Nano-Delivery System of Ethanolic Extract of Propolis Targeting Mycobacterium tuberculosis via Aptamer-Modified-Niosomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial and Cell Culture
2.3. Preparation of EEP Solution
2.4. Assessment of Total Phenolic Content
2.5. Optimization of Nano-Formulations
2.6. Conjugation of Apt to PEGNio/EEP
2.7. Physicochemical Characterization
2.7.1. Size Distribution and Zeta-Potential (ZP)
2.7.2. Entrapment Efficiency (EE)
2.7.3. Morphology of NPs
2.7.4. Fourier Transform Infrared (FT-IR) Spectrometry
2.8. In Vitro Release Profile
2.9. Examination of an Interaction between Apt-PEGNio/EEP and Mtb
2.10. Assessment of Anti-Mtb Activity by Resazurin Microtitre Assay (REMA) [27]
2.11. Cytotoxicity Test
2.12. Statistical Analysis
3. Results
3.1. Anti-Mtb Activity of EEP
3.2. Physicochemical Characteristics
3.3. Conjugation and Characterization of Apt-PEGNio/EEP
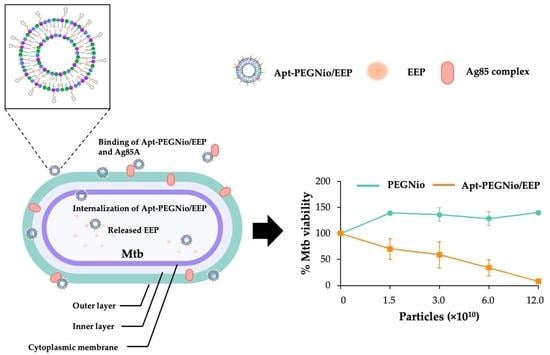
3.4. Binding Ability of Apt-PEGNio/EEP to Mtb
3.5. In Vitro Release of Apt-PEGNio/EEP
3.6. Anti-Mtb Activity and Cytotoxicity of Apt-PEGNio/EEP
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maiolini, M.; Gause, S.; Taylor, J.; Steakin, T.; Shipp, G.; Lamichhane, P.; Deshmukh, B.; Shinde, V.; Bishayee, A.; Deshmukh, R.R. The war against tuberculosis: A review of natural compounds and their derivatives. Molecules 2020, 25, 3011. [Google Scholar] [CrossRef]
- Bouzeyen, R.; Javid, B. Therapeutic vaccines for tuberculosis: An overview. Front. Immunol. 2022, 13, 878471. [Google Scholar] [CrossRef] [PubMed]
- Allue-Guardia, A.; Garcia, J.I.; Torrelles, J.B. Evolution of drug-resistant Mycobacterium tuberculosis strains and their adaptation to the human lung environment. Front. Microbiol. 2021, 12, 612675. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Klodzinska, S.N.; Wan, F.; Nielsen, H.M. Nanoparticle-mediated pulmonary drug delivery: State of the art towards efficient treatment of recalcitrant respiratory tract bacterial infections. Drug Deliv. Transl. Res. 2021, 11, 1634–1654. [Google Scholar] [CrossRef] [PubMed]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological properties and therapeutic applications of propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Sawicki, R.; Widelski, J.; Okinczyc, P.; Truszkiewicz, W.; Glous, J.; Sieniawska, E. Exposure to Nepalese propolis alters the metabolic state of Mycobacterium tuberculosis. Front. Microbiol. 2022, 13, 929476. [Google Scholar] [CrossRef]
- Ali, M.T.; Blicharska, N.; Shilpi, J.A.; Seidel, V. Investigation of the anti-TB potential of selected propolis constituents using a molecular docking approach. Sci. Rep. 2018, 8, 12238. [Google Scholar] [CrossRef]
- Scheller, S.; Dworniczak, S.; Waldemar-Klimmek, K.; Rajca, M.; Tomczyk, A.; Shani, J. Synergism between ethanolic extract of propolis (EEP) and anti-tuberculosis drugs on growth of mycobacteria. Z. Nat. C 1999, 54, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Leifer, F.; Rose, S.; Chun, D.Y.; Thaisz, J.; Herr, T.; Nashed, M.; Joseph, J.; Perkins, W.R.; DiPetrillo, K. Amikacin liposome inhalation suspension (ALIS) penetrates non-tuberculous Mycobacterial biofilms and enhances amikacin uptake into macrophages. Front. Microbiol. 2018, 9, 915. [Google Scholar] [CrossRef] [Green Version]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- PaweLczyk, J.; Kremer, L. The molecular genetics of mycolic acid biosynthesis. Microbiol. Spectr. 2014, 2, MGM2-0003-2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, J.; Ketkar, S.; Patil, S.; Fearnley, J.; Mahadik, K.R.; Paradkar, A.R. Potentiating antimicrobial efficacy of propolis through niosomal-based system for administration. Integr. Med. Res. 2015, 4, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent advances in aptamer discovery and applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef] [Green Version]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zheng, R.; Ma, Z.; Feng, Y.; Liu, Z.; Yang, H.; Wang, J.; Jin, R.; Lu, J.; Ding, Y.; et al. The selection and application of ssDNA aptamers against MPT64 protein in Mycobacterium tuberculosis. Clin. Chem. Lab. Med. 2009, 47, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Rotherham, L.S.; Maserumule, C.; Dheda, K.; Theron, J.; Khati, M. Selection and application of ssDNA aptamers to detect active TB from sputum samples. PLoS ONE 2012, 7, e46862. [Google Scholar] [CrossRef]
- Ansari, N.; Ghazvini, K.; Ramezani, M.; Shahdordizadeh, M.; Yazdian-Robati, R.; Abnous, K.; Taghdisi, S.M. Selection of DNA aptamers against Mycobacterium tuberculosis Ag85A, and its application in a graphene oxide-based fluorometric assay. Mikrochim. Acta 2017, 185, 21. [Google Scholar] [CrossRef]
- Iadnut, A.; Mamoon, K.; Thammasit, P.; Pawichai, S.; Tima, S.; Preechasuth, K.; Kaewkod, T.; Tragoolpua, Y.; Tragoolpua, K. In vitro antifungal and antivirulence activities of biologically synthesized ethanolic extract of propolis-loaded PLGA nanoparticles against Candida albicans. Evid.-Based Complement. Altern. Med. 2019, 2019, 3715481. [Google Scholar] [CrossRef] [Green Version]
- Mamoon, K.; Thammasit, P.; Iadnut, A.; Kitidee, K.; Anukool, U.; Tragoolpua, Y.; Tragoolpua, K. Unveiling the properties of Thai stingless bee propolis via diminishing cell wall-associated cryptococcal melanin and enhancing the fungicidal activity of macrophages. Antibiotics 2020, 9, 420. [Google Scholar] [CrossRef]
- Sangboonruang, S.; Semakul, N.; Obeid, M.A.; Ruano, M.; Kitidee, K.; Anukool, U.; Pringproa, K.; Chantawannakul, P.; Ferro, V.A.; Tragoolpua, K. Potentiality of melittin-loaded niosomal vesicles against vancomycin-intermediate Staphylococcus aureus and Staphylococcal skin infection. Int. J. Nanomed. 2021, 16, 7639–7661. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Hu, Y.; Duan, J.; Yuan, W.; Wang, C.; Xu, H.; Yang, X.D. Novel aptamer-nanoparticle bioconjugates enhances delivery of anticancer drug to MUC1-positive cancer cells in vitro. PLoS ONE 2011, 6, e24077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, Y.J.; McConville, J.T. Development of a standardized dissolution test method for inhaled pharmaceutical formulations. Int. J. Pharm. 2009, 382, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Andreu, N.; Fletcher, T.; Krishnan, N.; Wiles, S.; Robertson, B.D. Rapid measurement of antituberculosis drug activity in vitro and in macrophages using bioluminescence. J. Antimicrob. Chemother. 2012, 67, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Santos, K.; Lukka, P.B.; Grzegorzewicz, A.; Jackson, M.; Trivedi, A.; Pavan, F.; Chorilli, M.; Braunstein, M.; Hickey, A.; Meibohm, B.; et al. Primary lung dendritic cell cultures to assess efficacy of spectinamide-1599 against intracellular Mycobacterium tuberculosis. Front. Microbiol. 2018, 9, 1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grange, J.M.; Davey, R.W. Antibacterial properties of propolis (bee glue). J. R. Soc. Med. 1990, 83, 159–160. [Google Scholar] [CrossRef]
- Allend, S.O.; Volcao, L.; Canirlles, C.S.; Barbosa, I.; Biatobock, D.; Silva, P.E.A.; Ramos, D.F. Green propolis as an adjuvant against nontuberculous mycobacteria. Rodriguésia 2021, 72, e01562020.2021. [Google Scholar] [CrossRef]
- Machado, C.S.; Mokochinski, J.B.; de Lira, T.O.; de Oliveira Fde, C.; Cardoso, M.V.; Ferreira, R.G.; Sawaya, A.C.; Ferreira, A.G.; Pessoa, C.; Cuesta-Rubio, O.; et al. Comparative study of chemical composition and biological activity of yellow, green, brown, and red Brazilian propolis. Evid.-Based Complement. Altern. Med. 2016, 2016, 6057650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef]
- Kubiliene, L.; Laugaliene, V.; Pavilonis, A.; Maruska, A.; Majiene, D.; Karolina, B.; Kubilius, R.; Kasparaviciene, G.; Savickas, A. Alternative preparation of propolis extracts: Comparison of their composition and biological activities. BMC Complement. Altern. Med. 2015, 15, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delogu, G.; Sali, M.; Fadda, G. The biology of mycobacterium tuberculosis infection. Mediterr. J. Hematol. Infect. Dis. 2013, 5, e2013070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifacio, B.V.; Silva, P.B.; Ramos, M.A.; Negri, K.M.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar]
- Seleci, D.A.; Seleci, M.; Walter, J.G.; Stahl, F.; Scheper, T. Niosomes as nanoparticular drug carriers: Fundamental and recent applications. J. Nanomater. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rani, N.P.; Suriyaprakash, T.N.K.; Senthamarai, R. Formulation and evaluation of rifampicin and gatifloxacin niosomes on logarithmic phase cultures of Mycobacterium tuberculosis. Int. J. Pharm. Bio Sci. 2010, 1, 379–387. [Google Scholar]
- Singh, G.; Dwivedi, H.; Saraf, S.A. Niosomal delivery of isoniazid-development and characterization. Trop. J. Pharm. Res. 2011, 10, 203–210. [Google Scholar] [CrossRef]
- Nasiruddin, M.; Neyaz, m.k.; Das, S. Nanotechnology-based approach in tuberculosis treatment. Tuberc. Res. Treat. 2017, 2017, 4920209. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.K.; Jindal, N. Formulation of tyloxapol niosomes for encapsulation, stabilization and dissolution of anti-tubercular drugs. Colloids Surf. B Biointerfaces 2013, 101, 434–441. [Google Scholar] [CrossRef]
- Rahme, K.; Dagher, N. Chemistry routes for copolymer synthesis containing PEG for targeting, imaging, and drug delivery purposes. Pharmaceutics 2019, 11, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei Zadeh Babaki, M.; Soleimanpour, S.; Rezaee, S.A. Antigen 85 complex as a powerful Mycobacterium tuberculosis immunogene: Biology, immune-pathogenicity, applications in diagnosis, and vaccine design. Microb. Pathog. 2017, 112, 20–29. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. SELEX--a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 2007, 24, 381–403. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery-an overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef] [Green Version]
- Junyaprasert, V.B.; Singhsa, P.; Suksiriworapong, J.; Chantasart, D. Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int. J. Pharm. 2012, 423, 303–311. [Google Scholar] [CrossRef]
- Nowroozi, F.; Almasi, A.; Javidi, J.; Haeri, A.; Dadashzadeh, S. Effect of surfactant type, cholesterol content and various downsizing methods on the particle size of niosomes. Iran. J. Pharm. Res. 2018, 17, 1–11. [Google Scholar]
- Lin, T.; Fang, Q.; Peng, D.; Huang, X.; Zhu, T.; Luo, Q.; Zhou, K.; Chen, W. PEGylated non-ionic surfactant vesicles as drug delivery systems for Gambogenic acid. Drug Deliv. 2013, 20, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Yang, M.; Li, J.; Bi, X.; Li, G.; Xu, J.; Xie, S.; Dong, Y.; Li, D.; Du, Y. The enhancing antifungal effect of AD1 aptamer-functionalized amphotericin B-loaded PLGA-PEG nanoparticles with a low-frequency and low-intensity ultrasound exposure on C. albicans biofilm through targeted effect. NanoImpact 2021, 21, 100275. [Google Scholar] [CrossRef]
- Joseph, E.; Singhvi, G. Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. In Nanomaterials for Drug Delivery and Therapy; Grumezescu, A.M., Ed.; William and Andrew; Applied Science Publisher: Norwich, NY, USA, 2019; pp. 91–116. [Google Scholar]
- Cetin, E.O.; Salmanoglu, D.S.; Ozden, I.; Ors-Kumoglu, G.; Akar, S.; Demirozer, M.; Karabey, F.; Kilic, K.D.; Kirilmaz, L.; Uyanikgil, Y.; et al. Preparation of ethanol extract of propolis loaded niosome formulation and evaluation of effects on different cancer cell lines. Nutr. Cancer 2021, 74, 265–277. [Google Scholar] [CrossRef]
- Witika, B.A.; Bassey, K.E.; Demana, P.H.; Siwe-Noundou, X.; Poka, M.S. Current advances in specialised niosomal drug delivery: Manufacture, characterization and drug delivery applications. Int. J. Mol. Sci. 2022, 23, 9668. [Google Scholar] [CrossRef]
- Seleci, D.A.; Seleci, M.; Jochums, A.; Walter, J.G.; Stahl, F.; Scheper, T. Aptamer mediated niosomal drug delivery. RSC Adv. 2016, 6, 87910–87918. [Google Scholar] [CrossRef] [Green Version]
- Moosavian, S.A.; Sahebkar, A. Aptamer-functionalized liposomes for targeted cancer therapy. Cancer Lett. 2019, 448, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Rodrigues, B.; Mendes, A.; Correia-Neves, M.; Nobrega, C. Ag85-focused T-cell immune response controls Mycobacterium avium chronic infection. PLoS ONE 2018, 13, e0193596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.J.; Bell, H.; Hsieh, C.L.; Ptak, C.P.; Chang, Y.F. Novel mycobacteria antigen 85 complex binding motif on fibronectin. J. Biol. Chem. 2012, 287, 1892–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drowart, A.; De Bruyn, J.; Huygen, K.; Damiani, G.; Godfrey, H.P.; Stelandre, M.; Yernault, J.C.; Van Vooren, J.P. Isoelectrophoretic characterization of protein antigens present in mycobacterial culture filtrates and recognized by monoclonal antibodies directed against the Mycobacterium bovis BCG antigen 85 complex. Scand. J. Immunol. 1992, 36, 697–702. [Google Scholar] [CrossRef]
- Muller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery-a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]







| Formulations | SP 60:CHOL:DSPE-PEG (mM) | SP 60 (mg) | CHOL (mg) | DSPE-PEG (mg) | Total Phenolic Content of EEP (mg/mL GAE) |
|---|---|---|---|---|---|
| F1 | 7:3:0.1 | 7.5 | 2.88 | 0.75 | 0.175 |
| F2 | 5:5:0.1 | 5.4 | 4.75 | 0.75 | |
| F3 | 3:7:0.1 | 3.25 | 6.75 | 0.75 |
| Formulations | PEGNio | PEGNio/EEP | ||||||
|---|---|---|---|---|---|---|---|---|
| Size (nm) | PDI | ZP (mV) | Size (nm) | PDI | ZP (mV) | % EE | Total Phenolic Contents of EEP (mg/mL GAE) | |
| F1 | 222.51 ±11.75 | 0.48 ±0.05 | −16.33 ±1.08 | 266.87 ±13.92 | 0.44 ±0.02 | −20.48 ±3.10 | 38.83 ±0.66 | 0.06 ±0.004 |
| F2 | 161.13 ±6.09 | 0.31 ±0.05 | −20.69 ±1.26 | 176.14 ±13.88 | 0.21 ±0.04 | −21.86 ±0.36 | 80.38 *,# ±7.58 | 0.14 ±0.016 |
| F3 | 146.16 ±5.34 | 0.26 ±0.02 | −26.21 ±9.70 | 253.91 ±63.22 | 0.20 ±0.06 | −24.06 ±4.08 | 41.69 ±6.49 | 0.055 ±0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangboonruang, S.; Semakul, N.; Suriyaprom, S.; Kitidee, K.; Khantipongse, J.; Intorasoot, S.; Tharinjaroen, C.S.; Wattananandkul, U.; Butr-Indr, B.; Phunpae, P.; et al. Nano-Delivery System of Ethanolic Extract of Propolis Targeting Mycobacterium tuberculosis via Aptamer-Modified-Niosomes. Nanomaterials 2023, 13, 269. https://doi.org/10.3390/nano13020269
Sangboonruang S, Semakul N, Suriyaprom S, Kitidee K, Khantipongse J, Intorasoot S, Tharinjaroen CS, Wattananandkul U, Butr-Indr B, Phunpae P, et al. Nano-Delivery System of Ethanolic Extract of Propolis Targeting Mycobacterium tuberculosis via Aptamer-Modified-Niosomes. Nanomaterials. 2023; 13(2):269. https://doi.org/10.3390/nano13020269
Chicago/Turabian StyleSangboonruang, Sirikwan, Natthawat Semakul, Sureeporn Suriyaprom, Kuntida Kitidee, Jiaranai Khantipongse, Sorasak Intorasoot, Chayada Sitthidet Tharinjaroen, Usanee Wattananandkul, Bordin Butr-Indr, Ponrut Phunpae, and et al. 2023. "Nano-Delivery System of Ethanolic Extract of Propolis Targeting Mycobacterium tuberculosis via Aptamer-Modified-Niosomes" Nanomaterials 13, no. 2: 269. https://doi.org/10.3390/nano13020269