Insight into the Mechanisms of Nitride Films with Excellent Hardness and Lubricating Performance: A Review
Abstract
:1. Introduction
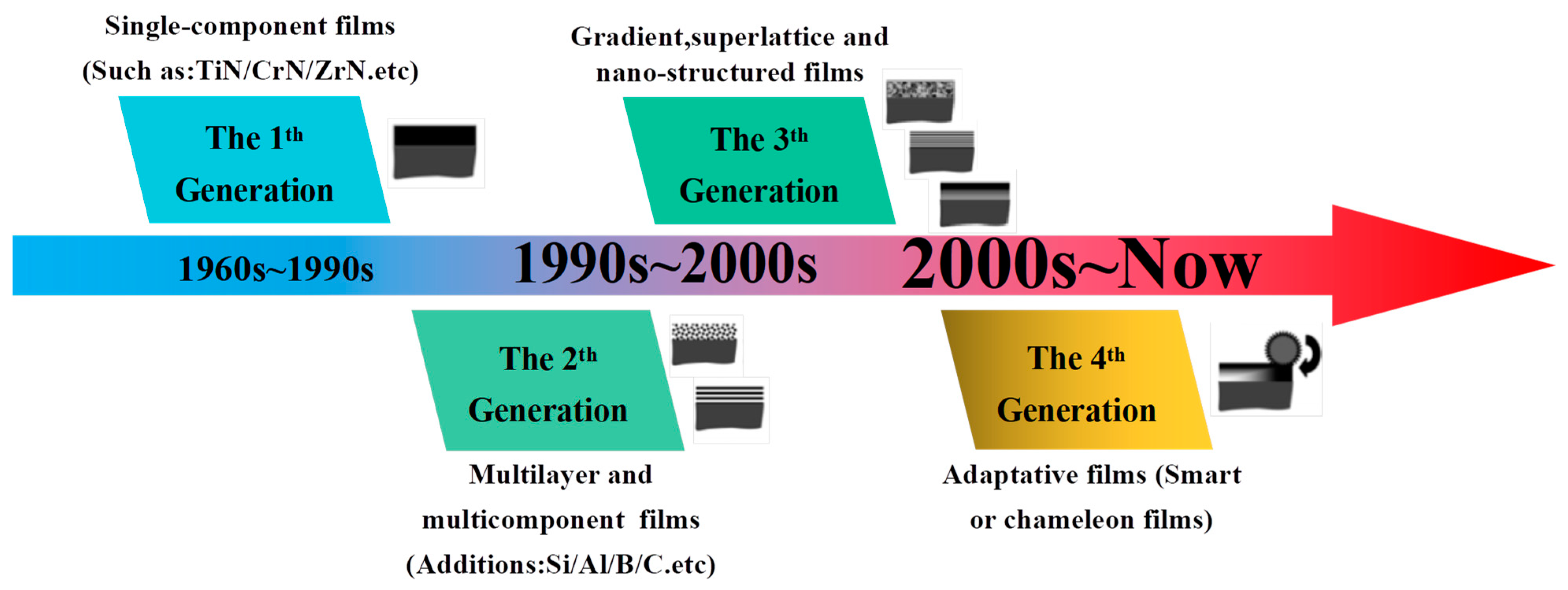
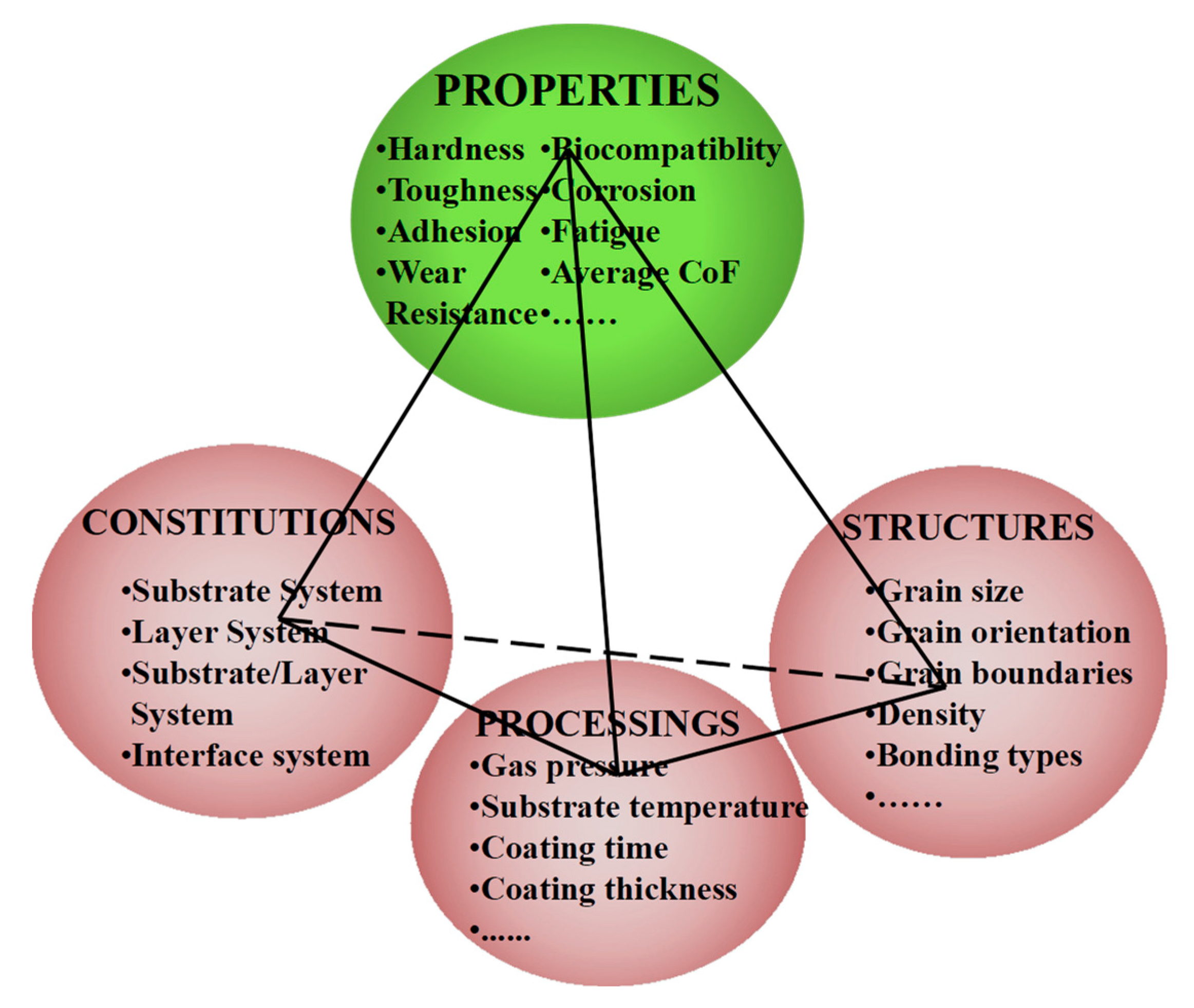
2. Design Considerations
- The two-phase formation model called hard phase/hard phase composite films, e.g., nc-Me1N/a-Me2N, etc. We denote the phase constitution model of hard/hard nanocomposites as Model I.
- The formation model of hard phase/soft phase composite films is referred to as Model II, e.g., nc-Me1N/Me3, etc.
2.1. The Components
2.1.1. The Amorphous Matrixes
2.1.2. The Composited Solid Lubricants
2.1.3. The Encapsulated Reinforcements
2.2. The Chemical Constitutions
2.2.1. The Matrix Film
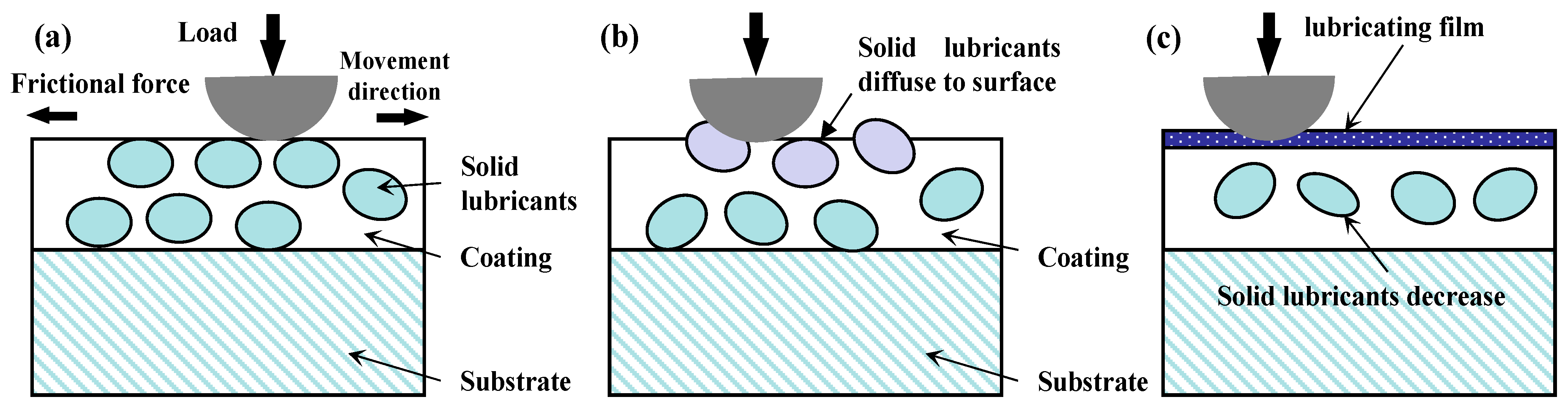
2.2.2. The Solid Lubricants
2.2.3. The Reinforcements
3. The Strengthening Mechanisms
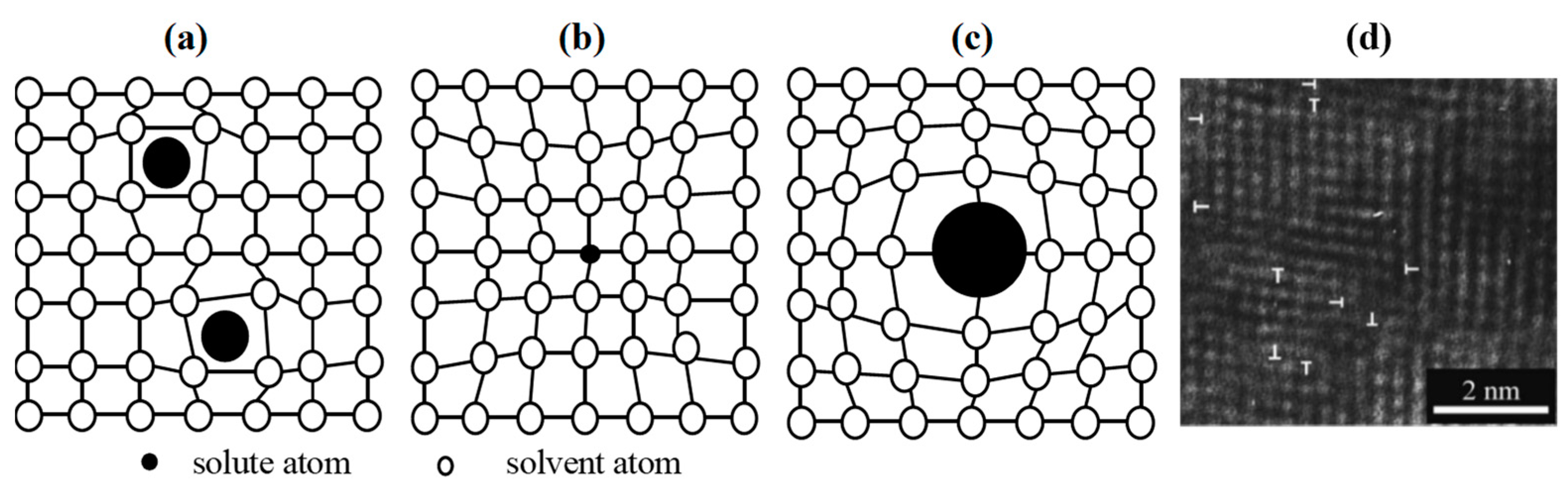
3.1. The Solid Solution Mechanisms
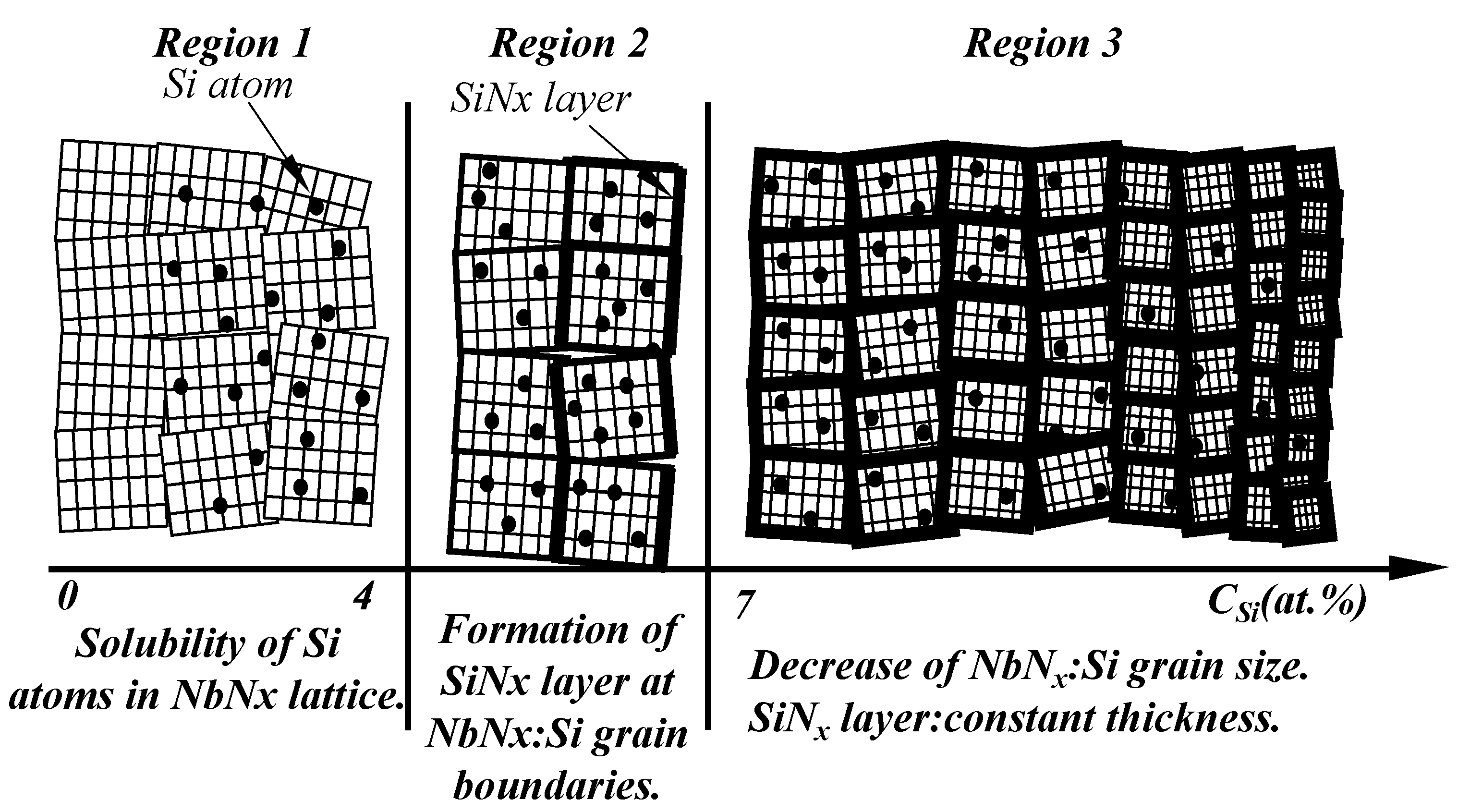
3.1.1. The Solid Solution Mechanisms Based on Solubility
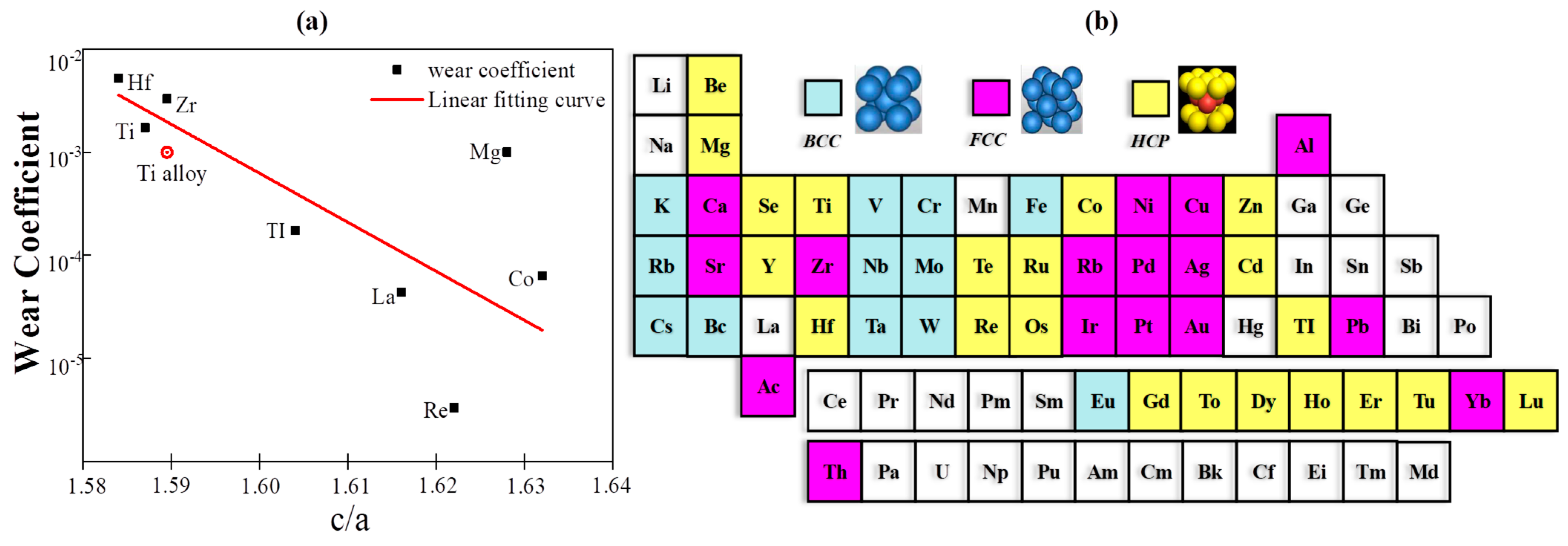
3.1.2. The Solid Solution Mechanisms Based on Deformation
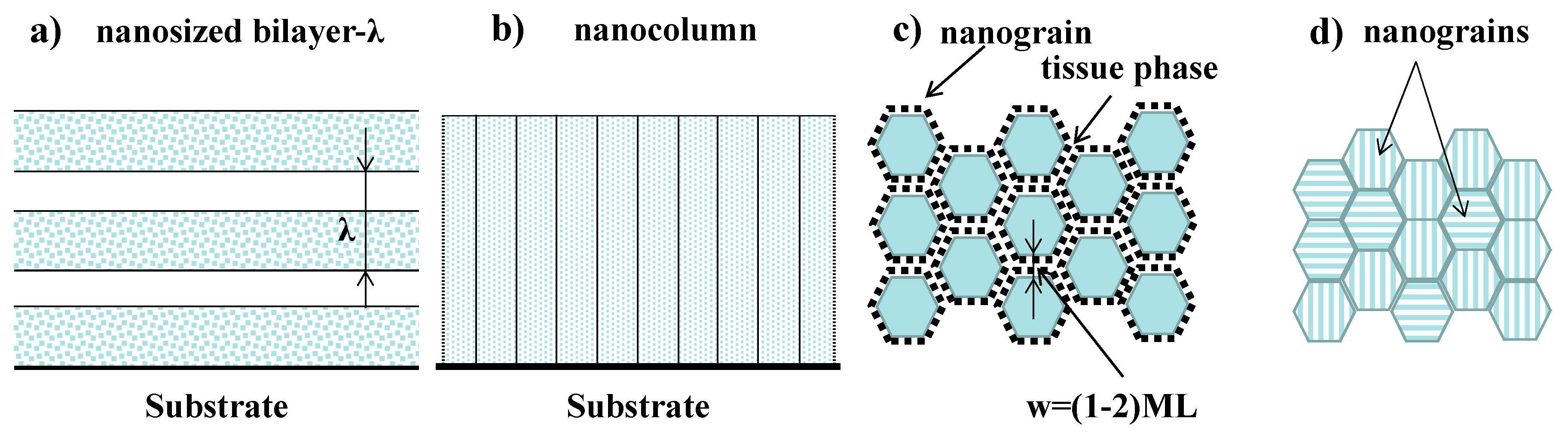
3.2. The Grain Size Effect
3.2.1. The Grain Refinement Mechanisms
- (1)
- The grains in the films are fine, making deformation more challenging within the grains.
- (2)
- The presence of numerous grain boundaries with 3D skeletal structures impedes the motion of dislocations, resulting in improved hardness and toughness. Additionally, the films can release deformations through the sliding of grain boundaries.
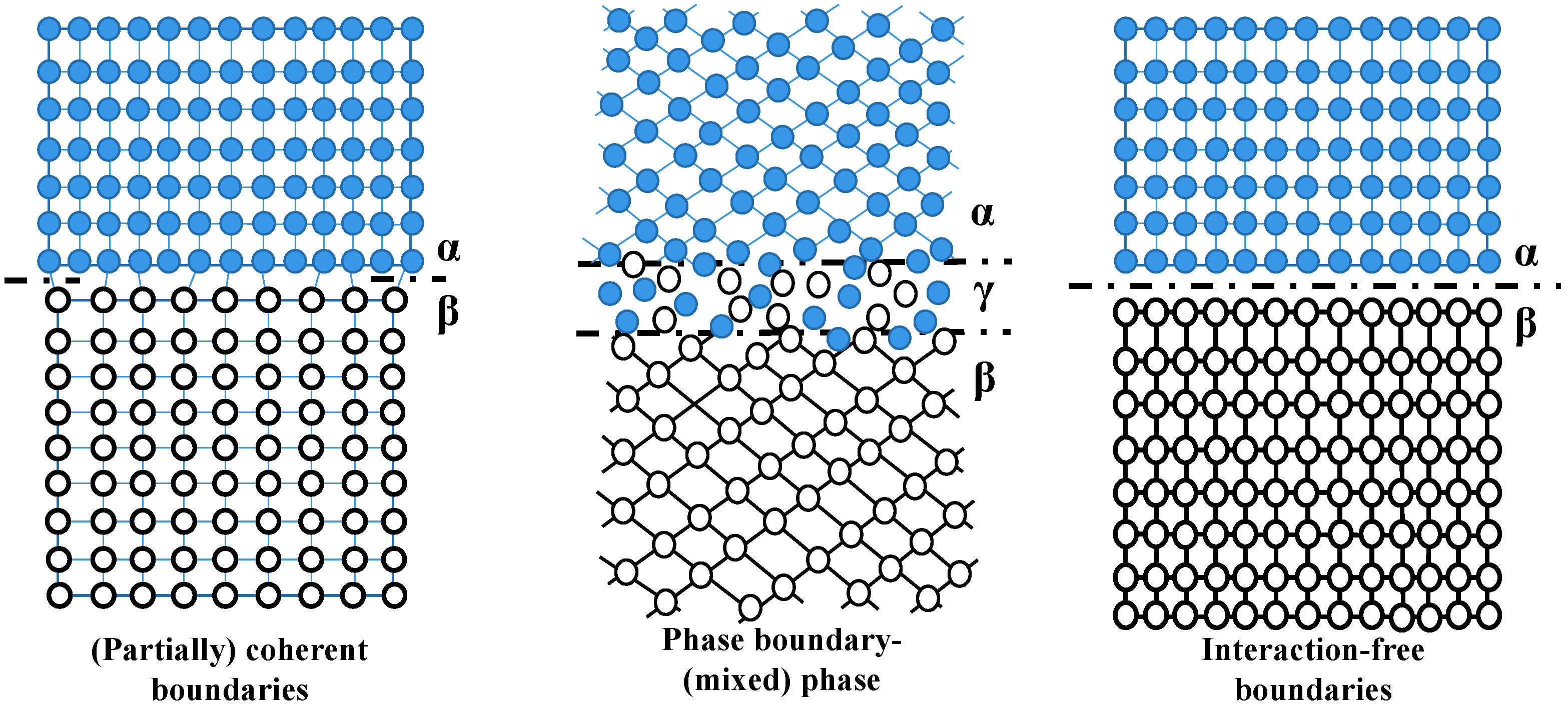
3.2.2. The Internal Interfaces
3.2.3. The Isotropic Effect
3.3. The Secondary Phase Strengthening Mechanisms
3.3.1. The Precipitations Forming during Deposition
3.3.2. The Precipitations Forming during Post-Deposition
3.4. The Strengthening Mechanisms Induced by Stress Field
3.4.1. Residual Stress
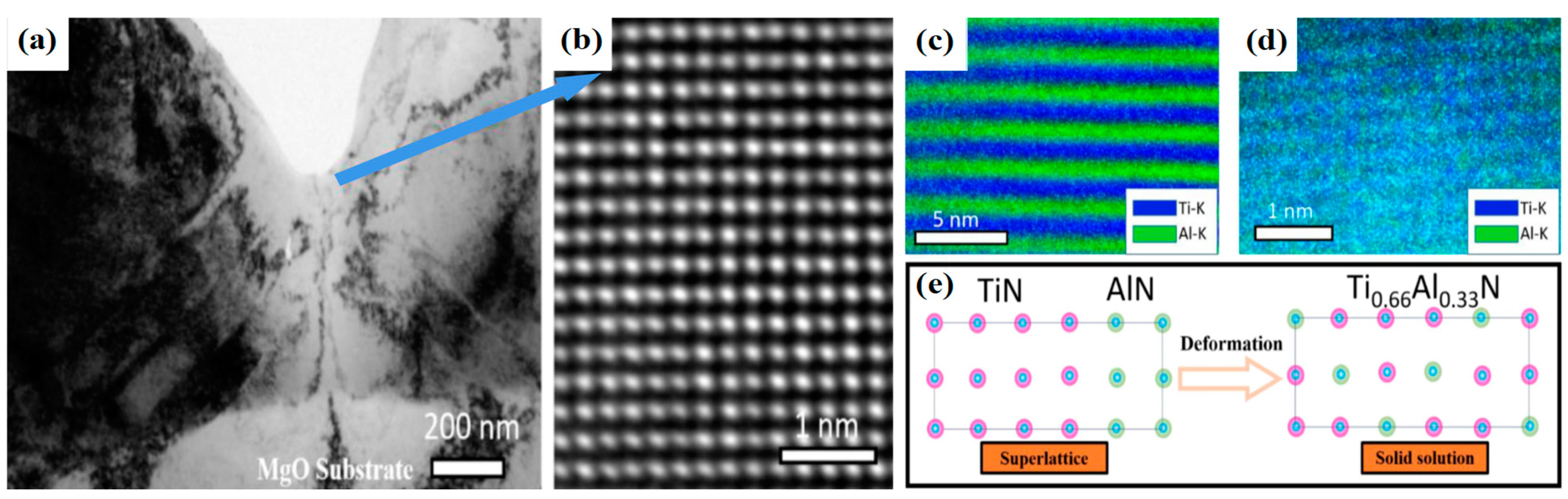
3.4.2. The Alternating Stress of Superlattice Structured Films
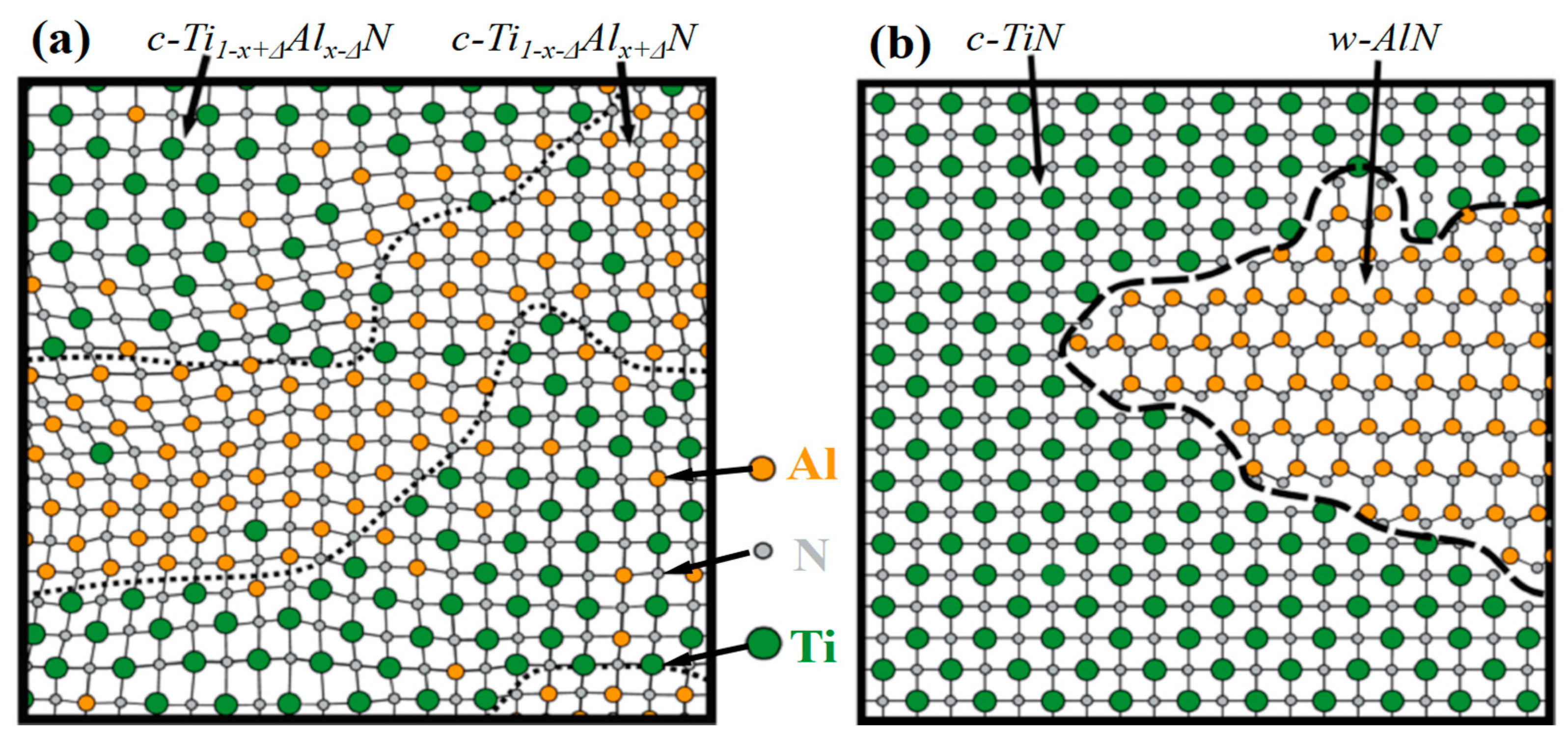
3.5. The Template Effect
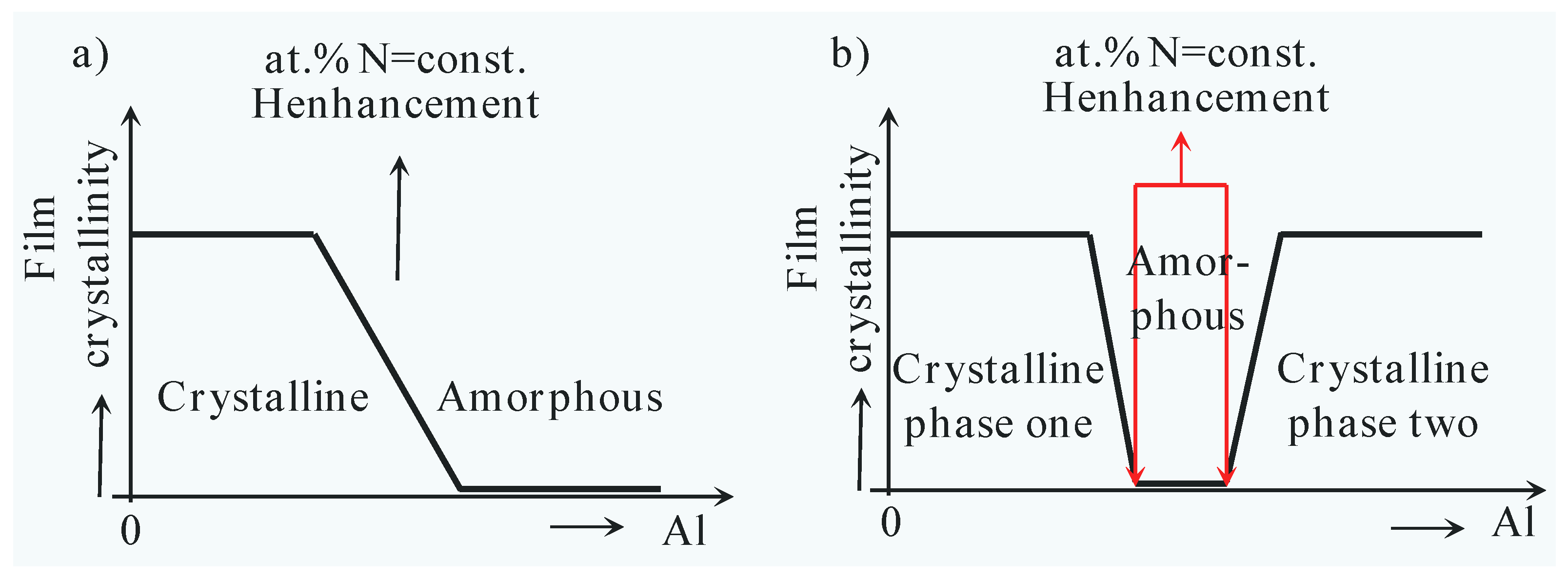
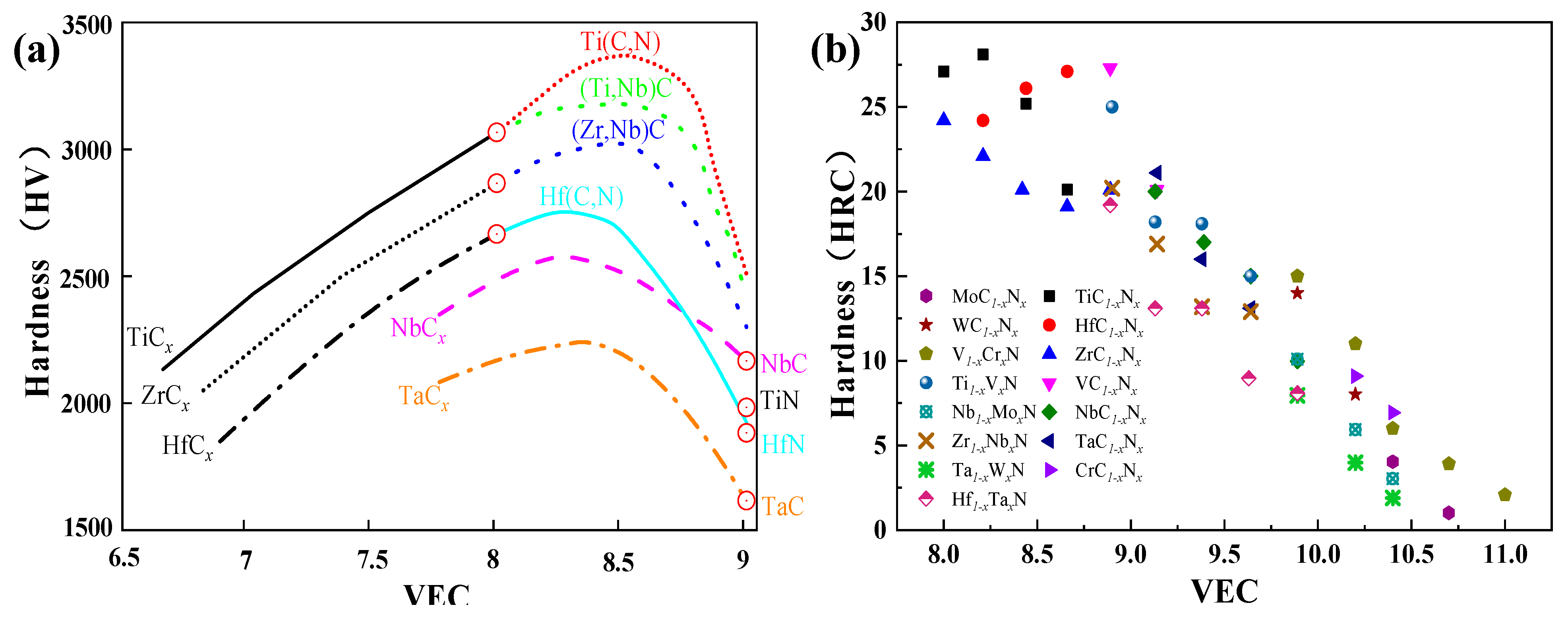
3.6. The Valence Electron Concentration Effect
3.7. The Synergistic Mechanism
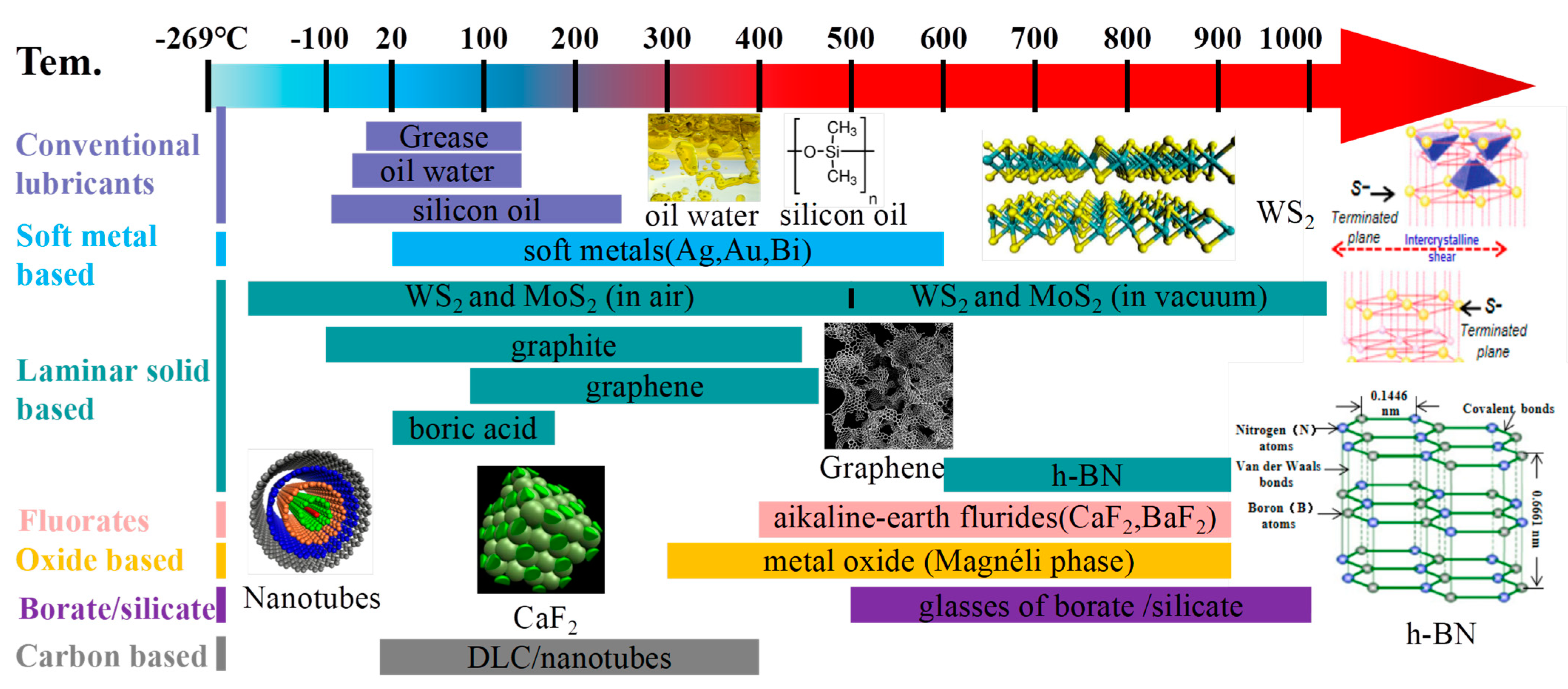
4. The Lubricating Mechanism
4.1. The Lubricating Mechanism Based on the Nature of Constituting Material
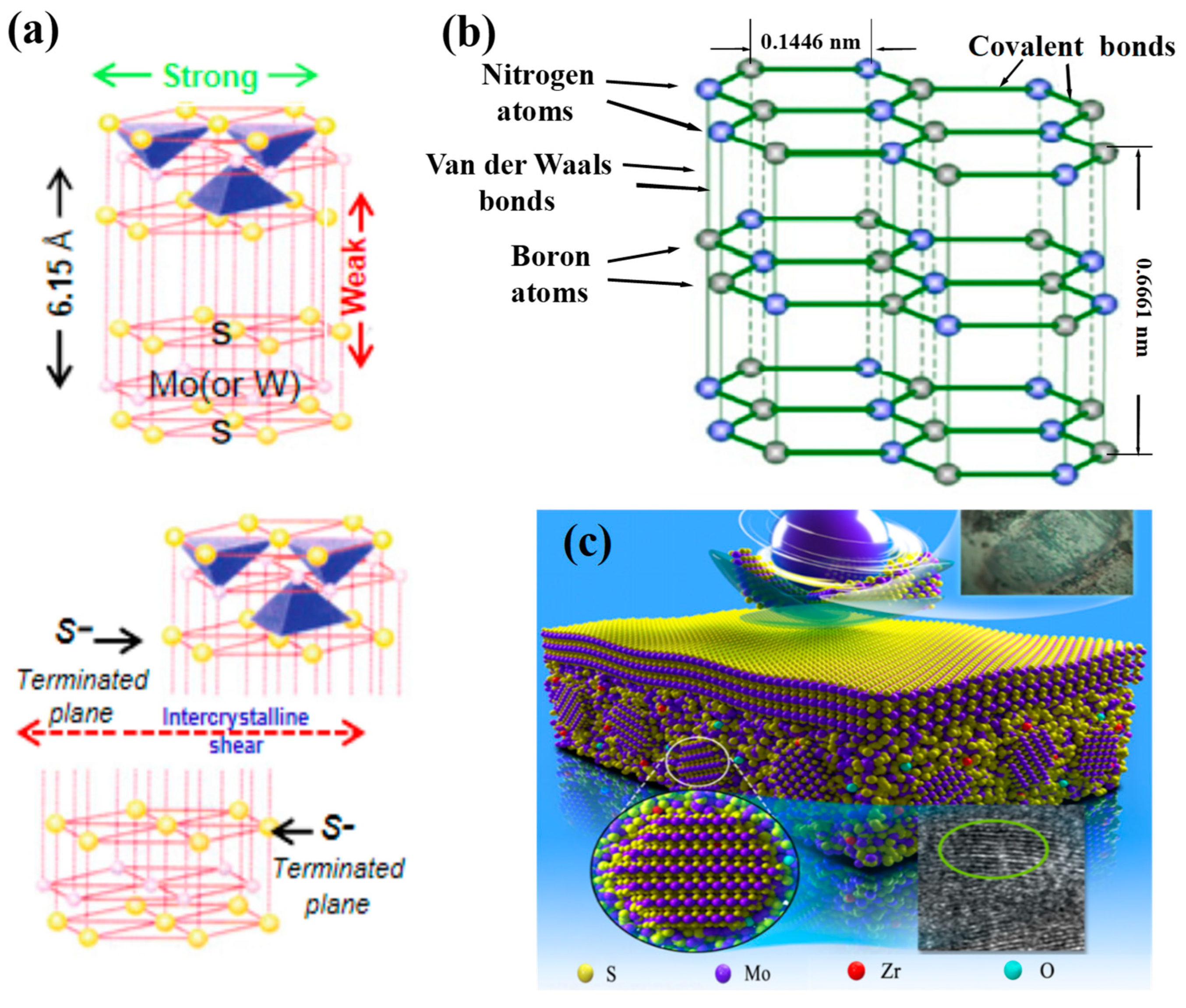
4.1.1. The Laminar Materials
4.1.2. The Soft Metal
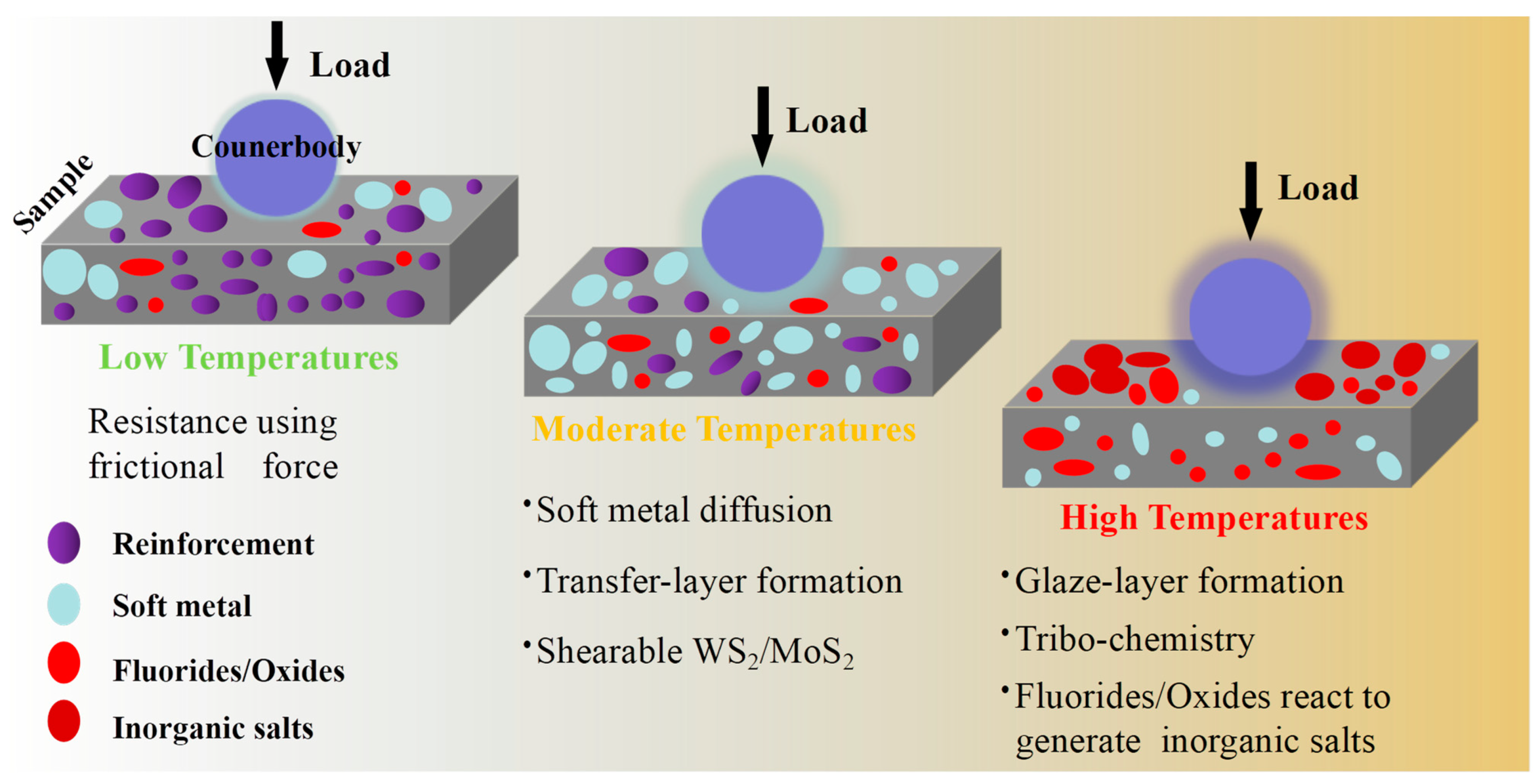
4.2. The Lubricating Mechanism Based on the Tribo-Chemical Reactions
4.2.1. The Lubricious Oxides
- (1)
- Oxides with low-shear-strength
- (2)
- Oxides with low melting points
- (3)
- Oxides with high-ionic potential
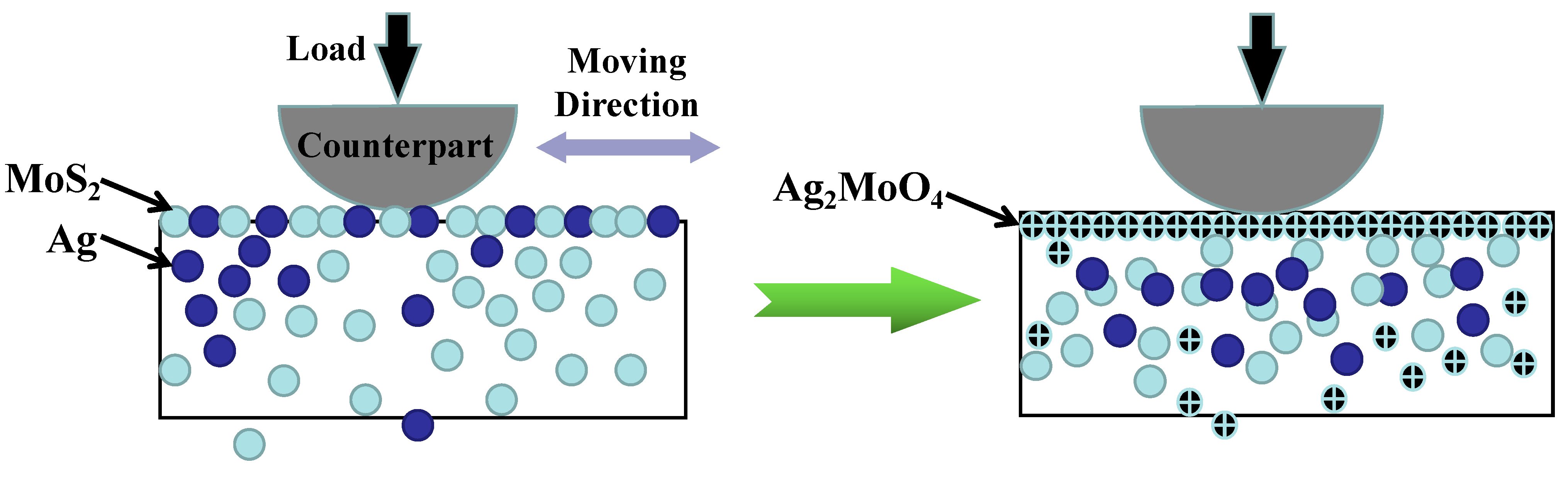
4.2.2. The Lubricious Inorganic Salts
4.3. The Lubricious Fluorides
4.4. The Lubricating Mechanism Based on Textures
4.5. The Lubricating Mechanism Based on the Synergistic Effect
5. Outlooks
5.1. Refine the Existing Theory
5.2. Application of Data-Driven Research
5.3. New Strategy in Guiding Design
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVD | Physical Vapor Deposition |
| CVD | Chemical Vapor Deposition |
| RT | Room Temperature |
| VEC | The valence electron concentration effect |
| TMN | Transition metal nitride |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| Eq | Equation |
| Ref | Reference |
| nc | Nanocrystalline |
| α | Amorphous |
| AIMD | Ab initio molecular dynamics |
| DFT | Density Functional Theory |
| MD | Molecular Dynamics |
| TEM | Transmission electron microscope |
| ML | Monolayer |
| H | Hardness |
| c | Crystalline |
| Ta | Annealing temperature |
| FCC | Face centered cubic |
| BCC | Body centered cubic |
| HCP | Hexagonal closed pack |
| HT | High temperature |
| MEMS | Micro Electro Mechanical Systems |
| NEMS | Nano-Electromechanical Systems |
| MMCs | Metal matrix composites |
References
- Kenneth, H.; Peter, A.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar]
- Meirong, C.; Ruisheng, G.; Feng, Z.; Liu, W.M. Lubricating a bright future: Lubrication contribution to energy saving and low carbon emission. Sci. China Technol. Sci. 2013, 56, 2888–2913. [Google Scholar]
- Voevodin, A.A.; Zabinski, J.S. Supertough wear-resistant coatings with ‘chameleon’ surface adaptation. Thin Solid Film. 2000, 370, 223–231. [Google Scholar] [CrossRef]
- Ardila-Téllez, L.C.; Orozco-Hernandez, G.; Estupiñan-Mongui, F.; Moreno-Téllez, C.M.; Olaya, J.J. Review of nitride-based multifunctional PVD-deposited coatings. Rev. Científica 2023, 46, 162–176. [Google Scholar] [CrossRef]
- Tibor, C. Application of Coatings for Tooling Quo Vadis 2005? Vac. Res. Pract. 2005, 17, 33–39. [Google Scholar]
- Ju, H.B.; Pei, J.; Junhua, X.; Lihua, Y.; Isaac, A.; Yaoxiang, G. Crystal structure and high temperature tribological behavior of niobium aluminum nitride films. Materialia 2018, 3, 202–211. [Google Scholar] [CrossRef]
- Ju, H.B.; Yu, L.H.; He, S.; Asempah, I.; Xu, J.H.; Hou, Y. The enhancement of fracture toughness and tribological properties of the titanium nitride films by doping yttrium. Surf. Coat. Technol. 2017, 321, 57–63. [Google Scholar] [CrossRef]
- Xu, J.H.; Ju, H.B.; Yu, L.H. Microstructure, oxidation resistance, mechanical and tribological properties of Mo–Al–N films by reactive magnetron sputtering. Vacuum 2014, 103, 21–27. [Google Scholar] [CrossRef]
- Yu, L.H.; Xu, J.H.; Dong, S.R.; Isao, K. Surface morphology and growth mechanisms for sputtered amorphous silicon nitride thin films. Thin Solid Films 2008, 516, 1781–1787. [Google Scholar] [CrossRef]
- Ju, H.B.; Xu, J.H. Microstructure, oxidation resistance, mechanical and tribological properties of Ti-Y-N films by reactive magnetron sputtering. Surf. Coat. Technol. 2015, 283, 311–317. [Google Scholar] [CrossRef]
- Mulligan, C.P.; Blanchet, T.A.; Gall, D. Control of lubricant transport by a CrN diffusion barrier layer during high-temperature sliding of a CrN-Ag composite coating. Surf. Coat. Technol. 2010, 205, 1350–1355. [Google Scholar] [CrossRef]
- Ju, H.B.; Yu, D.; Yu, L.H.; Ding, N.; Xu, J.H.; Zhang, X.D.; Zheng, Y.; Yang, L.; He, X.C. The influence of Ag contents on the microstructure, mechanical and tribological properties of ZrN-Ag films. Vacuum 2018, 148, 54–61. [Google Scholar] [CrossRef]
- Ju, H.B.; He, S.; Yu, L.H.; Asempah, I.; Xu, J.H. The improvement of oxidation resistance, mechanical and tribological properties of W2N films by doping silicon. Surf. Coat. Technol. 2017, 317, 158–165. [Google Scholar] [CrossRef]
- Ju, H.B.; Jia, P.; Xu, J.H.; Yu, L.H.; Geng, Y.X.; Chen, Y.H.; Liu, M.H.; Mater, T.W. The effects of adding aluminum on crystal structure, mechanical, oxidation resistance, friction and wear properties of nanocomposite vanadium nitride hard films by reactive magnetron sputtering. Chem. Phys. 2018, 215, 368–375. [Google Scholar] [CrossRef]
- Donnet, C.; Erdemir, A. Historical developments and new trends in tribological and solid lubricant coatings. Surf. Coat. Technol. 2004, 180–181, 76–84. [Google Scholar] [CrossRef]
- Saquib, H.M.; Syed, A.A.; Nahid, C.; Mohammad, A.M.; Amit, K.N. Applications of Nanocomposite Materials in Dentistry; Woodhead: Shaxton, UK, 2019; pp. 107–121. [Google Scholar]
- Bian, S.N.; Xu, J.H.; Yu, L.H.; Wang, P.K.; Jiang, Y.H.; Chen, C.Y. Structure regulation and property correlation of Hf-B-N thin films. Ceram. Int. 2023, 49, 25728–25743. [Google Scholar] [CrossRef]
- Musil, J.; Zeman, P.; Hrubý, H.; Mayrhofer, P.H. ZrN/Cu nanocomposite film-a novel superhard material. Surf. Coat. Technol. 1999, 120–121, 179–183. [Google Scholar] [CrossRef]
- Greene, J.E. Thin Film Nucleation, Growth, and Microstructural Evolution: An Atomic Scale View. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Peter, M.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 12, pp. 554–620. [Google Scholar]
- Abd-El-Rahman, A.M.; Wei, R.H. A comparative study of conventional magnetron sputter deposited and plasma enhanced magnetron sputter deposited Ti-Si-C-N nanocomposite coatings. Surf. Coat. Technol. 2014, 241, 74–79. [Google Scholar] [CrossRef]
- Muratore, C.; Andrey, A.V.; Hu, J.J.; Jeffrey, S.Z. Multilayered YSZ-Ag-Mo/TiN adaptive tribological nanocomposite coatings. Tribol. Lett. 2006, 24, 201–206. [Google Scholar] [CrossRef]
- Peng, L.J.; Zhang, J.; Wang, X.Y. Phase Composition, Hardness, and Thermal Shock Properties of AlCrTiN Hard Films with High Aluminum Content. Coatings 2023, 13, 547. [Google Scholar] [CrossRef]
- Yu, Z.; He, X.J.; Ren, P.; Sun, G.X.; Fan, X.F.; Singh, D.J.; Zhang, K.; Wen, M. Self-assemble Au@amorphous-C/fullerene-like-C core-shell nanocomposite film with near-elastic response. Mater. Charact. 2022, 193, 112268–112879. [Google Scholar]
- Sangiovanni, D.G.; Hultman, L.; Chirita, V. Supertoughening in B1 transition metal nitride alloys by increased valence electron concentration. Acta Mater. 2011, 59, 2121–2134. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, J.H.; Cheng, M.K.; Chang, Y.K.; Li, C.; Chang, C.L.; Liu, P.C. Effects of annealing on antiwear and antibacteria behaviors of TaN-Cu nanocomposite thin films. J. Vac. Sci. Technol. 2008, 26, 1093–1097. [Google Scholar] [CrossRef]
- Zhang, H.S.; Jose, L.E.; André, A. Comparative surface and nano-tribological characteristics of nanocomposite diamond-like carbon thin films doped by silver. Appl. Surf. Sci. 2008, 255, 2551–2556. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.C.; Wei, Y.Q.; Li, J.J.; Li, X.N.; Wang, K.L.; Li, K.; Sheldon, Q.S.; Li, J.Z.; Fang, Z. Development of a multifunctional nanocomposite film with record-high ultralow temperature toughness and unprecedented fatigue-resistance. Chem. Eng. J. 2022, 432, 134408–134419. [Google Scholar] [CrossRef]
- Wang, J. Recent Research and Applications of (Ti1−xAlx)N Thin Hard Coating. J. Cryst. Growth 2007, 36, 699–704. [Google Scholar]
- Deyab, M.A. Anticorrosion properties of nanocomposites coatings: A critical review. J. Mol. Liq. 2020, 313, 113533–113537. [Google Scholar] [CrossRef]
- Lu, Z.X.; Zhan, C.Z.; Chun, Z.; Siming, R.; Pu, J.B. A novel design by constructing MoS2/WS2 multilayer film doped with tantalum toward superior friction performance in multiple environment. J. Mater. Sci. 2021, 56, 17615–17631. [Google Scholar] [CrossRef]
- Chhowalla, M.; Gehan, A.J.A. Thin films of fullerene-like MoS2 nanoparticles with ultra-low friction and wear. Nature 2000, 407, 164–167. [Google Scholar] [CrossRef]
- Duan, Z.W.; Jiang, H.C.; Zhao, X.Y.; Li, Q.; Hu, M.; Wang, P.; Liu, W.M. MoS2 Nanocomposite Films with High Irradiation Tolerance and Self-Adaptive Lubrication. ACS Appl. Mater. Interfaces 2021, 13, 20435–20447. [Google Scholar] [CrossRef]
- Bobzin, K. High-performance coatings for cutting tools. CIRP J. Manuf. Sci. Technol. 2017, 18, 1–9. [Google Scholar] [CrossRef]
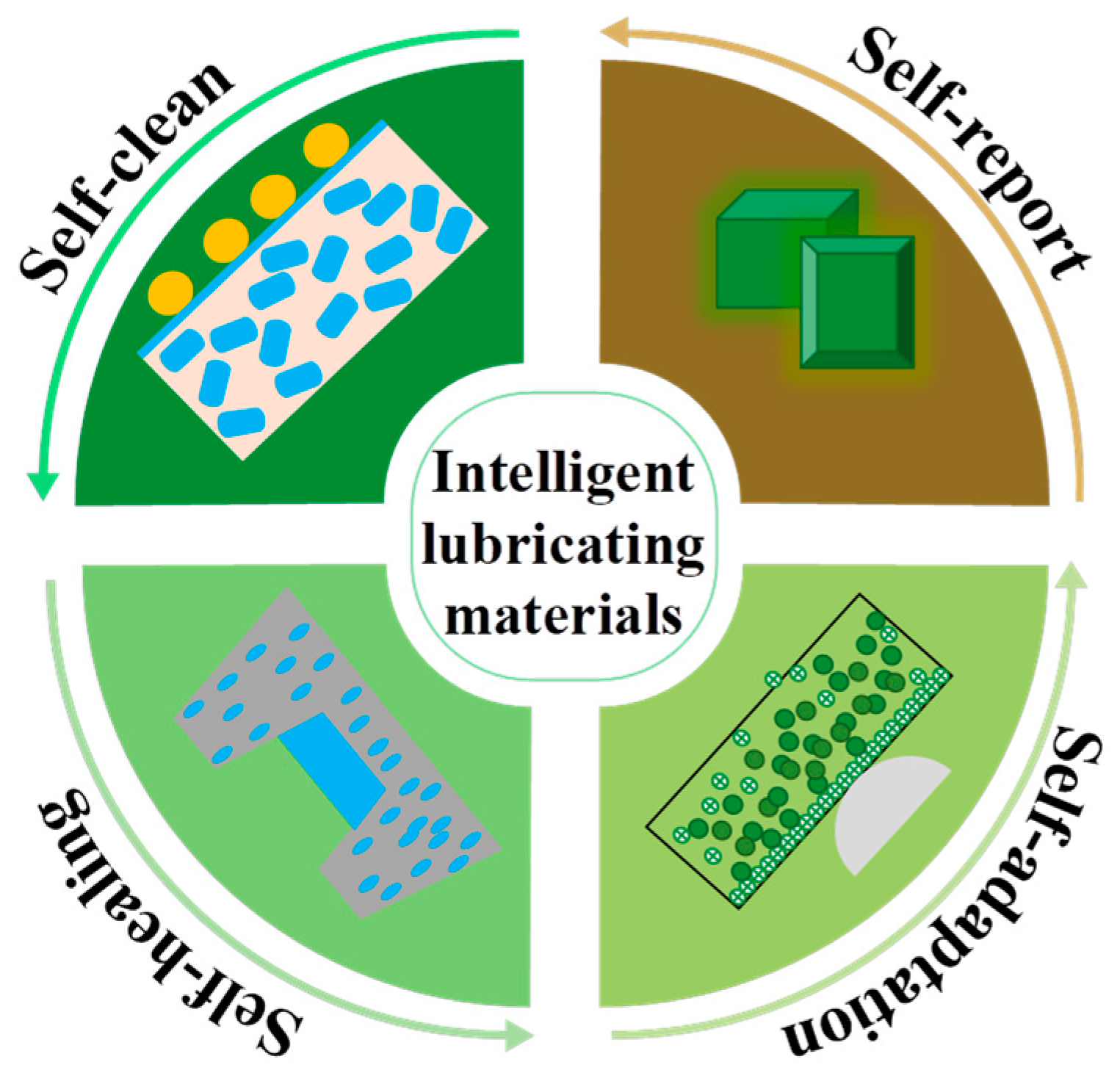
- Gong, H.J.; Yu, C.C.; Zhang, L.; Xie, G.X.; Guo, D.; Luo, J.B. Intelligent lubricating materials: A review. Compos. Part B Eng. 2020, 202, 108450–108467. [Google Scholar] [CrossRef]
- Baker, C.C.; Richard, R.C.; Kathryn, J.W.; Hu, J.J.; Andrey, A.V. Preparation of chameleon coatings for space and ambient environments. Thin Solid Film. 2006, 515, 6737–6743. [Google Scholar] [CrossRef]
- Baker, C.C.; Hu, J.J.; Andrey, A.V. Preparation of Al2O3/DLC/Au/MoS2 chameleon coatings for space and ambient environments. Surf. Coat. Technol. 2006, 201, 4224–4229. [Google Scholar] [CrossRef]
- Ju, H.B.; Huang, K.H.; Luan, J.; Geng, Y.X.; Yang, J.F.; Xu, J.H. Evaluation under temperature cycling of the tribological properties of Ag-SiNx films for green tribological applications. Ceram. Int. 2023, in press. [Google Scholar] [CrossRef]
- Musil, J. Hard nanocomposite coatings: Thermal stability, oxidation resistance and toughness. Surf. Coat. Technol. 2012, 207, 50–65. [Google Scholar] [CrossRef]
- Langlois, W.E.; Deville, M.O. Lubrication Theory. In Slow Viscous Flow; Springer: Cham, Switzerland, 2013; Volume 9, pp. 229–249. [Google Scholar]
- Davim, J.P. Tribology for Engineers; Woodhead: Shaxton, UK, 2011; pp. 33–63. [Google Scholar]
- Németh, A.K.; Mária, M.B. Wear Maps and Models in Tribological Damage Analysis of Si3N4 Ceramics with Special Attention to Tribochemical Wear. In Proceedings of the International Multidisciplinary Scientific Conference, Miskolc, Hungary, 20–21 April 2017. [Google Scholar]
- Evans, A.G.; Marshall, D.B. Fundamentals of Friction and Wear of Materials, Materials Park; ASM International: Metals Park, OH, USA, 1981; pp. 439–452. [Google Scholar]
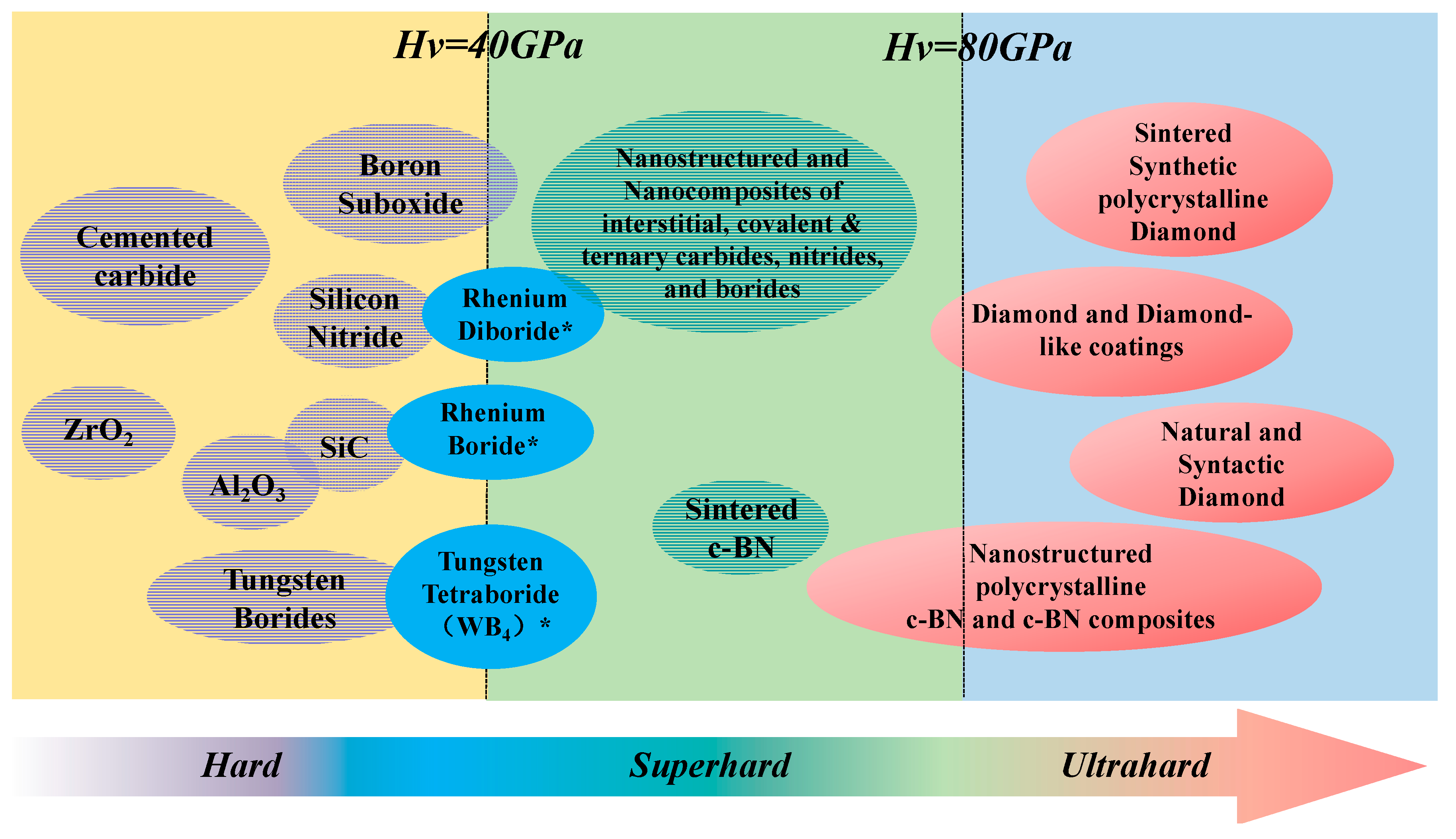
- Zhao, Z.S.; Xu, B.; Tian-Annu, Y.G. Recent Advances in Superhard Materials. Rev. Mater. Sci. 2016, 46, 383–406. [Google Scholar] [CrossRef]
- Ju, H.B.; Xu, L.Y.; Luan, J.; Geng, Y.X.; Xu, J.H.; Yu, L.H.; Yang, J.F.; Filipe, F. Enhancement on the hardness and oxidation resistance property of TiN/Ag composite films for high temperature applications by addition of Si. Vacuum 2023, 209, 111752–111758. [Google Scholar] [CrossRef]
- Vepřek, S.; Sieghard-Dipl, I.R. A concept for the design of novel superhard coatings. Thin Solid Film. 1995, 268, 64–71. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Filipe, F.; Fábio, F.; Albano, C. Tailoring the nanostructure of Ti-Si-N thin films by HiPIMS in deep oscillation magnetron sputtering (DOMS) mode. Surf. Coat. Technol. 2015, 264, 140–149. [Google Scholar] [CrossRef]
- Pan, S.H.; Jin, K.Y.; Wang, T.L.; Zhang, Z.N.; Zheng, L.L.; Umehara, N. Metal matrix nanocomposites in tribology: Manufacturing, performance, and mechanisms. Friction 2022, 10, 1596–1634. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Fitz, T.A.; Hu, J.J.; Jeffrey, S.Z. Nanocomposite tribological coatings with “chameleon” surface adaptation. J. Vac. Sci. Technol. 2002, 20, 1434–1444. [Google Scholar] [CrossRef]
- Ren, B.; Zhong, X.L.; Shi, L.; Cai, B.; Wang, M.X. Structure and properties of (AlCrMnMoNiZrB0.1)Nx coatings prepared by reactive DC sputtering. Appl. Surf. Sci. 2011, 257, 7172–7178. [Google Scholar] [CrossRef]
- Yu, L.H.; Chen, J.; Ju, H.B.; Dong, H.Z.; Zhao, H.J. Influence of Al content on microstructure, mechanical and tribological properties of Ti-W-Al-N composite films. Vacuum 2017, 137, 31–37. [Google Scholar] [CrossRef]
- Patscheider, J.; Thomas, N.Z.; Matthieu, D. Structure-performance relations in nanocomposite coatings. Surf. Coat. Technol. 2001, 146, 201–208. [Google Scholar] [CrossRef]
- Erdemir, A.; Andrey, A.V. Nanocomposite Coatings for Severe Applications, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 679–715. [Google Scholar]
- Ju, H.B.; Zhou, R.; Liu, S.J.; Yu, L.H.; Xu, J.H.; Geng, Y.X. Enhancement of the tribological behavior of self-lubricating nanocomposite Mo2N/Cu films by adding the amorphous SiNx. Surf. Coat. Technol. 2021, 423, 127565–127574. [Google Scholar] [CrossRef]
- Mulligan, C.P.; Thierry, A.B.; Daniel, G. CrN-Ag nanocomposite coatings: High-temperature tribological response. Wear 2010, 269, 125–131. [Google Scholar] [CrossRef]
- Musil, J.; Zeman, P. Hard a-Si3N4/MeNx Nanocomposite Coatings with High Thermal Stability and High Oxidation Resistance. Solid State Phenom. 2007, 127, 31–36. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Prasad, S.V.; Zabinski, J.S. Nanocrystalline carbide/amorphous carbon composites. J. Appl. Phys. 1997, 82, 855–858. [Google Scholar] [CrossRef]
- Yu, L.H.; Zhao, H.J.; Xu, J.J. Mechanical, tribological and corrosion performance of WBN composite films deposited by reactive magnetron sputtering. Appl. Surf. Sci. 2014, 315, 380–386. [Google Scholar] [CrossRef]
- Miyoshi, K. Durability evaluation of selected solid lubricating films. Wear 2001, 251, 1061–1067. [Google Scholar] [CrossRef] [Green Version]
- Ju, H.B.; Zhou, R.; Luan, J.; Yu, L.H.; Xu, J.H.; Zuo, B.; Yang, J.F.; Geng, Y.X.; Zhao, L.J.; Filipe, F. Multilayer Mo2N-Ag/SiNx films for demanding applications: Morphology, structure and temperature-cycling tribological properties. Mate. Design 2022, 223, 111128–111138. [Google Scholar]
- DellaCorte, C. Tribological Composition Optimization of Chromium-Carbide-Based Solid Lubricant Coatings for Foil Gas Bearings at Temperatures to 650 °C; NASA Contractor Report; NASA: Cleveland, OH, USA, 1987; p. 179649. [Google Scholar]
- DellaCorte, C. Experimental Evaluation of Chromium-Carbide-Based Solid Lubricant Coatings for Use to 760 °C; NASA Contractor Report; NASA: Cleveland, OH, USA, 1987; p. 180808. [Google Scholar]
- DellaCorte, C.; Laskowski, J.A. Tribological evaluation of PS300: A new chrome oxide-based solid lubricant coating sliding against Al2O3 from 25 to 650 °C. Tribol. Trans. 1997, 40, 163–167. [Google Scholar] [CrossRef]
- DellaCorte, C.; Fellenstein, J.A. The effect of compositional tailoring on the thermal expansion and tribological properties of PS300: A solid lubricant composite coating. Tribol. Trans. 1997, 40, 639–642. [Google Scholar] [CrossRef]
- DellaCorte, C.; Stanford, M.K.; Thomas, F.; Edmonds, B.J. The effect of composition on the surface finish of PS400: A new high temperature solid lubricant coating. In Proceedings of the 65th Annual Meeting and Exhibition, NASA/TM, Las Vegas, NV, USA, 1 September 2010; p. 216774. [Google Scholar]
- Heshmat, H.; Hryniewicz, P.; Walton, I.J.; Willis, J.P.; Jahanmir, S.; DellaCorte, C. Low friction wear-resistant coatings for high-temperature foil bearings. Tribol. Int. 2005, 38, 1059–1075. [Google Scholar] [CrossRef]
- Radil, K.; DellaCorte, C. The performance of PS400 subjected to sliding contact at temperatures from 260 to 927 °C. Tribol. Trans. 2017, 60, 957–964. [Google Scholar] [CrossRef]
- DellaCorte, C.; Edmonds, B.J. NASA PS400: A New High Temperature Solid Lubricant Coating for High Temperature Wear Applications; NASA: Cleveland, OH, USA, 2009; p. 215678. [Google Scholar]
- Zhu, S.Y.; Cheng, J.; Qiao, Z.H.; Yang, J. High temperature solid-lubricating materials: A review. Tribol. Int. 2019, 133, 206–223. [Google Scholar] [CrossRef]
- Andong, D.; Lucia, L.; Jarfors, A.E.W.; Zhou, J.; Zheng, J.C.; Wang, K.K.; Yu, G.G. The Influence of Ce, La, and SiC Particles Addition on the Formability of an Al-Si-Cu-Mg-Fe SiCp-MMC. Materials 2022, 15, 3789–3810. [Google Scholar]
- Lazarus, B.S.; Velasco-Hogan, A.; Gómez-del Río, T.; Meyers, M.A.; Iwona, M.J. A review of impact resistant biological and bioinspired materials and structures. J. Mater. Res. Technol. 2020, 9, 15705–15738. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Jeffrey, S.Z. Nanocomposite and nanostructured tribological materials for space applications. Compos. Sci. Technol. 2005, 65, 741–748. [Google Scholar] [CrossRef]
- Sproul, W.D. Reactive Sputter Deposition of Superhard Polycrystalline Nanolayered Coatings. MRS Online Proc. Libr. 1996, 434, 47–55. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Michael, A.C.; Safriet, A.J.; Michael, S.D.; Jeffrey, S.Z. Combined magnetron sputtering and pulsed laser deposition of carbides and diamond-like carbon films. Appl. Phys. Lett. 1996, 69, 188–190. [Google Scholar] [CrossRef]
- Sproul, W.D. Reactive sputter deposition of polycrystalline nitride and oxide superlattice coatings. Surf. Coat. Technol. 1996, 86, 170–176. [Google Scholar] [CrossRef]
- Vepřek, S.; Zhang, R.F.; Maritza, G.J.; Vepřek, H.; Sheng, S.H.; Argon, A.S. Superhard nanocomposites: Origin of hardness enhancement, properties and applications. Surf. Coat. Technol. 2010, 204, 1898–1906. [Google Scholar] [CrossRef]
- Vepřek, S.; Niederhofer, A.; Moto, K.; Tibor, B.; Männling, H.D.; Nesládek, P.; Dollinger, G.; Bergmaier, A. nanostructure and origin of the ultrahardness in nc-TiN/α-Si3N4/α- and nc-TiSi2 nanocomposites with HV = 80 to ≥ 105 GPa. Surf. Coat. Technol. 2000, 133134, 152–159. [Google Scholar] [CrossRef]
- Vepřek, S. The search for novel, superhard materials. J. Vac. Sci. Technol. 1999, 17, 2401–2420. [Google Scholar] [CrossRef]
- Vepřek, S. Recent search for new superhard materials: Go nano! J. Vac. Sci. Technol. 2013, 31, 50822–50854. [Google Scholar] [CrossRef]
- Vepřek, S.; Maritza, G.J.; Vepřek, H. The formation and role of interfaces in superhard nc-MenN/α-Si3N4 nanocomposites. Surf. Coat. Technol. 2007, 201, 6064–6070. [Google Scholar] [CrossRef]
- Vepřek, S.; Maritza, G.J.; Vepřek, H. Industrial applications of superhard nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 5063–5073. [Google Scholar] [CrossRef]
- Vepřek, S.; Maritza, G.J.; Vepřek, H. Limits to the preparation of superhard nanocomposites: Impurities, deposition and annealing temperature. Thin Solid Film. 2012, 522, 274–282. [Google Scholar] [CrossRef]
- Vepřek, S.; Zafar, I.; Sarott, F.A. A thermodynamic criterion of the crystalline-to-amorphous transition in silicon. Philos. Mag. Part B 1982, 45, 137–145. [Google Scholar] [CrossRef]
- Vepřek, S.; Weidmann, J.; Glatz, F. Plasma chemical vapor deposition and properties of hard C3N4 thin films. J. Vac. Sci. Technol. 1995, 13, 2914–2919. [Google Scholar] [CrossRef]
- Vepřek, S.; Haussmann, M.; Sieghard, D.I.R.; Li, S.Z.; Jia, D. Novel thermodynamically stable and oxidation resistant superhard coating materials. Surf. Coat. Technol. 1996, 86, 394–401. [Google Scholar] [CrossRef]
- Yang, L.N.; Wen, M.; Dai, X.; Cheng, G.; Zhang, K. Ultrafine Ceramic Grains Embedded in Metallic Glass Matrix: Achieving Superior Wear Resistance via Increase in Both Hardness and Toughness. ACS Appl. Mater. Interfaces 2018, 10, 16124–16132. [Google Scholar] [CrossRef]
- Ren, P.; Wen, M.; Zhang, K.; Du, S.X.; Zhang, Y.D.; Chen, J.H.; Zheng, W.T. Self-assembly of TaC@Ta core-shell-like nanocomposite film via solid-state dewetting: Toward superior wear and corrosion resistance. Acta. Mater. 2018, 160, 72–84. [Google Scholar] [CrossRef]
- Roth, S.V.; Helmut, W.; Manfred, B.; Christian, R.; Bruno, L.; Christian, G.S.; Marion, K.; Thomas, W.; Angelika, S.; Ralph, D.; et al. Combinatorial investigation of the isolated nanoparticle to coalescent layer transition in a gradient sputtered gold nanoparticle layer on top of polystyrene. Appl. Phys. Lett. 2006, 88, 21910–21913. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Lu, X.L.; Wang, C.; Sui, X.D.; Wang, Y.F.; Zhou, H.L.; Hao, J.Y. Tailoring the microstructure, mechanical and tribocorrosion performance of (CrNbTiAlV)Nx high-entropy nitride films by controlling nitrogen flow. J. Mater. Sci. Technol. 2021, 107, 172–182. [Google Scholar] [CrossRef]
- Rost, C.M.; Edward, S.; Trent, B.; Moballegh, A.; Elizabeth, C.D.; Hou, D.; Jacob, L.J.; Stefano, C.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements Novel Alloy Design. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, X.L.; Cheng, H.F.; Liu, J.W.; Shao, M.F.; Wei, M.; David, G.E.; Zhang, H.; Duan, X. Confined Synthesis of 2D Nanostructured Materials toward Electrocatalysis. Adv. Energy Mater. 2019, 10, 1900486–1900502. [Google Scholar] [CrossRef]
- Holleck, H. Material selection for hard coatings. J. Vac. Sci. Technol. A 1986, 4, 26612670. [Google Scholar] [CrossRef]
- Kanyanta, V. Microstructure-Property Correlations for Hard, Superhard, and Ultrahard Materials; Springer: Cham, Switzerland, 2016; pp. 75–103. [Google Scholar]
- Cheng, Y.; Yuan, W.X.; Xu, J.H.; Yu, L.H.; Hu, Y.X.; Huang, T.; Zhang, H. Ceramic particle-induced accelerated solution-aging behavior of spray-formed 7055 aluminum alloy TIG weld metal. J. Mater. Sci. 2023, 58, 802–817. [Google Scholar] [CrossRef]
- Bewilogua, K.; Clark, V.C.; Colin, S.; Schröder, J.; Wittorf, R.; Grischke, M. Effect of target material on deposition and properties of metal-containing DLC (Me-DLC) coatings. Surf. Coat. Technol. 2000, 127, 223–231. [Google Scholar] [CrossRef]
- McSkimming, B.; Catherine, C.; James, S.S. High active nitrogen flux growth of GaN by plasma assisted molecular beam epitaxy. J. Vac. Sci. Technol. 2015, 33, 128–136. [Google Scholar] [CrossRef]
- Devia, D.M.; Elisabeth, R.P.; Arango, P.J.; André, P.T.; Juan, M.V. TiAlN coatings deposited by triode magnetron sputtering varying the bias voltage. Appl. Surf. Sci. 2011, 257, 6181–6185. [Google Scholar] [CrossRef]
- Lewin, E. Multi-component and high-entropy nitride coatings-A promising field in need of a novel approach. J. Appl. Phys. 2020, 127, 160901–160913. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, D.; Fu, Y.Q.; Du, H.J. Recent advances of superhard nanocomposite coatings: A review. Surf. Coat. Technol. 2003, 167, 113–119. [Google Scholar] [CrossRef]
- Kumar, A.; Chung, Y.W.; Moore, J.J.; Smugeresky, J.E.E. Surface Engineering: Science and Technology I. Miner. Met. Mater. Ser. 1999, 1, 207–231. [Google Scholar]
- Zeman, P.; Michal, Z.; Zuzjakov, Š.; Radomír, Č. Amorphous Zr-Cu thin-film alloys with metallic glass behavior. J. Alloys Compd. 2017, 696, 1298–1306. [Google Scholar] [CrossRef]
- Červená, M.; Houška, J.; Čerstvý, R.; Zeman, P. On thermal stability and oxidation behavior of metastable W-Zr thin-film alloys. J. Alloys Compd. 2022, 925, 166599–166606. [Google Scholar] [CrossRef]
- Ju, H.B.; Ding, N.; Xu, J.H.; Yu, L.H.; Geng, Y.Y.; Yi, G.; Wei, T.Y. Improvement of tribological properties of niobium nitride films via copper addition. Vacuum 2018, 158, 1–5. [Google Scholar] [CrossRef]
- Bian, S.N.; Yu, L.H.; Xu, J.H.; Xu, J.D. Impact of Ag on the microstructure and tribological behaviors of adaptive ZrMoN–Ag composite lubricating films. J. Mater. Res. Technol. 2022, 19, 2346–2355. [Google Scholar] [CrossRef]
- Kumar, R.; Irina, H.; Ramin, R.; Maksim, A. Solid Lubrication at High-Temperatures-A Review. Materials 2022, 15, 1695. [Google Scholar] [CrossRef]
- Torres, H.; Manel, R.R.; Braham, P. Tribological behaviour of self-lubricating materials at high temperatures. Int. Mater. Rev. 2018, 63, 309–340. [Google Scholar] [CrossRef]
- Sohail, A.S. A critical review on self-lubricating ceramic-composite cutting tools. Ceram. Int. 2021, 47, 20745–20767. [Google Scholar]
- Erdemir, A.; Jean, M.M. Superior wear resistance of diamond and DLC coatings. Curr. Opin. Solid St. M. 2018, 22, 243–254. [Google Scholar] [CrossRef]
- Liu, A.H. Friction and Wear Behaviors of PVD Nitride Coatings at Elevated Temperature. Ph.D. Thesis, Shandong University, Jinan, China, December 2012. [Google Scholar]
- Li, B. Design and Development of In-Situ Reaction Self-Lubricating Ceramic Tools and Study on Their Antifriction Mechanisms. Ph.D. Thesis, Shandong University, Jinan, China, April 2010. [Google Scholar]
- Varenberg, M. Towards a unified classification of wear. Friction 2013, 1, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.X.; Zhang, H.; Xie, X.J.; Wang, E.D.; Lin, X.Y.; Song, Y.X.; Liu, G.D.; Chen, G.F. Effects of substrate pretreatment and annealing processes on AlN thin films prepared by EVPE. Mater. Sci. Semicond. Process. 2022, 150, 106975. [Google Scholar] [CrossRef]
- Azman, Z.; Nafarizal, N.; Ahmad, S.A.B.; Zamri, Y.; Mohamad, H.M.; Muliana, T.; Norain, B.S.; Mohd, Y.A. Deposition of AlN Thin Film by High-Power Impulse Magnetron Sputtering with Tilted Sputter Target at Different Working Pressure. In Proceedings of the 2020 IEEE Student Conference on Research and Development (SCOReD), Batu Pahat, Malaysia, 27–29 September 2020; pp. 1–5. [Google Scholar]
- Mohamed, N.A.; Javad, S.; Aznan, F.I.; Jailani, M.F.A.M.; Muhammad, N.K.; Mohamad, F.M.N.; Amin, A.; Nasir, S.N.S.; Jagdeep, S.S.; Mohd, A.M.T. The influences of post-annealing temperatures on fabrication graphitic carbon nitride, (g-C3N4) thin film. Appl. Surf. Sci. 2019, 489, 92–100. [Google Scholar] [CrossRef]
- Bhuvaneswari, H.B.; V-Rajagopal, R.; Chandramani, R.; Mohan, R.G. Annealing effects on zirconium nitride films. Appl. Surf. Sci. 2004, 230, 88–93. [Google Scholar] [CrossRef]
- Yu, L.H.; Dong, S.R.; Xu, J.H.; Isao, K. Microstructure and hardening mechanisms in a-Si3N4/nc-TiN nanostructured multilayers. Thin Solid Films 2008, 516, 1864–1870. [Google Scholar] [CrossRef]
- Hong, C.F.; Huan, Y.X.; Zhang, P.S.; Zhang, K.; Dai, P.Q. Effect of silver content on the microstructure, thermal stability and mechanical properties of CrNx/Ag nanocomposite films. Ceram. Int. 2021, 47, 25324–25336. [Google Scholar] [CrossRef]
- Du, X.Y.; Gao, B.; Li, Y.H.; Song, Z.X.; Zhu, X.D.; Chen, J. Fabrication of multiscale structured hydrophobic NiCrZrN coating with high abrasion resistance using multi-arc ion plating. J. Alloys Compd. 2020, 812, 152140–152147. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Anders, H.; Lennart, K.; Jacob, S.; Tommy, L.; Christian, M.; Lars, H. Self-organized nanostructures in the Ti-Al-N system. Appl. Phys. Lett. 2003, 83, 2049–2051. [Google Scholar] [CrossRef]
- Xu, J.H.; Ju, H.B.; Yu, L.H. Influence of silicon content on the microstructure, mechanical and tribological properties of magnetron sputtered Ti-Mo-Si-N films. Vacuum 2014, 110, 47–53. [Google Scholar] [CrossRef]
- Lou, B.S.; Wang, C.J.; Chen, Y.Y.; Hung, S.B.; Lin, Y.C.; Lee, J. Phase, mechanical property and corrosion resistance evaluation of W-Nb-Ta-Ti and W-Nb-Ta-Ti-N medium entropy alloy thin films. Surf. Coat. Technol. 2022, 442, 128339–128348. [Google Scholar] [CrossRef]
- Xu, J.H.; Chen, J.; Yu, L.H. Influence of Si content on the microstructure and mechanical properties of VSiN films deposited by reactive magnetron sputtering. Vacuum 2016, 131, 51–57. [Google Scholar] [CrossRef]
- Chen, Z.; Zhen, Y.H.; Huang, Y.; Gao, Z.C.; Sheng, H.P.; Matthias, B.; Paul, H.M.; Zhang, Z.L. Atomic-scale understanding of the structural evolution of TiN/AlN superlattice during nanoindentation—Part 1: Deformation. Acta Mater 2022, 234, 118008–118020. [Google Scholar] [CrossRef]
- Sandu, C.S.; Sanjinés, R.; Benkahoul, M.; Medjani, F.; Lévy, F. Formation of composite ternary nitride thin films by magnetron sputtering co-deposition. Surf. Coat. Technol. 2006, 201, 4083–4089. [Google Scholar] [CrossRef]
- Hugosson, H.W.; Jansson, U.; Johansson, B.; Eriksson, O. Restricting Dislocation Movement in Transition Metal Carbides by Phase Stability Tuning. Science 2001, 293, 2434–2437. [Google Scholar] [CrossRef]
- Xiang, H.; Li, H.; Fu, T.; Zhao, Y.; Huang, C.; Zhang, G.; Peng, X. Molecular dynamics simulation of AlN thin films under nanoindentation. Ceram. Int. 2017, 43, 4068–4075. [Google Scholar] [CrossRef]
- Koutná, N.; Löflfler, L.; Holec, D.; Chen, Z.; Zhang, Z.; Hultman, L.; Mayrhofer, P.H.; Sangiovanni, D.G. Atomistic mechanisms underlying plasticity and crack growth in ceramics: A case study of AlN/TiN superlattices. Acta Mater. 2022, 229, 117809–117821. [Google Scholar] [CrossRef]
- Sangiovanni, D.G.; Ferenc, T.; Lars, J.S.J.; Odén, M.; Igor, A.A. Strength, transformation toughening, and fracture dynamics of rocksalt-structure Ti1−xAlxN (0 ≤ x ≤ 0.75) alloys. Phys. Rev. Mater. 2020, 4, 33605–33616. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.F.; Veprek, S. Deformation paths and atomistic mechanism of B4→B1 phase transformation in aluminium nitride. Acta Mater. 2009, 57, 2259–2265. [Google Scholar] [CrossRef]
- Ivashchenko, V.; Patrice, E.A.T.; Shevchenko, R.V.; Leonid, G.; Jerzy, L. First-principles investigations of the pressure-induced phase transformations and properties of crystalline and amorphous AlN. Phys. Rev. Mater. 2020, 4, 113605–113614. [Google Scholar] [CrossRef]
- Zhuo, C.; Zhen, Y.H.; Huang, Y.; Gao, Z.M.; Sheng, H.P.; Bartosik, M.; Paul, H.M.; Zhang, Z.L. Atomic-scale understanding of the structural evolution in TiN/AlN superlattice during nanoindentation—Part 2. Acta Mater. 2022, 234, 118009–118021. [Google Scholar]
- Kumar, Y.; Wang, F.; Aboulfadl, H.; Jenifer, B.; Lina, R.; Emilio, J.P.; Frank, M.; Ferenc, T.; Magnus, O.; Naureen, G. Growth and thermal stability of TiN/ZrAlN: Effect of internal interfaces. Acta Mater. 2016, 121, 396–406. [Google Scholar]
- Sylwestrowicz, W.D.; Hall, E.O. The Deformation and Ageing of Mild Steel. III. Discussion of results. Proc. Phys. Soc. B 1951, 64, 747–753. [Google Scholar] [CrossRef]
- Li, X.; Li, X.T. A modified Hall-Petch relation for predicting size-induced weakening effect on yield strength of coarse-grained thin films and wires. Mater. Lett. 2023, 344, 134461–134464. [Google Scholar] [CrossRef]
- Huang, J.H.; Lin, C.G.; Yu, G.P. Texture evolution of vanadium nitride thin films. Thin Solid Films 2019, 688, 137415–137424. [Google Scholar] [CrossRef]
- Carsley, J.E.; Ning, J.; Milligan, W.W.; Hackney, S.A.; Aifantis, E.C. A simple, mixtures-based model for the grain size dependence of strength in nanophase metals. J. Nanomater. 1995, 5, 441–448. [Google Scholar] [CrossRef]
- Song, H.W.; Guo, S.R.; Hu, Z.Q. A coherent polycrystal model for the inverse Hall-Petch relation in nanocrystalline materials. J. Nanomater. 1999, 11, 203–210. [Google Scholar] [CrossRef]
- Hagel, W.C. Superalloys II: High-Temperature Materials for Aerospace and Industrial Power; John Wiley & Sons: New York, NY, USA, 1987; pp. 61–69. [Google Scholar]
- Cruz, R.; Rao, J.; Rose, T.; Lawson, K.; Nicholls, J.R. DLC-ceramic multilayers for automotive applications. Diam. Relat. Mater. 2006, 15, 2055–2060. [Google Scholar] [CrossRef]
- Abadias, G.; Dub, S.; Shmegera, R. Nanoindentation hardness and structure of ion beam sputtered TiN, W and TiN/W multilayer hard coatings. Surf. Coat. Technol. 2006, 200, 6538–6543. [Google Scholar] [CrossRef]
- Egami, T. Atomic Short-Range Ordering in Amorphous Metal Alloys. In Amorphous Metallic Alloys; Elsevier: Amsterdam, The Netherlands, 1983; pp. 100–113. [Google Scholar]
- Alan, J.A. Precipitation Hardening. Metall. Mater. Trans. A 1985, 16, 2131–2165. [Google Scholar]
- Shaginyan, L.R.; Alexander, V.K. Mechanisms for Hardening Film Materials: W-Ti-and TiN-Cu Systems as Examples. Powder Metall. Met. Ceram. 2005, 44, 161–168. [Google Scholar] [CrossRef]
- Subramanian, C.; Strafford, K.N. Review of multicomponent and multilayer coatings for tribological applications. Wear 1993, 165, 85–95. [Google Scholar] [CrossRef]
- Harrington, T.J.; Joshua, G.; Pranab, S.; Cormac, T.; Christina, M.R.; Olivia, F.D.; Cameron, M.; Kevin, K.; Eduardo, M.; Borowski, L.; et al. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Mate. 2019, 166, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Krug, M.E.; Mao, Z.; Seidman, D.N.; Dunand, D.C. Comparison between dislocation dynamics model predictions and experiments in precipitations trengthened Al-Li-Sc alloys. Acta Mater. 2014, 79, 382–395. [Google Scholar] [CrossRef] [Green Version]
- Booth-Morrison, C.; Seidman, D.N.; Dunand, D.C. Effect of Er additions on ambient and high-temperature strength of precipitation-strengthened Al-Zr-Sc-Si alloys. Acta Mater. 2012, 60, 3643–3654. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Jun, H.; Zhang, Y.D.; Ren, P.; Zhang, Z.; Chen, J.H.; Du, S.X.; Wang, M.J.; Wen, M. Spinodal decomposition in the Ta-Mo-Al-N films activated by Mo incorporation: Toward enhanced hardness and toughness. Ceram. Int. 2018, 44, 21358–21364. [Google Scholar]
- Rachbauer, R.; Mass, S.; Stergar, E.; Holec, D.; Kiener, D.; Keckes, J.; Patscheider, J.; Michael, S.; Harald, L.; Paul, H.M. Decomposition pathways in age hardening of Ti-Al-N films. J. Appl. Phys. 2011, 110, 23515–23524. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, L.; Anders, H.; Mats, P.J.; Lars, H.; Ganapathiraman, R. The influence of thermal annealing on residual stresses and mechanical properties of arc-evaporated TiCxN1−x (x = 0, 0.15 and 0.45) thin films. Acta Mater. 2002, 50, 5103–5114. [Google Scholar] [CrossRef]
- Bielawsk, M. Residual stress control in TiN/Si coatings deposited by unbalanced magnetron sputtering. Surf. Coat. Technol. 2006, 200, 3987–3995. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Yang, H.S.; Pang, X.L.; Gao, K.W.; Alex, A.V. Microstructure, residual stress, and fracture of sputtered TiN films. Surf. Coat. Technol. 2013, 224, 120–125. [Google Scholar] [CrossRef]
- Oettel, H.; Wiedemann, R. Residual stresses in PVD hard coatings. Surf. Coat. Technol. 1995, 76–77, 265–273. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Zhen, Y.J.; Sun, H.; Leng, Y.X.; Huang, N. Numerical and Experimental Study of Residual Stress of Multilayer Diamond-Like Carbon Films Prepared by Filtered Cathodic Vacuum Arc Deposition. IEEE Trans. Plasma Sci. 2012, 40, 2261–2266. [Google Scholar] [CrossRef]
- Rafaja, D.; Václav, V.; Anthony, J.P.; James, R.T. Depth profile of residual stress in metal-ion implanted TiN coatings. Surf. Coat. Technol. 1997, 92, 135–141. [Google Scholar] [CrossRef]
- Karlsson, L.; Lars, H.; Jan, E.S. Influence of residual stresses on the mechanical properties of TiCxN1−x (x = 0, 0.15, 0.45) thin films deposited by arc evaporation. Thin Solid Films 2000, 371, 167–177. [Google Scholar] [CrossRef]
- Martin, P.J.; Bendavid, A.; Kinde, T.J.; Wielunski, L. The deposition of TiN thin films by nitrogen ion assisted deposition of Ti from a filtered cathodic arc source. Surf. Coat. Technol. 1996, 86–87, 271–278. [Google Scholar] [CrossRef]
- Davis, C.A. A simple model for the formation of compressive stress in thin films by ion bombardment. Thin Solid Film. 1993, 226, 30–34. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Guido, T.; Christian, M. Microstructure and mechanical/thermal properties of Cr-N coatings deposited by reactive unbalanced magnetron sputtering. Surf. Coat. Technol. 2001, 142, 78–84. [Google Scholar] [CrossRef]
- Chang, Z.C. Structure and properties of duodenary (TiVCrZrNbMoHfTaWAlSi)N coatings by reactive magnetron sputtering. Mater. Chem. Phys. 2018, 220, 98–110. [Google Scholar] [CrossRef]
- Kristina, J.; Lars, R.; Stefan, F.; Erik, L. Multicomponent Hf-Nb-Ti-V-Zr nitride coatings by reactive magnetron sputter deposition. Surf. Coat. Technol. 2018, 349, 529–539. [Google Scholar]
- Wang, J.J.; Chang, S.Y.; Ouyang, F.Y. Effect of substrate bias on the microstructure and properties of (AlCrSiNbZr)Nx high entropy nitride thin film. Surf. Coat. Technol. 2020, 393, 125796–125806. [Google Scholar] [CrossRef]
- Lin, Y.C.; Hsu, S.Y.; Song, R.W.; Lo, W.L.; Lai, Y.T.; Tsai, S.Y.; Duh, J.G. Improving the hardness of high entropy nitride (Cr0.35Al0.25Nb0.12Si0.08V0.20)N coatings via tuning substrate temperature and bias for anti-wear applications. Surf. Coat. Technol. 2020, 403, 126417–126424. [Google Scholar] [CrossRef]
- Ding, J.C.; Mei, H.J.; Li, Q.G.; Zhao, Z.T.; Shen, Y.Q.; Cheng, L.X.; Wang, R.; Gong, W.P.; Wang, Q.M. Microstructure, mechanical and tribological properties of Mo–V–Cu–N coatings prepared by HIPIMS technique. Ceram. Int. 2022, 48, 10704–10712. [Google Scholar] [CrossRef]
- Cao, F.Y.; Paul, M.; Zhou, Z.F.; Xie, Z.H. Medium entropy alloy CoCrNi coatings: Enhancing hardness and damage-tolerance through a nanotwinned structuring. Surf. Coat. Technol. 2018, 335, 257–264. [Google Scholar] [CrossRef]
- Chang, H.W.; Huang, P.K.; Yeh, J.W.; Andrew, D.; Tsau, C.H.; Yang, C.C. Influence of substrate bias, deposition temperature and post-deposition annealing on the structure and properties of multi-principal-component (AlCrMoSiTi)N coatings. Surf. Coat. Technol. 2008, 202, 3360–3366. [Google Scholar] [CrossRef]
- Tsai, D.C.; Chang, Z.C.; Kuo, L.Y.; Lin, T.J.; Lin, T.N.; Shieu, F.S. Solid solution coating of (TiVCrZrHf)N with unusual structural evolution. Surf. Coat. Technol. 2013, 217, 84–87. [Google Scholar] [CrossRef]
- Ju, H.B.; Zhou, R.; Luan, J.; Kumar, C.S.; Yu, L.H.; Xu, J.H.; Yang, J.F.; Zhang, B.W.; Fernandes, F. Tribological performance under different environments of Ti-C-N composite films for marine wear-resistant parts. Int. J. Miner. Metall. Mater. 2023, 30, 144–155. [Google Scholar] [CrossRef]
- Li, H.; Jiang, N.; Li, J.L.; Huang, J.W.; Kong, J.; Xiong, D.S. Hard and tough (NbTaMoW)Nx high entropy nitride films with sub-stoichiometric nitrogen. J. Alloys Compd. 2021, 889, 161713–161722. [Google Scholar] [CrossRef]
- Chin, C.Z.; Tsa, D.C.; Chen, E.C. Structure and characteristics of reactive magnetron sputtered (CrTaTiVZr)N coatings. Mater. Sci. Semicond. Process. 2015, 39, 30–39. [Google Scholar]
- Cheng, K.H.; Weng, C.H.; La, C.H.; Lin, S.J. Study on adhesion and wear resistance of multi-element (AlCrTaTiZr)N coatings. Thin Solid Film. 2009, 517, 4989–4993. [Google Scholar] [CrossRef]
- Shinn, M.; Mirkarimi, P.B.; Barnett, S.A. Nucleation of lattice-mismatched transition-metal nitride films: Limitations on super-lattice growth. Surf Sci. 1993, 281, 1–9. [Google Scholar] [CrossRef]
- Ziebert, C.; Sven, U. Hard multilayer coatings containing TiN and/or ZrN: A review and recent progress in their nanoscale characterization. J. Vac. Sci. Technol. 2006, 24, 554–583. [Google Scholar] [CrossRef]
- Koehler, J.S. Attempt to Design a Strong Solid. Phys. Rev. B 1970, 2, 547–551. [Google Scholar] [CrossRef]
- Matthew, D.; Sandip, H.; Vignesh, K.R.; Jonathan, M.; Glenn, D.H.; Chandra, V.S. Size effects in strengthening of NiCo multilayers with modulated microstructures. Mat. Sci. Eng.-A Struct. 2020, 771, 138581–138591. [Google Scholar]
- Zuo, B.; Xu, J.H.; Lu, G.Y.; Ju, H.B.; Yu, L.H. Microstructures, mechanical properties and corrosion resistance of TiN/AlN multilayer films. Ceram. Int. 2022, 15, 11629–11635. [Google Scholar] [CrossRef]
- Cahoon, J. An improved equation relating hardness to ultimate strength. Metallurg. Mater. Trans. B 1972, 3, 3040. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.H.; Li, G.Y.; Gu, M.Y. The microstructures and superhardness effect of the polycrystalline TaN/TiN and TaWN/TiN superlattice films. Acta Metall. Sin. 1999, 35, 1214–1218. [Google Scholar]
- Ye, Y.W.; Yao, Y.R.; Chen, H.; Guo, S.D.; Li, J.L.; Wang, L.P. Structure, mechanical and tribological properties in seawater of multilayer TiSiN/Ni coatings prepared by cathodic arc method. Appl. Surf. Sci. 2019, 493, 1177–1186. [Google Scholar] [CrossRef]
- Hahn, R.; Bartosik, M.; Soler, R.; Kirchlechner, C.; Dehm, G.; Mayrhofer, P.H. Superlattice effect for enhanced fracture toughness of hard coatings. Scr. Mater. 2016, 124, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Hahn, R.; Bartosik, M.; Arndt, M.; Polcik, P.; Mayrhofer, P.H. Annealing effect on the fracture toughness of CrN/TiN superlattices. Int. J. Refract. Met. Hard Mater. 2018, 71, 352–356. [Google Scholar] [CrossRef]
- Xu, J.H.; Yu, L.H.; Dong, S.R.; Isao, K. Structure transition of BN layers and its influences on the mechanical properties of AlN/BN nanomultilayers. Thin Solid Films 2008, 516, 8640–8645. [Google Scholar] [CrossRef]
- Chen, Y.M.; Guo, T.; Wang, J.W.; Pang, X.L.; Qiao, L.J. Effects of orientation on microstructure and mechanical properties of TiN/AlN superlattice films. Scr. Mater 2021, 201, 113951–113955. [Google Scholar] [CrossRef]
- Fallmann, M.; Chen, Z.; Zhang, Z.L.; Mayrhofer, P.H.; Bartosik, M. Mechanical properties and epitaxial growth of TiN/AlN superlattices. Surf. Coat. Technol. 2019, 375, 1–7. [Google Scholar] [CrossRef]
- Schlögl, M.; Mayer, B.; Paulitsch, J.; Mayrhofer, P.H. Influence of CrN and AlN layer thickness on structure and mechanical properties of CrN/AlN superlattices. Thin Solid Films 2013, 545, 375–379. [Google Scholar] [CrossRef]
- Chen, Z.; David, H.; Matthias, B.; Paul, H.M.; Zhang, Z.L. Crystallographic orientation dependent maximum layer thickness of cubic AlN in CrN/AlN multilayers. Acta Mater. 2019, 168, 190–202. [Google Scholar] [CrossRef]
- Koutná, N.; Řehák, P.; Chen, Z.; Matthias, B.; Markus, F.; Černý, M.; Zhang, Z.L.; Friák, M.; Šob, M.; Paul, H.M.; et al. Correlating structural and mechanical properties of AlN/TiN superlattice films. Scr. Mater. 2019, 165, 159–163. [Google Scholar] [CrossRef]
- Lu, Z.P.; Jiang, Y.Z.; Yu, L.H.; Xu, J.H.; Peng, J.D.; Xiang, K.Y. The effect of minor scandium addition on microstructure evolution and mechanical properties of spray formed Al-Zn-Mg-Cu alloy. J. Alloys Compd. 2023, 948, 169710–169725. [Google Scholar] [CrossRef]
- Christopher, B.M.; David, C.D.; David, N.S. Coarsening resistance at 400 °C of precipitation-strengthened Al-Zr-Sc-Er alloys. Acta Mater. 2011, 59, 7029–7042. [Google Scholar]
- Keith, E.K.; Richard, A.K.; Constance, P.L.; David, C.D.; David, N.S. Precipitation evolution in Al-0.1Sc, Al-0.1Zr and Al-0.1Sc-0.1Zr (at.%) alloys during isochronal aging. Acta Mater. 2010, 58, 5184–5195. [Google Scholar]
- Liang, Y.C.; Gao, Z.B.; Qin, P.L.; Gao, L.; Tang, C. The mechanism of anomalous hardening in transition-metal monoborides. Nanoscale 2017, 9, 9112–9118. [Google Scholar] [CrossRef]
- Kindlund, H.; Davide, G.S.; Petrov, I.S.; Joseph, E.G.; Lars, H. A review of the intrinsic ductility and toughness of hard transition-metal nitride alloy thin films. Thin Solid Film. 2019, 688, 137479–137489. [Google Scholar] [CrossRef]
- Karthik, B.; Sanjay, V.K.; Daniel, G. Valence electron concentration as an indicator for mechanical properties in rocksalt structure nitrides, carbides and carbonitrides. Acta Mater. 2018, 152, 175–185. [Google Scholar]
- Han, K.C.; Liu, Y.Q.; Lin, G.Q.; Dong, C.; Tai, K.P.; Jiang, X. Study on atomic-scale strengthening mechanism of transition-metal nitride MNx (M = Ti,Zr,Hf) films with wide composition ranges. Acta Metall. Sin. 2016, 52, 1601–1609. [Google Scholar]
- Jhi, S.H.; Jisoon, I.; Steven, G.L.; Marvin, L.C. Electronic mechanism of hardness enhancement in transition-metal carbonitrides. Nature 1998, 399, 132–134. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, Y.Q.; Du, H.J.; Liu, Y.; Chen, T. Nanocomposite thin films for both mechanical and functional applications. Adv. Mater. Micro-Nano-Syst. 2004, 1721, 1–5. [Google Scholar]
- Kretschmer, A.; Kirnbauer, A.; Moraes, V.; Primetzhofer, D.; Kumar, Y.; Helmut, D.R.; Paul, H.M. Improving phase stability, hardness, and oxidation resistance of reactively magnetron sputtered (Al,Cr,Nb,Ta,Ti)N thin films by Si-alloying. Surf. Coat. Technol. 2021, 416, 127162–127173. [Google Scholar] [CrossRef]
- André, A. A structure zone diagram including plasma-based deposition and ion etching. Thin Solid Films 2010, 518, 4087–4090. [Google Scholar]
- Galvin, G.D. lubricats and lubrication. In Mechanical Engineer’s Reference Book, 11th ed.; Elsevier: Amsterdam, The Netherlands, 1973; Volume 14, pp. 2–30. [Google Scholar]
- Aouadi, S.M.; Singh, D.P.; Stone, D.S.; Polychronopoulou, K.; Nahif, F.; Rebholz, C.; Muratore, C.; Voevodin, A.A. Adaptive VN/Ag nanocomposite coatings with lubricious behavior from 25 to 1000 °C. Acta Mater. 2010, 58, 5326–5331. [Google Scholar] [CrossRef]
- Stone, D.S.; Harbin, S.; Mohseni, H.; Mogonye, J.E.; Scharf, T.W.; Muratore, C.; Voevodin, A.A.; Martini, A.; Aouadi, S.M. Lubricious silver tantalate films for extreme temperature applications. Surf. Coat. Technol. 2013, 217, 140–146. [Google Scholar] [CrossRef]
- Liu, C.K.; Ju, H.B.; Xu, J.H.; Yu, L.H.; Zhao, Z.T.; Geng, Y.X.; Zhao, Y. Influence of copper on the compositions, microstructure and room and elevated temperature tribological properties of the molybdenum nitride film. Surf. Coat. Technol. 2020, 395, 125811–125818. [Google Scholar] [CrossRef]
- Yao, Q.Q.; Jia, J.H.; Chen, T.J.; Xin, H.; Shi, Y.; He, N.R.; Feng, X.C.; Shi, P.Y.; Lu, C. High temperature tribological behaviors and wear mechanisms of NiAl-MoO3/CuO composite coatings. Surf. Coat. Technol. 2020, 395, 125910–125918. [Google Scholar] [CrossRef]
- Bian, S.N.; Yu, L.H.; Jia, P.; Xu, J.H. Study on microstructure, mechanical properties and corrosion resistance of NbCN-Cu composite films. Int. J. Refract. Hard Met. 2022, 107, 105885–105891. [Google Scholar] [CrossRef]
- Shi, X.R.; Tomasz, W.L.; Ben, D.B.; Chen, J.; Wang, C. Tribological performance of graphite-like carbon films with varied thickness. Tribol. Int. 2020, 149, 105586–105596. [Google Scholar] [CrossRef]
- Li, Y.L.; Rao, Y.; Mak, K.F.; You, Y.M.; Wang, S.Y.; Cory, R.D.; Tony, F.H. Probing symmetry properties of few-layer MoS2 and h-BN by optical second-harmonic generation. Nano Lett. 2013, 13, 3329–3333. [Google Scholar] [CrossRef]
- Yoshitsugu, K.; Toshiaki, W.; Kazumi, O.; Tetsuya, W.; Hiroshi, N. Boron nitride as a lubricant additive. Wear 1999, 232, 199–206. [Google Scholar]
- Scharf, T.W.; Somuri, V.P. Solid lubricants: A review. J. Mater. Sci. 2012, 48, 511–531. [Google Scholar] [CrossRef]
- Scharf, T.W.; Somuri, V.P.; Michael, T.D.; Paul, G.K.; Ronald, S.G.; Robert, K.G. Growth, structure, and tribological behavior of atomic layer-deposited tungsten disulphide solid lubricant coatings with applications to MEMS. Acta Mater. 2006, 54, 4731–4743. [Google Scholar] [CrossRef]
- Prasad, S.V.; Jeffrey, S.Z. Lubricants: Super slippery solids. Nature 1997, 387, 761–763. [Google Scholar] [CrossRef]
- Allam, I.M. Solid lubricants for applications at elevated temperatures. J. Mater. Sci. 1991, 26, 3977–3984. [Google Scholar] [CrossRef]
- Sliney, H.E. Lubricating Properties of Lead-Monoxide-Base Coatings of Various Compositions at Temperatures to 1250 F. Wear 1959, 2, 495. [Google Scholar]
- Prasad, S.V.; Neil, T.M.; Jeffrey, S.Z. Tribology of tungsten disulfide-nanocrystalline zinc oxide adaptive lubricant films from ambient to 500 °C. Wear 2000, 237, 186–196. [Google Scholar] [CrossRef]
- Walck, S.D.; Jeffrey, S.Z.; Neil, T.M.; John, E.B. Characterization of air-annealed, pulsed laser deposited ZnO-WS2 solid film lubricants by transmission electron microscopy. Thin Solid Film. 1997, 305, 130–143. [Google Scholar] [CrossRef]
- Majety, S.; Cao, X.K.; Dahal, R.; Pantha, B.N.; Li, J.; Lin, J.Y.; Jiang, H.X. Semiconducting hexagonal boron nitride for deep ultraviolet photonics. In Proceedings of the Quantum Sensing and Nanophotonic Devices IX, San Francisco, CA, USA, 21–26 January 2012; SPIE: Bellingham, WA, USA, 2012; Volume 8268. [Google Scholar]
- Zhang, H. Ultrathin Two-Dimensional Nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Kaewmaraya, T.; Pornjuk, S.; Tanveer, H.; Vittaya, A. Electronic Properties of h-BCN-Blue Phosphorene van der Waals Heterostructures. ChemPhysChem. 2018, 19, 612–618. [Google Scholar] [CrossRef]
- Pranav, D.S.; Charoo, M.S. Nano lubrication behaviour of Graphite, h-BN and Graphene Nano Platelets for reducing friction and wear. Mater. Today Proc. 2021, 44, 7–11. [Google Scholar]
- Yu, L.H.; Zhao, H.J.; Xu, J.H. Influence of silver content on structure, mechanical and tribological properties of WCN-Ag films. Mater. Charact. 2016, 114, 136–145. [Google Scholar] [CrossRef]
- Ju, H.B.; Xu, J.H. Microstructure and tribological properties of NbN-Ag composite films by reactive magnetron sputtering. Appl. Surf. Sci. 2015, 355, 878–883. [Google Scholar] [CrossRef]
- Ju, H.B.; Yu, L.H.; Yu, D.; Isaac, A.; Xu, J.H. Microstructure, mechanical and tribological properties of TiN-Ag films deposited by reactive magnetron sputtering. Vacuum 2017, 141, 82–88. [Google Scholar] [CrossRef]
- Wu, F.B.; Yu, L.H.; Ju, H.B.; Isaac, A.; Xu, J.H. Structural, Mechanical and Tribological Properties of NbCN-Ag Nanocomposite Films Deposited by Reactive Magnetron Sputtering. Coatings 2018, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- AL-Rjoub, A.; Cavaleiro, A.; Talha, B.Y.; Evaristo, M.; Figueiredo, N.M.; Fernandes, F. TiAlSiN(Ag) coatings for high temperature applications: The influence of Ag alloying on the morphology, structure, thermal stability and oxidation resistance. Surf. Coat. Technol. 2022, 442, 128087–128094. [Google Scholar] [CrossRef]
- Yu, L.H.; Zhao, H.J.; Ju, H.B.; Xu, J.H. Influence of Cu content on the structure, mechanical and tribological properties of W2N-Cu films. Thin Solid Film. 2017, 624, 144–151. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Muratore, C.; Aouadi, S.M. Hard coatings with high temperature adaptive lubrication and contact thermal management: Review. Surf. Coat. Technol. 2014, 257, 247–265. [Google Scholar] [CrossRef]
- Lesnevskiy, L.; Lyakhovetskiy, M.A.; Lozovan, A.A.; Nikolaev, I.A. Tribological properties of TiN-Pb system solid lubricant coatings with various morphologies. J. Phys. Conf. Ser. 2019, 1281, 12049–12054. [Google Scholar] [CrossRef]
- Lyakhovetskiy, M.A.; Lozovan, A.A.; Lesnevskiy, L.; Nikolaev, I.A.; Pavlov, Y.S. Tribological properties of solid lubricating coatings of the TiN-Pb system at various Pb content. J. Phys. Conf. Ser. 2020, 1713, 12029–12035. [Google Scholar] [CrossRef]
- Stoeva, S.I.; Bhagavatula, L.V.P.; Sitharaman, U.; Peter, K.S.; Vladimir, Z.; Christopher, M.S.; Kenneth, J.K. Face-Centered Cubic and Hexagonal Closed-Packed Nanocrystal Superlattices of Gold Nanoparticles Prepared by Different Methods. J. Phys. Chem. 2003, 107, 7441–7448. [Google Scholar] [CrossRef]
- Rabinowicz, E. Gaps in our knowledge of friction and wear. AIP Conf. Proc. 1976, 32, 165–174. [Google Scholar]
- Mikula, M.; Stela, U.; Tomáš, H.; Branislav, G.; Martin, T.; Tomáš, R.; Švec, P.; Leonid, S.; Čaplovičová, M.; Greczynski, G.; et al. Thermally induced structural evolution and age-hardening of polycrystalline V1-xMoxN (x ≈ 0.4) thin films. Surf. Coat. Technol. 2021, 405, 126723–126733. [Google Scholar] [CrossRef]
- Bak, S.H.; Lee, D.B. Oxidation of Nano-multilayered TiAlSiN Films at 800–900 °C in Air. Oxid. Met. 2015, 84, 345–352. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, J.J.; Yang, Z.B.; Qiu, S.Y.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Improved corrosion resistance of reactive gas pulse sputtered (TiTaNbZrNi)N high entropy alloy coatings with a hybrid architecture of multi-layered and compositionally graded structures. J. Nucl. Mater. 2021, 543, 152558–152566. [Google Scholar] [CrossRef]
- Cai, Q.; Li, S.X.; Pu, J.B.; Cai, Z.B.; Lu, X.; Cui, Q.F.; Wang, L.P. Effect of multicomponent doping on the structure and tribological properties of VN-based coatings. J. Alloys Compd. 2019, 806, 566–574. [Google Scholar] [CrossRef]
- Musil, J. Hard and superhard nanocomposite coatings. Surf. Coat. Technol. 2000, 125, 322–330. [Google Scholar] [CrossRef]
- Phuong, N.T.; Sami, R.; Claudiane, M.O.P. Nanomaterials-based coatings: An introduction. In Nanomaterials-Based Coatings, 1st ed.; Phuong, N.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 1–7. [Google Scholar]
- Wang, D.; Tan, H.; Chen, W.Y.; Zhu, S.G.; Cheng, J.; Yang, J. Tribological behavior of Ni3Al-Ag based self-lubricating alloy with Ag2MoO4 formed by high temperature tribo-chemical reaction. Tribol. Int. 2021, 153, 106659–106669. [Google Scholar] [CrossRef]
- Cao, X.Q.; Shang, L.L.; Liang, Y.M.; Lu, Z.B.; Zhang, G.G.; Xue, Q.J. The effect of tribo-chemical reactions of mating materials on tribological behaviors of the B4C film in various relative humidity environments. Ceram. Int. 2019, 45, 4581–4589. [Google Scholar] [CrossRef]
- Wu, Y.X.; Wang, F.X.; Cheng, Y.Q.; Chen, N.P. A study of the optimization mechanism of solid lubricant concentration in NiMoS2 self-lubricating composite. Wear 1997, 205, 64–70. [Google Scholar] [CrossRef]
- Vepřiek, S.; Niederhofer, A.; Keba, M.; Pavel, N.; Männling, H.D. Nanocomposites nc-TiN/α-Si3N4/a-and nc-TiSi2 with Hardness Exceeding 100 GPa and High Fracture Toughness. MRS Proc. 1999, 581, 321–326. [Google Scholar] [CrossRef]
- Menezes, P.L.; Rohatgi, P.K.; Omrani, E. (Eds.) Self-Lubricating Composites; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–29. [Google Scholar]
- Singer, I.L. Solid Lubricating Films for Extreme Environments. MRS Online Proc. Libr. 1988, 140, 215–226. [Google Scholar] [CrossRef]
- Yu, H.X.; Zheng, Z.W.; Chen, H.J.; Qiao, D.; Feng, D.P.; Gong, Z.B.; Dong, G.J. An investigation of tribochemical reaction kinetics from the perspective of tribo-oxidation. Tribol. Int. 2022, 165, 107289–107298. [Google Scholar] [CrossRef]
- Magnéli, A. Structures of the ReO3-type with recurrent dislocations of atoms: Homologous series of molybdenum and tungsten oxides. Acta Crystallogr. 1953, 6, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Minjeong, K.; Jaemin, C.; Woonghee, L.; Yong-Yoon, A.; Hangil, L.; Kangwoo, C.; Jaesang, L. Performance of Magnéli phase Ti4O7 and Ti3+ self-doped TiO2 as oxygen vacancy-rich titanium oxide anodes: Comparison in terms of treatment efficiency, anodic degradative pathways, and long-term stability. Appl. Catal. B-Environ. 2023, 337, 122993–123002. [Google Scholar]
- Gardos, M.N. Magnéli phases of anion-deficient rutile as lubricious oxides. Part I. Tribological behavior of single-crystal and polycrystalline rutile (TinO2n−1). Tribol. Lett. 2000, 8, 65–78. [Google Scholar] [CrossRef]
- Woydt, M.; Skopp, A.I.; Dörfel, K. Witke. Wear engineering oxides/anti-wear oxides. Wear 1998, 218, 84–95. [Google Scholar] [CrossRef]
- Chavanes, A.; Pauty, E.; Woydt, M. Titanium-molybdenum carbonitride as light-weight and wear resistant monolithic material. Wear 2004, 256, 647–656. [Google Scholar] [CrossRef]
- Reeswinkel, T.; Denis, M.; Jochen, M.S. Ab initio calculations of the structure and mechanical properties of vanadium oxides. J. Phys. Condens. Matter 2009, 21, 145404–145411. [Google Scholar] [CrossRef]
- Usui, E.; Takahiro, S.; Takeaki, K. Analytical prediction of cutting tool wear. Wear 1984, 100, 129–151. [Google Scholar] [CrossRef]
- Arno, P.M.; Laurence, D.M. Liquid-like tribology of gold studied by in situ TEM. Wear 2008, 265, 1864–1869. [Google Scholar]
- Yang, J.; Xia, Y.F.; Song, H.J.; Chen, B.B.; Zhang, Z.Z. Synthesis of the liquid-like graphene with excellent tribological properties. Tribol. Int. 2017, 105, 118–124. [Google Scholar] [CrossRef]
- Gulbiński, W.; Suszko, T.; Sienicki, W.; Warcholiński, B. Tribological properties of silver- and copper-doped transition metal oxide coatings. Wear 2003, 254, 129–135. [Google Scholar] [CrossRef]
- Fateh, N.; Fontalvo, G.A.; Mitterer, C. Tribological Properties of Reactive Magnetron Sputtered V2O5 and VN-V2O5 Coatings. Tribol. Let 2008, 30, 21–26. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Gao, H.; Martini, A.; Scharf, T.W.; Muratore, C. Lubricious oxide coatings for extreme temperature applications: A review. Surf. Coat. Technol. 2014, 257, 266–277. [Google Scholar] [CrossRef]
- Langlois, W.E.; Deville, M.O. Lubrication Theory; Springer: Cham, Switzerland, 2014; pp. 229–249. [Google Scholar]
- Zhu, L.L.; Dong, J.; Zeng, Q.F. High temperature solid/liquid lubrication behaviours of DLC films. Lubr. Sci. 2021, 33, 229–245. [Google Scholar] [CrossRef]
- Erdemir, A. A crystal-chemical approach to lubrication by solid oxides. Tribol. Lett. 2000, 8, 97–102. [Google Scholar] [CrossRef]
- Erdemir, A. A crystal chemical approach to the formulation of self-lubricating nanocomposite. Coat. Surf. Coat. Technol. 2005, 200, 1792–1796. [Google Scholar] [CrossRef]
- Erdemir, A.; Li, S.H.; Jin, Y.S. Relation of Certain Quantum Chemical Parameters to Lubrication Behavior of Solid Oxides. Int. J. Mol. Sci. 2005, 6, 203–218. [Google Scholar] [CrossRef]
- Jungho, S.; Wang, Q.M.; Kwang, H.K. Microstructural evolution and tribological behavior of Mo-Cu-N coatings as a function of Cu content. Mater. Chem. Phys. 2011, 130, 870–879. [Google Scholar]
- Öztürk, A.; Ezirmik, K.V.; Kazmanlı, K.; Ürgen, M.; Eryılmaz, O.L. Comparative tribological behaviors of TiN, CrN and MoNCu nanocomposite coatings. Tribol. Int. 2008, 41, 49–59. [Google Scholar] [CrossRef]
- Reeswinkel, T.; Denis, M.; Jochen, M.S. Coulomb-potential-dependent decohesion of Magnéli phases. J. Phys. Condens. Matter 2010, 22, 292203–292206. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.V.; Walck, S.D.; Zabinski, J.S. Microstructural evolution in lubricious ZnO films grown by pulsed laser deposition. Thin Solid Film. 2000, 360, 107–117. [Google Scholar] [CrossRef]
- Zabinski, J.S.; Jeffrey, H.S.; Josekutty, J.N.; Somuri, V.P. Lubrication using a microstructurally engineered oxide: Performance and mechanisms. Tribol. Lett. 2000, 8, 103–116. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Paudel, Y.; Simonson, W.J.; Ge, Q.; Kohli, P.; Muratore, C.; Voevodin, A.A. Tribological investigation of adaptive Mo2N/MoS2/Ag coatings with high sulfur content. Surf. Coat. Technol. 2009, 203, 1304–1309. [Google Scholar] [CrossRef]
- Gao, H.Y.; Donald, S.S.; Mohseni, H.; Sami, M.A.; Thomas, W.S.; Ashlie, M. Mechanistic studies of high temperature friction reduction in silver tantalate. Appl. Phys. Lett. 2013, 102, 121603–121605. [Google Scholar] [CrossRef]
- Walck, S.D.; Michael, S.D.; Jeffrey, S.Z.; Dyhouse, V.J. Characterization of pulsed laser deposited PbO/MoS2 by transmission electron microscopy. J. Mater. Res. 1994, 9, 236–245. [Google Scholar] [CrossRef]
- Mayur, A.M.; Patel, K.M. Improvement in friction and wear characteristics using CaF2 as a solid lubricant at different conditions. Met. Powder. Rep. 2021, 76, 55–65. [Google Scholar]
- Han, C.M.; Han, S.C.; Changhee, L.; Hyungjun, K.; Soon-Young, H. Evaluation of Solid Lubricant Coatings for Low Friction and Wear Resistance Using the Atmospheric Plasma Spray. Mater. Sci. Forum 2004, 449, 777–780. [Google Scholar] [CrossRef]
- Shiojiri, M.; Toshiyuki, I.; Ohta, M.; Nishio, K.; Hiroshi, S.; Yasufumi, Y.; Noboru, T. Structure of lubricant cerium fluoride powder. Phys. Status Solidi (A) 1994, 142, 9–17. [Google Scholar] [CrossRef]
- John, P.J.; Prasad, S.V.; Voevodin, A.A.; Zabinski, J.S. Calcium sulfate as a high temperature solid lubricant. Wear 1998, 219, 155–161. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Shi, C.C.; Liu, Z.G.; Wang, Y.M.; Wang, Y.J. Fabrication and high-temperature tribological properties of self-lubricating NiCr-BaMoO4 composites. Wear 2015, 330, 272–279. [Google Scholar] [CrossRef]
- Ulrika, P.; Staffan, J. Influence of surface texture on boundary lubricated sliding contacts. Tribol. Int. 2003, 36, 857–864. [Google Scholar]
- Fang, S.Q.; Luis, L.; Dirk, B. Laser surface texturing of a WC-CoNi cemented carbide grade: Surface topography design for honing application. Tribol. Int. 2018, 122, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.G.; Xu, J.; Jin, Z.M.; Braham, P.; Hu, Y.Z. A review of recent advances in tribology. Friction 2020, 8, 221–300. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Fan, H.Z.; Son, J.J.; Zhang, Y.S.; Hu, L.T. Surface engineering design of Al2O3/Mo self-lubricating structural ceramics—Part II: Continuous lubrication effects of a three-dimensional lubricating layer at temperatures from 25 to 800 °C. Wear 2016, 360, 97–103. [Google Scholar]
- Hu, T.C.; Hu, L.T.; Ding, Q. Effective solution for the tribological problems of Ti-6Al-4V: Combination of laser surface texturing and solid lubricant film. Surf. Coat. Technol. 2012, 206, 5060–5066. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Gao, F.; Wei, X.; Liu, G.L.; Hua, M.; Li, P.Y. Fabrication of textured composite surface and its tribological properties under starved lubrication and dry sliding conditions. Surf. Coat. Technol. 2018, 350, 313–322. [Google Scholar] [CrossRef]
- Mao, B.; Siddaiah, A.; Liao, Y.L.; Pradeep, L.M. Laser surface texturing and related techniques for enhancing tribological performance of engineering materials: A review. J. Manuf. Process. 2020, 53, 153–173. [Google Scholar] [CrossRef]
- Chen, L.X.; Liu, Z.Q.; Shen, Q. Enhancing tribological performance by anodizing micro-textured surfaces with nano-MoS2 coatings prepared on aluminum-silicon alloys. Tribol. Int. 2018, 122, 84–95. [Google Scholar] [CrossRef]
- Fan, H.Z.; Su, Y.F.; Song, J.J.; Wan, H.Q.; Hu, L.T.; Zhang, Y.S. Design of “double layer” texture to obtain superhydrophobic and high wear-resistant PTFE coatings on the surface of Al2O3/Ni layered ceramics. Tribol. Int. 2019, 136, 455–461. [Google Scholar] [CrossRef]
- Luster, B.; Stone, D.; Singh, D.P.; Baben, M.T.; Schneider, J.M.; Polychronopoulou, K.; Rebholz, C.; Kohli, P.; Aouadi, S.M. Textured VN coatings with Ag3VO4 solid lubricant reservoirs. Surf. Coat. Technol. 2011, 206, 1932–1935. [Google Scholar] [CrossRef]
- Shimizu, J.; Tomotaka, N.; Kouta, W.; Takeyuki, Y.; Teppei, O.; Hirotaka, O.; Zhou, L.B. Friction characteristics of mechanically microtextured metal surface in dry sliding. Tribol. Int. 2020, 149, 105634–105641. [Google Scholar] [CrossRef]










| Lubricants | Test Temp. (°C) | Load (kg) | Velocity (cm·s−1) | Coefficient of Friction (μ) |
|---|---|---|---|---|
| PbMoO4 | >700 | 7.7 | 0.76 | 0.29 |
| K2MoO4 | 0.20 | |||
| NiMoO4 | 0.29 | |||
| Ag2MoO4 | 0.28 | |||
| FeMoO4 | 0.42 | |||
| Na2WO4 | 0.17 | |||
| Pb2WO4 | 0.35 | |||
| CuWO4 | 0.41 | |||
| FeWO4 | 0.43 | |||
| CaWO4 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Jiang, Y.; Wu, T.; Zuo, B.; Bian, S.; Lu, K.; Zhao, L.; Yu, L.; Xu, J. Insight into the Mechanisms of Nitride Films with Excellent Hardness and Lubricating Performance: A Review. Nanomaterials 2023, 13, 2205. https://doi.org/10.3390/nano13152205
Wu X, Jiang Y, Wu T, Zuo B, Bian S, Lu K, Zhao L, Yu L, Xu J. Insight into the Mechanisms of Nitride Films with Excellent Hardness and Lubricating Performance: A Review. Nanomaterials. 2023; 13(15):2205. https://doi.org/10.3390/nano13152205
Chicago/Turabian StyleWu, Xinmeng, Yaohong Jiang, Tianhao Wu, Bin Zuo, Shunuo Bian, Kun Lu, Lijun Zhao, Lihua Yu, and Junhua Xu. 2023. "Insight into the Mechanisms of Nitride Films with Excellent Hardness and Lubricating Performance: A Review" Nanomaterials 13, no. 15: 2205. https://doi.org/10.3390/nano13152205





