Composition of Vapor–Liquid–Solid III–V Ternary Nanowires Based on Group-III Intermix
Abstract
:1. Introduction
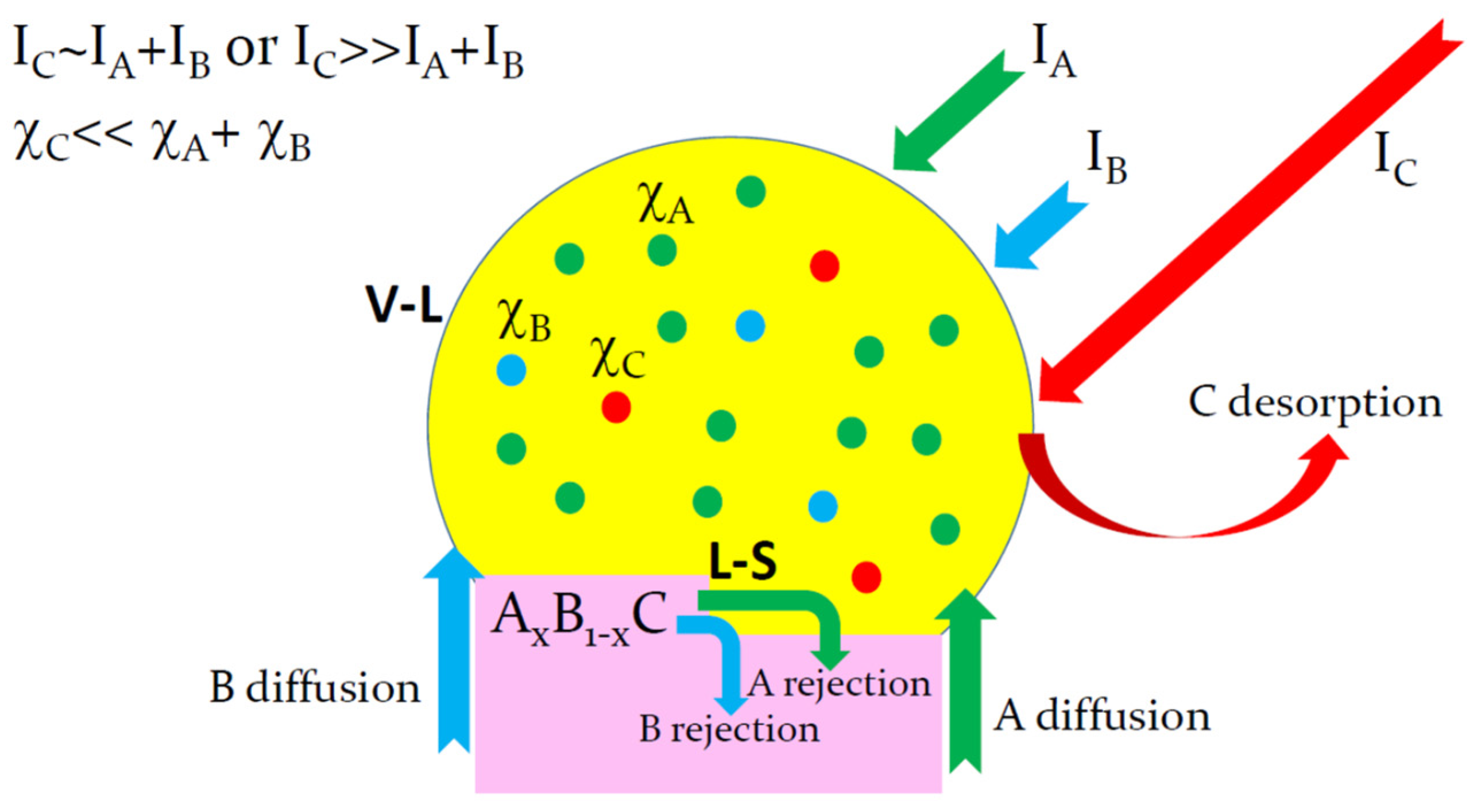
2. Limiting Steps of Group-III and Group-V Element Incorporation
3. Liquid–Solid Distribution
4. Vapor–Solid Distribution
5. Equilibrium Vapor–Solid Distribution
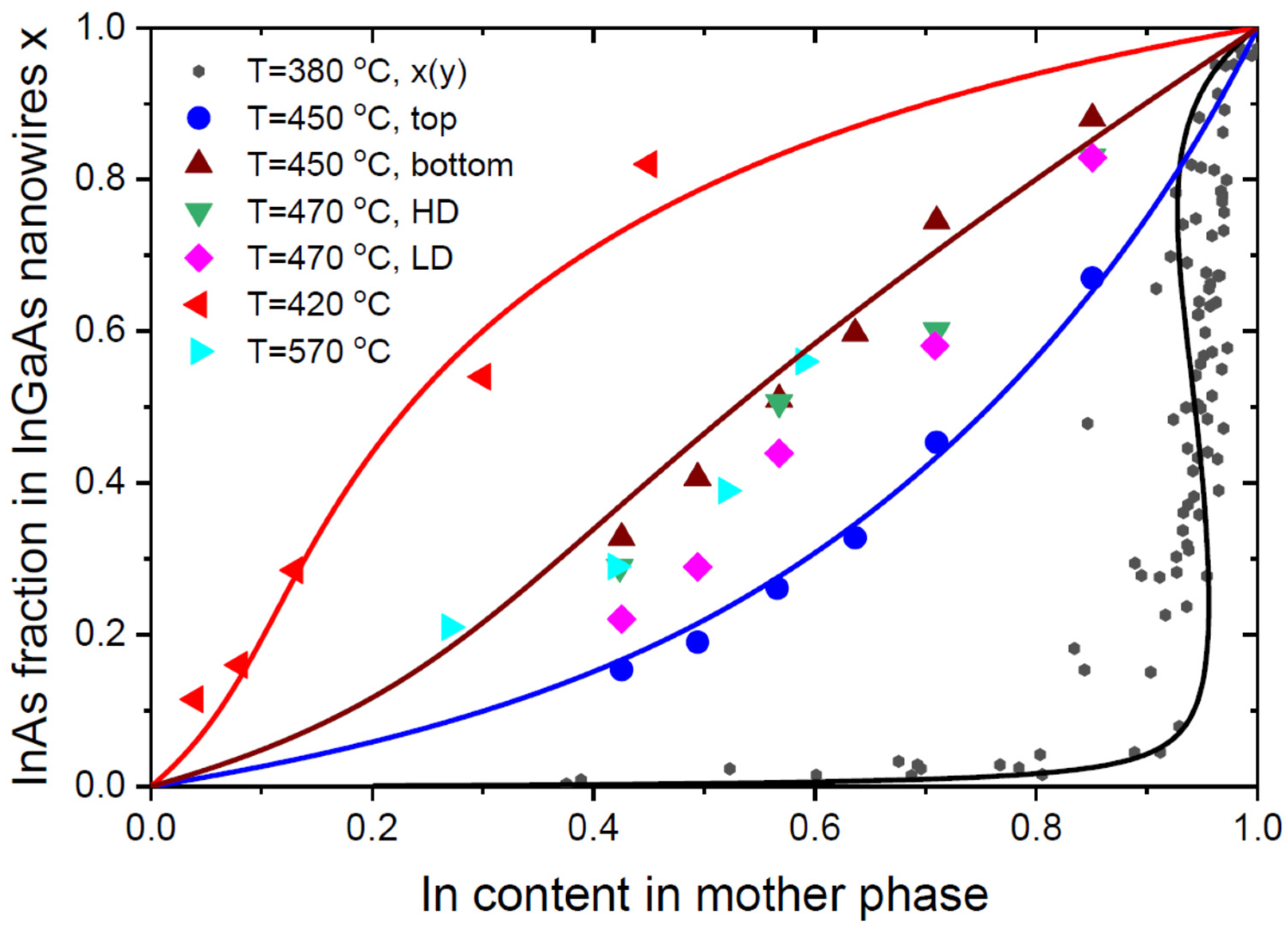
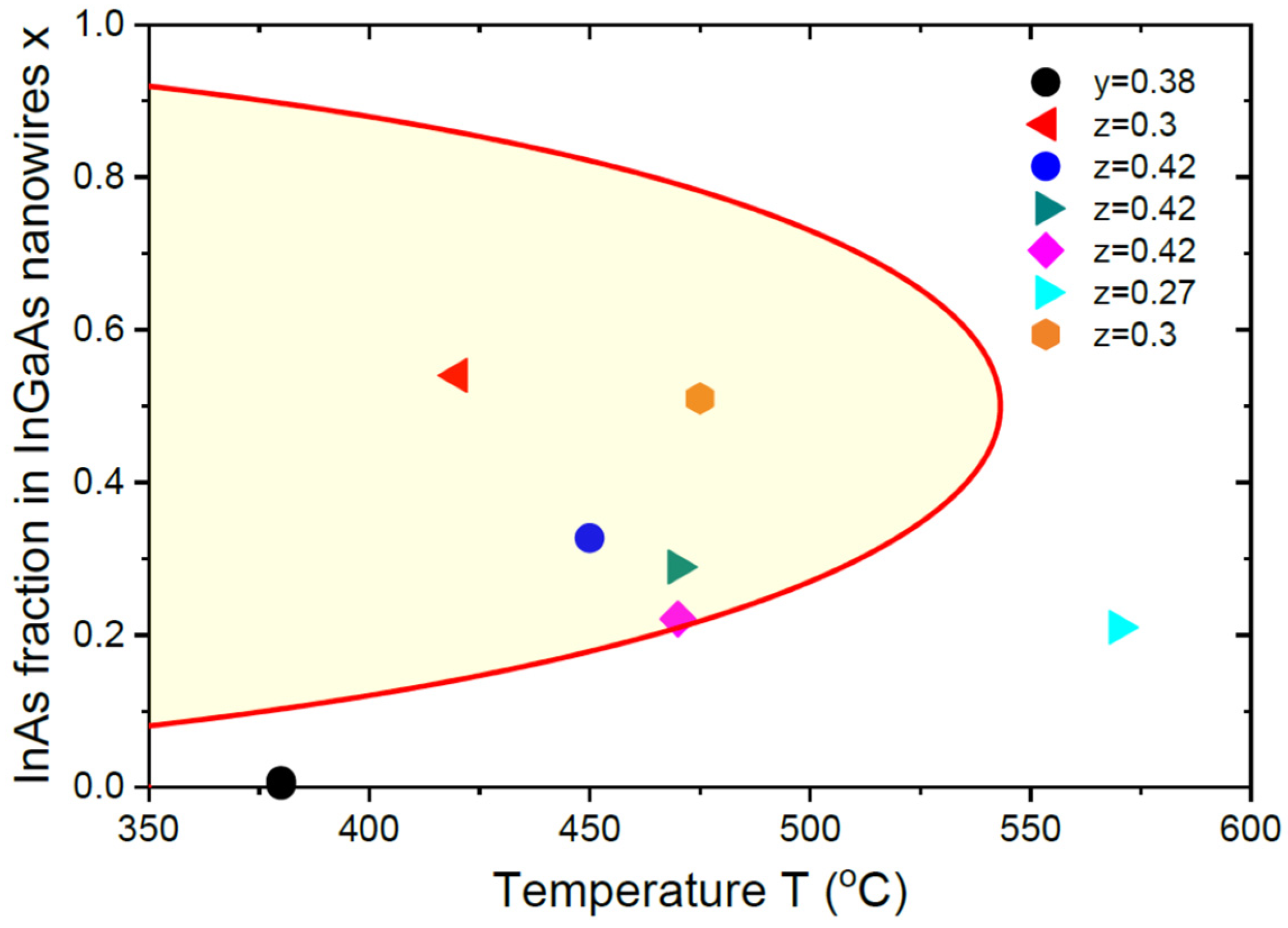
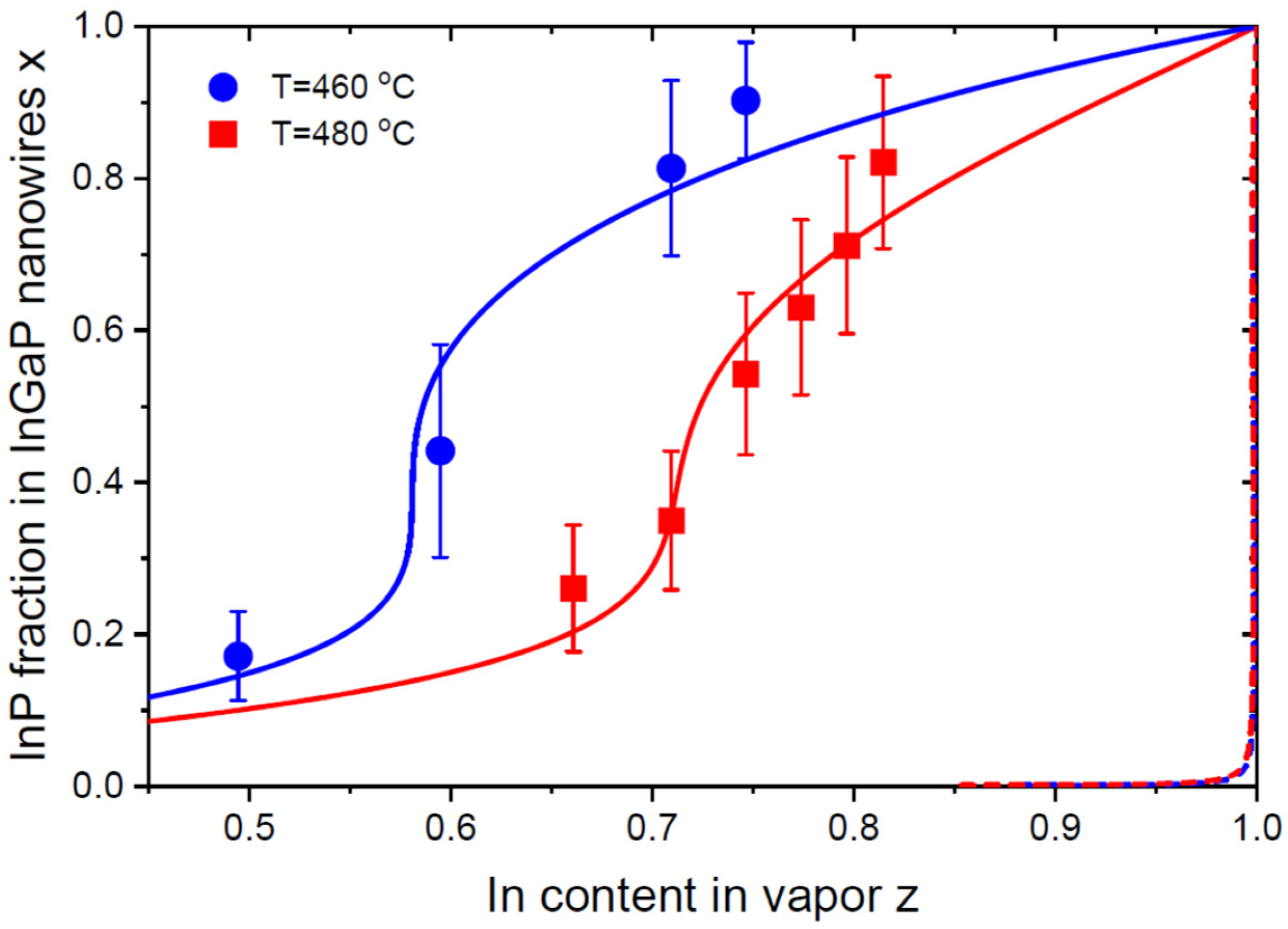
6. Results and Discussion
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ning, C.-Z.; Dou, L.; Yang, P. Bandgap engineering in semiconductor alloy nanomaterials with widely tunable compositions. Nat. Rev. Mater. 2017, 2, 17070. [Google Scholar] [CrossRef]
- McIntyre, P.C.; Fontcuberta i Morral, A. Semiconductor nanowires: To grow or not to grow? Mater. Today Nano 2020, 9, 100058. [Google Scholar] [CrossRef]
- Martelli, F. III–V ternary nanowires. In Advances in III–V Semiconductor Nanowires and Nanodevices; Li, J., Wang, D., LaPierre, R.R., Eds.; Bentham Science: Sharjah, United Arab Emirates, 2011; pp. 105–128. [Google Scholar] [CrossRef]
- Glas, F. Critical dimensions for the plastic relaxation of strained axial heterostructures in free-standing nanowires. Phys. Rev. B 2006, 74, 121302. [Google Scholar] [CrossRef]
- Chuang, L.C.; Moewe, M.; Chase, C.; Kobayashi, N.P.; Chang-Hasnain, C.; Crankshaw, S. Critical diameters for III–V nanowires grown on lattice-mismatched substrates. Appl. Phys. Lett. 2007, 90, 043115. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Cirlin, G.E.; Musikhin, Y.G.; Samsonenko, Y.B.; Tonkikh, A.A.; Polyakov, N.K.; Egorov, V.A.; Tsatsul’nikov, A.F.; Krizhanovskaya, N.A.; Ustinov, V.M.; et al. Effect of growth kinetics on the structural and optical properties of quantum dot ensembles. J. Cryst. Growth 2004, 267, 47. [Google Scholar] [CrossRef]
- Boras, G.; Yu, X.; Liu, H. III–V ternary nanowires on Si substrates: Growth, characterization and device applications. J. Semicond. 2019, 40, 101301. [Google Scholar] [CrossRef]
- Wagner, R.S.; Ellis, W.C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89. [Google Scholar] [CrossRef]
- Colombo, C.; Spirkoska, D.; Frimmer, M.; Abstreiter, G.; Fontcuberta i Morral, A. Ga-assisted catalyst-free growth mechanism of GaAs nanowires by molecular beam epitaxy. Phys. Rev. B 2008, 77, 155326. [Google Scholar] [CrossRef]
- Regolin, I.; Khorenko, V.; Prost, W.I.; Tegude, F.-J.; Sudfeld, D.; Kästner, J.; Dumpich, G. Composition control in metal-organic vapor-phase epitaxy grown InGaAs nanowhiskers. J. Appl. Phys. 2006, 100, 074321. [Google Scholar] [CrossRef]
- Wu, J.; Borg, B.M.; Jacobsson, D.; Dick, K.A.; Wernersson, L.-E. Control of composition and morphology in InGaAs nanowires grown by metalorganic vapor phase epitaxy. J. Cryst. Growth 2013, 383, 158. [Google Scholar] [CrossRef]
- Jung, C.S.; Kim, H.S.; Jung, G.B.; Gong, K.J.; Cho, Y.I.; Jang, S.Y.; Kim, C.H.; Lee, C.; Park, J. Composition and phase tuned InGaAs alloy nanowires. J. Phys. Chem. C 2011, 115, 7843. [Google Scholar] [CrossRef]
- Ameruddin, A.S.; Fonseka, H.A.; Caroff, P.; Wong-Leung, J.; Veld, R.O.H.; Boland, J.L.; Johnston, M.B.; Tan, H.H.; Jagadish, C. InxGa1-xAs nanowires with uniform composition, pure wurtzite crystal phase and taper-free morphology. Nan-Otechnology 2015, 26, 205604. [Google Scholar] [CrossRef]
- Ameruddin, A.S.; Caroff, P.; Tan, H.H.; Jagadish, C.; Dubrovskii, V.G. Understanding the growth and composition evolution of gold-seeded ternary InGaAs nanowires. Nanoscale 2015, 7, 16266. [Google Scholar] [CrossRef] [PubMed]
- Sjokvist, R.; Jacobsson, D.; Tornberg, M.; Wallenberg, R.; Leshchenko, E.D.; Johansson, J.; Dick, K.A. Compositional correlation between the nanoparticle and the growing Au-assisted InxGa1-xAs nanowire. J. Phys. Chem. Lett. 2021, 12, 7590. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, P.K.; Behnam, A.; Wood, J.D.; English, C.D.; Lyding, J.W.; Pop, E.; Li, X. InxGa1−xAs nanowire growth on graphene: Van der Waals epitaxy induced phase segregation. Nano Lett. 2013, 13, 1153. [Google Scholar] [CrossRef]
- Jacobsson, D.; Persson, J.M.; Kriegner, D.; Etzelstorfer, T.; Wallentin, J.; Wagner, J.B.; Stangl, J.; Samuelson, L.; Deppert, K.; Borgstrom, M.T. Particle-assisted GaxIn1-xP nanowire growth for designed bandgap structures. Nanotechnology 2012, 23, 245601. [Google Scholar] [CrossRef]
- Wu, Z.H.; Sun, M.; Mei, X.Y.; Ruda, H.E. Growth and photoluminescence characteristics of AlGaAs nanowires. Appl. Phys. Lett. 2004, 85, 657. [Google Scholar] [CrossRef]
- Cirlin, G.E.; Reznik, R.R.; Shtrom, I.V.; Khrebtov, A.I.; Soshnikov, I.P.; Kukushkin, S.A.; Leandro, L.; Kasama, T.; Akopian, N. AlGaAs and AlGaAs/GaAs/AlGaAs nanowires grown by molecular beam epitaxy on silicon substrates. J. Phys. D Appl. Phys. 2017, 50, 484003. [Google Scholar] [CrossRef]
- Priante, G.; Glas, F.; Patriarche, G.; Pantzas, K.; Oehler, F.; Harmand, J.C. Sharpening the interfaces of axial heterostructures in self-catalyzed AlGaAs nanowires: Experiment and theory. Nano Lett. 2016, 16, 1917. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Shtrom, I.V.; Reznik, R.R.; Samsonenko, Y.B.; Khrebtov, A.I.; Soshnikov, I.P.; Rouvimov, S.; Akopian, N.; Kasama, T.; Cirlin, G.E. Origin of spontaneous core–shell AlGaAs nanowires grown by molecular beam epitaxy. Cryst. Growth Des. 2016, 16, 7251. [Google Scholar] [CrossRef]
- Leandro, L.; Reznik, R.; Clement, J.D.; Repän, J.; Reynolds, M.; Ubyivovk, E.V.; Shtrom, I.V.; Cirlin, G.; Akopian, N. Wurtzite AlGaAs nanowires. Sci. Rep. 2020, 10, 735. [Google Scholar] [CrossRef]
- Boras, G.; Yu, X.; Aruni Fonseka, H.; Davis, G.; Velichko, A.V.; Gott, J.A.; Zeng, H.; Wu, S.; Parkinson, P.; Xu, X.; et al. Self-catalyzed AlGaAs nanowires and AlGaAs/GaAs nanowire-quantum dots on Si substrates. J. Phys. Chem. C 2021, 125, 14338. [Google Scholar] [CrossRef] [PubMed]
- Roche, E.; Andre, Y.; Avit, G.; Bougerol, C.; Castelluci, D.; Reveret, F.; Gil, E.; Medard, F.; Leymarie, J.; Jean, T.; et al. Circumventing the miscibility gap in InGaN nanowires emitting from blue to red. Nanotechnology 2018, 29, 465602. [Google Scholar] [CrossRef] [PubMed]
- Dubrovskii, V.G. Understanding the vapor-liquid-solid growth and composition of ternary III–V nanowires and nanowire heterostructures. J. Phys. D Appl. Phys. 2017, 50, 453001. [Google Scholar] [CrossRef]
- Ghasemi, M.; Leshchenko, E.D.; Johansson, J. Assembling your nanowire: An overview of composition tuning in ternary III–V nanowires. Nanotechnology 2021, 32, 072001. [Google Scholar] [CrossRef] [PubMed]
- Leshchenko, E.D.; Dubrovskii, V.G. An overview of modeling approaches for compositional control in III–V ternary nanowires. Nanomaterials 2023, 13, 1659. [Google Scholar] [CrossRef]
- Glas, F. Comparison of modeling strategies for the growth of heterostructures in III–V nanowires. Cryst. Growth Des. 2017, 17, 4785–4794. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Koryakin, A.A.; Sibirev, N.V. Understanding the composition of ternary III–V nanowires and axial nanowire heterostructures in nucleation-limited regime. Mat. Des. 2017, 132, 400. [Google Scholar] [CrossRef]
- Ghasemi, M.; Johansson, J. The composition of gold alloy seeded InGaAs nanowires in the nucleation limited regime. Cryst. Growth Des. 2017, 17, 1630. [Google Scholar]
- Leshchenko, E.D.; Ghasemi, M.; Dubrovskii, V.G.; Johansson, J. Nucleation-limited composition of ternary III–V nanowires forming from quaternary gold based liquid alloys. CrystEngComm 2018, 20, 1649. [Google Scholar] [CrossRef]
- Johansson, J.; Ghasemi, M. Kinetically limited composition of ternary III–V nanowires. Phys. Rev. Mater. 2017, 1, 040401. [Google Scholar] [CrossRef]
- Leshchenko, E.D.; Johansson, J. Role of thermodynamics and kinetics in the composition of ternary III–V nanowires. Nanomaterials 2020, 10, 2553. [Google Scholar] [CrossRef]
- Leshchenko, E.D.; Dubrovskii, V.G. Kinetic modeling of interfacial abruptness in axial nanowire heterostructures. Nanotechnology 2023, 34, 065602. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Leshchenko, E.D. Kinetically controlled composition of III–V ternary nanostructures. Phys. Rev. Mater. 2023, 7, 056001. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Leshchenko, E.D. Composition of III–V ternary materials under arbitrary material fluxes: The general approach unifying kinetics and thermodynamics. Phys. Rev. Mater. 2023, 7, 074603. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. Liquid-solid and vapor-solid distributions of vapor-liquid-solid III–V ternary nanowires. Phys. Rev. Mater. 2023. submitted. [Google Scholar]
- Biefeld, R.M. The preparation of InSb and InAs1−xSbx by metalorganic chemical vapor deposition. J. Cryst. Growth 1986, 75, 255. [Google Scholar] [CrossRef]
- Givargizov, E.I. Highly Anisotropic Crystals, 1st ed.; Springer: Dordrecht, The Netherlands, 1987. [Google Scholar]
- Dubrovskii, V.G.; Glas, F. Vapor–liquid–solid growth of semiconductor nanowires. In Fundamental Properties of Semiconductor Nanowires; Fukata, N., Rurali, R., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Cirlin, G.E.; Dubrovskii, V.G.; Sibirev, N.V.; Soshnikov, I.P.; Samsonenko, Y.B.; Tonkikh, A.A.; Ustinov, V.M. The diffusion mechanism in the formation of GaAs and AlGaAs nanowhiskers during the process of molecular-beam epitaxy. Semiconductors 2005, 39, 557. [Google Scholar] [CrossRef]
- Johansson, J.; Magnusson, M.H. From diffusion limited to incorporation limited growth of nanowires. J. Cryst. Growth 2019, 525, 125192. [Google Scholar] [CrossRef]
- Glas, F.; Ramdani, M.R.; Patriarche, G.; Harmand, J.C. Predictive modeling of self-catalyzed III–V nanowire growth. Phys. Rev. B 2013, 88, 195304. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Sibirev, N.V.; Cirlin, G.E.; Bouravleuv, A.D.; Samsonenko, Y.B.; Dheeraj, D.L.; Zhou, H.L.; Sartel, C.; Harmand, J.C.; Patriarche, G.; et al. Role of non-linear effects in nanowire growth and crystal phase. Phys. Rev. B 2009, 80, 205305. [Google Scholar] [CrossRef]
- Plante, M.C.; LaPierre, R.R. Analytical description of the metal-assisted growth of III–V nanowires: Axial and radial growths. J. Appl. Phys. 2009, 105, 114304. [Google Scholar] [CrossRef]
- Chen, C.; Shehata, S.; Fradin, C.; LaPierre, R.R.; Couteau, C.; Weihs, G. Self-directed growth of AlGaAs core-shell nanowires for visible light applications. Nano Lett. 2007, 7, 2584. [Google Scholar] [CrossRef]
- Kaiiyama, K. Vapor pressure dependence of the relative composition of IlI-V mixed crystals in vapor phase epitaxy. J. Electrochem. Soc. 1976, I23, 423. [Google Scholar]
- Adhikari, J.; Kofke, D. Molecular simulation study of miscibility of ternary and quaternary InGaAlN alloys. J. Appl. Phys. 2004, 95, 6129. [Google Scholar] [CrossRef]
- Kanagawa, Y.; Ito, T.; Kumagai, Y.; Koukitu, A. Thermodynamic study on compositional instability of InGaN/GaN and InGaN/InN during MBE. Appl. Surf. Sci. 2003, 216, 453. [Google Scholar] [CrossRef]

| Ref. | System | T (°C) | Distribution Type | ||||||
|---|---|---|---|---|---|---|---|---|---|
| [21] | Au-catalyzed AlGaAs NWs | 510 | , kinetic | - | - | 0 | - | 0.385 | - |
| [15] | Au-catalyzed InGaAs NWs | 380 | , equilibrium | 2.724 | 0.06 | - | - | - | - |
| [18] | Bottoms of Au-catalyzed InGaAs NWs | 450 | , kinetic | 2.373 | - | 0.15 | 0 | 0.39 | 0.39 |
| [18] | Tops of Au-catalyzed InGaAs NWs | 450 | , kinetic | 2.373 | - | 0.15 | 0 | 1.2 | 1.2 |
| [10] | Au-catalyzed InGaAs NWs | 420 | , kinetic | 2.475 | - | 0.2 | 0 | 4.1 | 1 |
| [17] | Au-catalyzed InGaP NWs | 460 | , kinetic | 2.514 | - | 0.52 | 0 | 4.2 | 4.2 |
| [17] | Au-catalyzed InGaP NWs | 480 | 2.438 | - | 0.65 | 0 | 2.2 | 2.2 | |
| [24] | Self-catalyzed InGaN NWs | 630–680 | , kinetic | - | - | 0 | - | Arrhenius, 3.29 eV | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubrovskii, V.G. Composition of Vapor–Liquid–Solid III–V Ternary Nanowires Based on Group-III Intermix. Nanomaterials 2023, 13, 2532. https://doi.org/10.3390/nano13182532
Dubrovskii VG. Composition of Vapor–Liquid–Solid III–V Ternary Nanowires Based on Group-III Intermix. Nanomaterials. 2023; 13(18):2532. https://doi.org/10.3390/nano13182532
Chicago/Turabian StyleDubrovskii, Vladimir G. 2023. "Composition of Vapor–Liquid–Solid III–V Ternary Nanowires Based on Group-III Intermix" Nanomaterials 13, no. 18: 2532. https://doi.org/10.3390/nano13182532





