Production of Exopolysaccharide-Based Porous Structures for Biomedical Applications: A Review
Abstract
:1. Introduction
2. EPS-Based Porous Devices Production
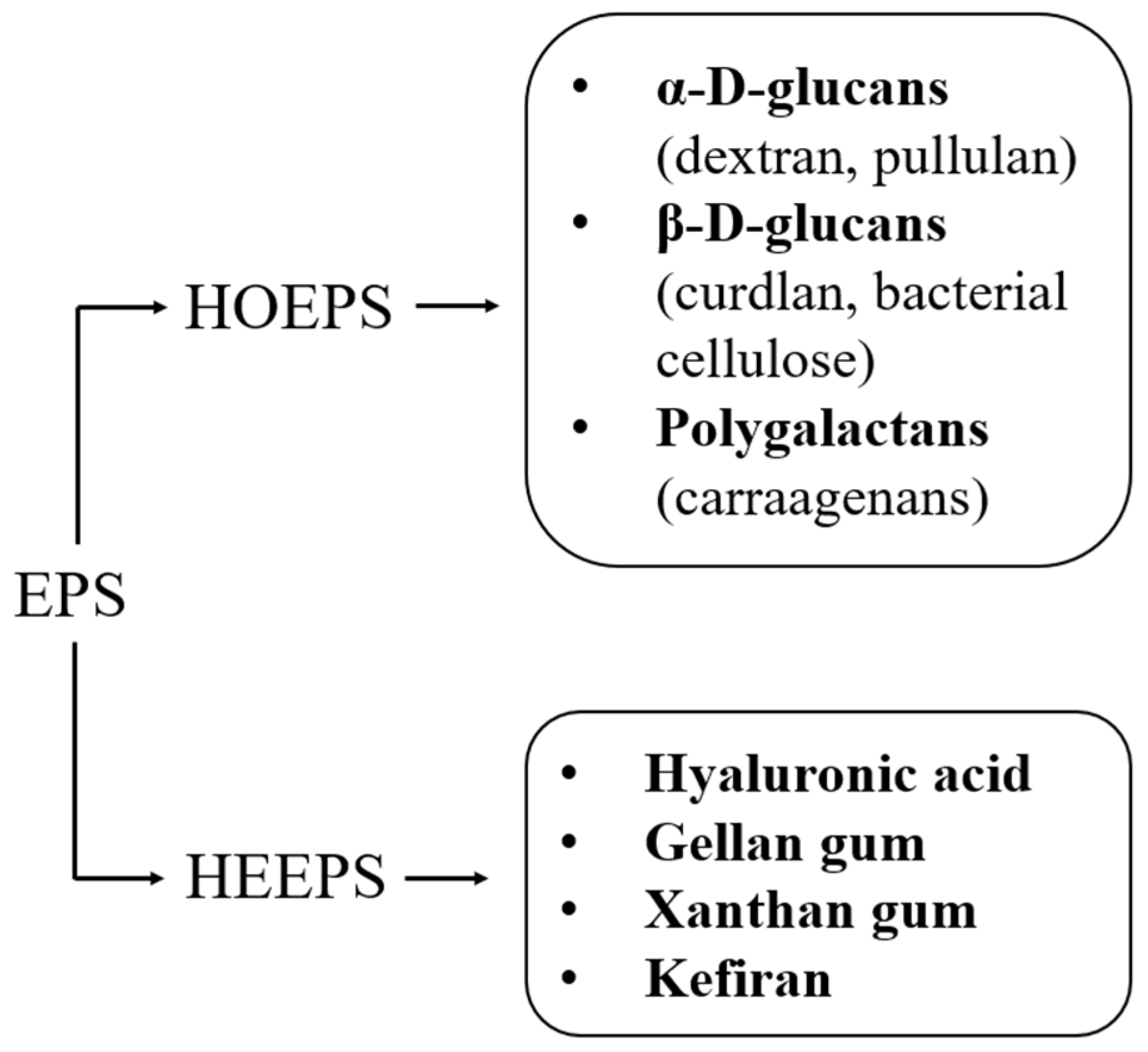
2.1. Homoexopolysaccharides
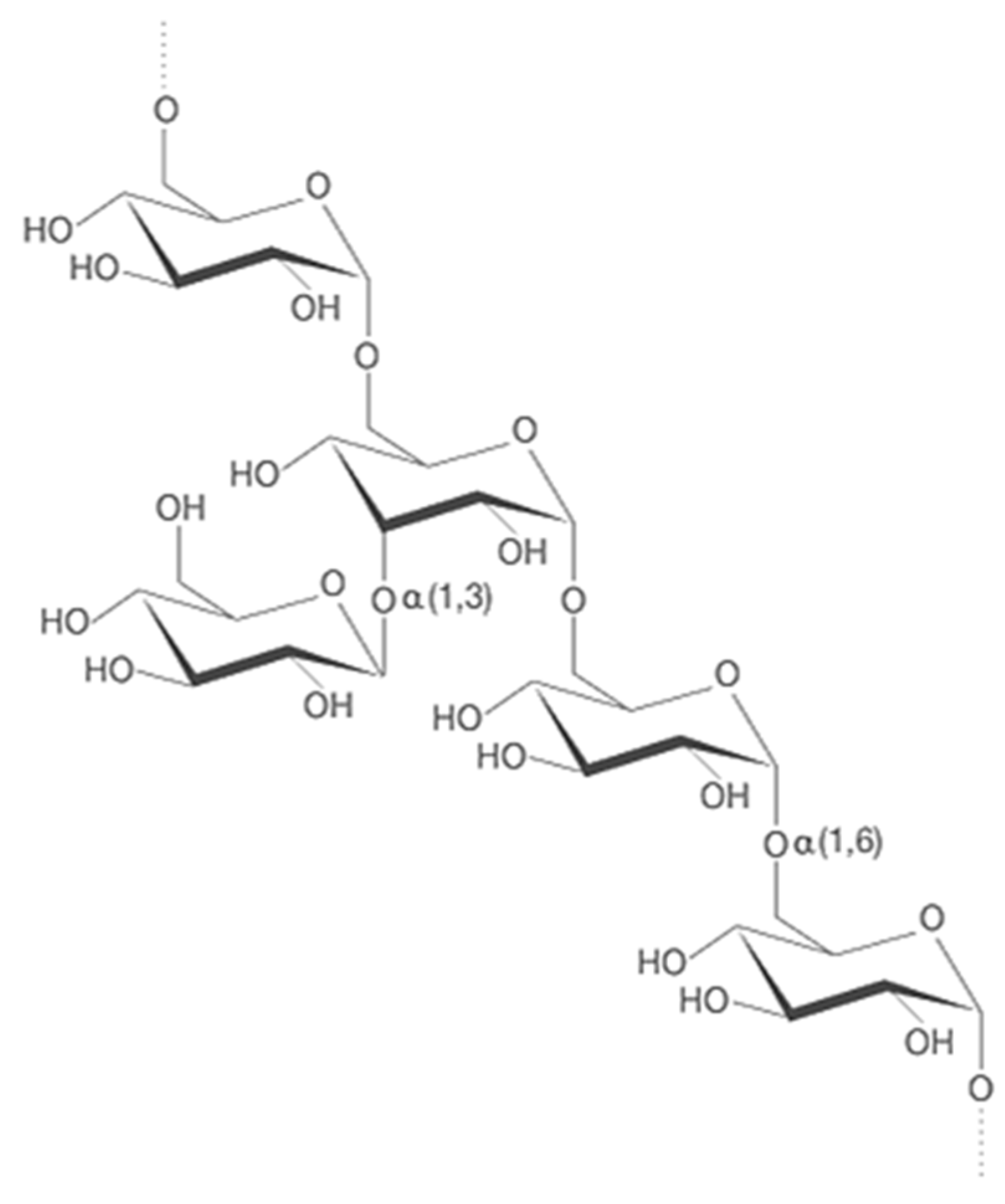
2.1.1. Alpha-D-Glucans
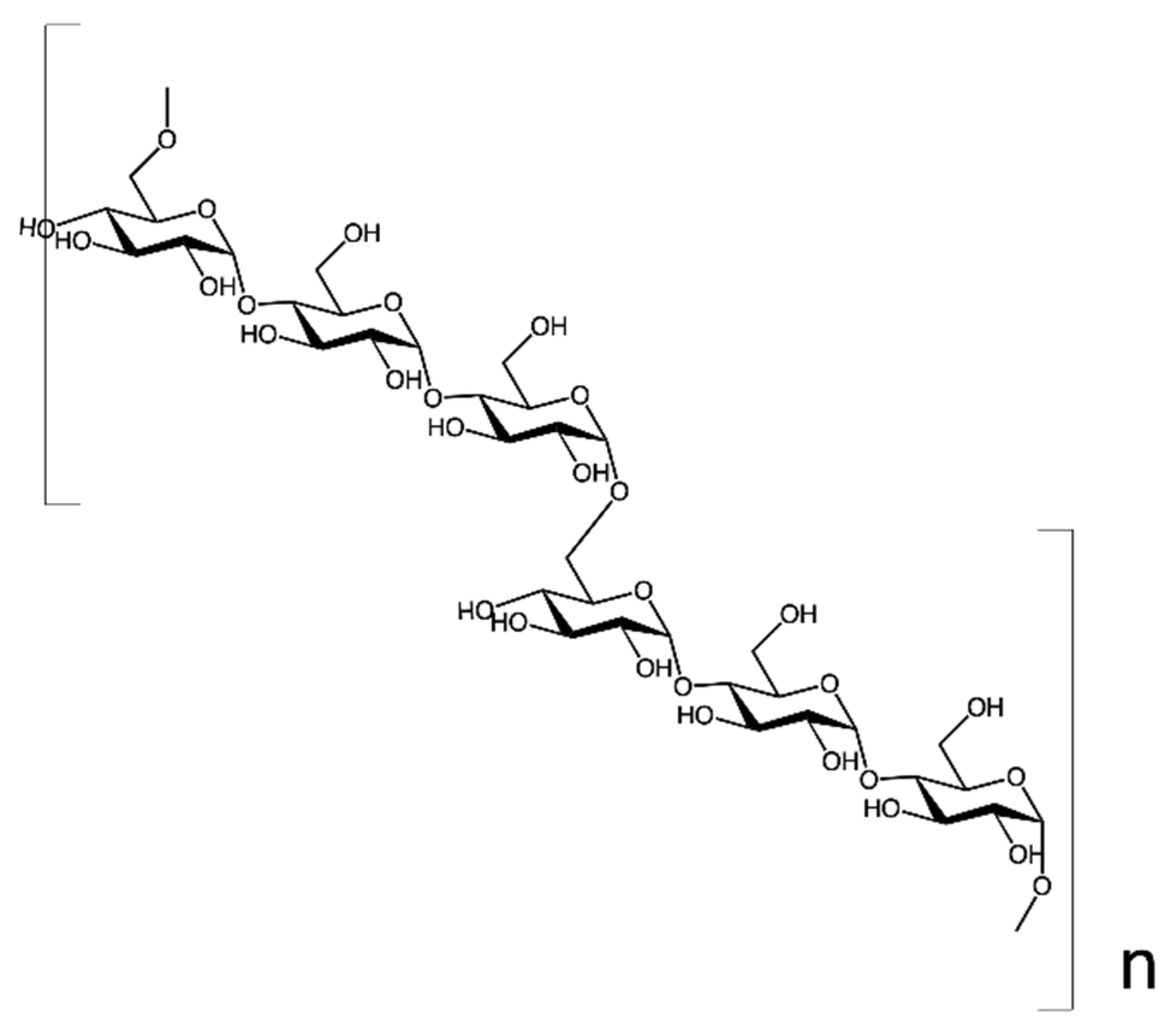
2.1.2. Beta-D-Glucans
2.1.3. Polygalactans
- Regardless of the HOEPS used, FD leads to macroporous devices, useful for nutrient exchange in TE but unable to mimic human tissues and to host nanoparticles without clustering;
- When working with HOEPSs, it is likely that cross-linkers will be needed to promote the formation of a stable, strong, and resilient polymeric network;
- SC-CO2 drying is the only technique that enables complete cross-linker removal during the process, thus improving biocompatibility;
- Aerogels often suffer from poor mechanical properties due to their high air volume: some fillers must be added to improve their characteristics;
- In general, HOEPSs are proven to be biocompatible and eligible for TE.
2.2. Heteroexopolysaccharides
- Hyaluronic acid (HA), a linear polysaccharide obtainable from Streptococcus thermophilus and composed of alternating N-acetylglucosamine and glucuronic acid [84]. This polysaccharide is now used in therapeutics, drug delivery, oncology, vascular tissue, cartilage, bone, and skin tissue engineering [85]. It is advantageous for scaffolding because of its biodegradability, biocompatibility, bioresorbability, abundance in connective tissues, presence of functional groups that enhance reactivity, etc. [86];
- Gellan gum (GG), obtained from Sphingomonas paucimobilis and made up of repeating units of monosaccharides, such as glucose, glucuronic acid, glucose, and rhamnose [87]. It is a linear anionic high-molecular-weight exopolysaccharide [88]. It is water-soluble, easy to fabricate, biocompatible, biodegradable, and allows hydrogel formation [89];
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohd Nadzir, M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical Applications of Bacterial Exopolysaccharides: A Review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef]
- Granato, E.T.; Smith, W.P.J.; Foster, K.R. Collective protection against the type VI secretion system in bacteria. ISME J. 2023, 17, 1052–1062. [Google Scholar] [CrossRef]
- Op De Beeck, M.; Persson, P.; Tunlid, A. Fungal extracellular polymeric substance matrices—Highly specialized microenvironments that allow fungi to control soil organic matter decomposition reactions. Soil Biol. Biochem. 2021, 159, 108304. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Du, C.; Mou, H.; Cui, J.; Guan, H.; Hwang, H.; Wang, P. Characterization of high yield exopolysaccharide produced by Phyllobacterium sp. 921F exhibiting moisture preserving properties. Int. J. Biol. Macromol. 2017, 101, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Holst, O.; Müller-Loennies, S. Microbial Polysaccharide Structures. In Comprehensive Glycoscience; Elsevier: Amsterdam, The Netherlands, 2007; Volume 1, pp. 123–179. [Google Scholar] [CrossRef]
- Finore, I.; Di Donato, P.; Mastascusa, V.; Nicolaus, B.; Poli, A. Fermentation Technologies for the Optimization of Marine Microbial Exopolysaccharide Production. Mar. Drugs 2014, 12, 3005–3024. [Google Scholar] [CrossRef] [PubMed]
- Dueholm, M.K.D.; Besteman, M.; Zeuner, E.J.; Riisgaard-Jensen, M.; Nielsen, M.E.; Vestergaard, S.Z.; Heidelbach, S.; Bekker, N.S.; Nielsen, P.H. Genetic potential for exopolysaccharide synthesis in activated sludge bacteria uncovered by genome-resolved metagenomics. Water Res. 2023, 229, 119485. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, Y.; Ahn, J.K.; Ka, K.H.; Park, S.Y. Factors influencing the production of endopolysaccharide and exopolysaccharide from Ganoderma applanatum. Enzyme Microb. Technol. 2007, 40, 249–254. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Llamas, M.G.; Iraporda, C.; Dueñas, M.T.; Abraham, A.G.; Garrote, G.L. Impact of growth temperature on exopolysaccharide production and probiotic properties of Lactobacillus paracasei strains isolated from kefir grains. Food Microbiol. 2018, 69, 212–218. [Google Scholar] [CrossRef]
- Lakra, U.; Sharma, S.R. Production of exopolysaccharides from Bacillus licheniformis, a thermophilic bacteria from hot spring of Jharkhand. Nat. Prod. Res. 2023, 1–10. [Google Scholar] [CrossRef]
- Decho, A.W. Microbial exopolymer secretions in ocean environments: Their role(s) in food webs and marine processes. Oceanogr. Mar. Biol. Annu. Rev. 1990, 28, 73–153. [Google Scholar]
- Jin, W.; Zhang, W.; Wang, J.; Yao, J.; Xie, E.; Liu, D.; Duan, D.; Zhang, Q. A study of neuroprotective and antioxidant activities of heteropolysaccharides from six Sargassum species. Int. J. Biol. Macromol. 2017, 67, 336–342. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Molecular Characterization and Biocompatibility of Exopolysaccharide Produced by Moderately Halophilic Bacterium Virgibacillus dokdonensis from the Saltern of Kumta Coast. Polymers 2022, 14, 3986. [Google Scholar] [CrossRef] [PubMed]
- Dilna, S.V.; Surya, H.; Aswathy, R.G.; Varsha, K.K.; Sakthikumar, D.N.; Pandey, A.; Nampoothiri, K.M. Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF4. LWT Food Sci. Technol. 2015, 64, 1179–1186. [Google Scholar] [CrossRef]
- Saha, I.; Datta, S. Bacterial exopolysaccharides in drug delivery applications. J. Drug Deliv. Sci. Technol. 2022, 74, 103557. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Soundarya, S.P.; Menon, A.H.; Chandran, S.V.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef]
- Zhang, F.; King, M.W. Biodegradable Polymers as the Pivotal Player in the Design of Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2020, 9, 1901358. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, T.; Shahroodi, A.; Ebrahimxadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 2, 90–99. [Google Scholar]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Bai, R.G.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 19, 5753–5783. [Google Scholar] [CrossRef]
- Yahya, E.B.; Amirul, A.A.; Abdul Khalil, H.P.S.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; Atty Sofea, A.K.; Adnan, A.S. Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S. Supercritical fluids in 3-D tissue engineering. J. Supercrit. Fluids 2012, 69, 97–107. [Google Scholar] [CrossRef]
- Hollister, S.J.; Maddox, R.D.; Taboas, J.M. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 2002, 23, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Guastaferro, M.; Reverchon, E.; Baldino, L. Polysaccharide-Based Aerogel Production for Biomedical Applications: A Comparative Review. Materials 2021, 14, 1631. [Google Scholar] [CrossRef] [PubMed]
- Cardea, S.; Baldino, L.; Scognamiglio, M.; Reverchon, E. 3D PLLA/Ibuprofen composite scaffolds obtained by a supercritical fluids assisted process. J. Mater. Sci. Mater. Med. 2014, 25, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Idumah, C.I.; Ezika, A.C.; Okpechi, V.U. Emerging trends in polymer aerogel nanoarchitectures, surfaces, interfaces and applications. Surf. Interfaces 2021, 25, 101258. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, drying process and medical application of polysaccharide-based aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef]
- Ahuja, V.; Bhatt, A.K.; Banu, J.R.; Kumar, V.; Kumar, G.; Yang, Y.-H.; Bhatia, S.K. Microbial Exopolysaccharide Composites in Biomedicine and Healthcare: Trends and Advances. Polymers 2023, 15, 1801. [Google Scholar] [CrossRef]
- Aburas, H.; İspirli, H.; Taylan, O.; Yilmaz, M.T.; Dertli, E. Structural and physicochemical characterisation and antioxidant activity of an α-D-glucan produced by sourdough isolate Weissella cibaria MED17. Int. J. Biol. Macromol. 2020, 161, 648–655. [Google Scholar] [CrossRef]
- Bounaix, M.-S.; Gabriel, V.; Morel, S.; Robert, H.; Rabier, P.; Remaud-Siméon, M.; Gabriel, B.; Fontagné-Faucher, C. Biodiversity of Exopolysaccharides Produced from Sucrose by Sourdough Lactic Acid Bacteria. J. Agric. Food Chem. 2009, 22, 10889–10897. [Google Scholar] [CrossRef]
- Lochhead, R. The Use of Polymers in Cosmetic Products. In Cosmetic Science and Technology: Theoretical Principles and Applications, 1st ed.; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 13, pp. 171–221. [Google Scholar]
- Kothari, D.; Das, D.; Patel, S.; Goyal, A. Dextran and food application. Polysacch. Bioactivity Biotechnol. 2015, 735–752. [Google Scholar]
- Song, E.-H.; Shang, J.; Ratner, D.M. Polymers in Biology and Medicine. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 9, pp. 137–155. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Antoniou, E.; Tsianou, M. Solution properties of dextran in water and in formamide. J. Appl. Polym. Sci. 2012, 125, 1681–1692. [Google Scholar] [CrossRef]
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Qiao, X.; Zhao, F.; Song, Q.; Zhou, Q.; Wang, Y.; Pan, L.; Han, Y.; Zhou, Z. Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohydr. Polym. 2018, 198, 529–536. [Google Scholar] [CrossRef]
- Padmanabham, P.A.; Kim, D.-S.; Pak, D.; Sim, S.J. Rheology and gelation of water-insoluble dextran from Leuconostoc mesenteroides NRRL B-523. Carbohydr. Polym. 2003, 53, 459–468. [Google Scholar] [CrossRef]
- McCurdy, R.D.; Goff, H.D.; Stanley, D.W.; Stone, A.P. Rheological properties of dextran related to food applications. Food Hydrocoll. 1994, 8, 609–623. [Google Scholar] [CrossRef]
- Nikpour, P.; Salimi-Kenari, H.; Fahimipour, F.; Rabiee, S.M.; Imani, M.; Dashtimoghadam, E.; Tayebi, L. Dextran hydrogels incorporated with bioactive glass-ceramic: Nanocomposite scaffolds for bone tissue engineering. Carbohydr. Polym. 2018, 190, 281–294. [Google Scholar] [CrossRef]
- Ghaffari, R.; Salimi-Kenari, H.; Fahimipour, F.; Rabiee, S.M.; Adeli, H.; Dashtimoghadam, E. Fabrication and characterization of dextran/nanocrystalline β-tricalcium phosphate nanocomposite hydrogel scaffolds. Int. J. Biol. Macromol. 2020, 148, 434–448. [Google Scholar] [CrossRef]
- El-Meliegy, E.; Abu-Elsaad, N.I.; El-Kady, A.M.; Ibrahim, M.A. Improvement of physico-chemical properties of dextran-chitosan composite scaffolds by addition of nano-hydroxyapatite. Sci. Rep. 2018, 8, 12180. [Google Scholar] [CrossRef]
- Pacelli, S.; Di Muzio, L.; Paolicelli, P.; Fortunati, V.; Petralito, S.; Trilli, J.; Casadei, M.A. Dextran-polyethylene glycol cryogels as spongy scaffolds for drug delivery. Int. J. Biol. Macromol. 2021, 161, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Budhwani, D.; Gurjar, P.; Telange, D.; Lambole, V. Pullulan based derivatives: Synthesis, enhanced physicochemical properties, and applications. Drug Deliv. 2022, 29, 3328–3339. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, A.; Cardea, S. Supercritical carbon dioxide techniques for processing microbial exopolysaccharides used in biomedical applications. Mat. Sci. Eng. C 2020, 112, 110940. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Kohyama, K.; Williams, P.A.; Phillips, G.O.; Burchard, W.; Ogino, K. Solution properties of pullulan. Macromolecules 1991, 24, 5590–5593. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, C.; Rao, J.; Li, J.; Yang, X.; Zhang, H.; Li, J.; Fawole, O.A.; Wu, D.; Chen, K. Biodegradable gelatin/pullulan aerogel modified by a green strategy: Characterization and antimicrobial activity. Food Packag. Shelf Life 2022, 34, 100957. [Google Scholar] [CrossRef]
- Zhao, S.; Emery, O.; Wohlhauser, A.; Koebel, M.M.; Adlhart, C.; Malfait, W.J. Merging flexibility with superinsulation: Machinable, nanofibrous pullulan-silica aerogel composites. Mater. Des. 2018, 160, 294–302. [Google Scholar] [CrossRef]
- Linhares, T.; Pessoa de Amorim, M.T.; Durães, L. Silica aerogel composites with embedded fibres: A review on their preparation, properties and applications. J. Mater. Chem. A 2019, 7, 22768–22802. [Google Scholar] [CrossRef]
- Synytsya, A.; Novák, M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef]
- Wang, Q.; Sheng, X.; Shi, A.; Hu, H.; Yang, Y.; Liu, L.; Fei, L.; Liu, H. β-Glucans: Relationships between Modification, Conformation and Functional Activities. Molecules 2017, 22, 257. [Google Scholar] [CrossRef]
- Zhang, R.; Edgar, K.J. Properties, Chemistry, and Applications of the Bioactive Polysaccharide Curdlan. Biomacromolecules 2014, 15, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, F. Review on the preparation, biological activities and applications of curdlan and its derivatives. Eur. Polym. J. 2020, 141, 110096. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, H. Recent progress on curdlan provided by functionalization strategies. Food Hydrocoll. 2017, 68, 128–135. [Google Scholar] [CrossRef]
- Przekora, A.; Penolazzi, L.; Kalisz, G.; Kazimierczak, P.; Canal, C.; Woijcik, R.; Piva, R.; Sroka-Bartnicka, A. Osteoclast-mediated acidic hydrolysis of thermally gelled curdlan component of the bone scaffolds: Is it possible? Carbohydr. Polym. 2022, 995, 119914. [Google Scholar] [CrossRef] [PubMed]
- Klimek, K.; Tarczynska, M.; Truszkiewicz, W.; Gaweda, K.; Douglas, T.E.L.; Ginalska, G. Freeze-Dried Curdlan/Whey Protein Isolate-Based Biomaterial as Promising Scaffold for Matrix-Associated Autologous Chondrocyte Transplantation—A Pilot In-Vitro Study. Cells 2022, 11, 282. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Abdelgawad, A.M.; Tripathi, A.; Rojas, O.J. Curdlan cryogels reinforced with cellulose nanofibrils for controlled release. J. Environ. Chem. Eng. 2017, 5, 5754–5761. [Google Scholar] [CrossRef]
- Baldino, L.; Concilio, S.; Cardea, S.; De Marco, I.; Reverchon, E. Complete glutaraldehyde elimination during chitosan hydrogel drying by SC-CO2 processing. J. Supercrit. Fluids 2015, 103, 70–76. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2016, 23, 57–91. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Yang, F.; Shao, Y.; Zhang, X.; Dai, K. Modification and evaluation of micro-nano structured porous bacterial cellulose scaffold for bone tissue engineering. Mater. Sci. Eng. C 2017, 75, 1034–1041. [Google Scholar] [CrossRef]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of aerogels, cryogels and xerogels of cellulose with hierarchical porous structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]
- Fleury, B.; Abraham, E.; De La Cruz, J.; Chandrasekar, V.S.; Senyuk, B.; Liu, Q.; Cherpak, S.; ten Hove, J.B.; Smalyukh, I.I. Aerogel from Sustainably Grown Bacterial Cellulose Pellicles as a Thermally Insulative Film for Building Envelopes. ACS Appl. Mater. Interfaces 2020, 12, 34115–34141. [Google Scholar] [CrossRef] [PubMed]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Halib, N.; Ahmad, I.; Grassi, M.; Grassi, G. The remarkable three-dimensional network structure of bacterial cellulose for tissue engineering applications. Int. J. Pharm. 2019, 566, 631–640. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef]
- Latiyan, S.; Kumar, T.S.S.; Doble, M.; Kennedy, J.F. Perspectives of nanofibrous wound dressings based on glucans and galactans—A review. Int. J. Biol. Macromol. 2023, 244, 125358. [Google Scholar] [CrossRef]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.Ș.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef]
- Caneira, I.; Machado-Moreira, B.; Dionísio, A.; Godinho, V.; Neves, O.; Dias, D.; Saiz-Jimenez, C.; Miller, A.Z. Application of exopolysaccharides to improve the performance of ceramic bodies in the unidirectional dry pressing process. In Proceedings of the EGU General Assembly, Vienna, Austria, 12–17 April 2015. [Google Scholar]
- Usov, A.I. Polysaccharides of the red algae. In Advances in Carbohydrate Chemistry and Biochemistry; Horton, D., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 65, pp. 115–217. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Mangione, M.R.; Giacomazza, D.; Bulone, D.; Martorana, V.; Cavallaro, G.; San Biagio, P.L. K+ and Na+ effects on the gelation properties of κ-Carrageenan. Biophys. Chem. 2005, 113, 129–135. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Mangione, M.R.; Giacomazza, D.; Bulone, D.; Martorana, V.; San Biagio, P.L. Thermoreversible gelation of κ-Carrageenan: Relation between conformational transition and aggregation. Biophys. Chem. 2003, 104, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Loukelis, K.; Papadogianni, D.; Chatzinikolaidou, M. Kappa-carrageenan/chitosan/gelatin scaffolds enriched with potassium chloride for bone tissue engineering. Int. J. Biol. Macromol. 2022, 209, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Raza, M.A.; Mehboob, H.; Kadir, M.R.A.; Razak, S.I.A.; Shah, S.A.; Iqbal, M.Z.; Amin, R. Development and in vitro evaluation of κ-carrageenan based polymeric hybrid nanocomposite scaffolds for bone tissue engineering. RSC Adv. 2020, 10, 40529–40542. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Valoppi, F.; Calligaris, S.; Andreatta, F.; Spilimbero, S.; Nicoli, M.C. Exploitation of κ-carrageenan aerogels as template for edible oleogel preparation. Food Hydrocoll. 2017, 71, 68–75. [Google Scholar] [CrossRef]
- Plazzotta, S.; Calligaris, S.; Manzocco, L. Structure of oleogels from κ-carrageenan templates as affected by supercritical-CO2-drying, freeze-drying and lettuce-filler addition. Food Hydrocoll. 2019, 96, 1–10. [Google Scholar] [CrossRef]
- Agostinho, D.A.S.; Paninho, A.I.; Cordeiro, T.; Nunes, A.V.M.; Fonseca, I.M.; Pereira, C.; Matias, A.; Ventura, M.G. Properties of κ-carrageenan aerogels prepared by using different dissolution media and its application as drug delivery systems. Mater. Chem. Phys. 2020, 253, 123290. [Google Scholar] [CrossRef]
- Ganesan, K.; Ratke, L. Facile preparation of monolithic κ-carrageenan aerogels. Soft Matter 2014, 10, 3218–3224. [Google Scholar] [CrossRef]
- Izawa, N.; Hanamizu, T.; Iizuka, R.; Sone, T.; Mizukoshi, H.; Kimura, K.; Chiba, K. Streptococcus thermophilus produces exopolysaccharides including hyaluronic acid. J. Biosci. Bioeng. 2009, 107, 119–123. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Salachna, P.; Mizielińska, M.; Soból, M. Exopolysaccharide Gellan Gum and Derived Oligo-Gellan Enhance Growth and Antimicrobial Activity in Eucomis Plants. Polymers 2018, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, T.; Song, J.E.; Khang, G. Biological Role of Gellan Gum in Improving Scaffold Drug Delivery, Cell Adhesion Properties for Tissue Engineering Applications. Molecules 2019, 24, 4514. [Google Scholar] [CrossRef] [PubMed]
- Hishamuddin, N.I.; Razali, M.H.; Mat Amin, K.A. Application of gellan gum biopolymer in biomedical applications: A review. Makara J. Sci. 2022, 26, 11–24. [Google Scholar] [CrossRef]
- Becker, A.; Katzen, F.; Püler, A.; Ielpi, L. Xanthan gum biosynthesis and application: A biochemical/genetic perspective. Appl. Microbiol. Biotechnol. 1998, 50, 145–152. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- Moradi, Z.; Kalanpour, N. Kefiran, a branched polysaccharide: Preparation, properties and applications: A review. Carbohydr. Polym. 2019, 223, 115100. [Google Scholar] [CrossRef]
- De Moreno de LeBlanc, A.; Matar, C.; Farnworth, E.; Perdigón, G. Study of Immune Cells Involved in the Antitumor Effect of Kefir in a Murine Breast Cancer Model. J. Dairy Sci. 2007, 90, 1920–1928. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Steinhoff, B.; Warren, H.; Clark, R.; in het Panhuis, M. Enhanced gelation properties of purified gellan gum. Carbohydr. Res. 2014, 388, 125–129. [Google Scholar] [CrossRef]
- Iijima, M.; Shinozaki, M.; Hatakeyama, T.; Takahashi, M.; Hatakeyama, H. AFM studies on gelation mechanism of xanthan gum hydrogels. Carbohydr. Polym. 2007, 68, 701–707. [Google Scholar] [CrossRef]
- Stevens, L.R.; Gilmore, K.J.; Wallace, G.G.; in het Panhuis, M. Tissue engineering with gellan gum. Biomater. Sci. 2016, 4, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Walimbe, T.; Panitch, A.; Sivasankar, P.M. A Review of Hyaluronic Acid and Hyaluronic Acid-based Hydrogels for Vocal Fold Tissue Engineering. J. Voice 2017, 4, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qi, Z.; Zheng, S.; Chang, Y.; Kong, W.; Fu, C.; Yu, Z.; Yang, X.; Pan, S. The Application of Hyaluronic Acid-Based Hydrogels in Bone and Cartilage Tissue Engineering. Adv. Mater. Sci. Eng. 2019, 2019, 3027303. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef]
- Suner, S.S.; Demirci, S.; Yetiskin, B.; Fakhrullin, R.; Naumenko, E.; Okay, O.; Ayyala, R.S.; Sahiner, N. Cryogel composites based on hyaluronic acid and halloysite nanotubes as scaffold for tissue engineering. Int. J. Biol. Macromol. 2018, 130, 627–635. [Google Scholar] [CrossRef]
- Najberg, M.; Mansor, M.H.; Taillé, T.; Bouré, C.; Molina-Peña, R.; Boury, F.; Cenis, J.L.; Garcion, E.; Alvarez-Lorenzo, C. Aerogel sponges of silk fibroin, hyaluronic acid and heparin for soft tissue engineering: Composition-properties relationship. Carbohydr. Polym. 2020, 237, 116107. [Google Scholar] [CrossRef]
- Aguilera-Bulla, D.; Legay, L.; Buwalda, S.J.; Budtova, T. Crosslinker-Free Hyaluronic Acid Aerogels. Biomacromolecules 2022, 23, 2838–2845. [Google Scholar] [CrossRef]
- Koivisto, J.T.; Joki, T.; Parraga, J.E.; Pääkkönen, R.; Ylä-Outinen, L.; Salonen, L.; Jönkkäri, I.; Peltola, M.; Ihalainen, T.O.; Narkilahti, S. Bioamine-crosslinked gellan gum hydrogel for neural tissue engineering. Biomed. Mater. 2017, 12, 025014. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Jiang, S.; Bratlie, K.M. Chemically Modified Gellan Gum Hydrogels with Tunable Properties for Use as Tissue Engineering Scaffolds. ACS Omega 2018, 3, 6998–7007. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mehdikhani, M.; Rafienia, M. Reduced graphene oxide–reinforced gellan gum thermoresponsive hydrogels as a myocardial tissue engineering scaffold. J. Bioact. Compat. Polym. 2019, 34, 331–345. [Google Scholar] [CrossRef]
- Gantar, A.; da Silva, L.P.; Oliveira, J.M.; Marques, A.P.; Correlo, V.M.; Novak, S.; Reis, R.L. Nanoparticulate bioactive-glass-reinforced gellan-gum hydrogels for bone-tissue engineering. Mater. Sci. Eng. C 2014, 43, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Cassanelli, M.; Prosapio, V.; Norton, I.; Mills, T. Role of the Drying Technique on the Low-Acyl Gellan Gum Gel Structure: Molecular and Macroscopic Investigations. Food Bioprocess Technol. 2019, 12, 313–324. [Google Scholar] [CrossRef] [PubMed]
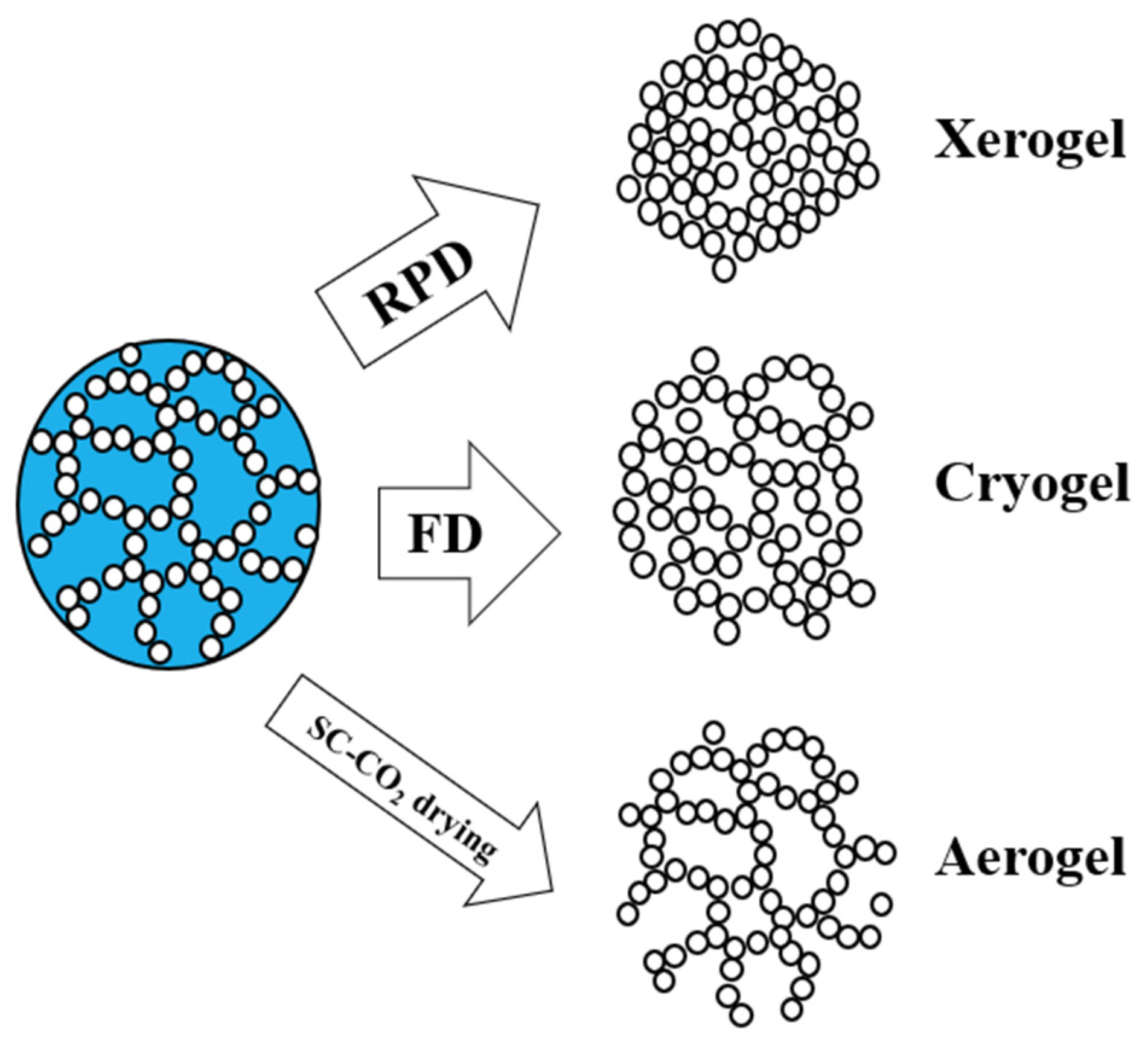
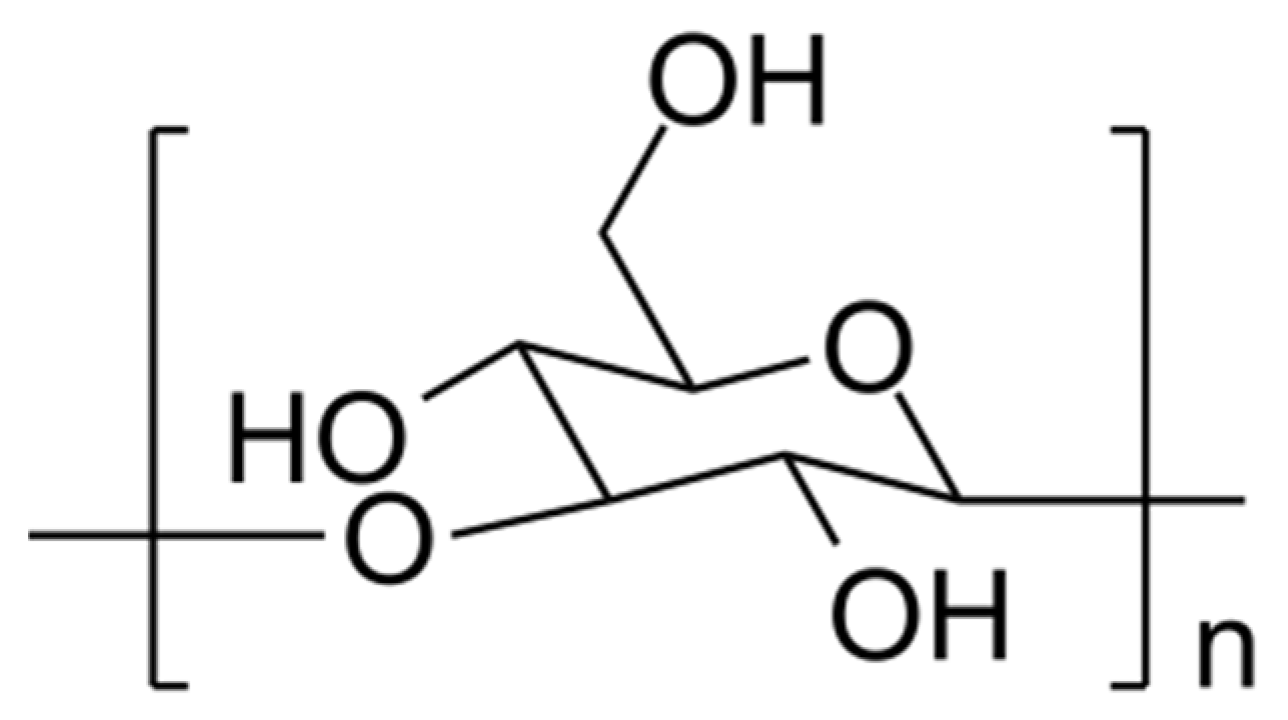
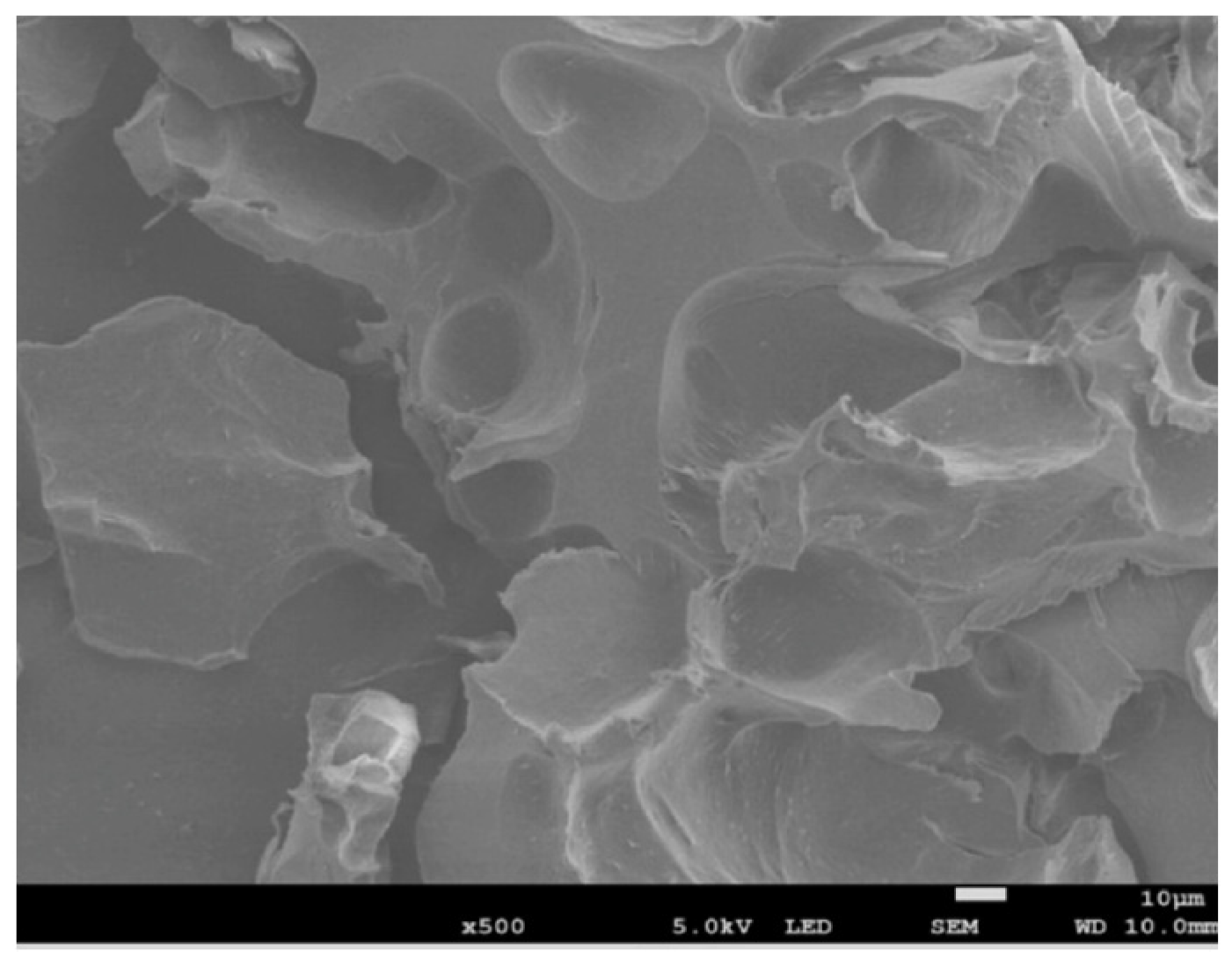
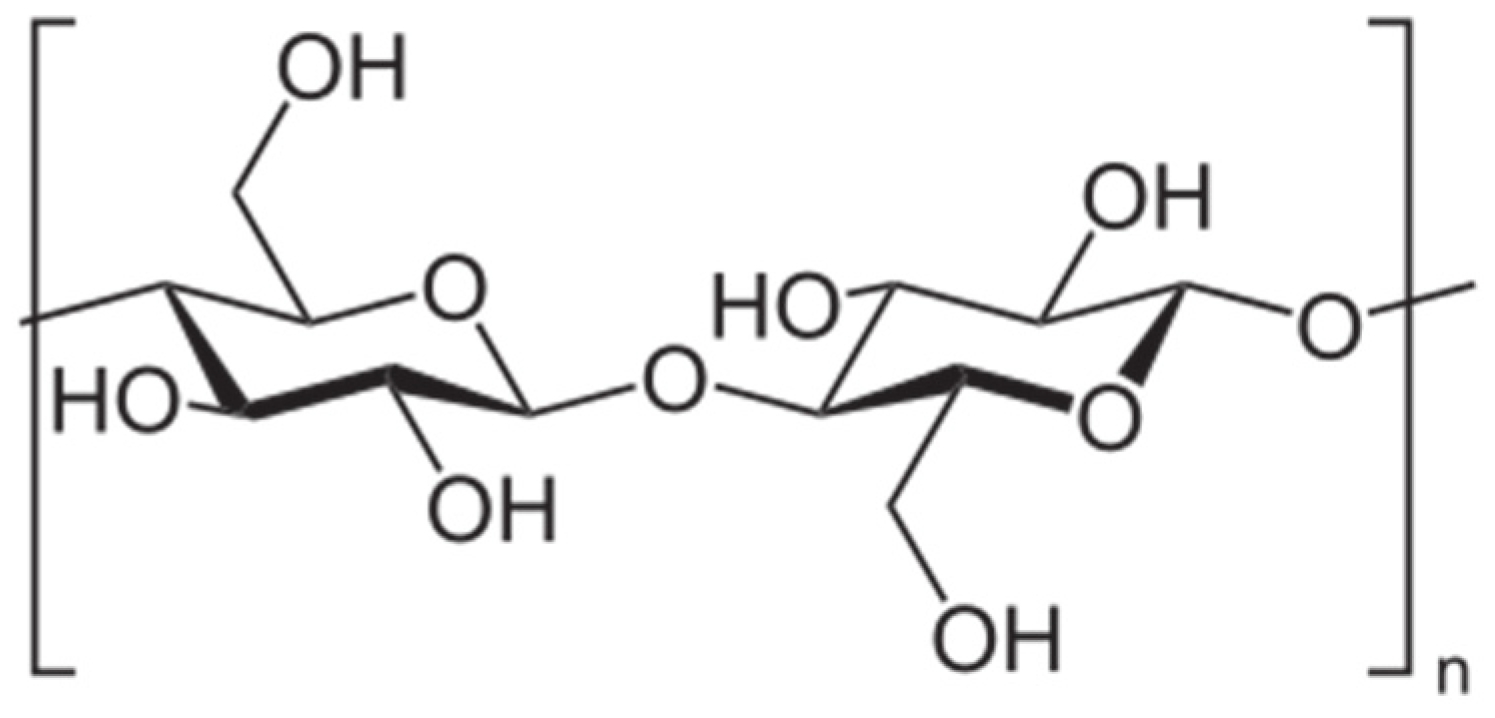
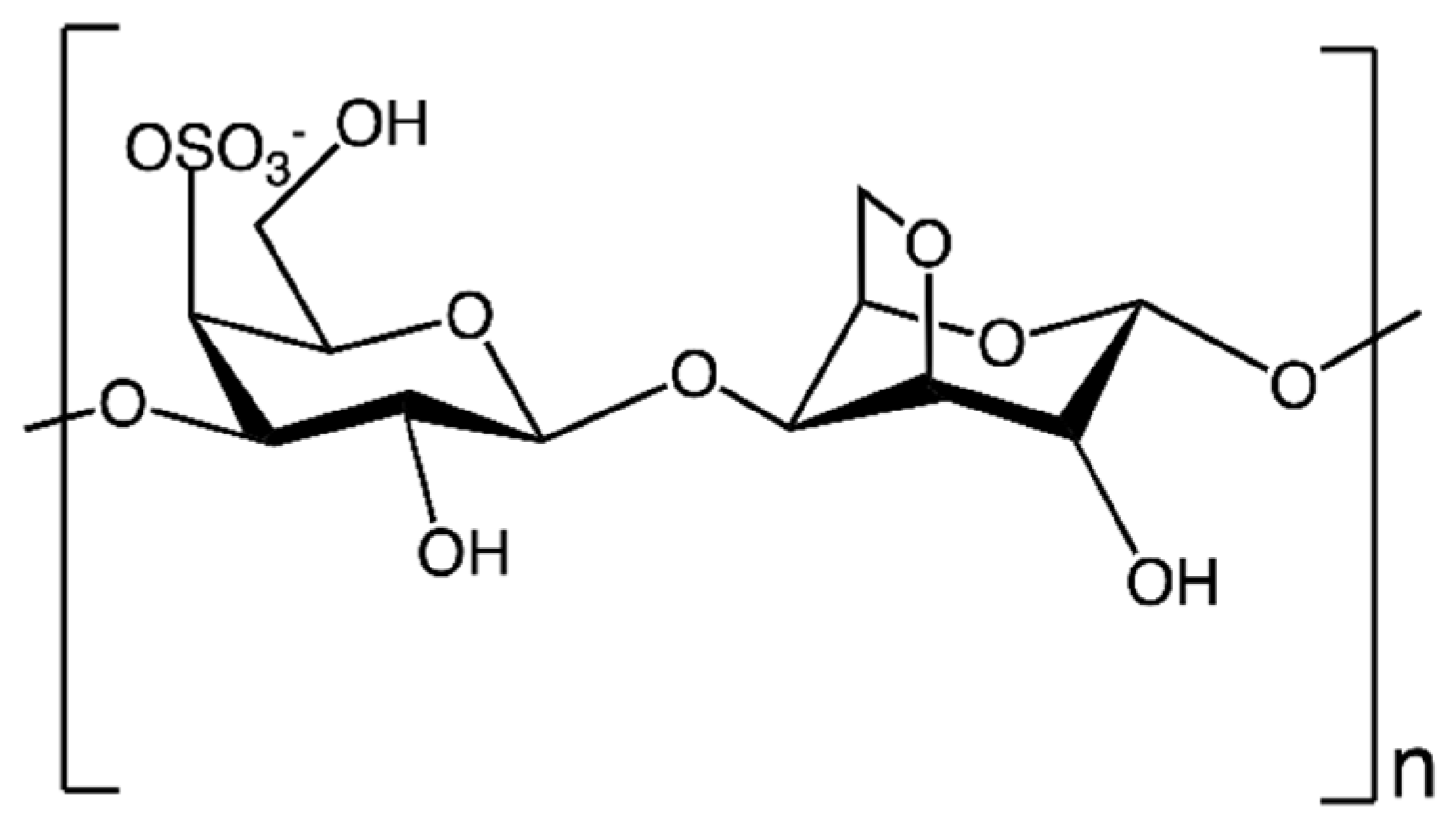
| HOEPS | Drying Technique | Advantages | Disadvantages | References |
|---|---|---|---|---|
| DEX | Freeze-drying | Ease of production; non-cytotoxic | Only macroporous structure; incomplete removal of toxic cross-linkers; non-homogeneous distribution of nanoadditives (clusters) | [41,42,43,44] |
| PUL | Freeze-drying | Ease of production; good mechanical properties | Week-long production; only macropores (100 μm) | [48] |
| PUL | SC-CO2 drying | Hierarchical morphology; intact nanostructure (50 nm pores); high SSA | Poor mechanical properties | [49] |
| CUR | Room pressure drying | Osteoclast proliferation; good biocompatibility | Closed and smooth surface | [57] |
| CUR | Freeze-drying | Good mechanical properties; cell proliferation | No nanostructure; incomplete removal of toxic cross-linkers | [58,59] |
| BC | Freeze-drying | Open interconnected macropores; cell proliferation and differentiation | Up to week-long process; wide pore size distribution; incomplete removal of cross-linkers; collapsed nanostructure | [64,65] |
| BC | SC-CO2 drying | Hierarchical morphology; regularity on microscale and nanoscale; few hours needed for complete drying; high SSA; open and interconnected structure | Poor mechanical properties; fillers needed | [65,66,67,68] |
| κ-CAR | Freeze-drying | Tunable mechanical properties; osteogenic proliferation | Absence of a hierarchical morphology; no nanostructure observed | [78,79] |
| κ-CAR | SC-CO2 drying | Complete removal of toxic cross-linkers; high SSA; fast process | Shrinkage occurrence | [80,81,82,83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanotti, A.; Baldino, L.; Reverchon, E. Production of Exopolysaccharide-Based Porous Structures for Biomedical Applications: A Review. Nanomaterials 2023, 13, 2920. https://doi.org/10.3390/nano13222920
Zanotti A, Baldino L, Reverchon E. Production of Exopolysaccharide-Based Porous Structures for Biomedical Applications: A Review. Nanomaterials. 2023; 13(22):2920. https://doi.org/10.3390/nano13222920
Chicago/Turabian StyleZanotti, Alessandra, Lucia Baldino, and Ernesto Reverchon. 2023. "Production of Exopolysaccharide-Based Porous Structures for Biomedical Applications: A Review" Nanomaterials 13, no. 22: 2920. https://doi.org/10.3390/nano13222920






