Ag Nanocluster Production through DC Magnetron Sputtering and Inert Gas Condensation: A Study of Structural, Kelvin Probe Force Microscopy, and Optical Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
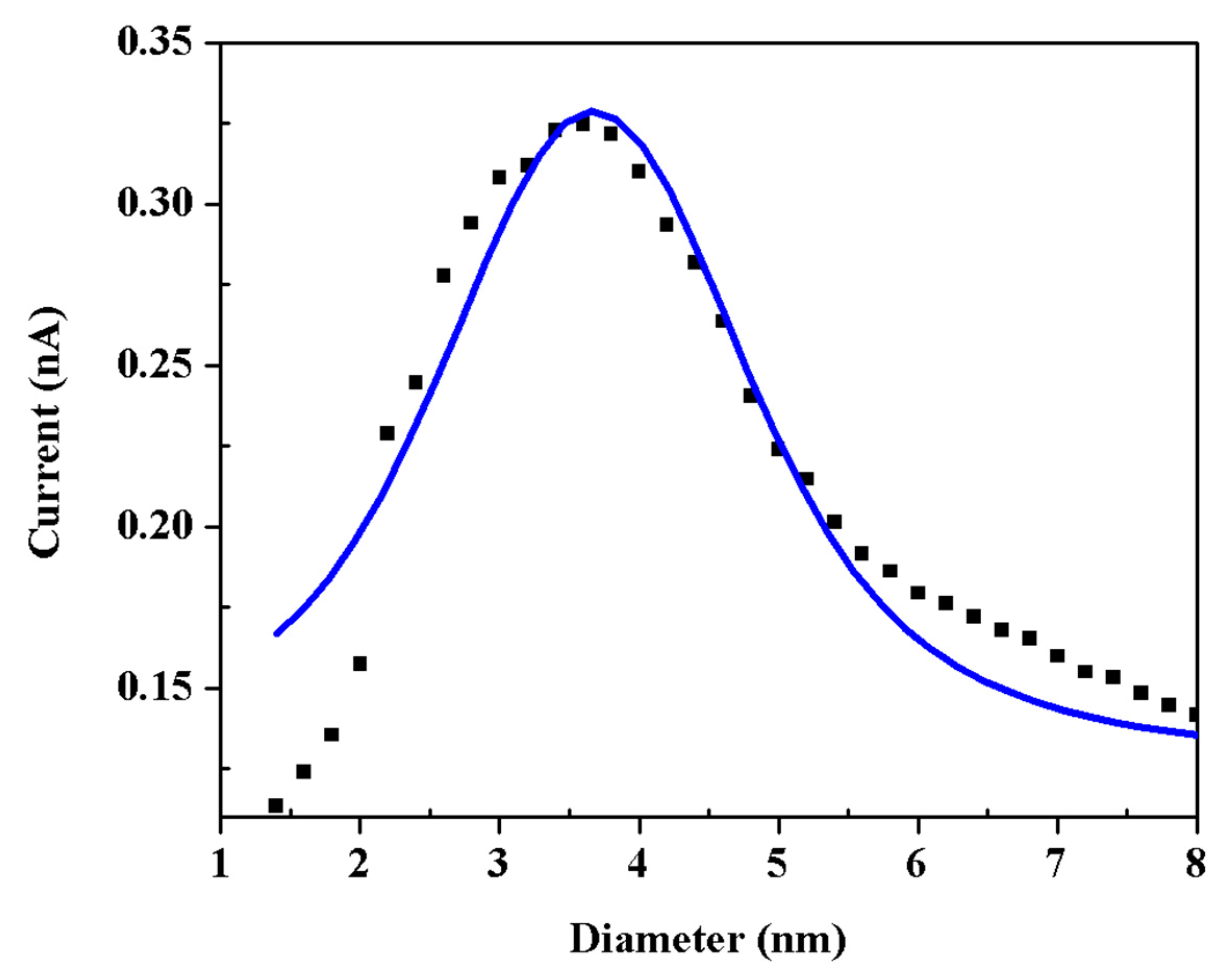
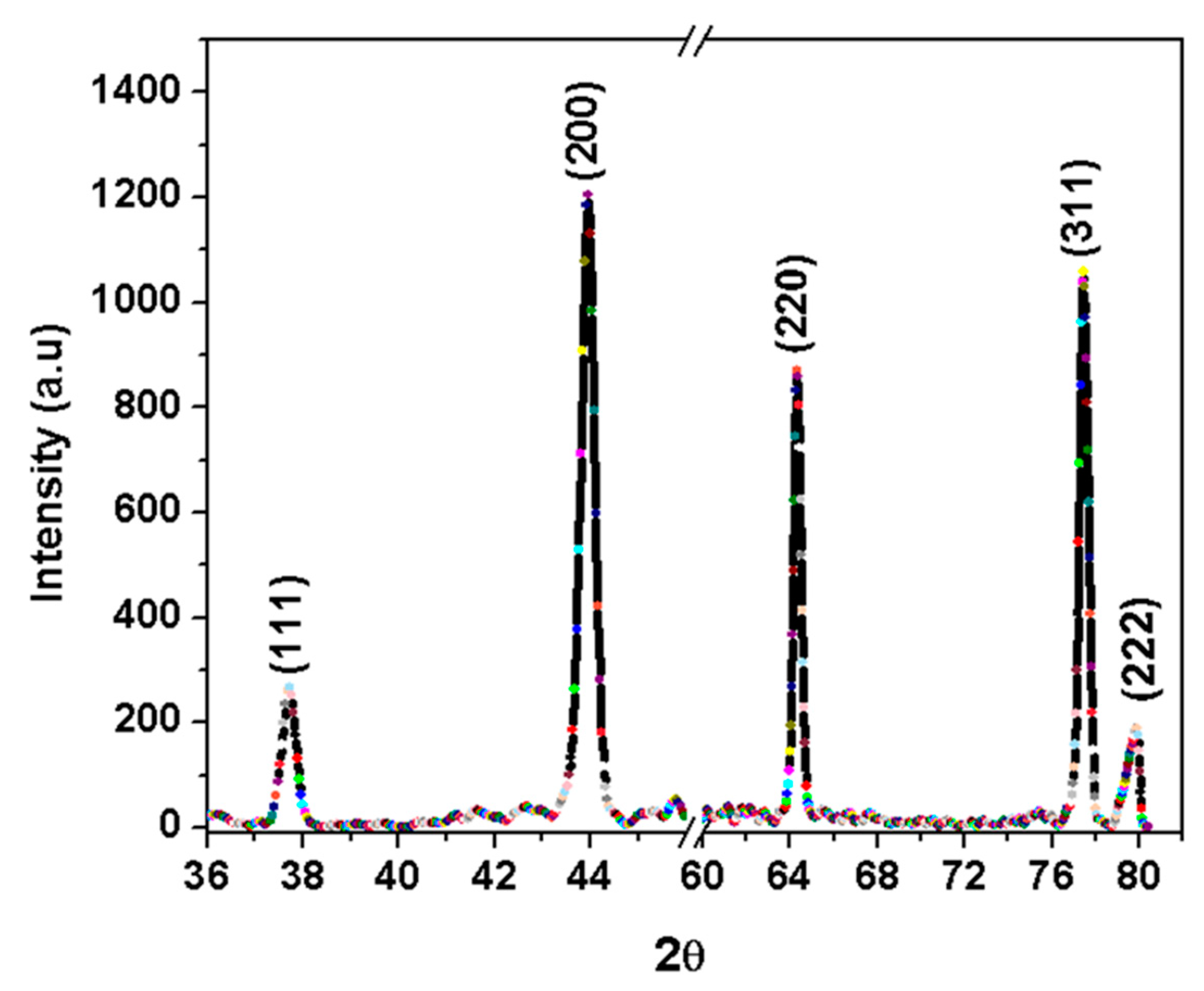
3.1. Morphology and Structure
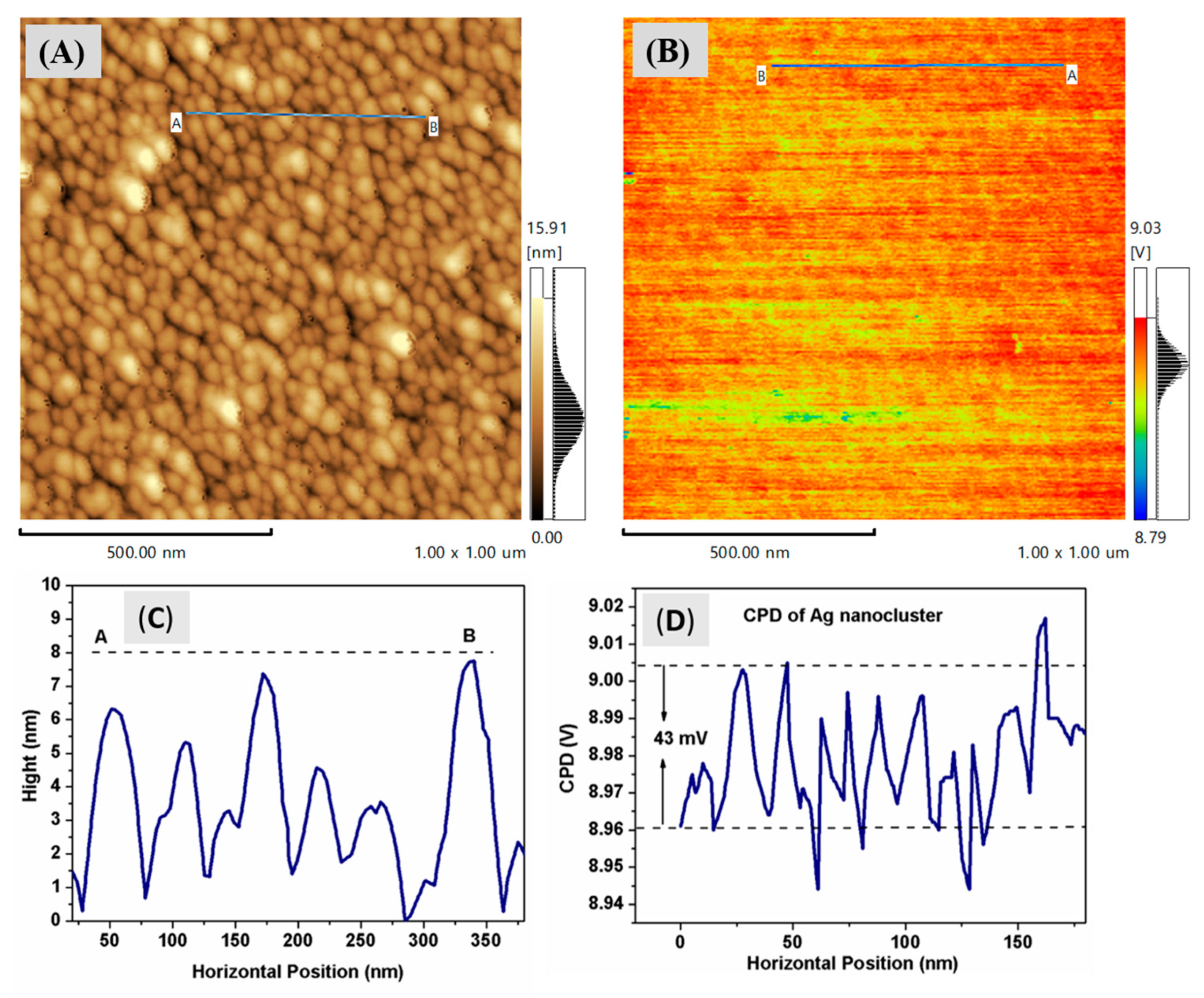
3.2. Kelvin Probe Force Microscopy
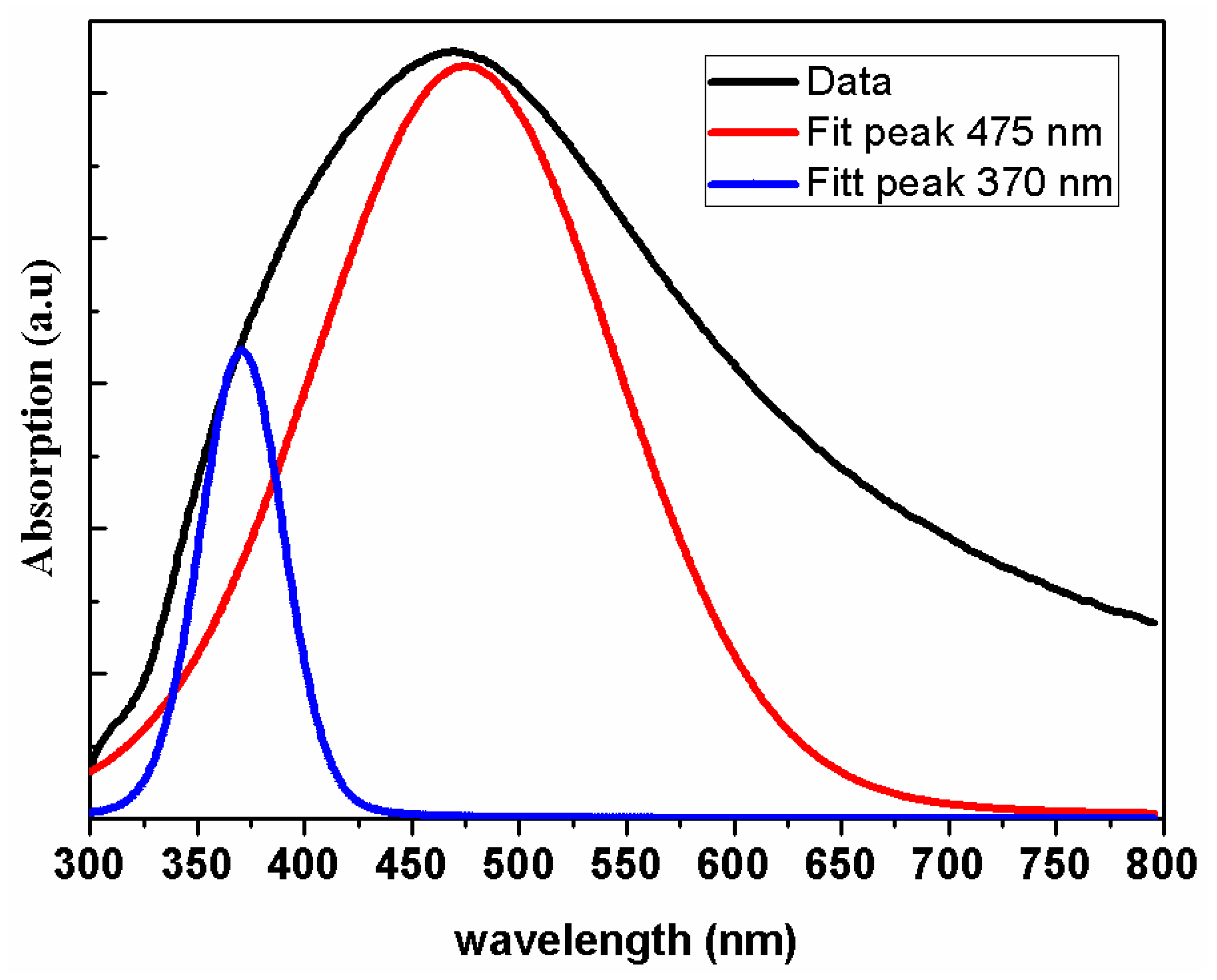
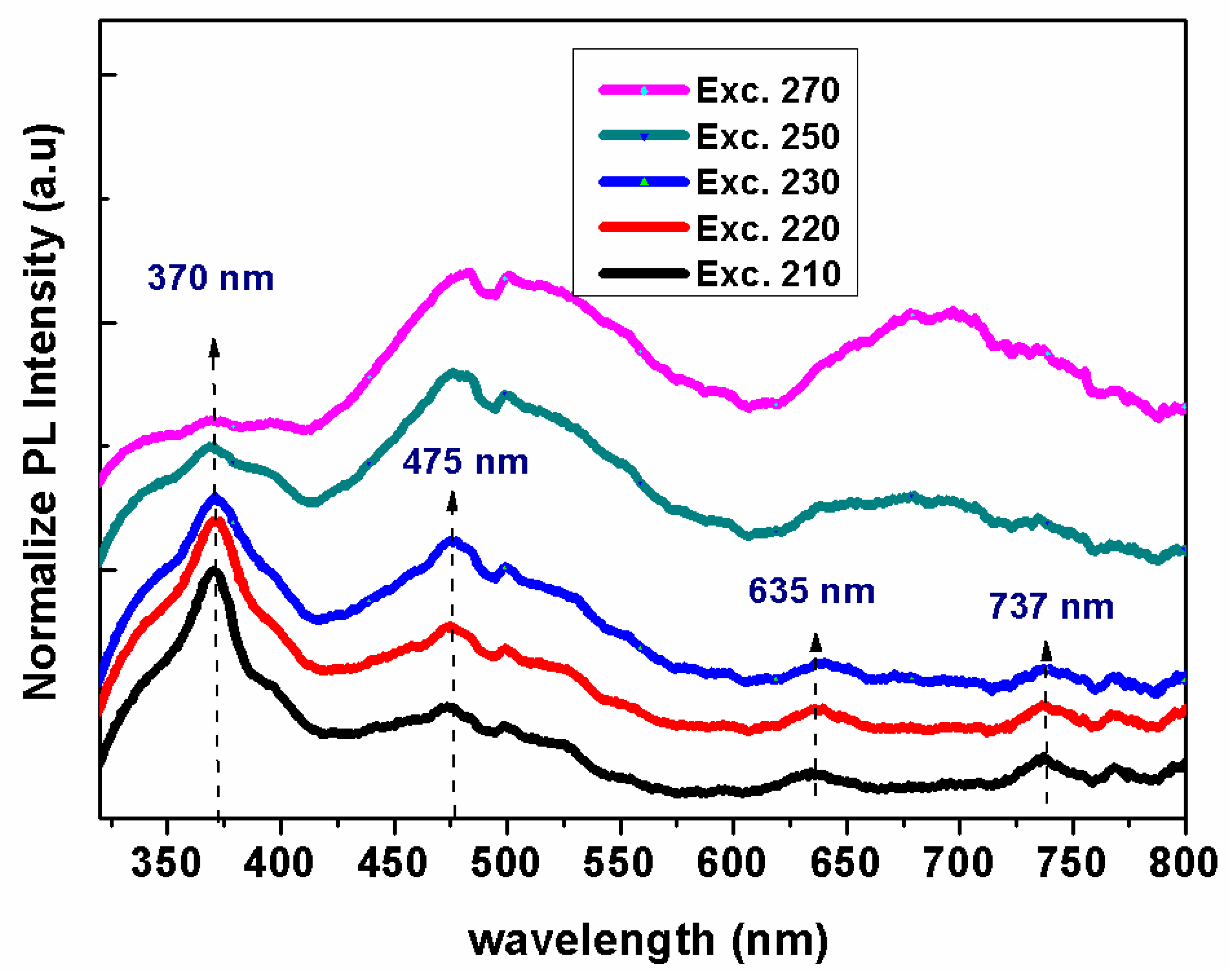
3.3. Optical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Shimizu, K.; Satsuma, A. Silver Cluster Catalysts for Green Organic Synthesis. J. Jpn. Petrol. Inst. 2011, 54, 347–360. [Google Scholar] [CrossRef]
- Albulym, O.; Hussien, M.S.A.; Kilany, M.; Zahran, H.Y.; Eid, R.A.; Yahia, I.S.; Abutalib, M.M. 5-Minute Synthesis of Gelatinous Silver Nanoparticles Using Microwave Radiation: Plasmonic Optical Spectroscopy and Antimicrobial Activity. Optik 2021, 228, 166139. [Google Scholar] [CrossRef]
- Chouhan, N. Silver Nanoparticles: Synthesis, Characterization and Applications. In Silver Nanoparticles; Maaz, K., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Chow, T.H.; Li, N.; Bai, X.; Zhuo, X.; Shao, L.; Wang, J. Gold Nanobipyramids: An Emerging and Versatile Type of Plasmonic Nanoparticles. Acc. Chem. Res. 2019, 52, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Beleño, Y.; Martinez-Nuñez, C.E.; Cortez-Valadez, M.; Flores-López, N.S.; Flores-Acosta, M. Optical Properties of Silver, Silver Sulfide and Silver Selenide Nanoparticles and Antibacterial Applications. Mater. Res. Bull. 2018, 99, 385–392. [Google Scholar] [CrossRef]
- Inwati, G.K.; Rao, Y.; Singh, M. In Situ Growth of Low-Dimensional Silver Nanoclusters with Their Tunable Plasmonic and Thermodynamic Behavior. ACS Omega 2017, 2, 5748–5758. [Google Scholar] [CrossRef]
- Eka Putri, G.; Rahayu Gusti, F.; Novita Sary, A.; Zainul, R. Synthesis of Silver Nanoparticles Used Chemical Reduction Method by Glucose as Reducing Agent. J. Phys. Conf. Ser. 2019, 1317, 012027. [Google Scholar] [CrossRef]
- Pyatenko, A.; Shimokawa, K.; Yamaguchi, M.; Nishimura, O.; Suzuki, M. Synthesis of Silver Nanoparticles by Laser Ablation in Pure Water. Appl. Phys. A 2004, 79, 803–806. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A Review on Silver Nanoparticles: Classification, Various Methods of Synthesis, and Their Potential Roles in Biomedical Applications and Water Treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Kylián, O.; Shelemin, A.; Solař, P.; Pleskunov, P.; Nikitin, D.; Kuzminova, A.; Štefaníková, R.; Kúš, P.; Cieslar, M.; Hanuš, J.; et al. Magnetron Sputtering of Polymeric Targets: From Thin Films to Heterogeneous Metal/Plasma Polymer Nanoparticles. Materials 2019, 12, 2366. [Google Scholar] [CrossRef]
- Straňák, V.; Block, S.; Drache, S.; Hubička, Z.; Helm, C.A.; Jastrabík, L.; Tichý, M.; Hippler, R. Size-Controlled Formation of Cu Nanoclusters in Pulsed Magnetron Sputtering System. Surf. Coat. Technol. 2011, 205, 2755–2762. [Google Scholar] [CrossRef]
- Musa, I.; Qamhieh, N.; Said, K. Germanium Antimony Quantum Dots Morphology and Raman Spectroscopy Fabricated by Inert Gas Condensation. Results Phys. 2019, 13, 102311. [Google Scholar] [CrossRef]
- Orozco-Montes, V.; Caillard, A.; Brault, P.; Chamorro-Coral, W.; Bigarre, J.; Sauldubois, A.; Andreazza, P.; Cuynet, S.; Baranton, S.; Coutanceau, C. Synthesis of Platinum Nanoparticles by Plasma Sputtering onto Glycerol: Effect of Argon Pressure on Their Physicochemical Properties. J. Phys. Chem. C 2021, 125, 3169–3179. [Google Scholar] [CrossRef]
- Yusuf, K.; Shekhah, O.; ALOthman, Z.; Eddaoudi, M. Metal-Organic Frameworks Characterization via Inverse Pulse Gas Chromatography. Appl. Sci. 2021, 11, 10243. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Du, Z.; Liu, F.; Qin, Y.; Mao, P.; Han, M. Gas Phase Fabrication of Morphology-Controlled ITO Nanoparticles and Their Assembled Conductive Films. Nanoscale 2023, 15, 3907–3918. [Google Scholar] [CrossRef]
- López-Martín, R.; Burgos, B.S.; Normile, P.S.; De Toro, J.A.; Binns, C. Gas Phase Synthesis of Multi-Element Nanoparticles. Nanomaterials 2021, 11, 2803. [Google Scholar] [CrossRef]
- Snellman, M.; Eom, N.; Ek, M.; Messing, M.E.; Deppert, K. Continuous Gas-Phase Synthesis of Core–Shell Nanoparticles via Surface Segregation. Nanoscale Adv. 2021, 3, 3041–3052. [Google Scholar] [CrossRef]
- Zhang, R.; Huo, Z.; Jiao, X.; Zhong, H.; Shi, Y. Deposition of Tungsten Thin Films on Flexible Polymer Substrates by Direct-Current Magnetron Sputtering. J. Elec. Mater. 2015, 44, 4557–4562. [Google Scholar] [CrossRef]
- Musa, I.; Qamhieh, N.; Said, K.; Mahmoud, S.T.; Alawadhi, H. Fabrication and Characterization of Aluminum Nitride Nanoparticles by RF Magnetron Sputtering and Inert Gas Condensation Technique. Coatings 2020, 10, 411. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Thaker, S.; Qamhieh, N.; Ghamlouche, H. Size-Controlled Pd Nanocluster Grown by Plasma Gas-Condensation Method. J. Nanopart. Res. 2011, 13, 1125–1131. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Qamhieh, N.; Ghamlouche, H.; Thaker, S.; El-Shaer, M. Fabrication of Size-Selected Pd Nanoclusters Using a Magnetron Plasma Sputtering Source. J. Appl. Phys. 2010, 107, 034317. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Qamhieh, N.; Mahmoud, S.T.; Alawadhi, H. Production of Size-Selected CuXSn1−X Nanoclusters. AMR 2011, 295–297, 70–73. [Google Scholar] [CrossRef]
- Gracia-Pinilla, M.; Martínez, E.; Vidaurri, G.S.; Pérez-Tijerina, E. Deposition of Size-Selected Cu Nanoparticles by Inert Gas Condensation. Nanoscale Res. Lett. 2010, 5, 180. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Tamai, T. Printed Silver Electrode for Silicon-Based Schottky Barrier Diode: High Rectification Ratio Enabled by Ag/p-Si Interface Exceeds Theoretical Barrier Height. ChemRxiv, 2020; preprint. [Google Scholar]
- Shameli, K.; Ahmad, M.; Shabanzadeh, P.; Zamanian, A.; Sangpour, P.; Abdollahi, Y.; Mohsen, Z. Green Biosynthesis of Silver Nanoparticles Using Curcuma Longa Tuber Powder. Int. J. Nanomed. 2012, 7, 5603. [Google Scholar] [CrossRef]
- Borodin, B.R.; Benimetskiy, F.A.; Nyapshaev, I.A.; Alekseev, P.A. Kelvin Probe Force Gradient Microscopy of WSe2 Monolayers on Ni. J. Phys. Conf. Ser. 2019, 1400, 055012. [Google Scholar] [CrossRef]
- Castanon, E.G.; Fernández Scarioni, A.; Schumacher, H.W.; Spencer, S.; Perry, R.; Vicary, J.A.; Clifford, C.A.; Corte-León, H. Calibrated Kelvin-Probe Force Microscopy of 2D Materials Using Pt-Coated Probes. J. Phys. Commun. 2020, 4, 095025. [Google Scholar] [CrossRef]
- Fernández Garrillo, P.A.; Grévin, B.; Chevalier, N.; Borowik, Ł. Calibrated Work Function Mapping by Kelvin Probe Force Microscopy. Rev. Sci. Instrum. 2018, 89, 043702. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y. Experimental Determination of Conduction and Valence Bands of Semiconductor Nanoparticles Using Kelvin Probe Force Microscopy. J. Nanopart. Res. 2013, 15, 1334. [Google Scholar] [CrossRef]
- Panchal, V.; Pearce, R.; Yakimova, R.; Tzalenchuk, A.; Kazakova, O. Standardization of Surface Potential Measurements of Graphene Domains. Sci. Rep. 2013, 3, 2597. [Google Scholar] [CrossRef]
- He, J.; Armstrong, J.; Cong, P.; Menagen, B.; Igaher, L.; Beale, A.M.; Etgar, L.; Avnir, D. Affecting an Ultra-High Work Function of Silver. Angew. Chem. Int. Ed. 2020, 59, 4698–4704. [Google Scholar] [CrossRef]
- Wang, P.; Okamoto, K.; Tamada, K. Tuning the Work Functions of 2D Silver Nanoparticle Sheets Using Local Oxidation Nanolithography. Adv. Mater. Interfaces 2014, 1, 1400268. [Google Scholar] [CrossRef]
- Beaton, G.C.; Bottomley, A.J.; Prezgot, D.; Ianoul, A.; Stamplecoskie, K.G. Shape Control of Silver Nanoparticles and Their Stability on Al2O3. J. Mater. Chem. C 2020, 8, 10755–10760. [Google Scholar] [CrossRef]
- Ashraf, J.M.; Ansari, M.A.; Khan, H.M.; Alzohairy, M.A.; Choi, I. Green Synthesis of Silver Nanoparticles and Characterization of Their Inhibitory Effects on AGEs Formation Using Biophysical Techniques. Sci. Rep. 2016, 6, 20414. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Petrova, H.; Sekkinen, A.R.; Chen, J.; McLellan, J.M.; Li, Z.-Y.; Marquez, M.; Li, X.; Xia, Y.; Hartland, G.V. Optical Properties of Au–Ag Nanoboxes Studied by Single Nanoparticle Spectroscopy. J. Phys. Chem. B 2006, 110, 19923–19928. [Google Scholar] [CrossRef]
- Zhao, Y.; Lim, M.; Choi, S.; Yu, J. Excitation Spectrum, Nanoparticles, and Their Applications in Cellular Optical Imaging. J. Biol. Educ. 2022, 56, 122–129. [Google Scholar] [CrossRef]
- Tafur, G.; Benndorf, C.; Acosta, D.; Asencios, J.; Talledo, A. Optical Properties of Silver Nanoparticles Embedded in Dielectric Films Produced by Dc and Rf Magnetron Sputtering. J. Phys. Conf. Ser. 2019, 1173, 012004. [Google Scholar] [CrossRef]
- Dhara, S. Origin of Shifts in the Surface Plasmon Resonance Frequencies for Au and Ag Nanoparticles. In Reviews in Plasmonics 2015; Geddes, C.D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 275–294. ISBN 978-3-319-24606-2. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, I.; Qamhieh, N.; Mahmoud, S.T. Ag Nanocluster Production through DC Magnetron Sputtering and Inert Gas Condensation: A Study of Structural, Kelvin Probe Force Microscopy, and Optical Properties. Nanomaterials 2023, 13, 2758. https://doi.org/10.3390/nano13202758
Musa I, Qamhieh N, Mahmoud ST. Ag Nanocluster Production through DC Magnetron Sputtering and Inert Gas Condensation: A Study of Structural, Kelvin Probe Force Microscopy, and Optical Properties. Nanomaterials. 2023; 13(20):2758. https://doi.org/10.3390/nano13202758
Chicago/Turabian StyleMusa, Ishaq, Naser Qamhieh, and Saleh T. Mahmoud. 2023. "Ag Nanocluster Production through DC Magnetron Sputtering and Inert Gas Condensation: A Study of Structural, Kelvin Probe Force Microscopy, and Optical Properties" Nanomaterials 13, no. 20: 2758. https://doi.org/10.3390/nano13202758




