Na0.1Mn0.95Fe2O4 | Ca0.1Mn0.9Fe2O4 | Cd0.1Mn0.9Fe2O4 | La0.1MnFe1.9O4 | Errors |
|---|---|---|---|---|---|---|---|---|
| Ms (emu/g) | 800 | 21.5 | 21.0 | 6.4 | 19.5 | 17.5 | 20.1 | ±1.1 |
| 1200 | 23.3 | 26.3 | 30.7 | 32.2 | 29.9 | 31.7 | ±2.8 | |
| MR (emu/g) | 800 | 5.9 | 4.4 | 1.0 | 4.6 | 4.4 | 3.4 | ±0.4 |
| 1200 | 6.5 | 5.4 | 6.2 | 5.8 | 5.5 | 5.6 | ±0.6 | |
| Hc (Oe) | 800 | 116 | 112 | 100 | 105 | 110 | 113 | ±10 |
| 1200 | 160 | 145 | 120 | 125 | 140 | 156 | ±15 | |
| K·103 (erg/cm3) | 800 | 1.57 | 1.48 | 0.40 | 1.29 | 1.21 | 1.43 | ±0.10 |
| 1200 | 2.34 | 2.39 | 3.68 | 2.53 | 2.63 | 3.11 | ±0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dippong, T.; Levei, E.A.; Petean, I.; Deac, I.G.; Cadar, O. A Strategy for Tuning the Structure, Morphology, and Magnetic Properties of MnFe2O4/SiO2 Ceramic Nanocomposites via Mono-, Di-, and Trivalent Metal Ion Doping and Annealing. Nanomaterials 2023, 13, 2129. https://doi.org/10.3390/nano13142129
Dippong T, Levei EA, Petean I, Deac IG, Cadar O. A Strategy for Tuning the Structure, Morphology, and Magnetic Properties of MnFe2O4/SiO2 Ceramic Nanocomposites via Mono-, Di-, and Trivalent Metal Ion Doping and Annealing. Nanomaterials. 2023; 13(14):2129. https://doi.org/10.3390/nano13142129
Chicago/Turabian StyleDippong, Thomas, Erika Andrea Levei, Ioan Petean, Iosif Grigore Deac, and Oana Cadar. 2023. "A Strategy for Tuning the Structure, Morphology, and Magnetic Properties of MnFe2O4/SiO2 Ceramic Nanocomposites via Mono-, Di-, and Trivalent Metal Ion Doping and Annealing" Nanomaterials 13, no. 14: 2129. https://doi.org/10.3390/nano13142129






