Microstructure and Superconducting Properties of Bi-2223 Synthesized via Co-Precipitation Method: Effects of Graphene Nanoparticle Addition
Abstract
:1. Introduction
2. Materials and Methods
3. Results
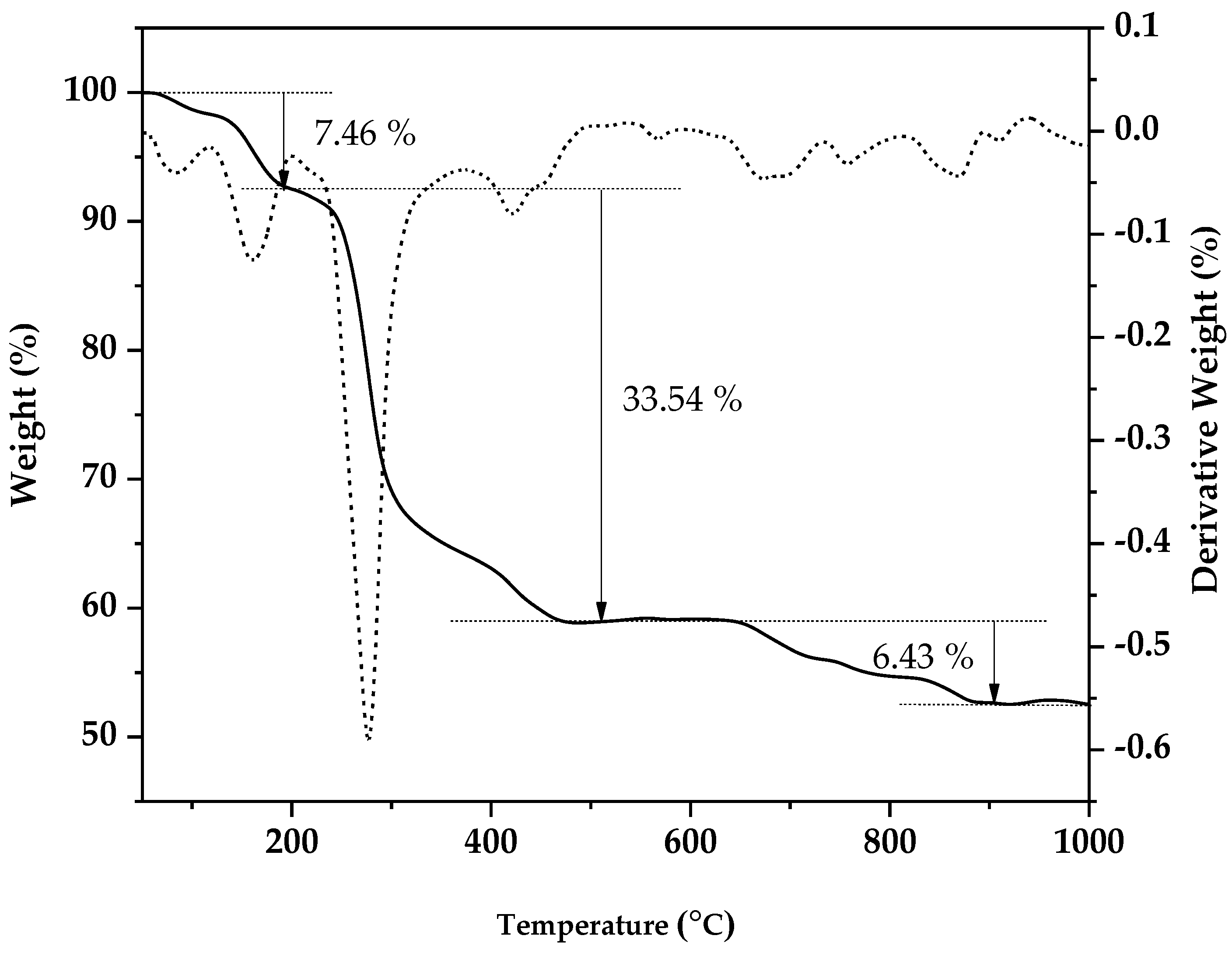
3.1. Thermogravimetric Analysis
3.2. X-ray Diffraction (XRD) Analysis
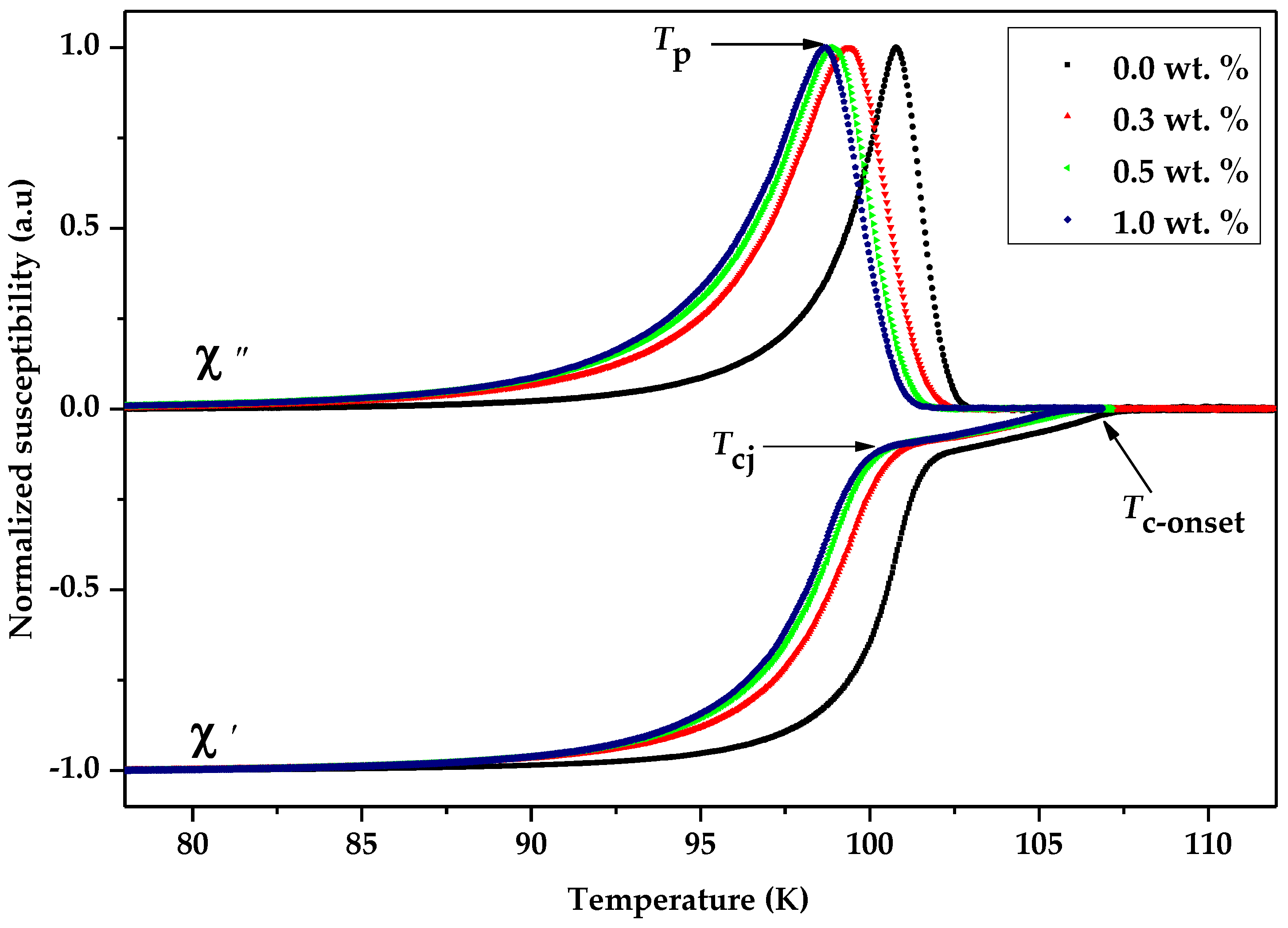
3.3. Critical Temperature, Tc Analysis
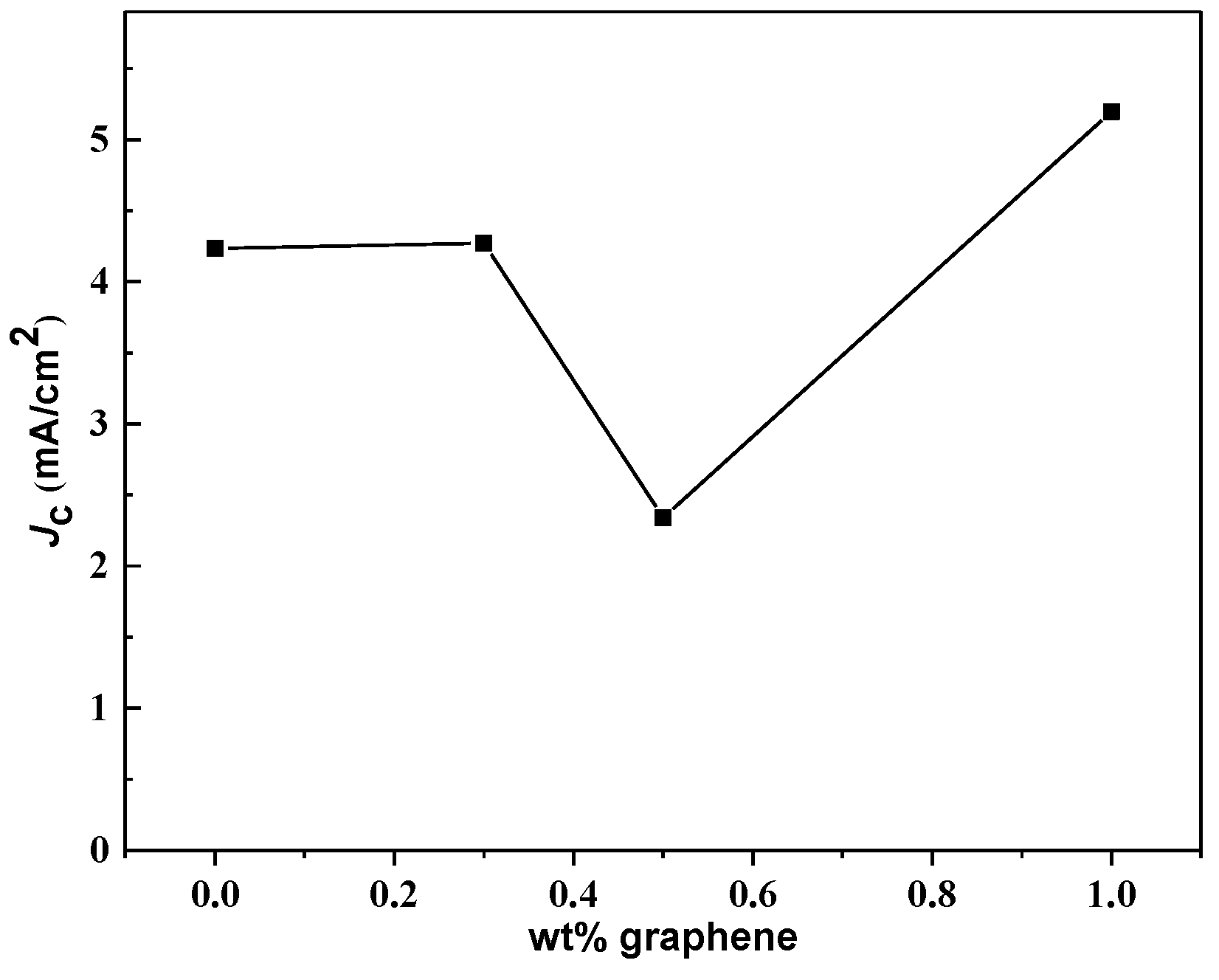
3.4. Critical Current Density, Jc Analysis
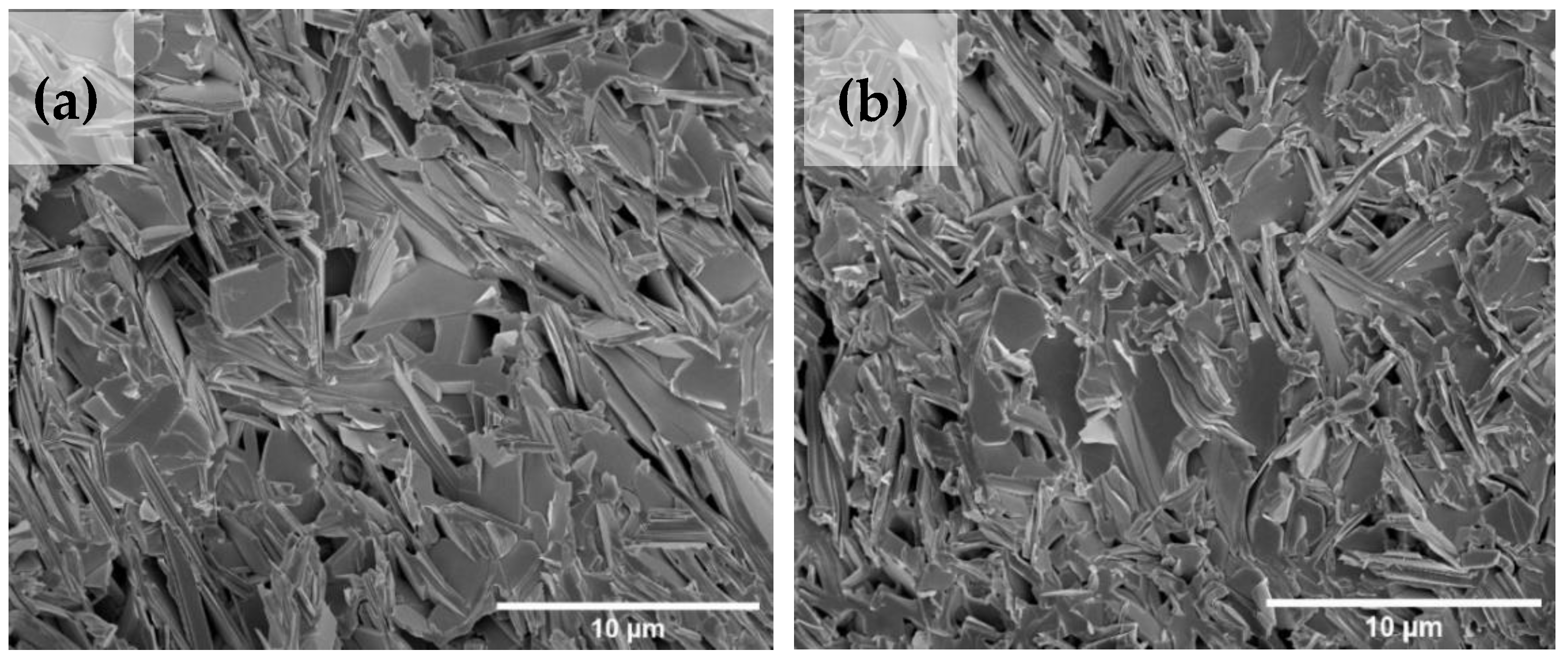
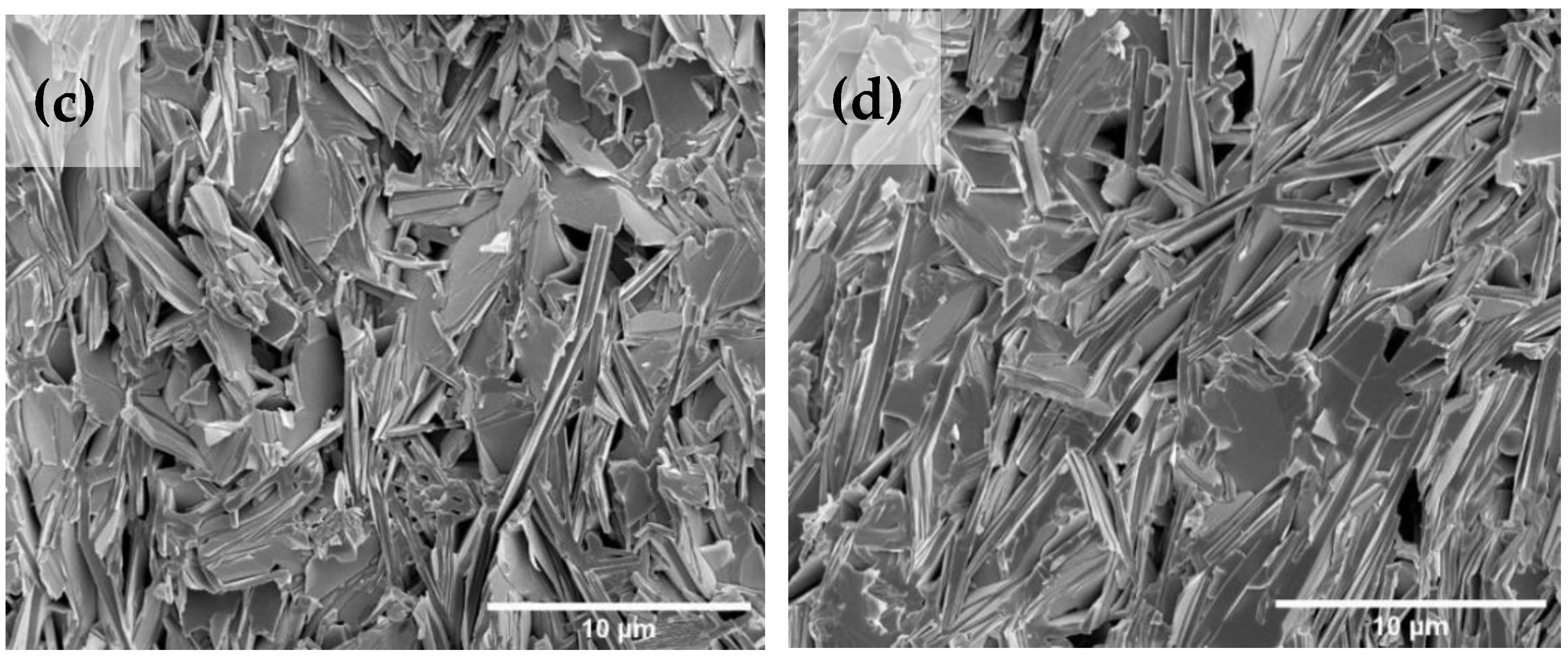
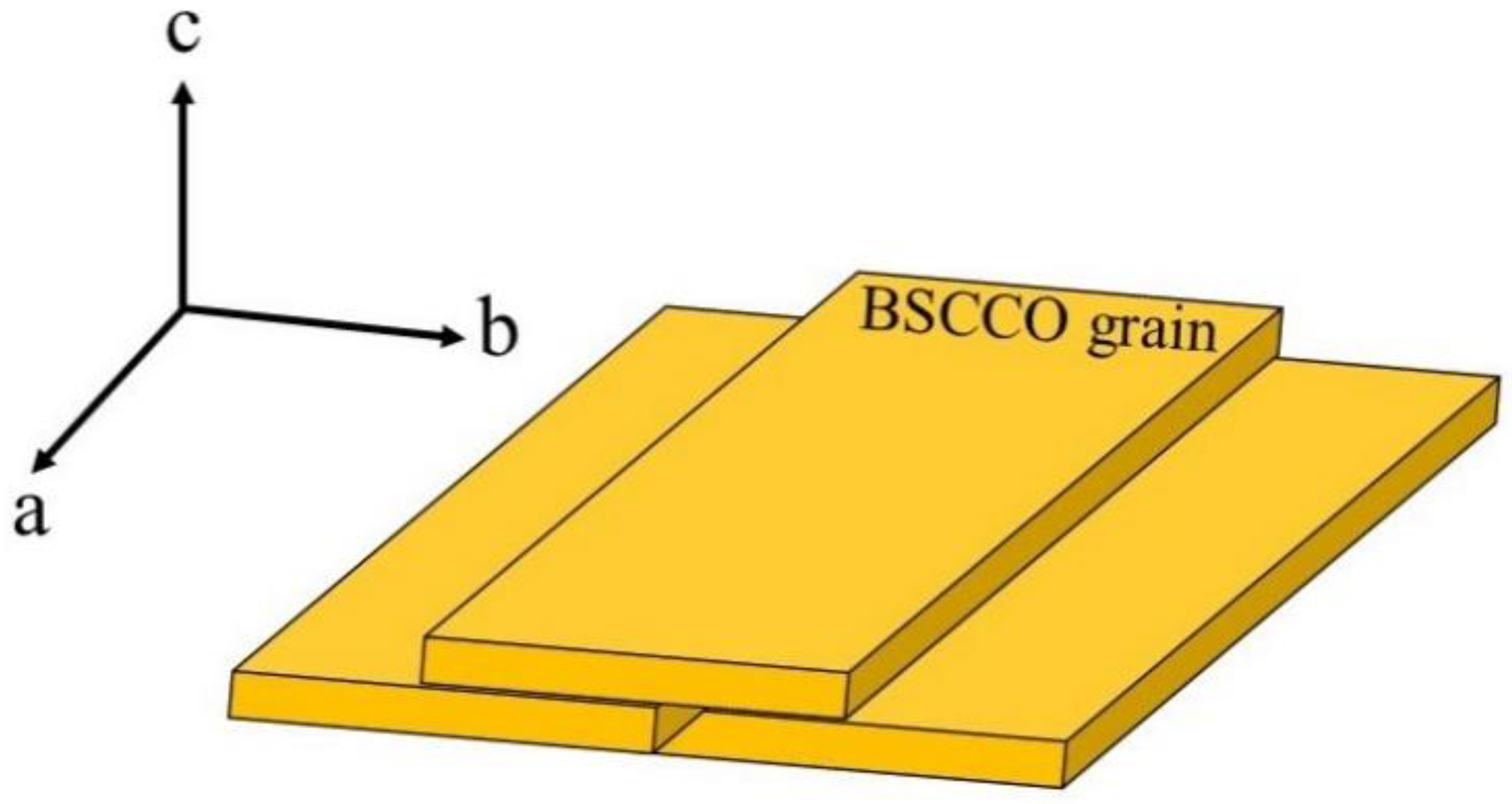
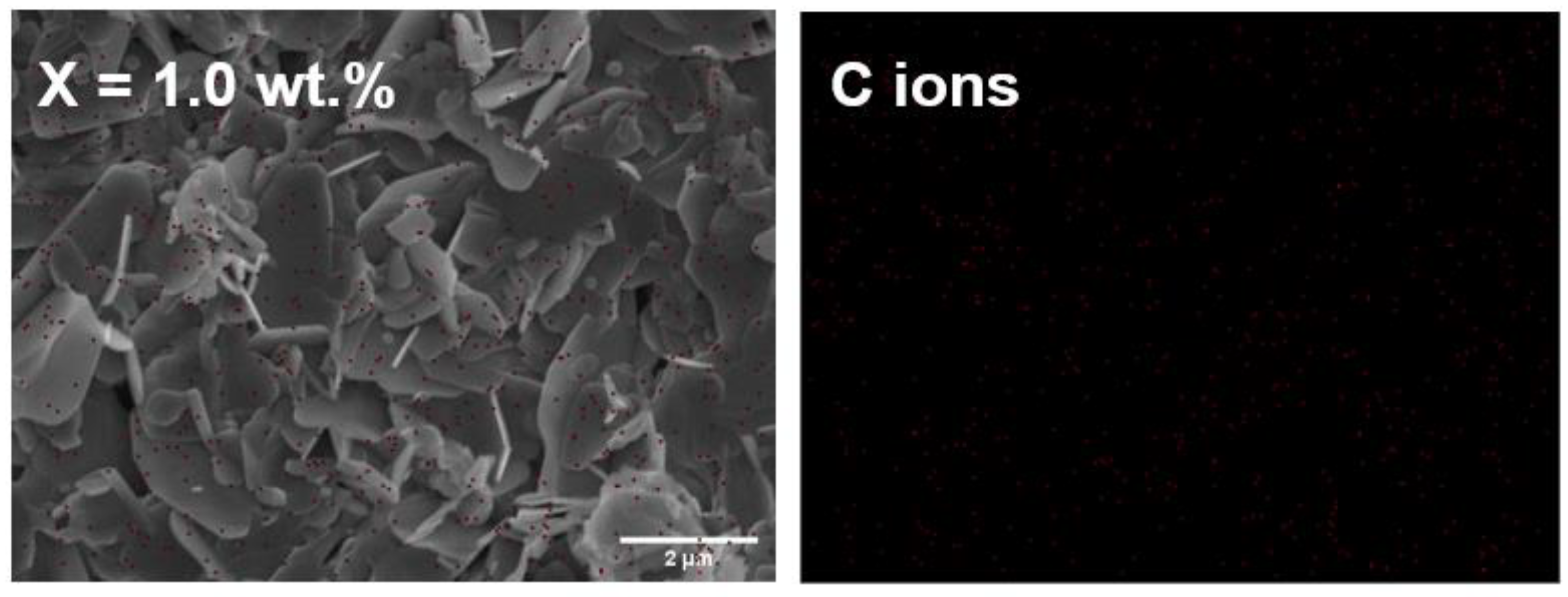
3.5. Microstructure Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maeda, H.; Tanaka, Y.; Fukutomi, M.; Asano, T.A. New High-Tc Oxide Superconductor without a Rare Earth Element. In Ten Years of Superconductivity: 1980–1990. Perspectives in Condensed Matter Physics; Ott, H.R., Ed.; A Critical Reprint Series; Springer: Berlin/Heidelberg, Germany, 1993; pp. 303–304. [Google Scholar]
- Tarascon, J.M.; McKinnon, W.R.; Barboux, P.; Hwang, D.M.; Bagley, B.G.; Greene, L.H. Preparation, structure, and properties of the superconducting compound series Bi2Sr2Can-1CunOy with n=1,2, and 3. Phys. Rev. B Condens. Matter. 1988, 38, 8885–8892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, J.O.; Charles, P.P.J. The New Superconductors; Stuart Wolf Naval Research Laboratory: Washington, DC, USA, 1996. [Google Scholar]
- Hayashi, K. Commercialization of Bi-2223 Superconducting Wires and Their Applications. Sei Tech. Rev. 2020, 91, 68–74. [Google Scholar]
- Kikuchi, M.; Ayai, N.; Ishida, T.; Tatamidani, K.; Hayashi, K.; Kobayashi, S. Development of new types of DI-BSCCO wire. Sei Tech. Rev. 2008, 66, 73–80. [Google Scholar]
- Yumura, H.; Ashibe, Y.; Itoh, H.; Ohya, M.; Watanabe, M.; Masuda, T. Phase II of the Albany HTS cable project. IEEE Trans. Appl. Supercond. 2009, 19, 1698–1701. [Google Scholar] [CrossRef]
- Yumura, H.; Ashibe, Y.; Ohya, M.; Itoh, H.; Watanabe, M.; Masuda, T. Update of Yokohama HTS cable project. IEEE Trans. Appl. Supercond. 2013, 23, 5402306. [Google Scholar] [CrossRef]
- Elschner, S.; Demencik, E.; Douine, B.; Grilli, F.; Kudymow, A.; Stemmle, M. New experimental method for investigating AC losses in concentric HTS power cables. IEEE Trans. Appl. Supercond. 2014, 25, 5900105. [Google Scholar] [CrossRef] [Green Version]
- Sytnikov, V.E.; Bemert, S.E.; Ivanov, Y.V.; Kopylov, S.I.; Krivetskiy, I.V.; Rimorov, D.S. HTS DC Cable line project: On-Going activities in Russia. IEEE Trans. Appl. Supercond. 2013, 23, 5401904. [Google Scholar] [CrossRef]
- Tomita, M.; Fukumoto, Y.; Ishihara, A.; Suzuki, K.; Akasaka, T.; Caron, H. Train Running Test Transmitted by Superconducting Feeder Cable and Study as an Example of Line in Japan and France. IEEE Trans. Appl. Supercond. 2019, 30, 5400107. [Google Scholar] [CrossRef]
- Sato, K.I.; Kobayashi, S.I.; Nakashima, T. Present status and future perspective of bismuth-based high-temperature superconducting wires realizing application systems. Jpn. J. Appl. Phys. 2011, 51, 010006. [Google Scholar] [CrossRef]
- Yamasaki, H.; Endo, K.; Kosaka, S.; Utneda, M.; Misawa, S.; Yoshida, S. Magnetic-Field Angle Dependence of the Critical Current Density in High-Quality Bi2Sr2Ca2Cu30X Thin Films. IEEE Trans. Appl. Supercond. 1993, 1, 1536–1539. [Google Scholar] [CrossRef]
- Patnaik, S.; Feldmann, D.M.; Polyanskii, A.A.; Yuan, Y.; Jiang, J.; Cai, X.Y. Local Measurement of Current Density by Magneto-Optical Current Reconstruction in Normally and Overpressure Processed Bi-2223 Tapes. IEEE Trans. Appl. Supercond. 2003, 13, 2930–2933. [Google Scholar] [CrossRef]
- Arlina, A.; Halim, S.A.; Awang Kechik, M.M.; Chen, S.K. AC Losses in YBCO-Added Bi1.6Pb0.4Sr2Ca2Cu3Oδ Superconductors. J. Supercond. Nov. Magn. 2015, 28, 1953–1958. [Google Scholar] [CrossRef]
- Bagiah, H.; Halim, S.A.; Chen, S.K.; Lim, K.P.; Awang Kechik, M.M. Effects of rare earth nanoparticles (M = Sm2O3, Ho2O3, Nd2O3) addition on the microstructure and superconducting transition of Bi1.6Pb0.4Sr2Ca2Cu3O10+δ Ceramics. Sains Malays. 2016, 45, 643–651. [Google Scholar]
- Azman, N.J.; Abdullah, H.; Abd-Shukor, R. Transport critical current density of (Bi1.6Pb0.4)Sr2Ca2Cu3O10 ceramic superconductor with different nanosized Co3O4 addition. Adv. Condens. Matter Phys. 2014, 2014, 498747. [Google Scholar] [CrossRef]
- Abd-Shukor, R.; Awang Kechik, M.M.; Halim, S.A. Transport critical current density of Bi-Sr-Ca-Cu-O/Ag superconductor tapes with addition of Fe3O4 as flux pinning center. J. Phys. Conf. Ser. 2008, 97, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Khalid, N.A.; Kong, W.; Kong, I.; Kong, C.; Awang Kechik, M.M.; Abd-Shukor, R. Significance of Cobalt Ferrite Nanoparticles on Superconducting Properties of Tl-1212 High Temperature Superconductor. Nano Hybrids Compos. 2021, 31, 1–5. [Google Scholar] [CrossRef]
- Khalid, N.A.; Awang Kechik, M.M.; Baharuddin, N.A.; Chen, S.K.; Baqiah, H.; Lim, K.P. Carbon nanofibers addition on transport and superconducting properties of bulk YBa2Cu3O7−δ material prepared via co-precipitation. J. Mater. Sci. Mater. Electron. 2020, 31, 16983–16990. [Google Scholar] [CrossRef]
- Wei, K.; Abd-Shukor, R. Superconducting and transport properties of (Bi-Pb)-Sr-Ca-Cu-O with nano-Cr2O3 additions. J. Electron. Mater. 2007, 36, 1648–1651. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.J.; Shin, D.; Ahn, J.H.; Hong, B.H. Towards industrial applications of graphene electrodes. Phys. Scr. 2012, T146, 014024. [Google Scholar] [CrossRef]
- Sato, S. Application of graphene to electronic devices. In Proceedings of the International Workshop on Active-Matrix Flatpanel Displays and Devices (AM-FPD), Kyoto, Japan, 4–7 July 2017; pp. 90–93. [Google Scholar]
- Singh, S.; Hasan, M.R.; Sharma, P.; Narang, J. Graphene nanomaterials: The wondering material from synthesis to applications. Sens. Int. 2022, 3, 100190. [Google Scholar] [CrossRef]
- Sahoo, B.; Routray, K.L.; Samal, D.; Behera, D. Effect of artificial pinning centers on YBCO high temperature superconductor through substitution of graphene nano-platelets. Mater. Chem. Phys. 2019, 223, 784–788. [Google Scholar] [CrossRef]
- Maio, A.; Pibiri, I.; Morreale, M.; La Mantia, F.P.; Scaffaro, R. An Overview of Functionalized Graphene Nanomaterials for Advanced Applications. Nanomaterials 2021, 11, 1717. [Google Scholar] [CrossRef] [PubMed]
- Dadras, S.; Falahati, S.; Dehghani, S. Effects of graphene oxide doping on the structural and superconducting properties of YBa2Cu3O7−δ. Phys. C Supercond. Appl. 2018, 548, 65–67. [Google Scholar] [CrossRef]
- Shoushtari, M.Z.; Akbari, M.; Hajati, Y. Study of YBa2Cu3O7−δ Superconductor/Graphene Oxide Composite. J. Supercond. Nov. Magn. 2018, 31, 2733–2739. [Google Scholar] [CrossRef]
- Falahati, S.; Dadras, S.; Mosqueira, J. Investigation of the Magnetic and Transport Properties of YBa2Cu3O7-δ High-Temperature Superconductor Doped with Graphene Oxide. J. Supercond. Nov. Magn. 2019, 32, 3755–3760. [Google Scholar] [CrossRef]
- Kamarudin, A.N.; Awang Kechik, M.M.; Abdullah, S.N.; Baqiah, H.; Chen, S.K.; Karim, M.K.A.; Ramli, A.; Lim, K.P.; Halim, S.A.; Miryala, M.; et al. Effect of Graphene Nanoparticles Addition on Superconductivity of YBa2Cu3O7~δ Synthesized via the Thermal Treatment Method. Coatings 2020, 12, 91. [Google Scholar] [CrossRef]
- Gaffoor, M.Z.; Jarvis, A.L.L.; Young, E.A.; Dorrell, D. Comparison of the Effect of Graphene and Graphene Oxide Doping on YBCO. J. Phys. Conf. Ser. 2020, 1559, 012028. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Liu, C.; Zhang, X. Improvement of the pinning property in YBa2Cu3O7-X films below 35 K by doping with graphene oxide. AIP Adv. 2019, 9, 015118. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.; Ing, K.; Hamdan, M.S.; Radiman, S.; Abd-Shukor, R. AC Susceptibility and Superconducting Properties of Graphene Added YBa2Cu3O7−d. J. Supercond. Nov. Magn. 2018, 31, 2699–2703. [Google Scholar] [CrossRef]
- Sahoo, B.; Singh, A.K.; Behera, D. Graphene oxide modified superconducting and elastic parameters of YBCO superconductor. Mater. Chem. Phys. 2020, 240, 122252. [Google Scholar] [CrossRef]
- Sudesh, K.N.; Das, S.; Bernhard, C.; Varma, G.D. Effect of graphene oxide doping on superconducting properties of bulk MgB2. Supercond. Sci. Technol. 2013, 26, 095008. [Google Scholar] [CrossRef] [Green Version]
- Sudesh, K.N.; Das, S.; Bernhard, C.; Varma, G.D. Enhanced superconducting properties of rare-earth oxides and graphene oxide added MgB2. Phys. C Supercond. Appl. 2014, 505, 32–38. [Google Scholar] [CrossRef]
- De Silva, K.S.B.; Gambhir, S.; Wang, X.L.; Xu, X.; Li, W.X.; Officer, D.L. The effect of reduced graphene oxide addition on the superconductivity of MgB2. J. Mater. Chem. A 2012, 22, 13941–13946. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.; Kong, I.; Awang Kechik, M.M.; Abd-Shukor, R. Effect of graphene addition on the transport critical current density of bulk (Tl0.85Cr0.15)Sr2CaCu2O7-δ superconductor. Mater. Today 2018, 5, 3176–3184. [Google Scholar] [CrossRef]
- Bilgili, Ö.; Yurddaskal, M. Effects of Graphene Oxide Doping on Magnetic and Structural Properties of Bi1.6Pb0.4Sr2Ca2Cu3Oy Superconductor. J. Electron. Mater. 2021, 50, 4999–5006. [Google Scholar] [CrossRef]
- Azhan, H.; Hawa, J.S.; Azman, K.; Hidayah, H.N.; Yusainee, S.Y.S. Superconducting properties of Bi1.6Pb0.4Sr2Ca2-xDyxCu3Oy prepared via co-precipitation method. Adv. Mater. Res. 2013, 622, 177–181. [Google Scholar]
- Azhan, H.; Hawa, J.S.; Azura, C.M.; Azman, K.; Syamsyir, S.A. Structural and electrical properties of high and low-density Yb-doped Bi(Pb)-2223 superconductor. J. Teknol. 2016, 78, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Hapipi, N.M.; Chen, S.K.; Shaari, A.H.; Awang Kechik, M.M.; Tan, K.B.; Lim, K.P. Superconductivity of Y2O3 and BaZrO3 nanoparticles co-added YBa2Cu3O7−δ bulks prepared using co-precipitation method. J. Mater. Sci. Mater. Elec. 2018, 29, 18684–18692. [Google Scholar] [CrossRef]
- Cui, L.J.; Zhang, P.X.; Li, J.S.; Yan, G.; Liu, X.H.; Feng, Y. Optimization of Bi-2223 precursor powder prepared by co-precipitation method. In Proceedings of the 2015 IEEE International Conference on Applied Superconductivity and Electromagnetic Devices (ASEMD), Shanghai, China, 20–23 November 2015; pp. 617–618. [Google Scholar]
- Hamadneh, I.; Halim, S.A.; Lee, C.K. Characterization of Bi1.6Pb0.4Sr2Ca2Cu3Oy ceramic superconductor prepared via coprecipitation method at different sintering time. J. Mater. Sci. 2006, 41, 5526–5530. [Google Scholar]
- Nur Haleeda, M.; Awang Kechik, M.M.; Abd-Shukor, R. Effect of Yb2O3 Nanoparticle Addition on Superconducting Properties of BSCCO (2223)/Ag Tapes by Acetate Precipitation Method. Pertanika J. Sci. Technol. 2016, 2, 1–11. [Google Scholar]
- Hapipi, N.M.; Lim, J.K.; Chen, S.K.; Lee, O.J.; Shaari, A.H.; Awang Kechik, M.M. Comparative study on AC susceptibility of YBa2Cu3O7−δ added with BaZrO3 nanoparticles prepared via solid-state and co-precipitation method. Crystals 2019, 9, 655. [Google Scholar] [CrossRef] [Green Version]
- Hapipi, N.M.; Chen, S.K.; Shaari, A.H.; Awang Kechik, M.M.; Tan, K.B.; Lim, K.P. AC Susceptibility of BaZrO3 Nanoparticles Added YBa2Cu3O7−δ Superconductor Prepared via Coprecipitation Method. J. Supercond. Nov. Magn. 2019, 32, 1191–1198. [Google Scholar] [CrossRef]
- Knauf, N.; Harnischmacher, J.; Müller, R.; Borowski, R.; Roden, B.; Wohlleben, D. Preparation and characterisation of single-phase Bi-Pb-Sr-Ca-Cu-O high temperature superconductors. Phys. C Supercond. 1991, 173, 414–424. [Google Scholar] [CrossRef]
- Popa, M.; Calderon-Moreno, J.M.; Crisan, D.; Zaharescu, M. Study of Fe addition on the thermal decomposition of coprecipitated oxalates for the Bi-based superconductor synthesis. J. Therm. Anal. Calorim. 2000, 62, 633–645. [Google Scholar] [CrossRef]
- Durrani, S.K.; Qureshi, A.H.; Qayyum, S.; Arif, M. Development of superconducting phases in BSCCO and Ba-BSCCO by sol spray process. J. Therm. Anal. Calorim. 2009, 95, 87–91. [Google Scholar] [CrossRef]
- Polasek, A.; Majewski, P.; Serra, E.T.; Rizzo, F.; Aldinger, F. Phase relations study on the melting and crystallization regions of the Bi-2223 high temperature superconductor. Mater. Res. 2004, 7, 393–408. [Google Scholar] [CrossRef] [Green Version]
- Torsoni, G.B.; Cena, C.R.; Freitas, G.Q.; Carvalho, C.L. Synthesis of (Bi, Pb)-2223 superconductor powder by Pechini method. Rev. Bras. Física Tecnológica Apl. 2018, 5, 42–53. [Google Scholar] [CrossRef]
- Van Driessche, I.; Buekenhoudt, A.; Konstantinov, K.; Bruneel, E.; Hoste, S. Evaluation of the phase composition of BPSCCO bulk samples by XRD- and susceptibility analysis. IEEE Trans. Appl. Super. 1996, 4, 185–190. [Google Scholar] [CrossRef]
- Matsushita, T.; Suzuki, A.; Kishida, T.; Okuda, M.; Naito, H. The effect of Ag on the superconductivity of Bi2-xPbxSr2Ca2Cu3Oy superconductors prepared by an optimum thermal procedure. Supercond. Sci. Technol. 1994, 7, 222–226. [Google Scholar] [CrossRef]
- Halim, S.A.; Khawaldeh, S.A.; Mohamed, S.B.; Azhan, H. Superconducting properties of Bi2-xPbxSr2Ca2Cu3Oy system derived via sol-gel and solid-state routes. Mater. Chem. Phys. 1999, 61, 251–259. [Google Scholar] [CrossRef]
- Kamarudin, A.N.; Kechik, M.M.A.; Muralidhar, M.; Pinmangkorn, S.; Murakami, M.; Chen, S.K.; Baqiah, H.; Ramli, A.; Lim, K.P.; Halim, S.A. Microstructural, Phase Formation, and Superconducting Properties of Bulk YBa2Cu3Oy Superconductors Grown by Infiltration Growth Process Utilizing the YBa2Cu3Oy + ErBa2Cu3Oy + Ba3Cu5O8 as a Liquid Source. Coatings 2021, 11, 377. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Mote, V.D.; Purushotham, Y.; Dole, B. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar]
- Kameli, P.; Salamati, H.; Eslami, M. The effect of sintering temperature on the intergranular properties of Bi2223 superconductors. Solid State Commun. 2006, 137, 30–35. [Google Scholar] [CrossRef]
- Deac, I.; Burzo, E.; Pop, A.; Pop, V.; Tetean, R.; Kovacs, D. Intergranular properties of (Y1-x-yZrxCay)Ba2Cu3O7-δ compounds. Int. J. Mod. Phys. B 1999, 13, 1645–1654. [Google Scholar] [CrossRef]
- Ambegaokar, V.; Baratoff, A. Tunneling Between Superconductors. Phys. Rev. Lett. 1963, 10, 486–489. [Google Scholar] [CrossRef]
- Clem, J.R. Granular and superconducting-glass properties of the high-temperature superconductors. Phys. C Supercond. 1988, 153–155, 50–55. [Google Scholar] [CrossRef]
- Gao, W.; Li, Z.; Sammes, N. An Introduction to Electronic Materials for Engineers; World Scientific Publishing Company: Hackensack, NJ, USA, 2011. [Google Scholar]
- Bulaevskii, L.N.; Clem, J.R.; Glazman, L.I.; Malozemoff, A.P. Model for the low-temperature transport of Bi-based high-temperature superconducting tapes. Phys. Rev. B 1992, 45, 2545–2548. [Google Scholar] [CrossRef]
- Bulaevskii, L.N.; Daemen, L.L.; Maley, M.P.; Coulter, J.Y. Limits to the critical current in high-Tc superconducting tapes. Phys. Rev. B 1993, 48, 13798–13816. [Google Scholar] [CrossRef]
- Hensel, B.; Grivel, J.C.; Jeremie, A.; Perin, A.; Pollini, A.; Flükiger, R. A model for the critical current in (Bi,Pb)2Sr2Ca2Cu3Ox silver-sheathed tapes. The role of small-angle c-axis grain boundaries and of the texture. Phys. C Supercond. 1993, 205, 329–337. [Google Scholar] [CrossRef]
- Hensel, B.; Grasso, G.; Flükiger, R. Limits to the critical transport current in superconducting (Bi,Pb)2Sr2Ca2Cu3O10 silver-sheathed tapes: The railway-switch model. Phys. Rev. B 1995, 51, 15456–15473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oz, Y.; Jiang, J.; Matras, M.; Oloye, T.A.; Kametani, F.; Hellstrom, E.E. Conundrum of strongly coupled supercurrent flow in both under- and overdoped Bi-2212 round wires. Phys. Rev. Mater. 2021, 5, 074803. [Google Scholar] [CrossRef]
- Kametani, F.; Jiang, J.; Matras, M.; Abraimov, D.; Hellstrom, E.E.; Larbalestier, D.C. Comparison of growth texture in round Bi2212 and flat Bi2223 wires and its relation to high critical current density development. Sci. Rep. 2015, 5, 8285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parinov, I.A. Microstructure and Properties of High-Temperature Superconductors; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
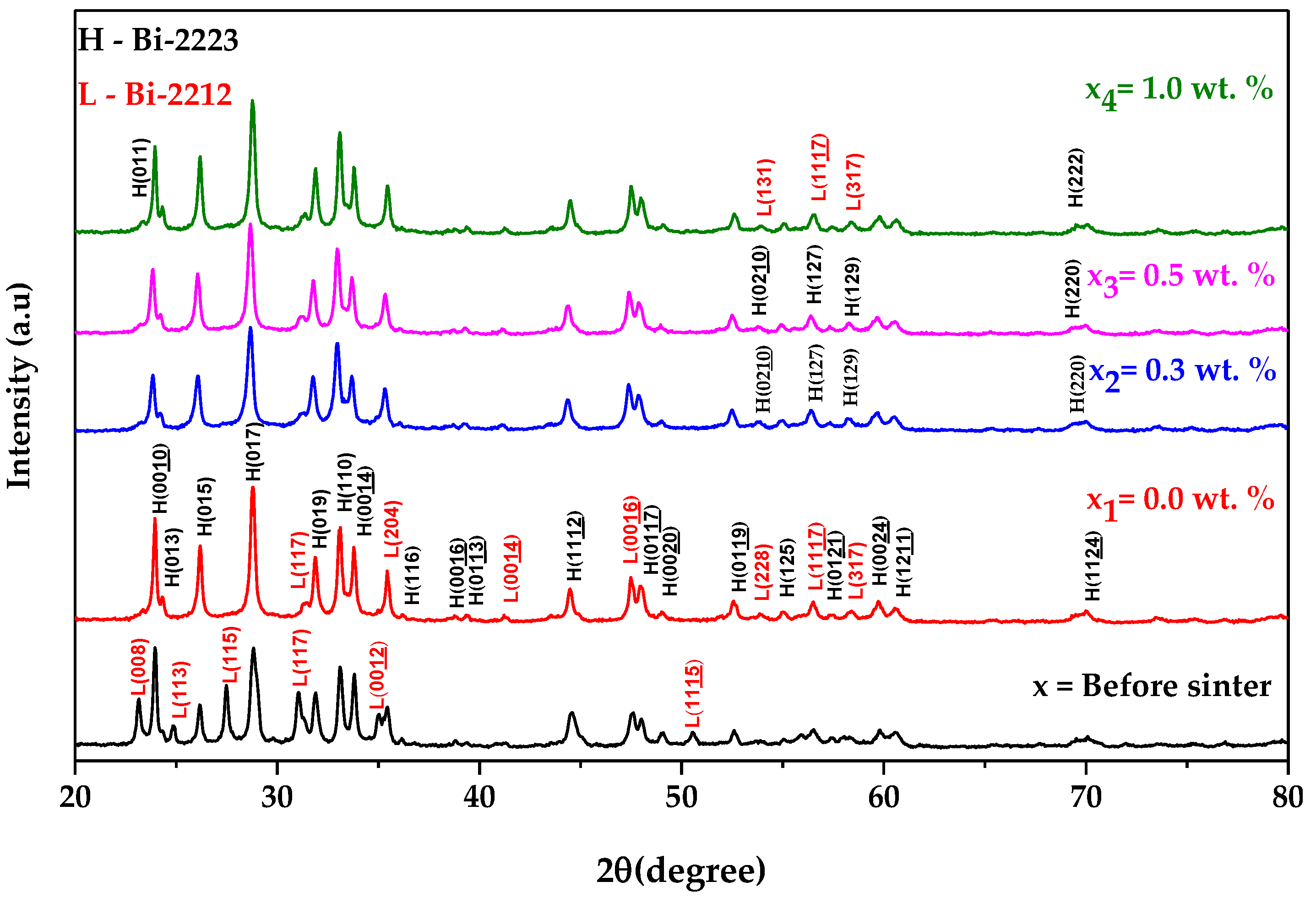


| Bi-2223 + x wt.% of Graphene | Intensity Fraction (%) | |
|---|---|---|
| Bi-2223 | Bi-2212 | |
| 0.0 | 85.17 | 14.83 |
| 0.3 | 87.43 | 12.57 |
| 0.5 | 88.25 | 11.75 |
| 1.0 | 84.00 | 16.00 |
| Bi-2223 + x wt.% of Graphene | Lattice Parameter (Å) | Crystallite Size (nm) | Unit Cell Volume (Å3) | Lattice Strain (%) | |||
|---|---|---|---|---|---|---|---|
| a-Axis | b-Axis | c-Axis | Scherrer | Williamson–Hall | |||
| 0.0 | 3.826 ± 0.000659 | 3.826 ± 0.000659 | 37.104 ± 0.008048 | 46.26 | 397 | 543.083 | 0.175 |
| 0.3 | 3.824 ± 0.000760 | 3.824 ± 0.000760 | 37.064 ± 0.009404 | 39.09 | 74.5 | 541.859 | 0.070 |
| 0.5 | 3.823 ± 0.000703 | 3.823± 0.000703 | 37.062 ± 0.008636 | 46.25 | 182 | 541.768 | 0.179 |
| 1.0 | 3.823 ± 0.000635 | 3.823 ± 0.000635 | 37.066 ± 0.007766 | 46.26 | 109 | 541.791 | 0.073 |
| Bi-2223 + x wt.% of Graphene | Tp (K) | Tc-onset (K) | Tp/Tc-onset | Tcj (K) | Io (µA) |
|---|---|---|---|---|---|
| 0.0 | 100.78 | 107.69 | 0.936 | 101.91 | 31.50 |
| 0.3 | 99.10 | 107.21 | 0.924 | 100.94 | 28.80 |
| 0.5 | 98.92 | 106.60 | 0.928 | 100.23 | 28.01 |
| 1.0 | 98.78 | 106.54 | 0.927 | 99.90 | 26.84 |
| Bi-2223 + x wt.% of Graphene | Ic (A) | Jc (mA/cm2) |
|---|---|---|
| 0.0 | 0.270 | 4235 |
| 0.3 | 0.240 | 4271 |
| 0.5 | 0.140 | 2340 |
| 1.0 | 0.320 | 5197 |
| Bi-2223 + x wt.% of Graphene (wt.%) | Elements (%) | ||||||
|---|---|---|---|---|---|---|---|
| Bi | Pb | Sr | Ca | Cu | O | C | |
| 0.0 (2.0:0.8:2.2:2.0:3.5:6.3) | 11.98 | 4.86 | 12.93 | 12.11 | 20.79 | 37.33 | - |
| 0.3 (2.0:0.3:1.8:1.7:3.5:5.4) | 9.23 | 1.39 | 8.53 | 7.68 | 16.34 | 24.77 | 32.07 |
| 0.5 (2.0:0.4:2.0:1.7:3.5:6.0) | 8.51 | 1.54 | 8.31 | 7.41 | 14.87 | 25.71 | 33.66 |
| 1.0 (2.0:0.4:2.0:1.8:3.4:6.6) | 7.5 | 1.52 | 7.31 | 6.71 | 12.84 | 24.83 | 39.29 |
| Bi-2223 + x wt.% of Graphene (wt.%) | Elements (%) | ||||||
|---|---|---|---|---|---|---|---|
| Bi | Pb | Sr | Ca | Cu | O | C | |
| 0.0 | 35.54 | 14.28 | 16.08 | 6.89 | 18.75 | 8.48 | - |
| 0.3 | 37.90 | 5.64 | 14.68 | 6.04 | 20.39 | 7.78 | 7.57 |
| 0.5 | 36.43 | 6.52 | 14.91 | 6.08 | 19.35 | 8.42 | 8.28 |
| 1.0 | 35.02 | 7.04 | 14.30 | 6.01 | 18.22 | 8.87 | 10.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, S.N.; Kechik, M.M.A.; Kamarudin, A.N.; Talib, Z.A.; Baqiah, H.; Kien, C.S.; Pah, L.K.; Abdul Karim, M.K.; Shabdin, M.K.; Shaari, A.H.; et al. Microstructure and Superconducting Properties of Bi-2223 Synthesized via Co-Precipitation Method: Effects of Graphene Nanoparticle Addition. Nanomaterials 2023, 13, 2197. https://doi.org/10.3390/nano13152197
Abdullah SN, Kechik MMA, Kamarudin AN, Talib ZA, Baqiah H, Kien CS, Pah LK, Abdul Karim MK, Shabdin MK, Shaari AH, et al. Microstructure and Superconducting Properties of Bi-2223 Synthesized via Co-Precipitation Method: Effects of Graphene Nanoparticle Addition. Nanomaterials. 2023; 13(15):2197. https://doi.org/10.3390/nano13152197
Chicago/Turabian StyleAbdullah, Siti Nabilah, Mohd Mustafa Awang Kechik, Aliah Nursyahirah Kamarudin, Zainal Abidin Talib, Hussein Baqiah, Chen Soo Kien, Lim Kean Pah, Muhammad Khalis Abdul Karim, Muhammad Kashfi Shabdin, Abdul Halim Shaari, and et al. 2023. "Microstructure and Superconducting Properties of Bi-2223 Synthesized via Co-Precipitation Method: Effects of Graphene Nanoparticle Addition" Nanomaterials 13, no. 15: 2197. https://doi.org/10.3390/nano13152197








