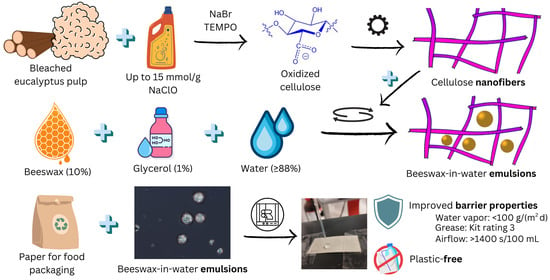
Stabilization of Beeswax-In-Water Dispersions Using Anionic Cellulose Nanofibers and Their Application in Paper Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of TEMPO-Oxidized Cellulose Nanofibers
2.3. Preparation and Visual Inspection of BW-In-Water Emulsions
2.4. Analysis of BW/TOCNF/Glycerol/Water Systems
2.5. Coating and Visualization of Paper
2.6. Characterization of BW-Coated Sheets
3. Results and Discussion
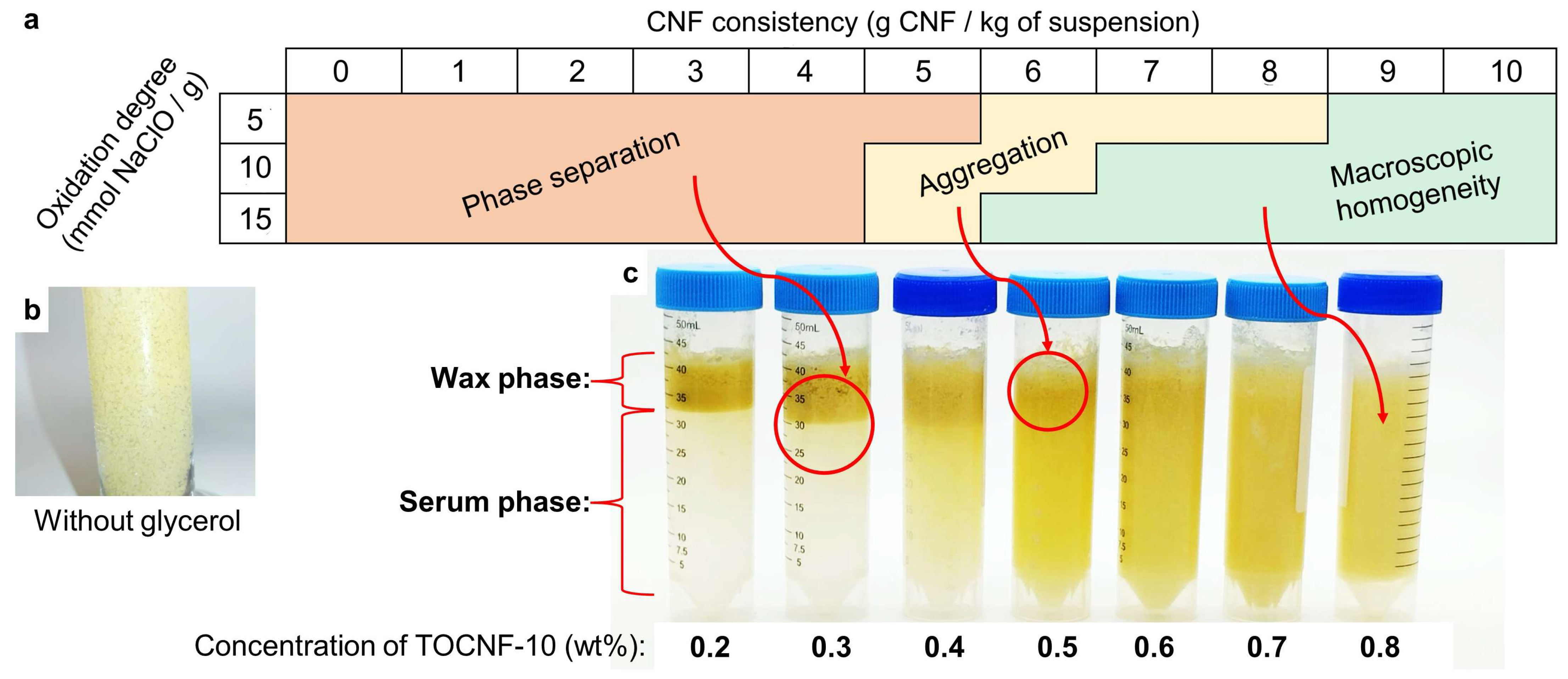
3.1. Pickering Stabilization: Macroscopic Features
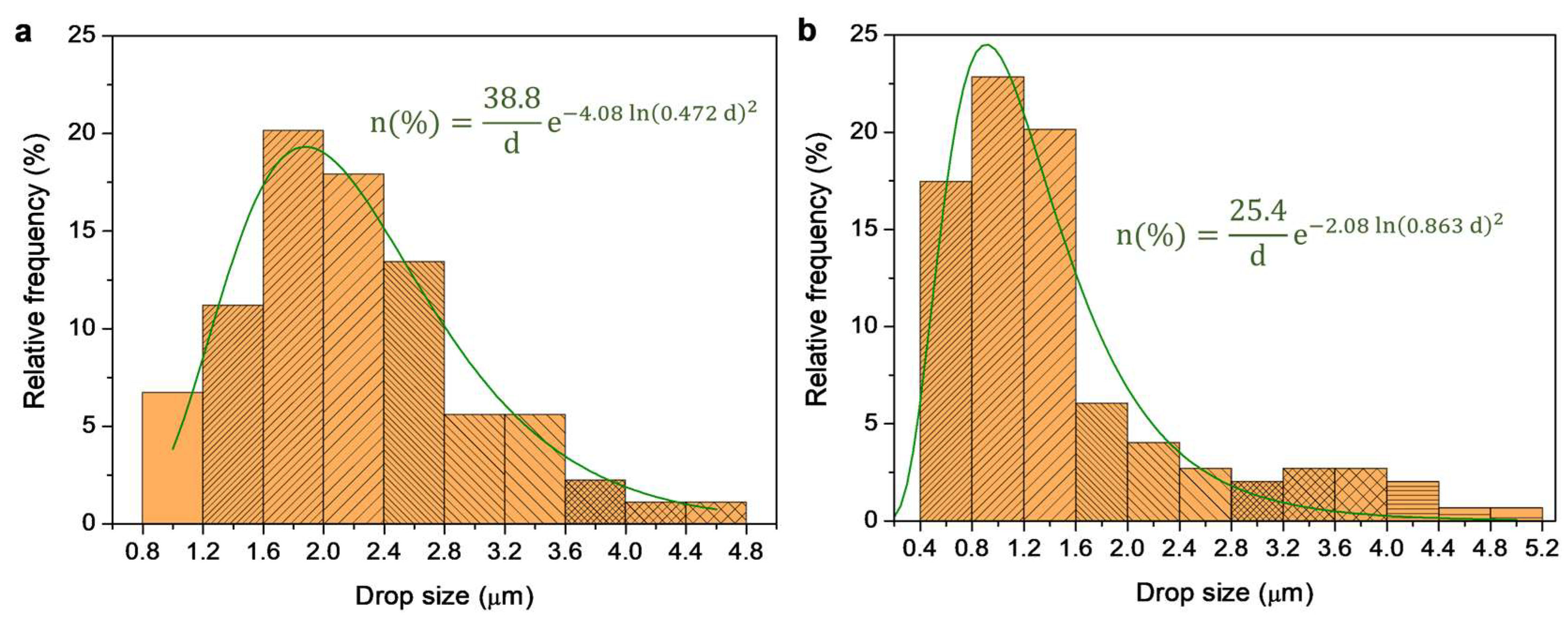
3.2. Assessment of Stable BW-In-Water Dispersions
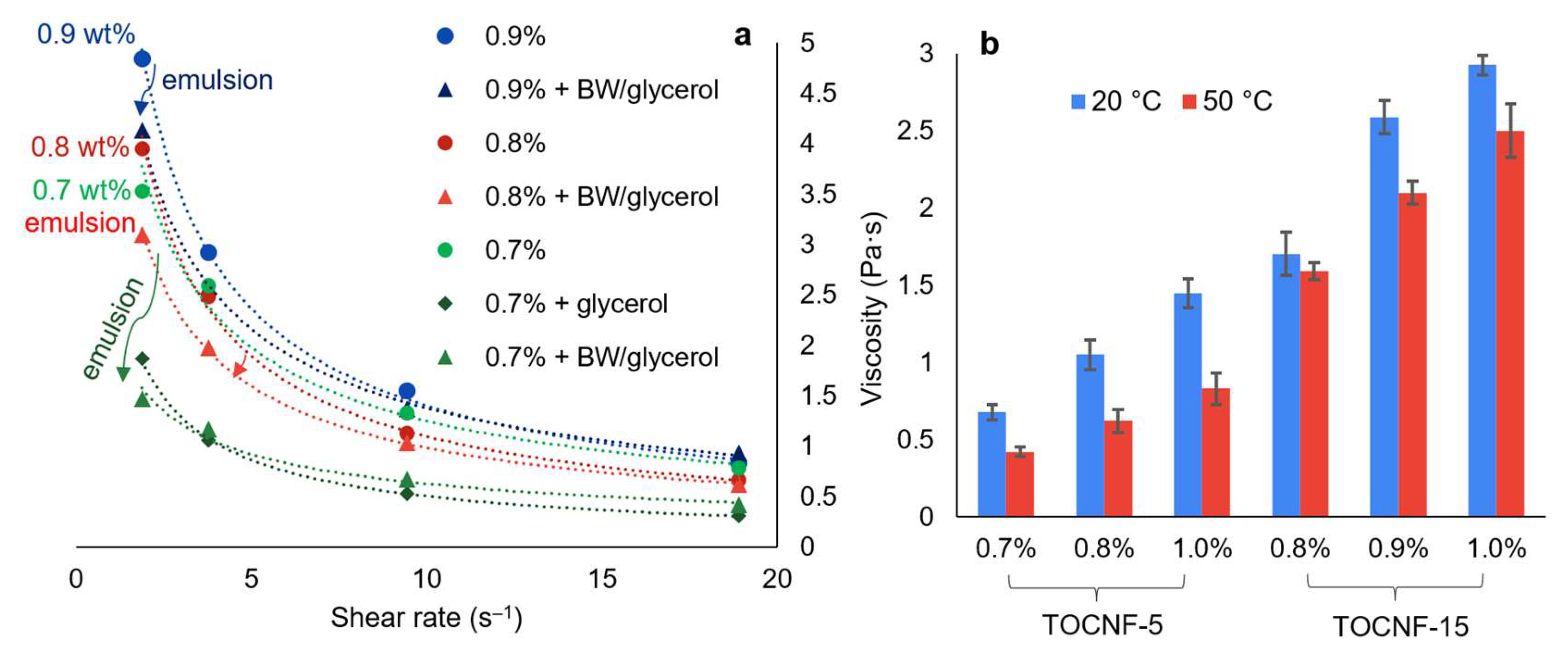
3.3. Rheology of BW/TOCNF/Glycerol/Water Systems
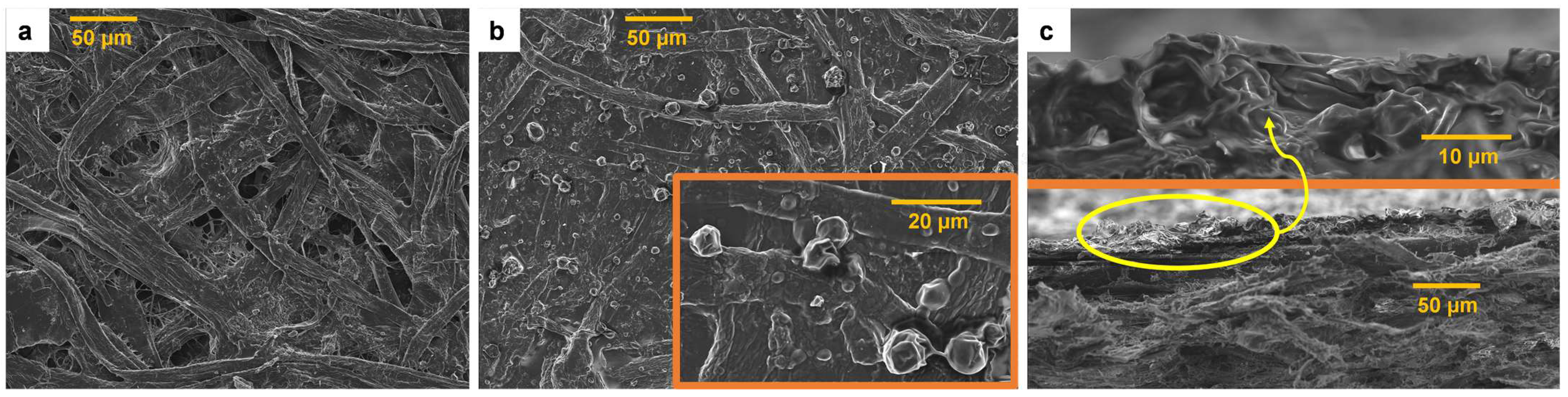
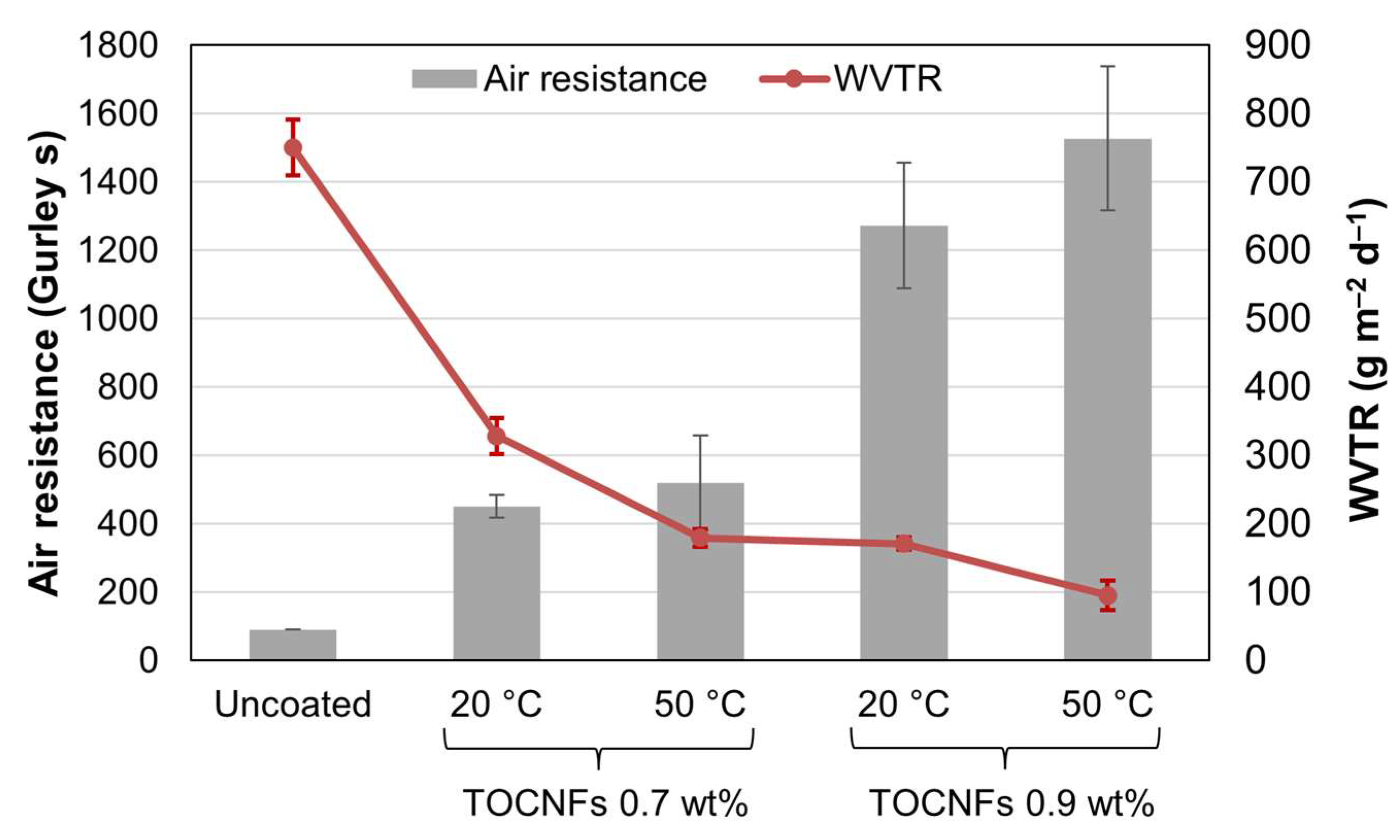
3.4. Properties of BW-Coated Papers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguado, R.J.; Mazega, A.; Tarrés, Q.; Delgado-Aguilar, M. The Role of Electrostatic Interactions of Anionic and Cationic Cellulose Derivatives for Industrial Applications: A Critical Review. Ind. Crops Prod. 2023, 201, 116898. [Google Scholar] [CrossRef]
- Zheng, S.X.; Chen, H.S. Correlations of Rheological Methods to Coatings’ Performance. Prog. Org. Coat. 2023, 177, 107403. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Monte, M.C.; Batchelor, W.; Garnier, G.; Negro, C.; Blanco, A. Characterizing Highly Fibrillated Nanocellulose by Modifying the Gel Point Methodology. Carbohydr. Polym. 2020, 227, 115340. [Google Scholar] [CrossRef]
- Hatchell, D.; Song, W.; Daigle, H. Effect of Interparticle Forces on the Stability and Droplet Diameter of Pickering Emulsions Stabilized by PEG-Coated Silica Nanoparticles. J. Colloid Interface Sci. 2022, 626, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Aguado, R.; Mazega, A.; Fiol, N.; Tarrés, Q.; Mutjé, P.; Delgado-Aguilar, M. Durable Nanocellulose-Stabilized Emulsions of Dithizone/Chloroform in Water for Hg2+ Detection: A Novel Approach for a Classical Problem. ACS Appl. Mater. Interfaces 2023, 15, 12580–12589. [Google Scholar] [CrossRef] [PubMed]
- Tarrés, Q.; Aguado, R.; Zoppe, J.O.; Mutjé, P.; Fiol, N.; Delgado-Aguilar, M. Dynamic Light Scattering Plus Scanning Electron Microscopy: Usefulness and Limitations of a Simplified Estimation of Nanocellulose Dimensions. Nanomaterials 2022, 12, 4288. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-Oxidized Cellulose Nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A. Cellulose Nanofibers: Recent Progress and Future Prospects. J. Fiber Sci. Technol. 2020, 76, 310–326. [Google Scholar] [CrossRef]
- Mazega, A.; Santos, A.F.; Aguado, R.; Tarrés, Q.; Fiol, N.; Pèlach, M.À.; Delgado-Aguilar, M. Kinetic Study and Real-Time Monitoring Strategy for TEMPO-Mediated Oxidation of Bleached Eucalyptus Fibers. Cellulose 2023, 30, 1421–1436. [Google Scholar] [CrossRef]
- Koochi, H.; Mac Intyre, J.; Viitanen, L.; Puisto, A.; Maleki-Jirsaraei, N.; Alava, M. Local Time-Dependent Microstructure of Aging TEMPO Nanofibrillated Cellulose Gel. Cellulose 2023, 30, 61–74. [Google Scholar] [CrossRef]
- Szumała, P.; Luty, N. Effect of Different Crystalline Structures on W/O and O/W/O Wax Emulsion Stability. Colloids Surf. A Physicochem. Eng. Asp. 2016, 499, 131–140. [Google Scholar] [CrossRef]
- Trinh, B.M.; Smith, M.; Mekonnen, T.H. A Nanomaterial-Stabilized Starch-Beeswax Pickering Emulsion Coating to Extend Produce Shelf-Life. Chem. Eng. J. 2022, 431, 133905. [Google Scholar] [CrossRef]
- Liu, D.; Duan, Y.; Wang, S.; Gong, M.; Dai, H. Improvement of Oil and Water Barrier Properties of Food Packaging Paper by Coating with Microcrystalline Wax Emulsion. Polymers 2022, 14, 1786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Simpson, B.K.; Dumont, M.-J. Effect of Beeswax and Carnauba Wax Addition on Properties of Gelatin Films: A Comparative Study. Food Biosci. 2018, 26, 88–95. [Google Scholar] [CrossRef]
- Sigwarth, T.; Büchner, J.; Wistuba, M.P. Bio-Degradable Wax to Modify Asphalt Binder for Warm Mix Asphalt. Sustainability 2022, 14, 10219. [Google Scholar] [CrossRef]
- Jeong, M.-J.; Bogolitsyna, A.; Jo, B.-M.; Kang, K.-Y.; Rosenau, T.; Potthast, A. Deterioration of Ancient Korean Paper (Hanji), Treated with Beeswax: A Mechanistic Study. Carbohydr. Polym. 2014, 101, 1249–1254. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A Minireview of Its Antimicrobial Activity and Its Application in Medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of Chitosan-Beeswax Edible Coatings on the Quality of Fresh Strawberries (Fragaria Ananassa Cv Camarosa) under Commercial Storage Conditions. LWT-Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Reshmi, C.R.; Sundaran, S.P.; Juraij, A.; Athiyanathil, S. Fabrication of Superhydrophobic Polycaprolactone/Beeswax Electrospun Membranes for High-Efficiency Oil/Water Separation. RSC Adv. 2017, 7, 2092–2102. [Google Scholar] [CrossRef]
- Garrido-Romero, M.; Aguado, R.; Moral, A.; Brindley, C.; Ballesteros, M. From Traditional Paper to Nanocomposite Films: Analysis of Global Research into Cellulose for Food Packaging. Food Packag. Shelf Life 2022, 31, 100788. [Google Scholar] [CrossRef]
- Triantafillopoulos, N.; Koukoulas, A.A. The Future of Single-Use Paper Coffee Cups: Current Progress and Outlook. Bioresources 2020, 15, 7260–7287. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, X.; Luo, X.; Wang, Y.; Li, Y.; Li, B.; Liu, S. Edible Coating Based on Beeswax-in-Water Pickering Emulsion Stabilized by Cellulose Nanofibrils and Carboxymethyl Chitosan. Food Chem. 2020, 331, 127108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, H.; Qian, L. Beeswax–Chitosan Emulsion Coated Paper with Enhanced Water Vapor Barrier Efficiency. Appl. Surf. Sci. 2014, 300, 80–85. [Google Scholar] [CrossRef]
- Arola, S.; Kou, Z.; Rooijakkers, B.J.M.; Velagapudi, R.; Sammalkorpi, M.; Linder, M.B. On the Mechanism for the Highly Sensitive Response of Cellulose Nanofiber Hydrogels to the Presence of Ionic Solutes. Cellulose 2022, 29, 6109–6121. [Google Scholar] [CrossRef]
- Guancheng, J. Chapter 2—Evaluation Methods and Influencing Factors of Gas Wettability. In Gas Wettability of Reservoir Rock Surfaces with Porous Media; Guancheng, J., Ed.; Gulf Professional Publishing: Oxford, UK, 2018; pp. 29–84. ISBN 978-0-12-815150-1. [Google Scholar]
- ASTM E96; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: Conshohocken, PA, USA, 2017.
- TAPPI T559; Grease Resistance Test for Paper and Paperboard. TAPPI Standards, Technical Information Papers, and Useful Methods. Technical Association of the Pulp & Paper Industry: New York, NY, USA, 2020.
- ISO TC/6; Paper, Board and Pulps. International Standardization Organization: Geneva, Switzerland, 2011.
- Souza, A.G.; de Ferreira, R.R.; Aguilar, E.S.F.; Zanata, L.; Rosa, D.D.S. Cinnamon Essential Oil Nanocellulose-Based Pickering Emulsions: Processing Parameters Effect on Their Formation, Stabilization, and Antimicrobial Activity. Polysaccharides 2021, 2, 608–625. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Blanco, A.; Negro, C. Pickering Emulsions Containing Cellulose Microfibers Produced by Mechanical Treatments as Stabilizer in the Food Industry. Appl. Sci. 2019, 9, 359. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Ilyin, S.O. Morphology and Rheology of Heavy Crude Oil/Water Emulsions Stabilized by Microfibrillated Cellulose. Energy Fuels 2021, 35, 6527–6540. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Pinem, M.P.; Kustiningsih, I.; Agustina, S.; Oudet, F.; Lefebvre, C.; Clausse, D.; Saleh, K.; Guénin, E. Cellulose Nanocrystals to Improve Stability and Functional Properties of Emulsified Film Based on Chitosan Nanoparticles and Beeswax. Nanomaterials 2019, 9, 1707. [Google Scholar] [CrossRef]
- Sultan, M.; Hafez, O.M.; Saleh, M.A.; Youssef, A.M. Smart Edible Coating Films Based on Chitosan and Beeswax-Pollen Grains for the Postharvest Preservation of Le Conte Pear. RSC Adv. 2021, 11, 9572–9585. [Google Scholar] [CrossRef]
- Olarte-Paredes, A.; Salgado-Delgado, A.M.; García-Fuentes, J.J.; Salgado-Delgado, R.; Cedillo-Valverde, G.; López-Lara, T.; Hernández-Zaragoza, J.B.; Castaño, V.M. Synthesis and Characterization of a Polyester Based on Citric Acid/Ethylene Glycol/Glycerol. J. Macromol. Sci. Part A 2021, 58, 890–898. [Google Scholar] [CrossRef]
- Du, Y.; Liu, J.; Wang, J.; Wang, B.; Li, H.; Su, Y. Starch-Based Bio-Latex Redistribution during Paper Coating Consolidation. Prog. Org. Coat. 2017, 106, 155–162. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, X.; Xiao, Y.; Hu, L.; Eggersdorfer, M.; Chen, D.; Yang, Z.; Weitz, D.A. Pickering Emulsions Stabilized by Colloidal Surfactants: Role of Solid Particles. Particuology 2022, 64, 153–163. [Google Scholar] [CrossRef]
- Goi, Y.; Fujisawa, S.; Saito, T.; Yamane, K.; Kuroda, K.; Isogai, A. Dual Functions of TEMPO-Oxidized Cellulose Nanofibers in Oil-in-Water Emulsions: A Pickering Emulsifier and a Unique Dispersion Stabilizer. Langmuir 2019, 35, 10920–10926. [Google Scholar] [CrossRef]
- Coppock, R.W. Chapter 47—Bee Products as Nutraceuticals to Nutraceuticals for Bees. In Nutraceuticals, 2nd ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 813–833. ISBN 978-0-12-821038-3. [Google Scholar]
- Yadykova, A.Y.; Ilyin, S.O. Nanocellulose-Stabilized Bitumen Emulsions as a Base for Preparation of Nanocomposite Asphalt Binders. Carbohydr. Polym. 2023, 313, 120896. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Pham, K.A.; Breedveld, V. TEMPO-CNF Suspensions in the Viscoelastic Regime: Capturing the Effect of Morphology and Surface Charge with a Rheological Parameter. Cellulose 2021, 28, 813–827. [Google Scholar] [CrossRef]
- Ferreira, A.G.M.; Egas, A.P.V.; Fonseca, I.M.A.; Costa, A.C.; Abreu, D.C.; Lobo, L.Q. The Viscosity of Glycerol. J. Chem. Thermodyn. 2017, 113, 162–182. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Liu, R. Effect of Particle Size Distribution and Shear Rate on Relative Viscosity of Concentrated Suspensions. Rheol. Acta 2021, 60, 763–774. [Google Scholar] [CrossRef]
- Konijn, B.J.; Sanderink, O.B.J.; Kruyt, N.P. Experimental Study of the Viscosity of Suspensions: Effect of Solid Fraction, Particle Size and Suspending Liquid. Powder Technol. 2014, 266, 61–69. [Google Scholar] [CrossRef]
- Tarrés, Q.; Aguado, R.; Pèlach, M.À.; Mutjé, P.; Delgado-Aguilar, M. Electrospray Deposition of Cellulose Nanofibers on Paper: Overcoming the Limitations of Conventional Coating. Nanomaterials 2022, 12, 79. [Google Scholar] [CrossRef]
- Tyagi, P.; Lucia, L.A.; Hubbe, M.A.; Pal, L. Nanocellulose-Based Multilayer Barrier Coatings for Gas, Oil, and Grease Resistance. Carbohydr. Polym. 2019, 206, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, W.; Zhang, H.; Dai, Y.; Dong, H.; Kong, L.; Hou, H. Effects of Preparation Conditions on the Properties of Agar/Maltodextrin-Beeswax Pseudo-Bilayer Films. Carbohydr. Polym. 2020, 236, 116029. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liang, H.; Nasrallah, J.; Chen, L.; Huang, L.; Ni, Y. Preparation of the CNC/Ag/Beeswax Composites for Enhancing Antibacterial and Water Resistance Properties of Paper. Carbohydr. Polym. 2016, 142, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Nechita, P. The Influence of Drying Conditions of Clay-Based Polymer Coatings on Coated Paper Properties. Coatings 2021, 11, 12. [Google Scholar] [CrossRef]
- Tyagi, P.; Hubbe, M.A.; Lucia, L.; Pal, L. High Performance Nanocellulose-Based Composite Coatings for Oil and Grease Resistance. Cellulose 2018, 25, 3377–3391. [Google Scholar] [CrossRef]
- Mazega, A.; Tarrés, Q.; Aguado, R.; Pèlach, M.À.; Mutjé, P.; Ferreira, P.J.T.; Delgado-Aguilar, M. Improving the Barrier Properties of Paper to Moisture, Air, and Grease with Nanocellulose-Based Coating Suspensions. Nanomaterials 2022, 12, 3675. [Google Scholar] [CrossRef]

| Stabilizer | Concentration of Stabilizer | Concentration of Beeswax | Purpose | Ref. |
|---|---|---|---|---|
| Cellulose nanocrystals | 0.03–0.15 wt% | 1.5 wt% | Starch-supported films | [13] |
| Carboxymethyl chitosan with unmodified CNFs | 1–5 wt% | 10 vol% | Edible coating for food | [23] |
| Chitosan | 1–3 wt% | 10–30 wt% | Paper coating | [24] |
| Sample | Carboxyl Group Content (mmol/g) | Surface Charge Density (meq/g) |
|---|---|---|
| TOCNF-5 | 0.76 ± 0.04 | −1.30 ± 0.00 |
| TOCNF-10 | 1.11 ± 0.09 | −1.64 ± 0.10 |
| TOCNF-15 | 1.39 ± 0.10 | −1.92 ± 0.16 |
| TOCNF-15 Concentration (wt%) | CNF Suspension | BW Emulsion | ||
|---|---|---|---|---|
| K (Pa sn) | n | K (Pa sn) | n | |
| 0.7 | 5.8 ± 0.4 | 0.66 ± 0.06 | 2.2 ± 0.2 | 0.55 ± 0.06 |
| 0.8 | 6.7 ± 0.3 | 0.79 ± 0.04 | 4.92 ± 0.08 | 0.70 ± 0.01 |
| 0.9 | 8.0 ± 0.2 | 0.76 ± 0.02 | 6.2 ± 0.1 | 0.65 ± 0.01 |
| 1.0 | 8.05 ± 0.09 | 0.72 ± 0.01 | 9.8 ± 0.3 | 0.84 ± 0.03 |
| Coating | T (°C) | Coat Weight (g m−2) | Coat Thickness (µm) | Static Contact Angle (°) | Kit Rating | |
|---|---|---|---|---|---|---|
| Water | Oil | |||||
| None | -- | -- | -- | Drop absorbed | 25.5 ± 5.0 | 1 |
| 10% BW, 1% glycerol | 20 | 6.6 ± 1.9 | 5.9 ± 0.7 | 71.3 ± 0.5 | 65.5 ± 0.6 | 5 |
| 0.7% TOCNF-15 | 50 | 5.3 ± 1.1 | 8.5 ± 1.1 | 66.1 ± 9.6 | 64.8 ± 0.5 | 5 |
| 10% BW, 1% glycerol | 20 | 8.3 ± 1.2 | 8.5 ± 1.5 | 79.8 ± 0.8 | 67.1 ± 4.4 | 5 |
| 0.9% TOCNF-15 | 50 | 5.2 ± 1.7 | 8.7 ± 1.8 | 96.1 ± 3.9 | 62.6 ± 0.7 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayés, G.; Aguado, R.J.; Tarrés, Q.; Planella, J.; Delgado-Aguilar, M. Stabilization of Beeswax-In-Water Dispersions Using Anionic Cellulose Nanofibers and Their Application in Paper Coating. Nanomaterials 2023, 13, 2353. https://doi.org/10.3390/nano13162353
Bayés G, Aguado RJ, Tarrés Q, Planella J, Delgado-Aguilar M. Stabilization of Beeswax-In-Water Dispersions Using Anionic Cellulose Nanofibers and Their Application in Paper Coating. Nanomaterials. 2023; 13(16):2353. https://doi.org/10.3390/nano13162353
Chicago/Turabian StyleBayés, Genís, Roberto J. Aguado, Quim Tarrés, Jaume Planella, and Marc Delgado-Aguilar. 2023. "Stabilization of Beeswax-In-Water Dispersions Using Anionic Cellulose Nanofibers and Their Application in Paper Coating" Nanomaterials 13, no. 16: 2353. https://doi.org/10.3390/nano13162353