Human Dental Pulp Mesenchymal Stem Cell-Derived Soluble Factors Combined with a Nanostructured Scaffold Support the Generation of a Vascular Network In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scaffold
2.2. Cell Isolation and Maintenance
2.3. Conditioned Medium Preparation and Characterization
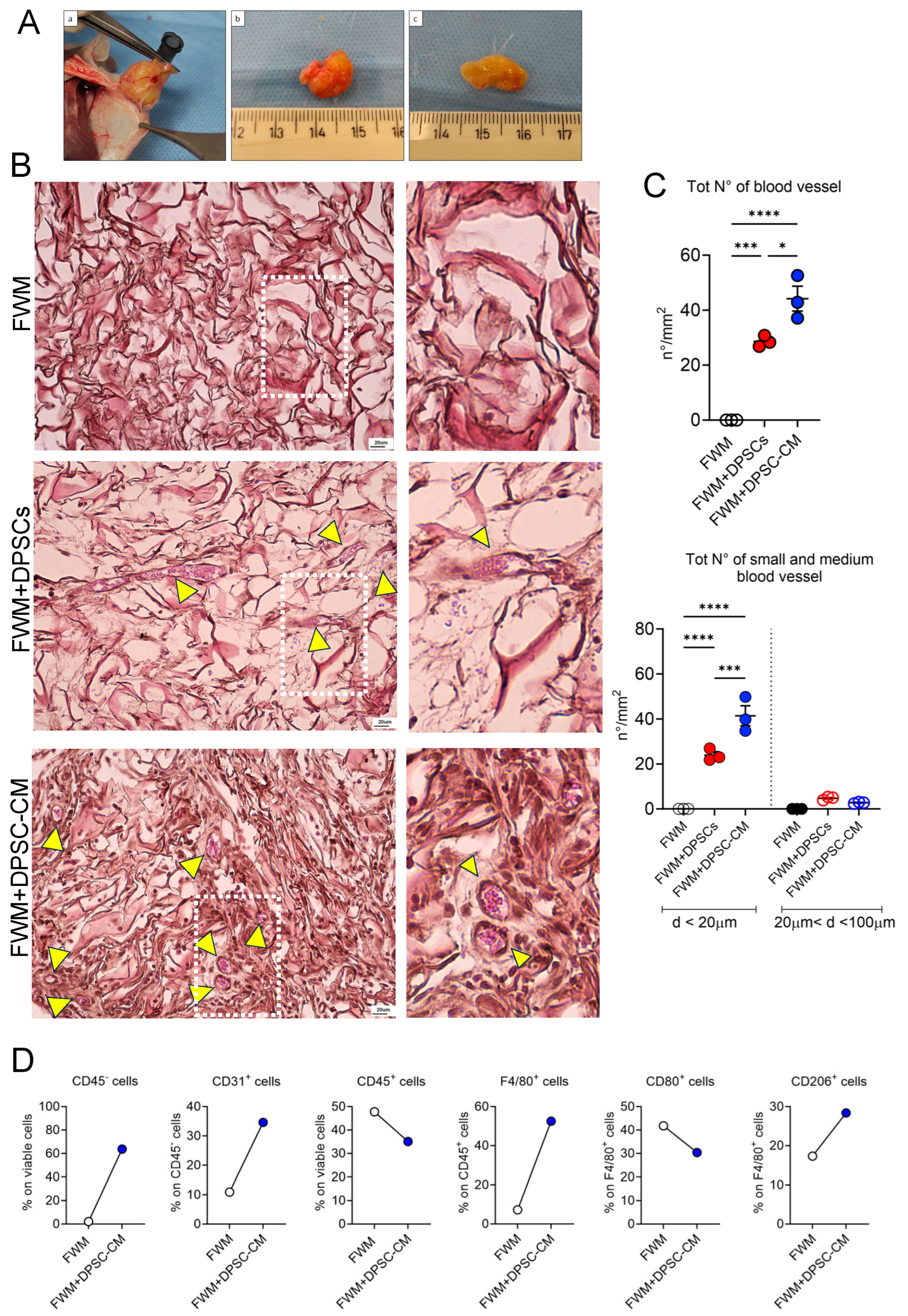
2.4. In Vivo FWM Assay
2.5. Gross Examination of Scaffold
2.6. Sample Collection
2.7. Microscopy Analysis and Blood Vessel Assessment
2.8. RNA Extraction, Reverse Transcription, and Real-Time PCR
2.9. Flow Cytometry
2.10. Statistical Analysis
3. Results
3.1. Characterization of DPSCs Prior to In Vivo Engraftment
3.2. CM from DPSCs Efficiently Induces a Vascular Network In Vivo
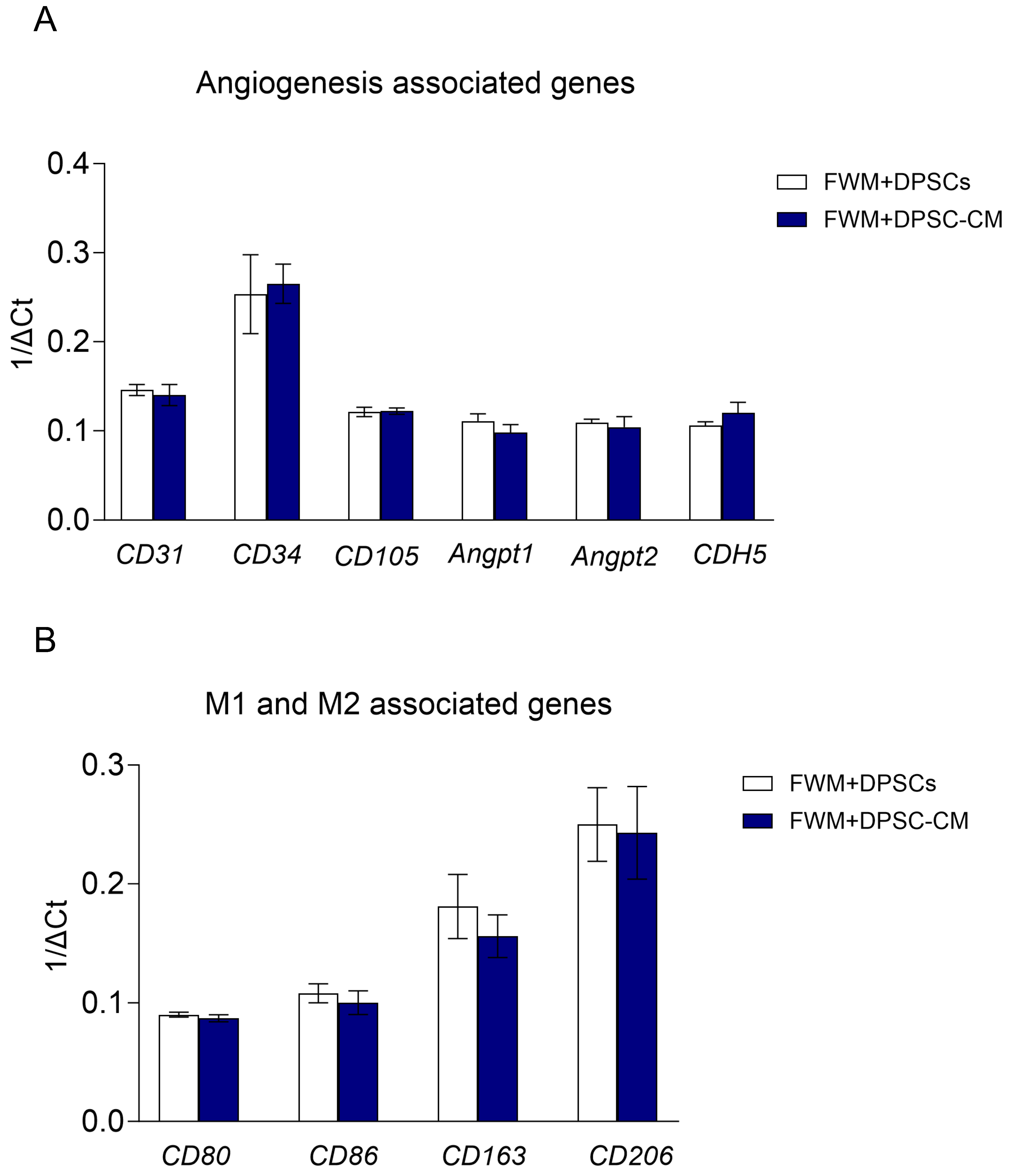
3.3. Scaffolds with DPSCs or DPSC–CM Show Similar Pro-Angiogenic Signatures at Gene Expression Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Wu, D.; Zhao, X.; Pakvasa, M.; Tucker, A.B.; Luo, H.; Qin, K.H.; Hu, D.A.; Wang, E.J.; Li, A.J.; et al. Stem Cell-Friendly Scaffold Biomaterials: Applications for Bone Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 598607. [Google Scholar] [CrossRef]
- Krishna, L.; Dhamodaran, K.; Jayadev, C.; Chatterjee, K.; Shetty, R.; Khora, S.S.; Das, D. Nanostructured scaffold as a determinant of stem cell fate. Stem Cell Res. Ther. 2016, 7, 188. [Google Scholar] [CrossRef]
- Golchin, A.; Farzaneh, S.; Porjabbar, B.; Sadegian, F.; Estaji, M.; Ranjbarvan, P.; Kanafimahbob, M.; Ranjbari, J.; Salehi-Nik, N.; Hosseinzadeh, S. Regenerative Medicine Under the Control of 3D Scaffolds: Current State and Progress of Tissue Scaffolds. Curr. Stem Cell Res. Ther. 2021, 16, 209–229. [Google Scholar] [CrossRef] [PubMed]
- Borgese, M.; Barone, L.; Rossi, F.; Raspanti, M.; Papait, R.; Valdatta, L.; Bernardini, G.; Gornati, R. Effect of Nanostructured Scaffold on Human Adipose-Derived Stem Cells: Outcome of In Vitro Experiments. Nanomaterials 2020, 10, 1822. [Google Scholar] [CrossRef]
- Khan, F.; Tanaka, M. Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci. 2017, 19, 17. [Google Scholar] [CrossRef]
- Huynh, N.P.T.; Brunger, J.M.; Gloss, C.C.; Moutos, F.T.; Gersbach, C.A.; Guilak, F. Genetic Engineering of Mesenchymal Stem Cells for Differential Matrix Deposition on 3D Woven Scaffolds. Tissue Eng. Part A 2018, 24, 1531–1544. [Google Scholar] [CrossRef]
- Liu, H.; MacQueen, L.A.; Usprech, J.F.; Maleki, H.; Sider, K.L.; Doyle, M.G.; Sun, Y.; Simmons, C.A. Microdevice arrays with strain sensors for 3D mechanical stimulation and monitoring of engineered tissues. Biomaterials 2018, 172, 30–40. [Google Scholar] [CrossRef]
- Cherubino, M.; Valdatta, L.; Balzaretti, R.; Pellegatta, I.; Rossi, F.; Protasoni, M.; Tedeschi, A.; Accolla, R.S.; Bernardini, G.; Gornati, R. Human adipose-derived stem cells promote vascularization of collagen-based scaffolds transplanted into nude mice. Regen. Med. 2016, 11, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wei, K.; Loebel, C.; Zhang, K.; Feng, Q.; Li, R.; Wong, S.H.D.; Xu, X.; Lau, C.; Chen, X.; et al. Enhanced mechanosensing of cells in synthetic 3D matrix with controlled biophysical dynamics. Nat. Commun. 2021, 12, 3514. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Gao, P.L.; Wang, K.; Wang, J.Y.; Zhong, Y.; Luo, Y. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials 2017, 141, 74–85. [Google Scholar] [CrossRef]
- Rabelink, T.J.; Little, M.H. Stromal cells in tissue homeostasis: Balancing regeneration and fibrosis. Nat. Rev. Nephrol. 2013, 9, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Barone, L.; Palano, M.T.; Gallazzi, M.; Cucchiara, M.; Rossi, F.; Borgese, M.; Raspanti, M.; Zecca, P.A.; Mortara, L.; Papait, R.; et al. Adipose mesenchymal stem cell-derived soluble factors, produced under hypoxic condition, efficiently support in vivo angiogenesis. Cell Death Discov. 2023, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]
- Merino-Gonzalez, C.; Zuniga, F.A.; Escudero, C.; Ormazabal, V.; Reyes, C.; Nova-Lamperti, E.; Salomon, C.; Aguayo, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Angiogenesis: Potencial Clinical Application. Front. Physiol. 2016, 7, 24. [Google Scholar] [CrossRef]
- Newman, A.C.; Nakatsu, M.N.; Chou, W.; Gershon, P.D.; Hughes, C.C. The requirement for fibroblasts in angiogenesis: Fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol. Biol. Cell 2011, 22, 3791–3800. [Google Scholar] [CrossRef]
- Saraswati, S.; Marrow, S.M.W.; Watch, L.A.; Young, P.P. Identification of a pro-angiogenic functional role for FSP1-positive fibroblast subtype in wound healing. Nat. Commun. 2019, 10, 3027. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Gieseck, R.L., 3rd; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76. [Google Scholar] [CrossRef]
- Minton, K. Connecting angiogenesis and autoimmunity. Nat. Rev. Immunol. 2019, 19, 596–597. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Pagani, A.; Pulze, L.; Albini, A.; Dallaglio, K.; Noonan, D.M.; Mortara, L. Orchestration of angiogenesis by immune cells. Front. Oncol. 2014, 4, 131. [Google Scholar] [CrossRef] [PubMed]
- Frantz, S.; Vincent, K.A.; Feron, O.; Kelly, R.A. Innate immunity and angiogenesis. Circ. Res. 2005, 96, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bhagwani, A.; Thompson, A.A.R.; Farkas, L. When Innate Immunity Meets Angiogenesis-The Role of Toll-Like Receptors in Endothelial Cells and Pulmonary Hypertension. Front. Med. 2020, 7, 352. [Google Scholar] [CrossRef]
- Varricchi, G.; Loffredo, S.; Galdiero, M.R.; Marone, G.; Cristinziano, L.; Granata, F.; Marone, G. Innate effector cells in angiogenesis and lymphangiogenesis. Curr. Opin. Immunol. 2018, 53, 152–160. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. Immune cells and angiogenesis. J. Cell. Mol. Med. 2009, 13, 2822–2833. [Google Scholar] [CrossRef]
- Parisi, L.; Gini, E.; Baci, D.; Tremolati, M.; Fanuli, M.; Bassani, B.; Farronato, G.; Bruno, A.; Mortara, L. Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? J. Immunol. Res. 2018, 2018, 8917804. [Google Scholar] [CrossRef]
- Cassetta, L.; Cassol, E.; Poli, G. Macrophage polarization in health and disease. Sci. World J. 2011, 11, 2391–2402. [Google Scholar] [CrossRef]
- Biswas, S.K.; Chittezhath, M.; Shalova, I.N.; Lim, J.Y. Macrophage polarization and plasticity in health and disease. Immunol. Res. 2012, 53, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Doyle, A.D.; Lu, J. Cell-3D matrix interactions: Recent advances and opportunities. Trends Cell Biol. 2022, 32, 883–895. [Google Scholar] [CrossRef]
- Barone, L.; Rossi, F.; Valdatta, L.; Cherubino, M.; Papait, R.; Binelli, G.; Romano, N.; Bernardini, G.; Gornati, R. Human Adipose-Derived Stem Cell-Conditioned Medium Promotes Vascularization of Nanostructured Scaffold Transplanted into Nude Mice. Nanomaterials 2022, 12, 1521. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, A.; Garcia-Sanchez, D.; Dotta, M.; Rodriguez-Rey, J.C.; Perez-Campo, F.M. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J. Stem Cells 2020, 12, 1529–1552. [Google Scholar] [CrossRef] [PubMed]
- Costela-Ruiz, V.J.; Melguizo-Rodriguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. Int. J. Mol. Sci. 2022, 23, 6356. [Google Scholar] [CrossRef]
- Krivanek, J.; Soldatov, R.A.; Kastriti, M.E.; Chontorotzea, T.; Herdina, A.N.; Petersen, J.; Szarowska, B.; Landova, M.; Matejova, V.K.; Holla, L.I.; et al. Dental cell type atlas reveals stem and differentiated cell types in mouse and human teeth. Nat. Commun. 2020, 11, 4816. [Google Scholar] [CrossRef]
- Li, B.; Ouchi, T.; Cao, Y.; Zhao, Z.; Men, Y. Dental-Derived Mesenchymal Stem Cells: State of the Art. Front. Cell Dev. Biol. 2021, 9, 654559. [Google Scholar] [CrossRef]
- Ledesma-Martinez, E.; Mendoza-Nunez, V.M.; Santiago-Osorio, E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Ratushnyy, A.; Ezdakova, M.; Buravkova, L. Secretome of Senescent Adipose-Derived Mesenchymal Stem Cells Negatively Regulates Angiogenesis. Int. J. Mol. Sci. 2020, 21, 1802. [Google Scholar] [CrossRef] [PubMed]
- Marcozzi, C.; Frattini, A.; Borgese, M.; Rossi, F.; Barone, L.; Solari, E.; Valli, R.; Gornati, R. Paracrine effect of human adipose-derived stem cells on lymphatic endothelial cells. Regen. Med. 2020, 15, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Bernardini, G.; Bonfanti, P.; Colombo, A.; Prati, M.; Gornati, R. Effects of TCDD on spermatogenesis related factor-2 (SRF-2): Gene expression in Xenopus. Toxicol. Lett. 2009, 191, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Palombella, S.; Pirrone, C.; Cherubino, M.; Valdatta, L.; Bernardini, G.; Gornati, R. Identification of reference genes for qPCR analysis during hASC long culture maintenance. PLoS ONE 2017, 12, e0170918. [Google Scholar] [CrossRef]
- Phelps, E.A.; Garcia, A.J. Engineering more than a cell: Vascularization strategies in tissue engineering. Curr. Opin. Biotechnol. 2010, 21, 704–709. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Tayebi, L. Vascularization strategies in tissue engineering approaches for soft tissue repair. J. Tissue Eng. Regen. Med. 2021, 15, 747–762. [Google Scholar] [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef]
- Dellaquila, A.; Le Bao, C.; Letourneur, D.; Simon-Yarza, T. In Vitro Strategies to Vascularize 3D Physiologically Relevant Models. Adv. Sci. 2021, 8, e2100798. [Google Scholar] [CrossRef]
- Fonsatti, E.; Sigalotti, L.; Arslan, P.; Altomonte, M.; Maio, M. Emerging role of endoglin (CD105) as a marker of angiogenesis with clinical potential in human malignancies. Curr. Cancer Drug Targets 2003, 3, 427–432. [Google Scholar] [CrossRef]
- Sauteur, L.; Krudewig, A.; Herwig, L.; Ehrenfeuchter, N.; Lenard, A.; Affolter, M.; Belting, H.G. Cdh5/VE-cadherin promotes endothelial cell interface elongation via cortical actin polymerization during angiogenic sprouting. Cell Rep. 2014, 9, 504–513. [Google Scholar] [CrossRef]
- Siemerink, M.J.; Klaassen, I.; Vogels, I.M.; Griffioen, A.W.; Van Noorden, C.J.; Schlingemann, R.O. CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis 2012, 15, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Bourdeau, A.; Letarte, M.; Zuniga-Pflucker, J.C. Expression and function of CD105 during the onset of hematopoiesis from Flk1+ precursors. Blood 2001, 98, 3635–3642. [Google Scholar] [CrossRef] [PubMed]
- Jadhao, M.; Chen, C.L.; Liu, W.; Deshmukh, D.; Liao, W.T.; Chen, J.Y.; Urade, R.; Tsai, E.M.; Hsu, S.K.; Wang, L.F.; et al. Endoglin Modulates TGFbetaR2 Induced VEGF and Proinflammatory Cytokine Axis Mediated Angiogenesis in Prolonged DEHP-Exposed Breast Cancer Cells. Biomedicines 2022, 10, 417. [Google Scholar] [CrossRef]
- ten Dijke, P.; Goumans, M.J.; Pardali, E. Endoglin in angiogenesis and vascular diseases. Angiogenesis 2008, 11, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Alt, A.; Miguel-Romero, L.; Donderis, J.; Aristorena, M.; Blanco, F.J.; Round, A.; Rubio, V.; Bernabeu, C.; Marina, A. Structural and functional insights into endoglin ligand recognition and binding. PLoS ONE 2012, 7, e29948. [Google Scholar] [CrossRef]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef]
- Felcht, M.; Luck, R.; Schering, A.; Seidel, P.; Srivastava, K.; Hu, J.; Bartol, A.; Kienast, Y.; Vettel, C.; Loos, E.K.; et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J. Clin. Investig. 2012, 122, 1991–2005. [Google Scholar] [CrossRef]
- Yuan, H.T.; Khankin, E.V.; Karumanchi, S.A.; Parikh, S.M. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol. Cell. Biol. 2009, 29, 2011–2022. [Google Scholar] [CrossRef]
- Xie, J.Y.; Wei, J.X.; Lv, L.H.; Han, Q.F.; Yang, W.B.; Li, G.L.; Wang, P.X.; Wu, S.B.; Duan, J.X.; Zhuo, W.F.; et al. Angiopoietin-2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell Commun. Signal. 2020, 18, 46. [Google Scholar] [CrossRef]


| Gene Name | Sequence 5′-3′ | Tm (°C) | Accession Number |
|---|---|---|---|
| Mm_β-Actin | Fw GCCCAGAGCAAGAGAGGTA Rv TAGAAGGTGTGGTGCCAGAT | 65.0 | NM_007393.5 |
| 64.9 | |||
| Mm_GAPDH | Fw ACCTGCCAAGTATGATGAC Rv GGAGTTGCTGTTGAAGTC | 64.0 | NM_008084.3 |
| 59.7 | |||
| Mm_CD80 | Fw TTATCATCCTGGGCCTGGTC Rv GTGTCTGCAGATGGGTTTCC | 65.4 | NM_001359898.1 |
| 65.2 | |||
| Mm_CD86 | Fw TGCTGCTCATCATTGTATGT Rv GGTTCAAGTTCCTTCAGGTT | 61.5 | NM_019388.3 |
| 61.9 | |||
| Mm_CD163 | Fw GGTGCTGGATCTCCTGGTTG Rv CAGGAGCGTTAGTGACAGCA | 66.8 | NM_001170395.1 |
| 66.3 | |||
| Mm_CD206 | Fw GGCTGATTACGAGCAGTGGA Rv CATCACTCCAGGTGAACCCC | 66.2 | NM_008625.2 |
| 66.5 | |||
| Mm_CD31 | Fw AACAGAGCCAGCAGTATGA Rv ATGACAACCACCGCAATG | 62.6 | NM_008816.3 |
| 62.5 | |||
| Mm_CD34 | Fw CTGCTCCGAGTGCCATTA Rv CTCCTCACAACTAGATGCTTCA | 63.3 | NM_133654.3 |
| 63.7 | |||
| Mm_CD105 | Fw CGATAGCAGCACTGGATGAC Rv TGGCAAGCACAAGAATGGT | 64.7 | NM_007932.2 |
| 64.5 | |||
| Mm_Angpt1 | Fw GGAAGATGGAAGCCTGGATT Rv ACTGCCTCTGACTGGTTATTG | 65.1 | NM_009640.4 |
| 65.2 | |||
| Mm_Angpt2 | Fw CGACTACGACGACTCAGT Rv TCTCCACCATCTCCTTCTTC | 63.7 | NM_007426.4 |
| 63.8 | |||
| Mm_CDH5 | Fw CAGAGTCCATCGCAGAGT Rv AGCCAGCATCTTGAACCT | 64.1 | NM_009868.4 |
| 64.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, L.; Gallazzi, M.; Rossi, F.; Papait, R.; Raspanti, M.; Zecca, P.A.; Buonarrivo, L.; Bassani, B.; Bernardini, G.; Bruno, A.; et al. Human Dental Pulp Mesenchymal Stem Cell-Derived Soluble Factors Combined with a Nanostructured Scaffold Support the Generation of a Vascular Network In Vivo. Nanomaterials 2023, 13, 2479. https://doi.org/10.3390/nano13172479
Barone L, Gallazzi M, Rossi F, Papait R, Raspanti M, Zecca PA, Buonarrivo L, Bassani B, Bernardini G, Bruno A, et al. Human Dental Pulp Mesenchymal Stem Cell-Derived Soluble Factors Combined with a Nanostructured Scaffold Support the Generation of a Vascular Network In Vivo. Nanomaterials. 2023; 13(17):2479. https://doi.org/10.3390/nano13172479
Chicago/Turabian StyleBarone, Ludovica, Matteo Gallazzi, Federica Rossi, Roberto Papait, Mario Raspanti, Piero Antonio Zecca, Luca Buonarrivo, Barbara Bassani, Giovanni Bernardini, Antonino Bruno, and et al. 2023. "Human Dental Pulp Mesenchymal Stem Cell-Derived Soluble Factors Combined with a Nanostructured Scaffold Support the Generation of a Vascular Network In Vivo" Nanomaterials 13, no. 17: 2479. https://doi.org/10.3390/nano13172479
APA StyleBarone, L., Gallazzi, M., Rossi, F., Papait, R., Raspanti, M., Zecca, P. A., Buonarrivo, L., Bassani, B., Bernardini, G., Bruno, A., & Gornati, R. (2023). Human Dental Pulp Mesenchymal Stem Cell-Derived Soluble Factors Combined with a Nanostructured Scaffold Support the Generation of a Vascular Network In Vivo. Nanomaterials, 13(17), 2479. https://doi.org/10.3390/nano13172479







