Ag Sinter Bonding to Si Substrate via Temporal Formation and Decomposition of Ag Carboxylate
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
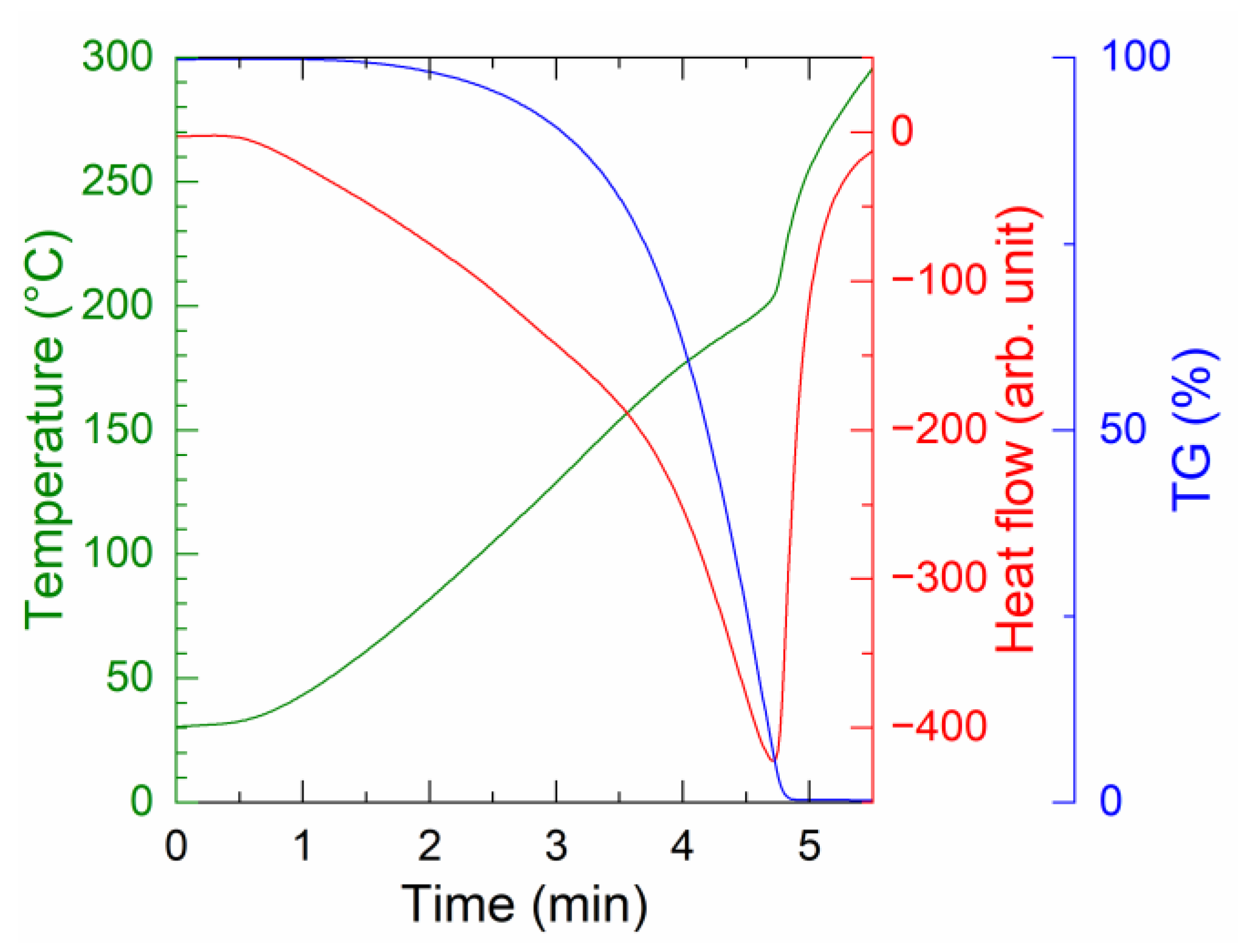
3.1. Low-Temperature Decomposition-Induced Sinter Bonding
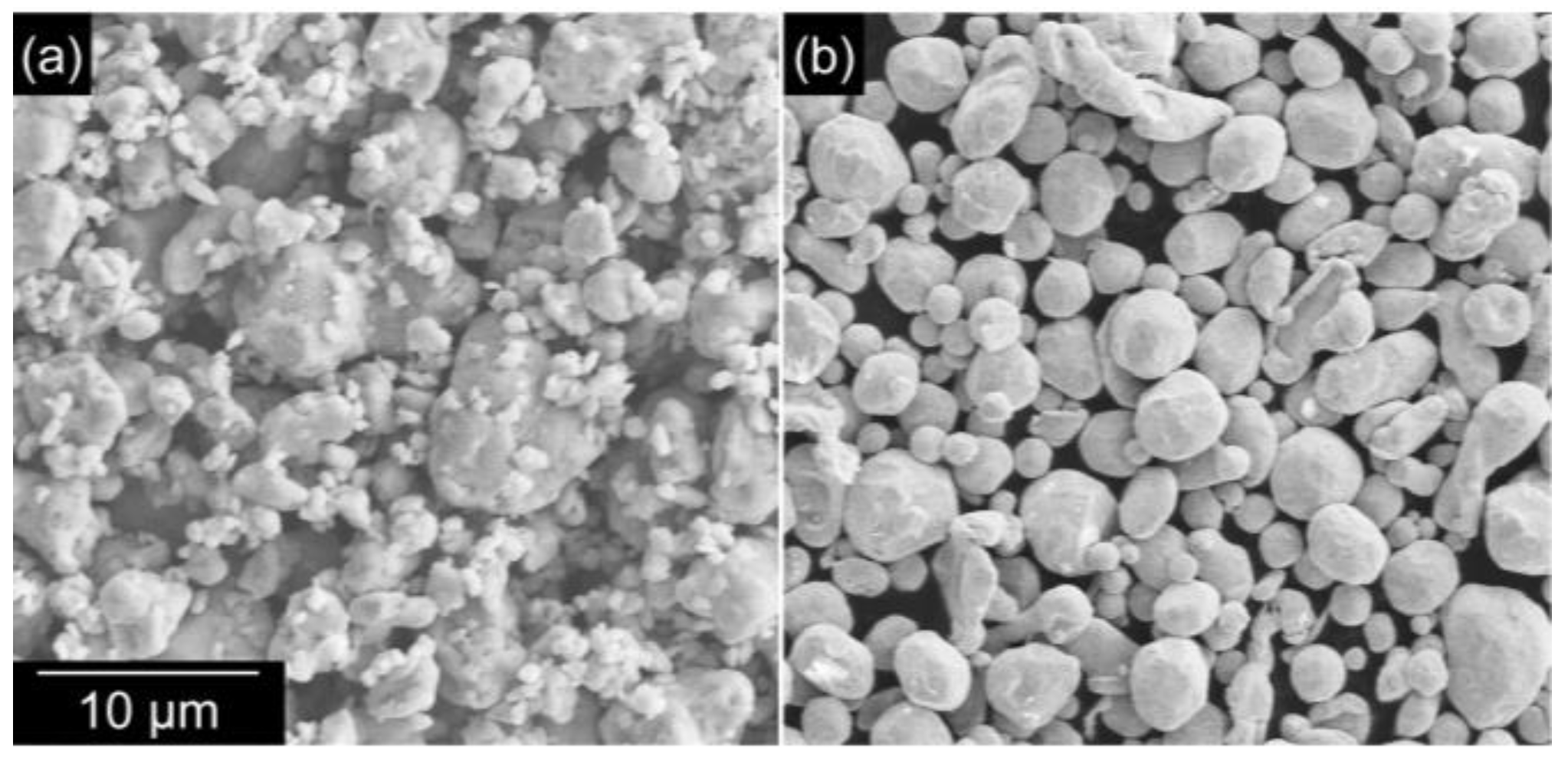
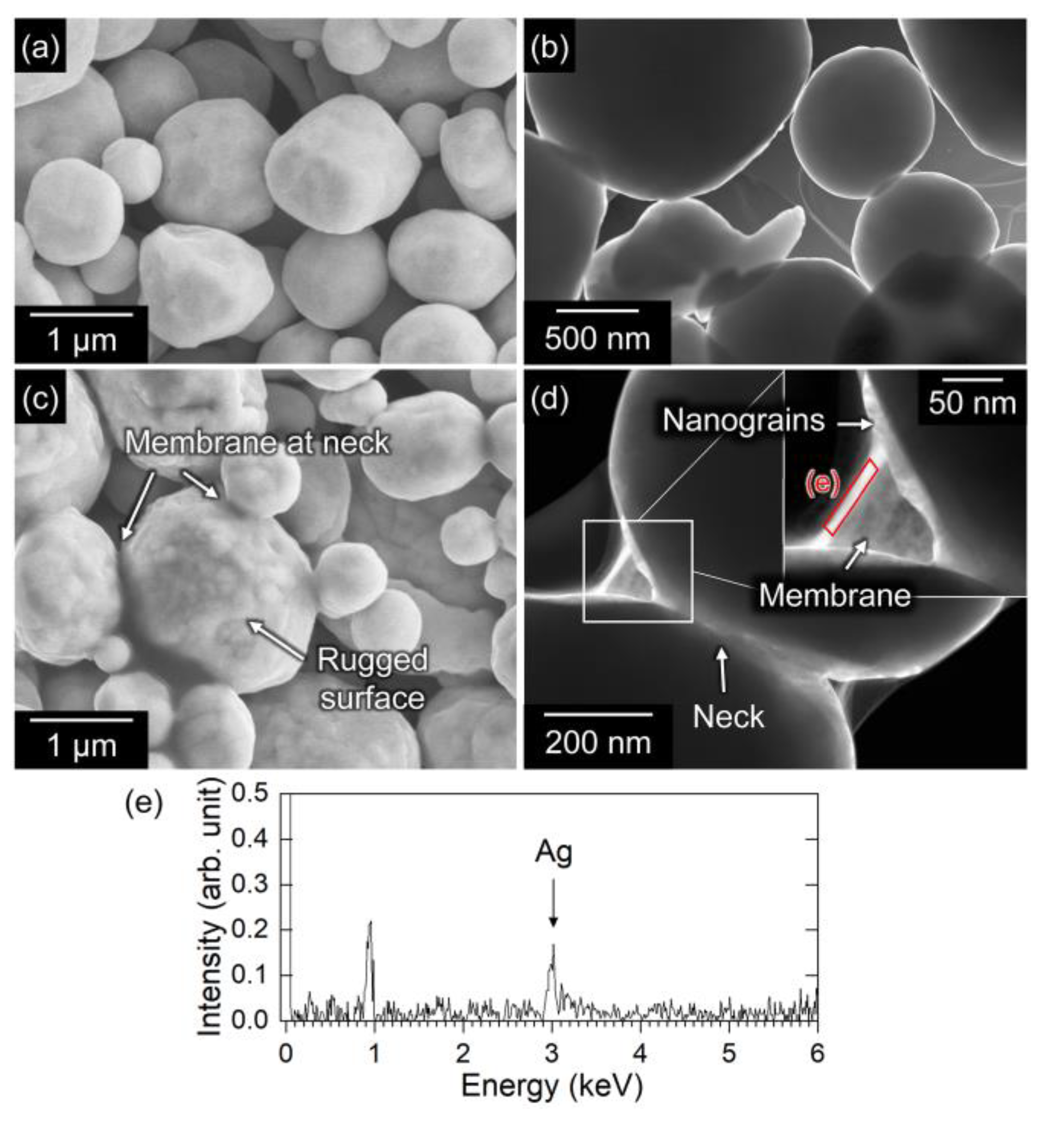
3.2. Bonding via the Reaction between Ag and Carboxylic Acid
4. Conclusions
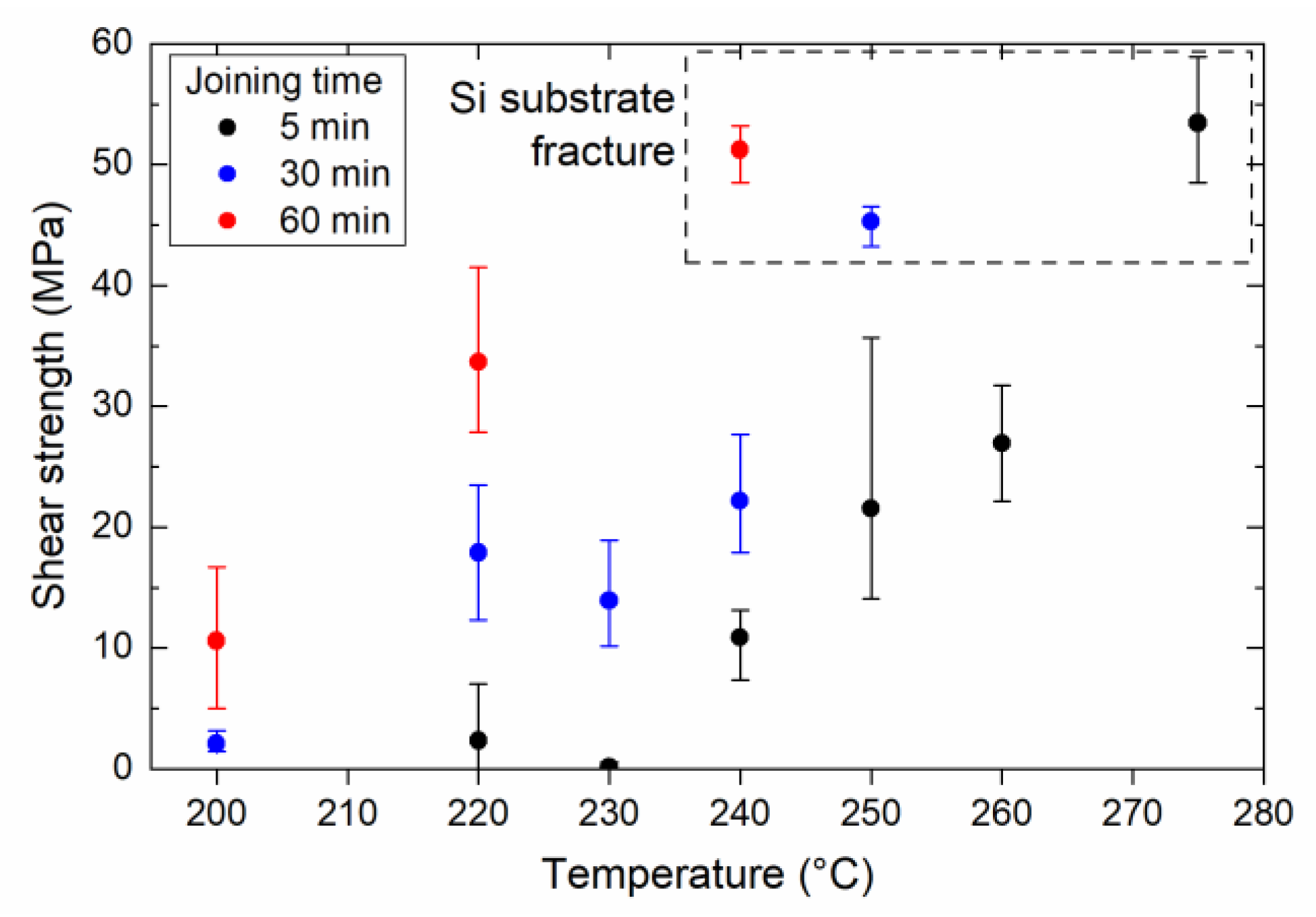
- Ag2O–EG paste was used to lower the bonding temperature to 220 °C, while simultaneously achieving a bond strength above 30 MPa.
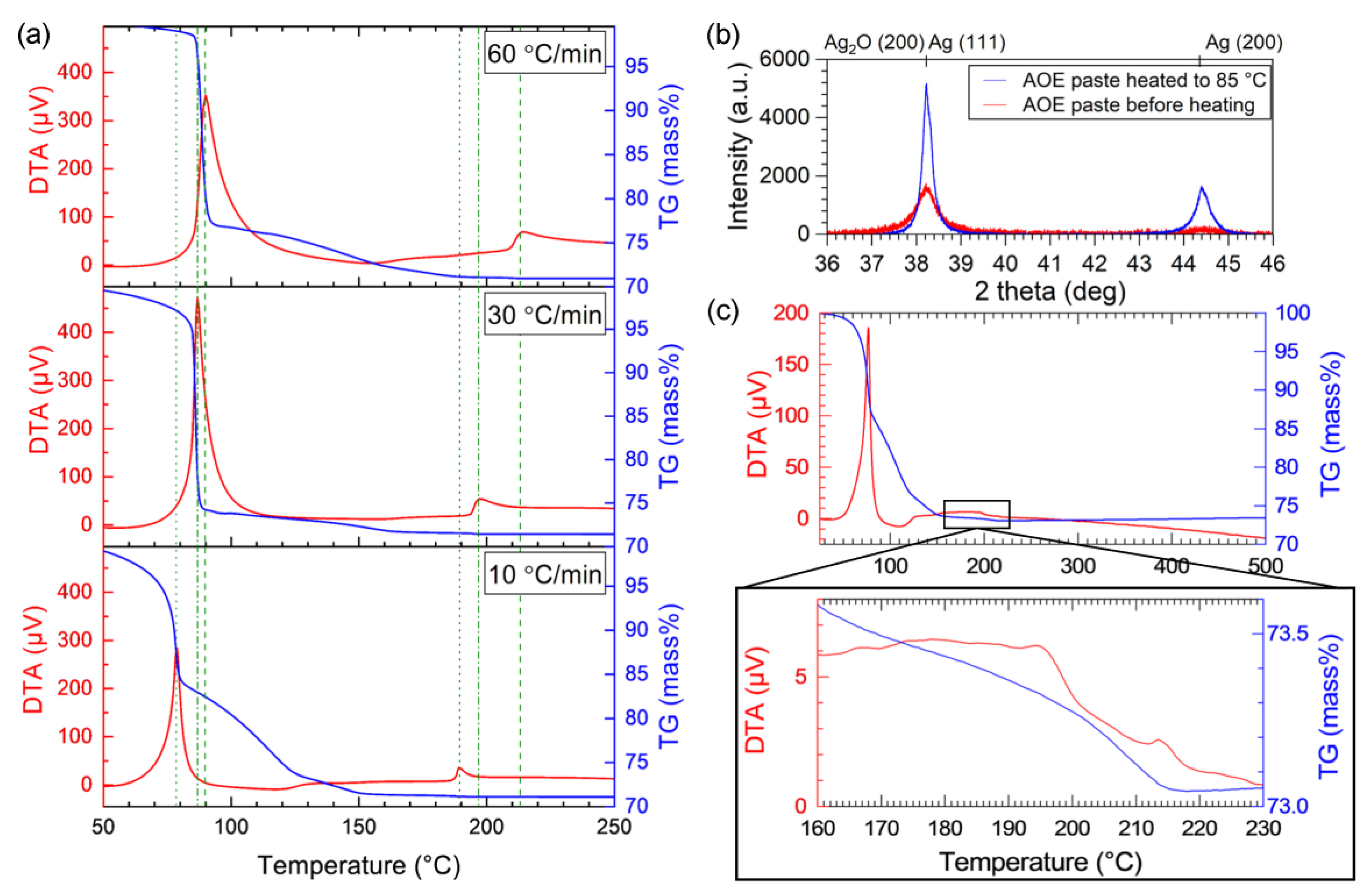
- TG-DTA showed that the organic residue remaining after the redox reaction between Ag2O and EG decomposes at approximately 215 °C, which occurs in the presence of Ag produced by the redox reaction. Spectroscopy analyses indicated the formation of carboxylic acid and Ag salts in the presence of Ag after the redox reaction.
- Typically, it is not possible to achieve direct sinter bonding to a Si substrate using Ag microparticles. However, the addition of oxalic acid resulted in the formation of Ag nanoparticles on the surface of the original microparticles, which enhanced sintering (evidenced by the observation of necks between the particles). The enhanced sintering achieved by the addition of oxalic acid resulted in a significant increase in the bond strength. Therefore, bonding using the in situ formation and decomposition of Ag salts is a promising process because it can offer localized sinter bonding.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ide, E.; Angata, S.; Hirose, A.; Kobayashi, K.F. Metal-Metal Bonding Process Using Ag Metallo-Organic Nanoparticles. Acta Mater. 2005, 53, 2385–2393. [Google Scholar] [CrossRef]
- Peng, P.; Hu, A.; Gerlich, A.P.; Zou, G.; Liu, L.; Zhou, Y.N. Joining of Silver Nanomaterials at Low Temperatures: Processes, Properties, and Applications. ACS Appl. Mater. Interfaces 2015, 7, 12597–12618. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.L.; Bayles, R.A.; Gile, W.W.; Jesser, W.A. Small Particle Melting of Pure Metals. Thin Solid Films 1986, 144, 297–308. [Google Scholar] [CrossRef]
- Goldstein, A.N.; Echer, C.M.; Alivisatos, A.P. Melting in Semiconductor Nanocrystals. Science 1992, 256, 1425–1427. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.G.; Yin, J.; Zhang, Z.; Lu, G.Q.; van Wyk, J.D. High-Temperature Operation of SiC Power Devices by Low-Temperature Sintered Silver Die-Attachment. IEEE Trans. Adv. Packag. 2007, 30, 506–510. [Google Scholar] [CrossRef]
- Lin, S.K.; Nagao, S.; Yokoi, E.; Oh, C.; Zhang, H.; Liu, Y.C.; Lin, S.G.; Suganuma, K. Nano-Volcanic Eruption of Silver. Sci. Rep. 2016, 6, 34769. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Inoue, D.; Zalar, P.; Jin, H.; Matsuba, Y.; Itoh, A.; Yokota, T.; Hashizume, D.; Someya, T. Printable Elastic Conductors by in Situ Formation of Silver Nanoparticles from Silver Flakes. Nat. Mater. 2017, 16, 834–840. [Google Scholar] [CrossRef]
- Matsuda, T.; Inami, K.; Motoyama, K.; Sano, T.; Hirose, A. Silver Oxide Decomposition Mediated Direct Bonding of Silicon-Based Materials. Sci. Rep. 2018, 8, 10472. [Google Scholar] [CrossRef] [Green Version]
- Inami, K.; Matsuda, T.; Kawabata, R.; Sano, T.; Hirose, A. Lowering Bonding Temperature for Silver Sintering to Silicon and Silicon Carbide Using Silver Oxide Decomposition. J. Mater. Sci. Mater. Electron. 2020, 31, 16511–16518. [Google Scholar] [CrossRef]
- Asama, K.; Matsuda, T.; Ogura, T.; Sano, T.; Takahashi, M.; Hirose, A. Low-Temperature Metal-to-Alumina Direct Bonding Process Utilizing Redox Reaction between Silver Oxide and Organic Agent. Mater. Sci. Eng. A 2017, 702, 398–405. [Google Scholar] [CrossRef]
- Motoyama, K.; Matsuda, T.; Sano, T.; Hirose, A. AlN-to-Metal Direct Bonding Process Utilizing Sintering of Ag Nanoparticles Derived from the Reduction of Ag2O. J. Electron. Mater. 2018, 47, 5780–5787. [Google Scholar] [CrossRef]
- Hirose, A.; Tatsumi, H.; Takeda, N.; Akada, Y.; Ogura, T.; Ide, E.; Morita, T. A Novel Metal-to-Metal Bonding Process through in-Situ Formation of Ag Nanoparticles Using Ag2O Microparticles. J. Phys. Conf. Ser. 2009, 165, 012074. [Google Scholar] [CrossRef]
- Kawabata, R.; Matsuda, T.; Seo, R.; Hirose, A. Metallization-Free Silver Sinter Bonding to Silicon via in Situ Decomposition of Silver Oxalate. Mater. Lett. 2021, 300, 130205. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.H. Low-Temperature and High-Speed Pressure-Assisted Sinter Bonding Using Ag Derived by the Redox Reaction of Ethylene Glycol-Based Ag2O Paste. Electron. Mater. Lett. 2022, 18, 94–103. [Google Scholar] [CrossRef]
- Fievet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.Y.; Sicard, L.; Viau, G. The Polyol Process: A Unique Method for Easy Access to Metal Nanoparticles with Tailored Sizes, Shapes and Compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Titkov, A.I.; Gerasimov, E.Y.; Shashkov, M.V.; Logutenko, O.A.; Bulina, N.V.; Yukhin, Y.M.; Lyakhov, N.Z. Specific Features of Polyol Synthesis of Silver Nanoparticles with the Use of Solid Carboxylates as Precursors. Colloid J. 2016, 78, 515–524. [Google Scholar] [CrossRef]
- Yasuda, Y.; Ide, E.; Morita, T. Low-Temperature Bonding Using Silver Nanoparticles Stabilized by Short-Chain Alkylamines. Jpn. J. Appl. Phys. 2009, 48, 125004. [Google Scholar] [CrossRef]
- Wakuda, D.; Hatamura, M.; Suganuma, K. Novel Method for Room Temperature Sintering of Ag Nanoparticle Paste in Air. Chem. Phys. Lett. 2007, 441, 305–308. [Google Scholar] [CrossRef]
- Ansell, R.J.; Barrett, S.A.; Meegan, J.E.; Warriner, S.L. On the Interactions of Alkyl 2-Hydroxycarboxylic Acids with Alkoxysilanes: Selective Esterification of Simple 2-Hydroxycarboxylic Acids. Chem.-A Eur. J. 2007, 13, 4654–4664. [Google Scholar] [CrossRef]
- Williamson, K.L.; Hasan, M.U.; Clutter, D.R. Conformational Analysis by NMR. 13C Nuclear Magnetic Resonance Spectra of Saturated and Unsaturated Carboxylic Acids and Their Corresponding Esters and Anhydrides. J. Magn. Reson. 1978, 30, 367–383. [Google Scholar] [CrossRef]
- Lu, D.; Wong, C.P. Thermal Decomposition of Silver Flake Lubricants. J. Therm. Anal. Calorim. 2000, 61, 3–12. [Google Scholar] [CrossRef]
- Moskovits, M.; Suh, J.S. Conformation of Mono- and Dicarboxylic Acids Adsorbed on Silver Surfaces. J. Am. Chem. Soc. 1985, 107, 6826–6829. [Google Scholar] [CrossRef]
- Schulte, J.P.; Grass, S.; Treuel, L. Adsorption of Dicarboxylic Acids onto Nano-Structured Silver Surfaces-Surface-Enhanced Raman Scattering Studies of PH-Dependent Adsorption Geometries. J. Raman Spectrosc. 2013, 44, 247–254. [Google Scholar] [CrossRef]
- Lu, D.; Tong, Q.K.; Wong, C.P. A Study of Lubricants on Silver Flakes for Microelectronics Conductive Adhesives. IEEE Trans. Compon. Packag. Technol. 1999, 22, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Xue, L.; Su, W.; Lin, Z. Mechanism of Silver- and Copper-Catalyzed Decarboxylation Reactions of Aryl Carboxylic Acids. Dalt. Trans. 2011, 40, 11926–11936. [Google Scholar] [CrossRef]
- Cornella, J.; Sanchez, C.; Banawa, D.; Larrosa, I. Silver-Catalysed Protodecarboxylation of Ortho-Substituted Benzoic Acids. Chem. Commun. 2009, 46, 7176–7178. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Yang, J.; Cao, H.; Zhang, Y. Reaction Mechanism and Metal Ion Transformation in Photocatalytic Ozonation of Phenol and Oxalic Acid with Ag+/TiO2. J. Environ. Sci. 2014, 26, 662–672. [Google Scholar] [CrossRef]
- Patel, N.R.; Flowers, R.A. Mechanistic Study of Silver-Catalyzed Decarboxylative Fluorination. J. Org. Chem. 2015, 80, 5834–5841. [Google Scholar] [CrossRef]
- Fields, E.K.; Meyerson, S. Thermal and Photochemical Decomposition of Silver Carboxylates. J. Org. Chem. 1976, 41, 916–920. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, W.; Moon, K.S.; Wong, C.P. Fast Preparation of Printable Highly Conductive Polymer Nanocomposites by Thermal Decomposition of Silver Carboxylate and Sintering of Silver Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 2637–2645. [Google Scholar] [CrossRef]
- Le Trong, H.; Kiryukhina, K.; Gougeon, M.; Baco-Carles, V.; Courtade, F.; Dareys, S.; Tailhades, P. Paramagnetic Behaviour of Silver Nanoparticles Generated by Decomposition of Silver Oxalate. Solid State Sci. 2017, 69, 44–49. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, T.; Kawabata, R.; Okamoto, T.; Hirose, A. Ag Sinter Bonding to Si Substrate via Temporal Formation and Decomposition of Ag Carboxylate. Nanomaterials 2023, 13, 2292. https://doi.org/10.3390/nano13162292
Matsuda T, Kawabata R, Okamoto T, Hirose A. Ag Sinter Bonding to Si Substrate via Temporal Formation and Decomposition of Ag Carboxylate. Nanomaterials. 2023; 13(16):2292. https://doi.org/10.3390/nano13162292
Chicago/Turabian StyleMatsuda, Tomoki, Rei Kawabata, Takuya Okamoto, and Akio Hirose. 2023. "Ag Sinter Bonding to Si Substrate via Temporal Formation and Decomposition of Ag Carboxylate" Nanomaterials 13, no. 16: 2292. https://doi.org/10.3390/nano13162292




