Photocatalytic Synthesis of Coumarin Derivatives Using Visible-Light-Responsive Strawberry Dye-Sensitized Titanium Dioxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
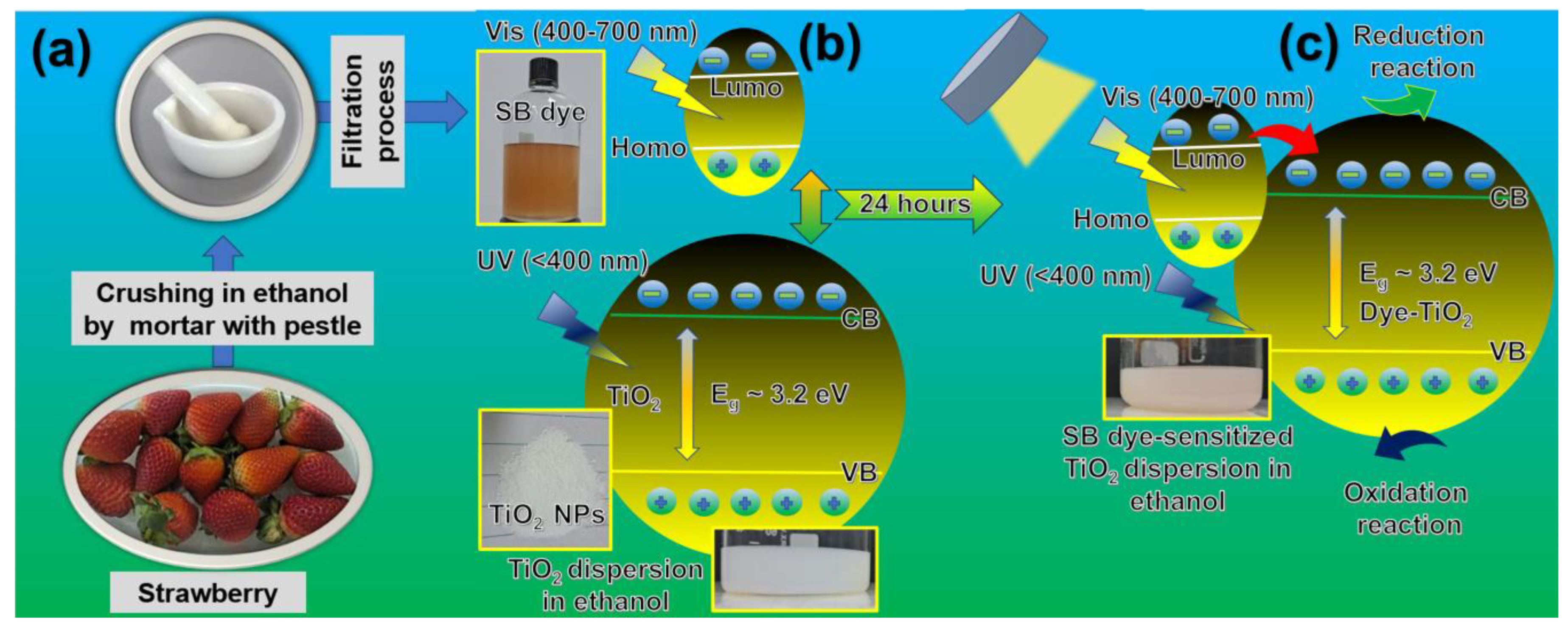
2.2. Preparation of Strawberry Dye
2.3. Preparation of Strawberry Dye-Sensitized TiO2
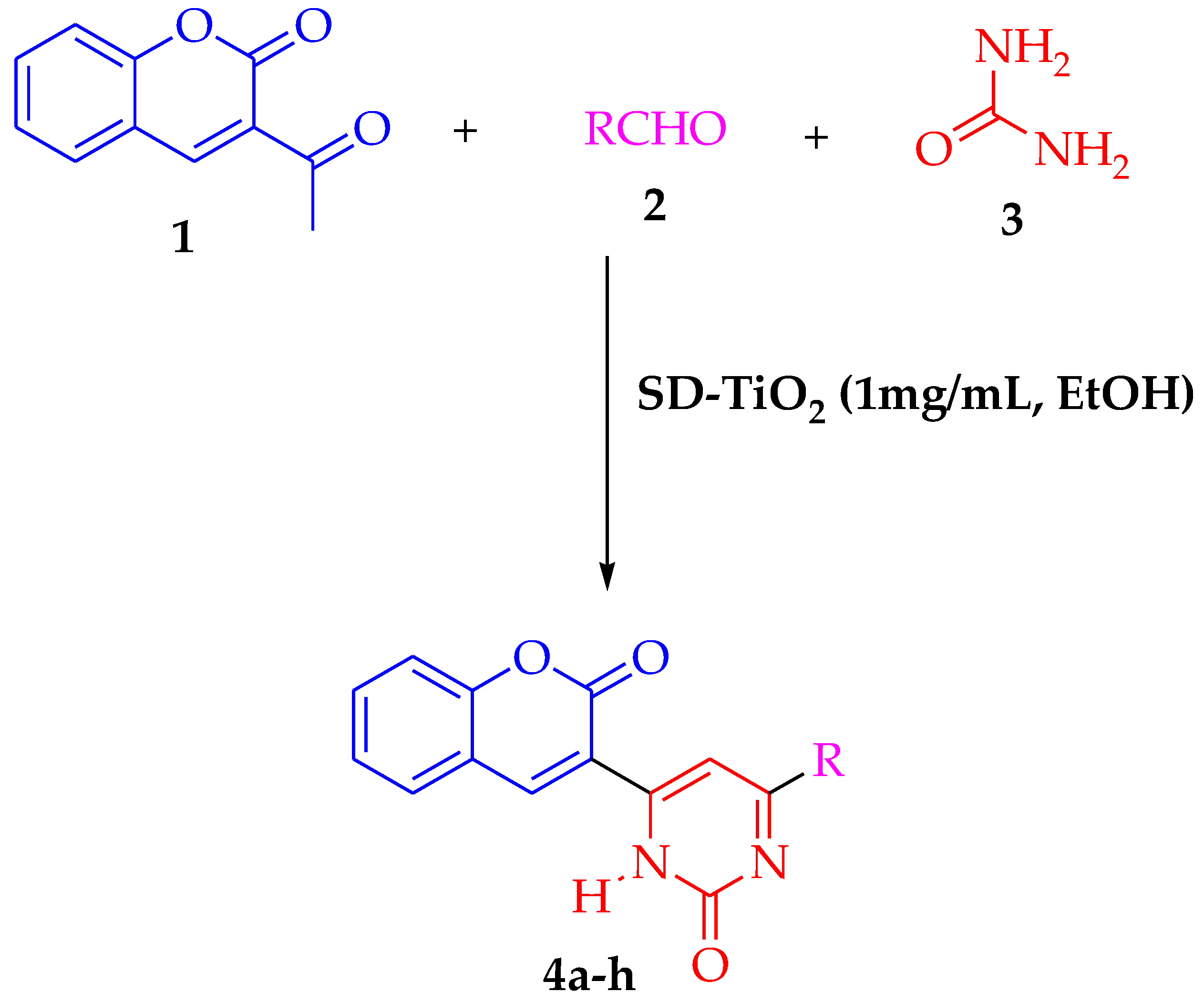
2.4. Photocatalytic Synthesis of Coumarin Derivatives
2.5. Characterization
3. Results and Discussion
3.1. Photocatalysts Characterization
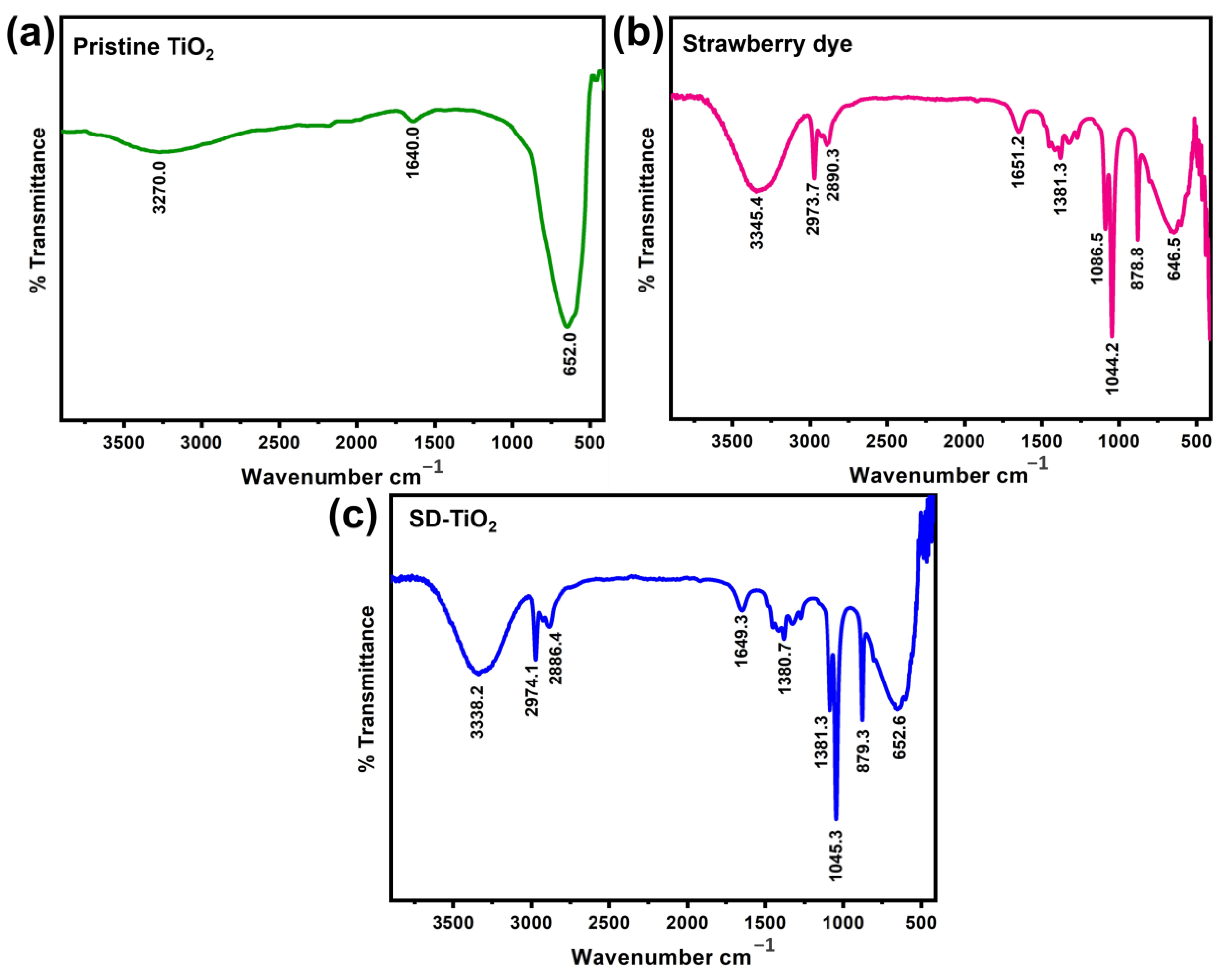
3.1.1. FTIR Analysis
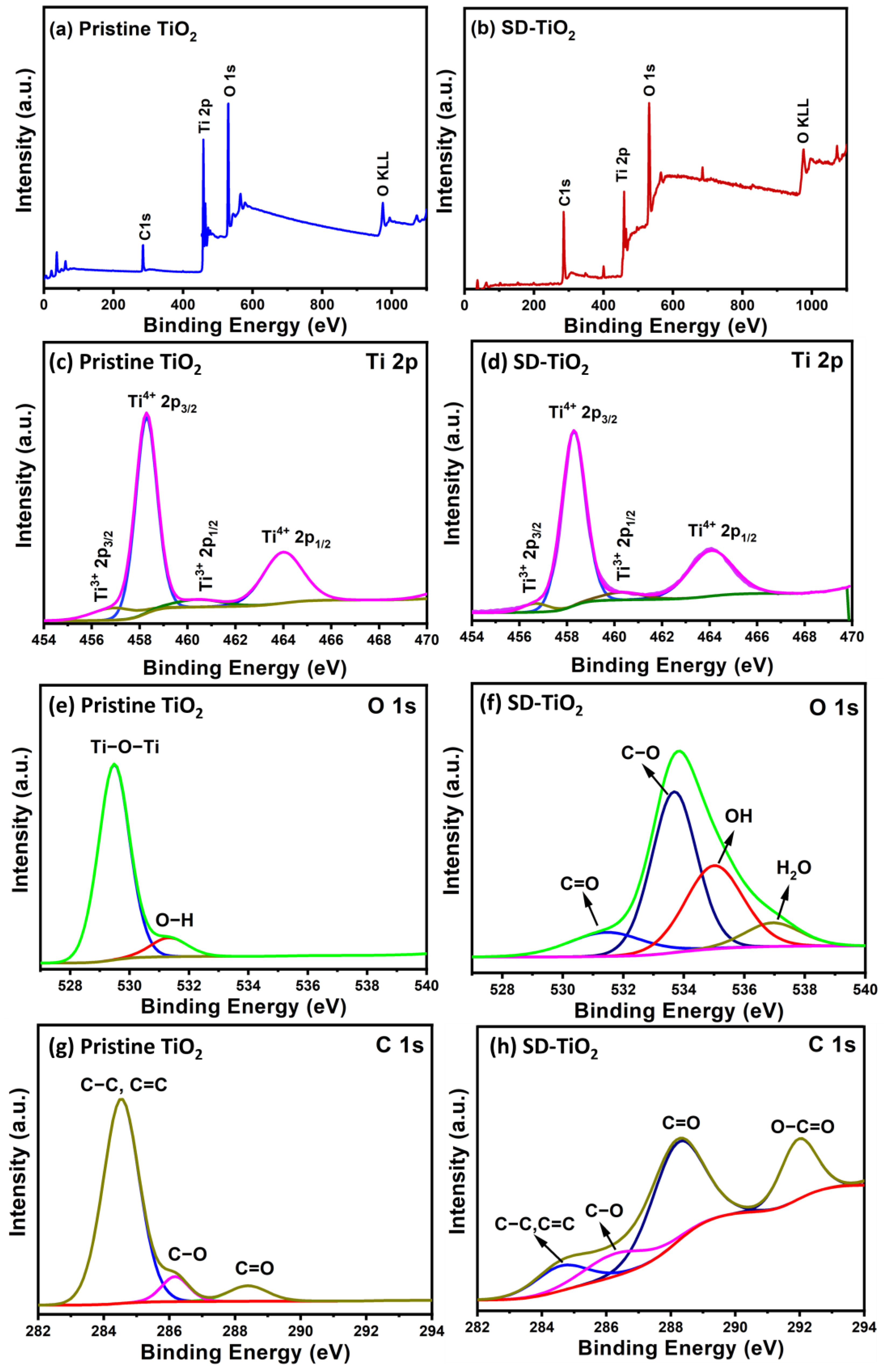
3.1.2. XPS Analysis
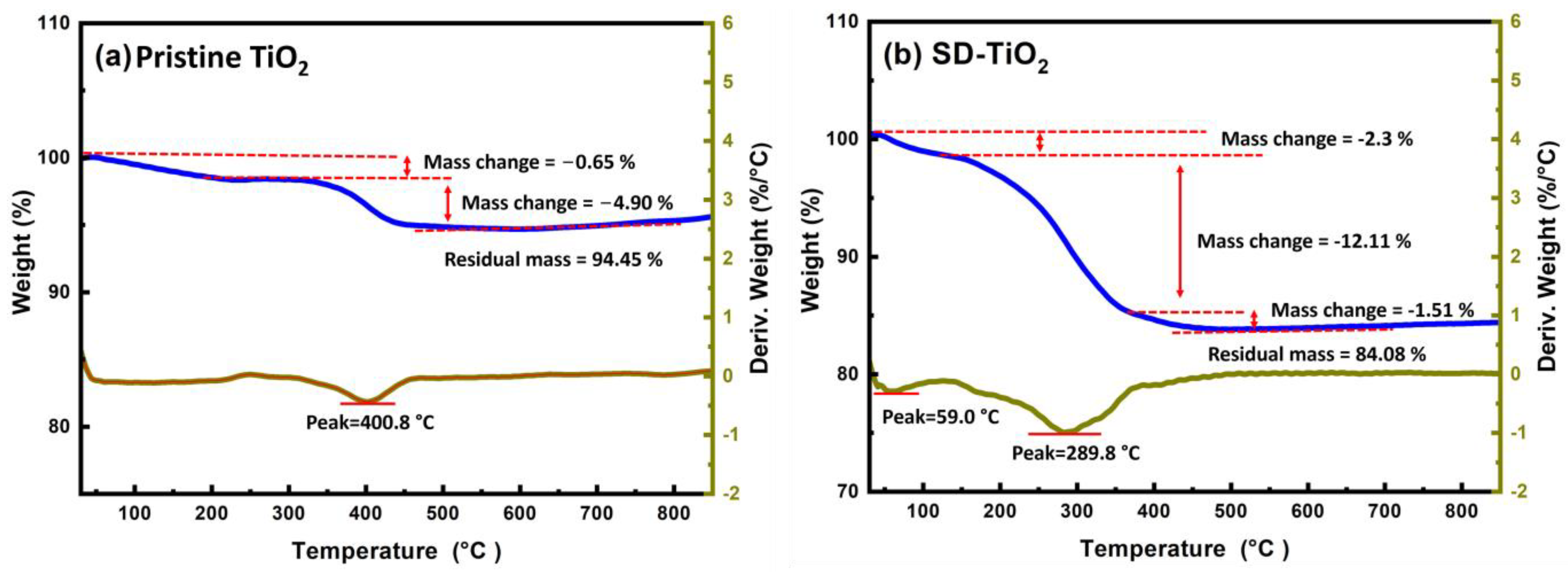
3.1.3. TGA/DTA
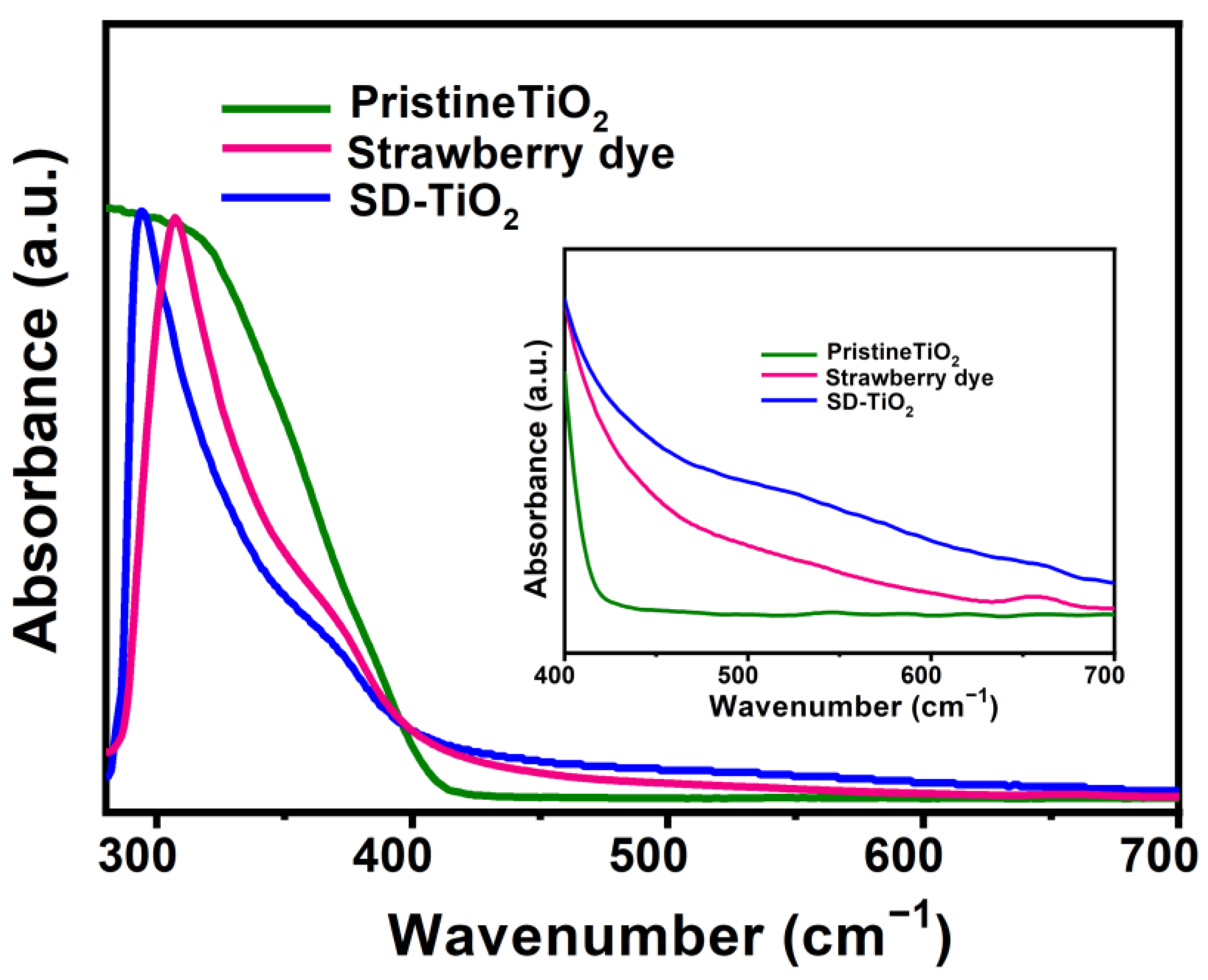
3.1.4. UV-Vis Absorption Spectra
3.1.5. XRD
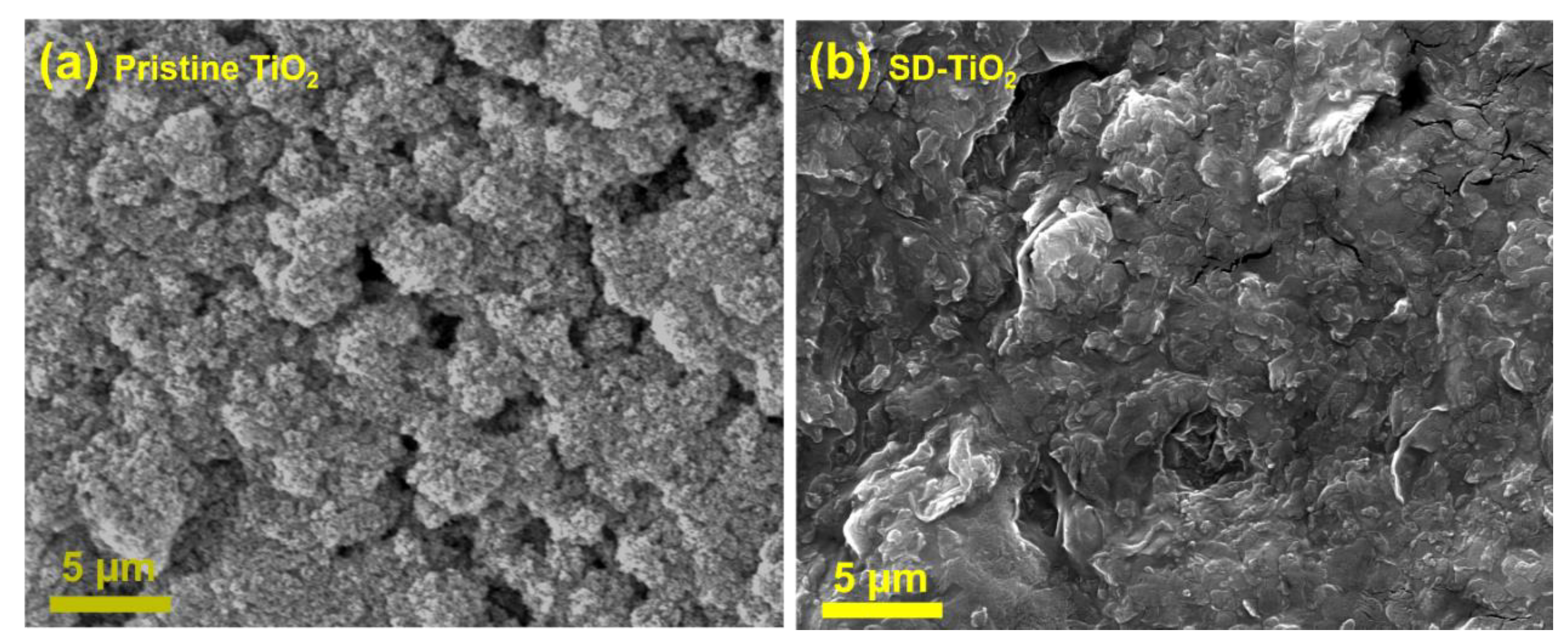
3.1.6. SEM
3.1.7. BET
3.2. Photocatalytic Activity
3.3. Photocatalyst Reusability
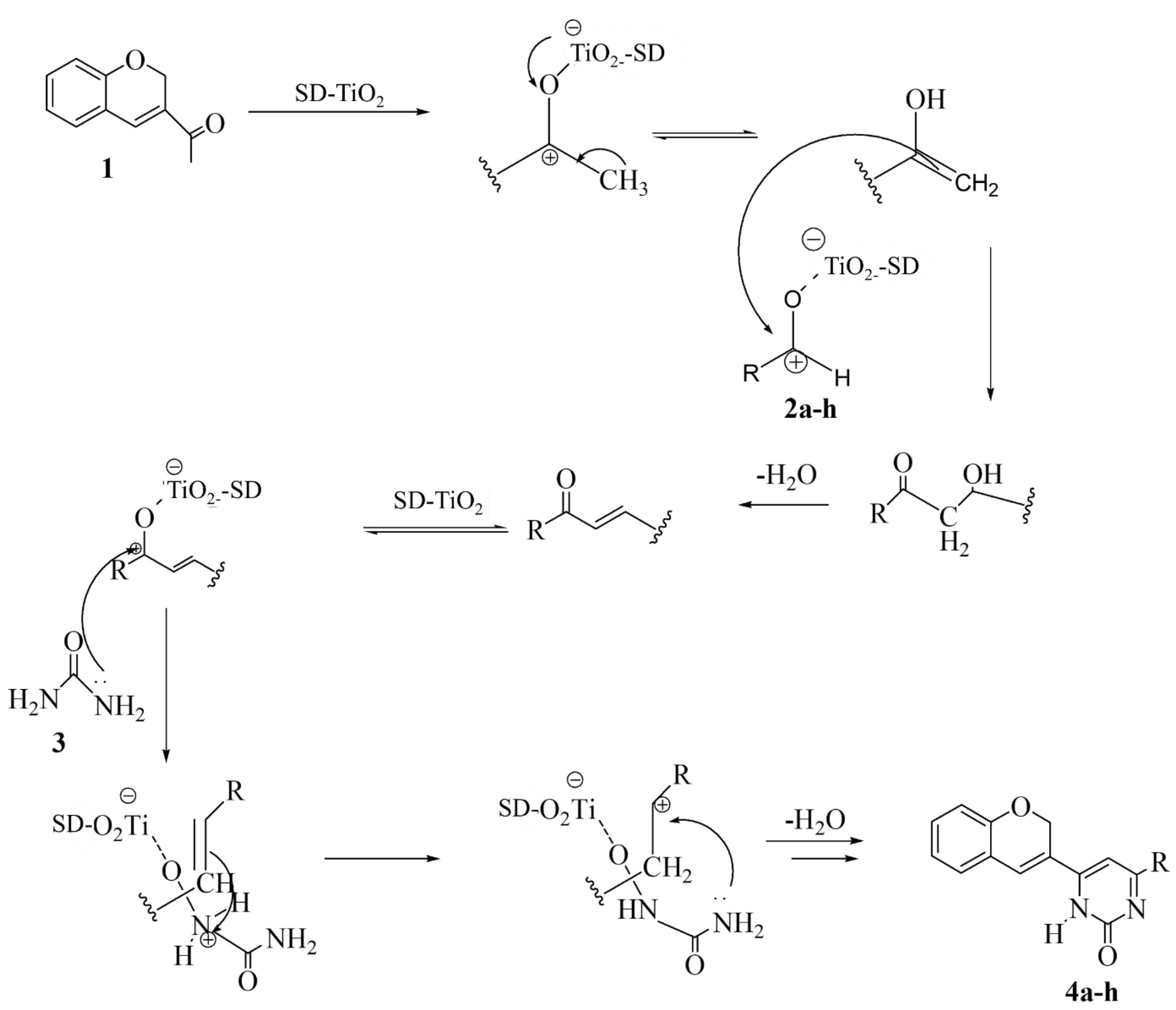
3.4. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dömling, A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Aghaei, M.; Ghamari, N. Facile synthesis of tetrahydrobenzoxanthenones via a one-pot three-component reaction using an eco-friendly and magnetized biopolymer chitosan-based heterogeneous nanocatalyst. Appl. Organomet. Chem. 2016, 30, 939–942. [Google Scholar] [CrossRef]
- Maleki, A.; Azadegan, S. Preparation and characterization of silica-supported magnetic nanocatalyst and application in the synthesis of 2-amino-4H-chromene-3-carbonitrile derivatives. Inorg. Nano-Met. Chem. 2017, 47, 917–924. [Google Scholar] [CrossRef]
- Al-Bayati, R.I.; Al-Amiery, A.A.H.; Al-Majedy, Y.K. Design, synthesis and bioassay of novel coumarins. Afr. J. Pure Appl. Chem. 2010, 4, 74–86. [Google Scholar]
- Cravotto, G.; Nano, G.M.; Palmisano, G.; Tagliapietra, S. An asymmetric approach to coumarin anticoagulants via hetero-Diels–Alder cycloaddition. Tetrahedron Asymmetry 2001, 12, 707–709. [Google Scholar] [CrossRef]
- Wang, C.-J.; Hsieh, Y.-J.; Chu, C.-Y.; Lin, Y.-L.; Tseng, T.-H. Inhibition of cell cycle progression in human leukemia HL-60 cells by esculetin. Cancer Lett. 2002, 183, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Kolodziej, H. Antibacterial Activity of Extracts and Constituents of Pelargonium sidoides and Pelargonium reniforme. Planta Med. 1997, 63, 508–510. [Google Scholar] [CrossRef]
- Pradhan, R.; Khandelwal, K.; Panda, S.J.; Purohit, C.S.; Bag, B.P.; Singhal, R.; Liu, W.; Zhu, X.; Sharma, G.D.; Mishra, A. Achieving High-Efficiency in Binary Organic Solar Cells by the Structural Fine-Tuning of Coumarin-Based Donor. Sol. RRL 2023, 7, 2300032. [Google Scholar] [CrossRef]
- Rattanawan, R.; Promarak, V.; Sudyoadsuk, T.; Namuangruk, S.; Kungwan, N.; Yuan, S.; Jungsuttiwong, S. Theoretical design of coumarin derivatives incorporating auxiliary acceptor with D-π-A-π-A configuration for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2016, 322–323, 16–26. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Gong, Y.; Yu, T.; Zhao, Y. Synthesis and characterization of SFX-based coumarin derivatives for OLEDs. Dye. Pigment. 2021, 185, 108969. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Atta, S.; Singh, N.D.P.; Jacob, C. Influence of functional derivatives of an amino-coumarin/MWCNT composite organic hetero-junction on the photovoltaic characteristics. Mater. Sci. Semicond. Process. 2014, 25, 279–285. [Google Scholar] [CrossRef]
- Heravi, M.M.; Khaghaninejad, S.; Mostofi, M. Chapter one—Pechmann reaction in the synthesis of coumarin derivatives. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 112, pp. 1–50. [Google Scholar] [CrossRef]
- Sharma, D.; Dhayalan, V.; Manikandan, C.; Dandela, R. Recent Methods for Synthesis of Coumarin Derivatives and Their New Applications; IntechOpen: London, UK, 2022; Available online: https://www.intechopen.com/online-first/84910 (accessed on 12 July 2023).
- Shaabani, A.; Ghadari, R.; Rahmati, A.; Rezayan, A.H. Coumarin synthesis via Knoevenagel condensation reaction in 1,1,3,3-N,N,N′,N′-tetramethylguanidinium trifluoroacetate ionic liquid. JICS 2009, 6, 710–714. [Google Scholar] [CrossRef]
- Kumbar, M.N.; Kamble, R.R.; Kamble, A.A.; Salian, S.R.; Kumari, S.; Nair, R.; Kalthur, G.; Adiga, S.K.; Prasad, D.J. Design and Microwave Assisted Synthesis of Coumarin Derivatives as PDE Inhibitors. Int. J. Med. Chem. 2016, 2016, 9890630. [Google Scholar] [CrossRef] [PubMed]
- Eng Oi, L.; Choo, M.-Y.; Voon Lee, H.; Chyuan Ong, H.; Abd Hamid, S.B.; Ching Juan, J. Recent advances of titanium dioxide (TiO 2) for green organic synthesis. RSC Adv. 2016, 6, 108741–108754. [Google Scholar] [CrossRef]
- Din, I.U.; Alotaibi, M.A.; Alharthi, A.I.; Al-Shalwi, M.N.; Alshehri, F. Green synthesis approach for preparing zeolite based Co-Cu bimetallic catalysts for low temperature CO2 hydrogenation to methanol. Fuel 2022, 330, 125643. [Google Scholar] [CrossRef]
- Kader, D.A.; Mohammed, S.J. Emerging developments in dye-sensitized metal oxide photocatalysis: Exploring the design, mechanisms, and organic synthesis applications. RSC Adv. 2023, 13, 26484. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.h.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrog. Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Gholami, P.; Heidari, A.; Khataee, A.; Ritala, M. Oxygen and nitrogen plasma modifications of ZnCuCo LDH-graphene nanocomposites for photocatalytic hydrogen production and antibiotic degradation. Sep. Purif. Technol. 2023, 325, 124706. [Google Scholar] [CrossRef]
- Gholami, P.; Khataee, A.; Ritala, M. Template-free hierarchical trimetallic oxide photocatalyst derived from organically modified ZnCuCo layered double hydroxide. J. Clean. Prod. 2022, 366, 132761. [Google Scholar] [CrossRef]
- Darbandi, M.; Shaabani, B.; Schneider, J.; Bahnemann, D.; Gholami, P.; Khataee, A.; Yardani, P.; Hosseini, M.G. TiO2 nanoparticles with superior hydrogen evolution and pollutant degradation performance. Int. J. Hydrogen Energy 2019, 44, 24162–24173. [Google Scholar] [CrossRef]
- Kowalska, E.; Artelska, A.; Albrecht, A. Visible Light-Driven Reductive Azaarylation of Coumarin-3-carboxylic Acids. J. Org. Chem. 2022, 87, 9645–9653. [Google Scholar] [CrossRef]
- Kim, J.K.; Liu, Y.; Gong, M.; Li, Y.; Huang, M.; Wu, Y. A facile visible-light-induced one-pot synthesis of 3-alkyl coumarins from simple salicylaldehydes. Tetrahedron 2023, 132, 133249. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of Titanium Dioxide (TiO2) Modification on Its Application to Pollution Treatment—A Review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Y.; Bu, X.; Wu, Q.; Hang, Z.; Dong, Z.; Wu, X. Facile one-step synthesis of TiO2/Ag/SnO2 ternary heterostructures with enhanced visible light photocatalytic activity. Sci. Rep. 2018, 8, 10532. [Google Scholar] [CrossRef] [PubMed]
- Higashida, Y.; Takizawa, S.; Yoshida, M.; Kato, M.; Kobayashi, A. Hydrogen Production from Hydrophobic Ruthenium Dye-Sensitized TiO2 Photocatalyst Assisted by Vesicle Formation. ACS Appl. Mater. Interfaces 2023, 15, 27277–27284. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, J.-L.; Hao, H.; Lang, X. Visible light-induced selective oxidation of alcohols with air by dye-sensitized TiO2 photocatalysis. Appl. Catal. B Environ. 2018, 232, 260–267. [Google Scholar] [CrossRef]
- Reginato, G.; Zani, L.; Calamante, M.; Mordini, A.; Dessì, A. Dye-Sensitized Heterogeneous Photocatalysts for Green Redox Reactions. Eur. J. Inorg. Chem. 2020, 2020, 899–917. [Google Scholar] [CrossRef]
- Tiwari, A.; Mondala, I.; Pal, U. Visible light induced hydrogen production over thiophenothiazine-based dye sensitized TiO2 photocatalyst in neutral water. RSC Adv. 2015, 5, 31415–31421. [Google Scholar] [CrossRef]
- Ahmad, I.; Kan, C. Visible-Light-Driven, Dye-Sensitized TiO2 Photo-Catalyst for Self-Cleaning Cotton Fabrics. Coatings 2017, 7, 192. [Google Scholar] [CrossRef]
- Dil, A.M.; Haghighatzadeh, A.; Mazinani, B. Photosensitization effect on visible-light-induced photocatalytic performance of TiO2/chlorophyll and flavonoidnano structures: Kinetic and isotherm studies. Bull. Mater. Sci. 2019, 42, 248. [Google Scholar] [CrossRef]
- Akın, S.; Açıkgöz, S.; Gülen, M.; Akyürek, C.; Sönmezoğlu, S. Investigation of the photoinduced electron injection processes for natural dye-sensitized solar cells: The impact of anchoring groups. RSC Adv. 2016, 6, 85125–85134. [Google Scholar] [CrossRef]
- Richhariya, G.; Kumar, A.; Tekasakul, P.; Gupta, B. Natural dyes for dye sensitized solar cell: A review. Renew. Sustain. Energy Rev. 2017, 69, 705–718. [Google Scholar] [CrossRef]
- Goulart, S.; Jaramillo Nieves, L.J.; Dal Bó, A.G.; Bernardin, A.M. Sensitization of TiO2 nanoparticles with natural dyes extracts for photocatalytic activity under visible light. Dye. Pigment. 2020, 182, 108654. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green. Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Ilhan, H.; Durmaz Cayci, G.B.; Aksoy, E.; Diker, H.; Varlikli, C. Photocatalytic activity of dye-sensitized and non-sensitized GO-TiO2 nanocomposites under simulated and direct sunlight. Int. J. Appl. Ceram. Technol. 2022, 19, 425–435. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; L’Quiles, J.; Bompadre, S.; Mezzetti, B.; et al. An anthocyanin-rich strawberry extract protects against oxidative stress damage and improves mitochondrial functionality in human dermal fibroblasts exposed to an oxidizing agent. Food Funct. 2014, 5, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Shriwastav, A. Application of TiO2 nanoparticles sensitized with natural chlorophyll pigments as catalyst for visible light photocatalytic degradation of methylene blue. J. Environ. Chem. Eng. 2021, 9, 104699. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Xie, E.; Duan, H.; Han, W.; Zhao, J. A simple method to prepare uniform-size nanoparticle TiO2 electrodes for dye-sensitized solar cells. J. Power Sources 2009, 189, 1256–1263. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Comparison between Maceration and Microwave Extraction Techniques of Strawberry Fruit (Fragaria sp.) and Antioxidant Activity Test—IOPscience n.d. Available online: https://iopscience.iop.org/article/10.1088/1757-899X/523/1/012024/meta (accessed on 12 July 2023).
- Komtchou, S.; Delegan, N.; Dirany, A.; Drogui, P.; Robert, D.; El Khakani, M.A. Removal of atrazine by photoelectrocatalytic process under sunlight using WN-codoped TiO2 photoanode. J. Appl. Electrochem. 2018, 48, 1353–1361. [Google Scholar] [CrossRef]
- Didwal, P.N.; Chikate, P.R.; Bankar, P.K.; More, M.A.; Devan, R.S. Intense field electron emission source designed from large area array of dense rutile TiO2 nanopillars. J. Mater. Sci. Mater. Electron. 2019, 30, 2935–2941. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Lu, Y.; Wang, D.; Wang, C.; Li, J. One-Step Synthesis of Eu3+-Modified Cellulose Acetate Film and Light Conversion Mechanism. Polymers 2021, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lv, J.-J.; Zhou, D.-L.; Bao, N.; Xu, Y.; Wang, A.-J.; Feng, J.J. One-pot green synthesis of nitrogen-doped carbon nanoparticles as fluorescent probes for mercury ions. RSC Adv. 2013, 3, 21691–21696. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Li, T.; Ma, P.; Zhang, X.; Xia, B.; Chen, M.; Du, M.; Dong, W. Skin bioinspired anti-ultraviolet melanin/TiO2 nanoparticles without penetration for efficient broad-spectrum sunscreen. Colloid. Polym. Sci. 2021, 299, 1797–1805. [Google Scholar] [CrossRef]
- Vinutha, K.V.; Kb, N.K.; Tejas, M.K.; Kumar, J.; Kumar, S.; Mahesh, H.M. Natural Dye Sensitized Solar Cells Using Anthocyanin Pigment of Strawberry as Sensitizers. Imp. J. Interdiscip. Res. 2016, 2, 1011–1016. [Google Scholar]
- Velmurugan, R.; Krishnakumar, B.; Kumar, R.; Swaminathan, M. Solar active nano-TiO2 for mineralization of Reactive Red 120 and Trypan Blue: 1st Nano Update. Arab. J. Chem. 2012, 5, 447–452. [Google Scholar] [CrossRef]
- McKay, G.; Parthasarathy, P.; Sajjad, S.; Saleem, J.; Alherbawi, M. 12—Dye removal using biochars. In Sustainable Biochar for Water and Wastewater Treatment; Mohan, D., Pittman, C.U., Mlsna, T.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 429–471. [Google Scholar] [CrossRef]
- Zhou, W.; Li, W.; Wang, J.-Q.; Qu, Y.; Yang, Y.; Xie, Y.; Zhang, K.; Wang, L.; Fu, H.; Zhao, D. Ordered Mesoporous Black TiO2 as Highly Efficient Hydrogen Evolution Photocatalyst. J. Am. Chem. Soc. 2014, 136, 9280–9283. [Google Scholar] [CrossRef]
- Bakht, M.A.; Alotaibi, M.; Alharthi, A.I.; Geesi, M.H.; Ud Din, I.; Imran, M.; Thabet, H.K. Zn:Cr Mixed Oxide Catalyzed Facile Construction of 4-Aryl-6-(3-Coumarinyl)Pyrimidin-2(1H)-Ones Derivatives under the Solvent-Free Condition. Polycycl. Aromat. Compd. 2021, 48, 4922–4936. [Google Scholar] [CrossRef]
- Kong, R.; Han, S.-B.; Wei, J.-Y.; Peng, X.-C.; Xie, Z.-B.; Gong, S.-S.; Sun, Q. Highly Efficient Synthesis of Substituted 3,4-Dihydropyrimidin-2-(1H)-ones (DHPMs) Catalyzed by Hf(OTf)4: Mechanistic Insights into Reaction Pathways under Metal Lewis Acid Catalysis and Solvent-Free Conditions. Molecules 2019, 24, 364. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, D.; Vo, P.P.; Doan, H.N.; Pham, H.T.; Hoang, V.H.; Le, K.T.; Kinashi, K.; Huynh, V.T.; Nguyen, P.T. The roles of ethanol and isopropanol as hole scavengers in the photoreduction reaction of graphene oxide by TiO2: A competition of oxygenated groups removal and carbon defects invasion. J. Mol. Liq. 2023, 381, 121831. [Google Scholar] [CrossRef]

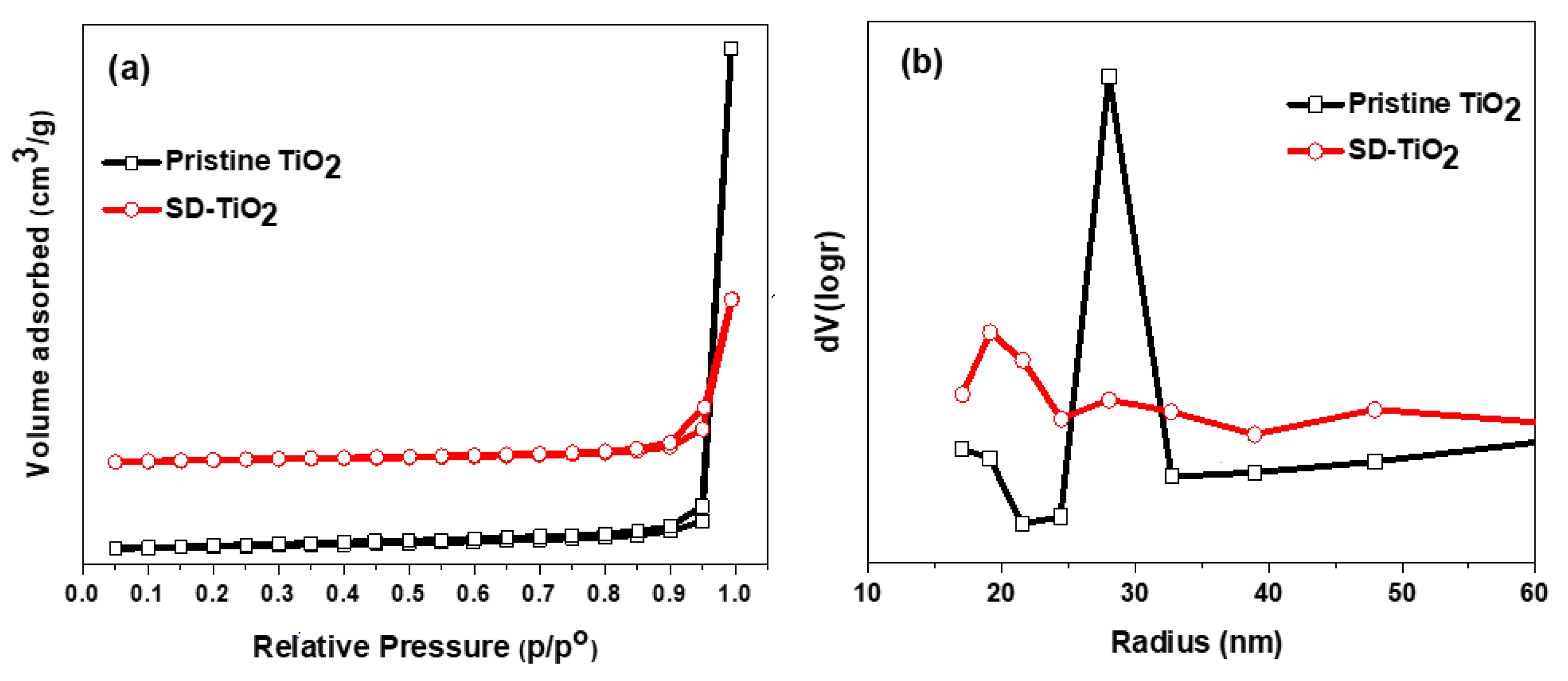

| Catalyst | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Radius (nm) |
|---|---|---|---|
| Pristine TiO2 | 50.60 | 1.80 | 28 nm |
| SD-TiO2 | 34.20 | 0.58 | 19 nm |
| Entry | Photocatalyst (mg/mL) | Time (min) | Yield (%) b |
|---|---|---|---|
| SD-TiO2 | |||
| 1 | 0 | 30 | 20 |
| 2 | 1 | 30 | 96 |
| 3 | 2 | 60 | 95 |
| 4 | 3 | 90 | 92 |
| 5 | 4 | 120 | 88 |
| 6 | 5 | 150 | 85 |
| Entry | Time (Min) | Yield (%) | |
|---|---|---|---|
| SD-TiO2 | Pristine TiO2 | ||
| 1 | 30 | 96 | 85 |
| 2 | 60 | 95 | 82 |
| 3 | 90 | 92 | 80 |
| 4 | 120 | 88 | 77 |
| 5 | 150 | 85 | 71 |
| Entry | Intensity (mW cm−2) | Yield (%) |
|---|---|---|
| 1 | 20 | 68 |
| 2 | 39 | 78 |
| 3 | 58 | 85 |
| 4 | 75 | 92 |
| 5 | 100 | 96 |
| 6 | 120 | 94 |
| Entry | Time (min) | Yield (%) | |
|---|---|---|---|
| Photocatalyst | Thermocatalyst | ||
| 1 | 30 | 96 | 72 |
| 2 | 60 | 95 | 70 |
| 3 | 90 | 92 | 66 |
| 4 | 120 | 88 | 64 |
| 5 | 150 | 85 | 63 |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | Product | Time (min) | Yield (%) a,b | M.P (°C) | M.P (°C) | Ref. No. |
| Observed | Reported | ||||||
| 1 |  | 4a | 30 | 96 | 277–278 | 278–280 | [52] |
| 2 | 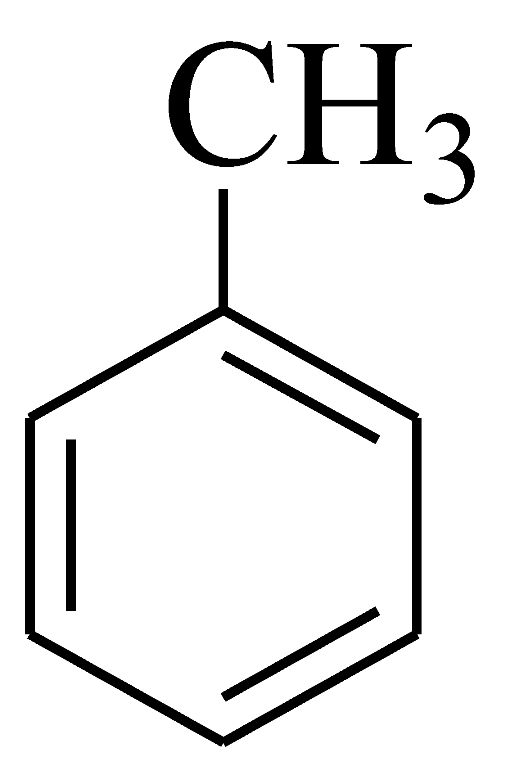 | 4b | 40 | 91 | 208–210 | 202–204 | [53] |
| 3 |  | 4c | 45 | 95 | 230–233 | - | - |
| 4 | 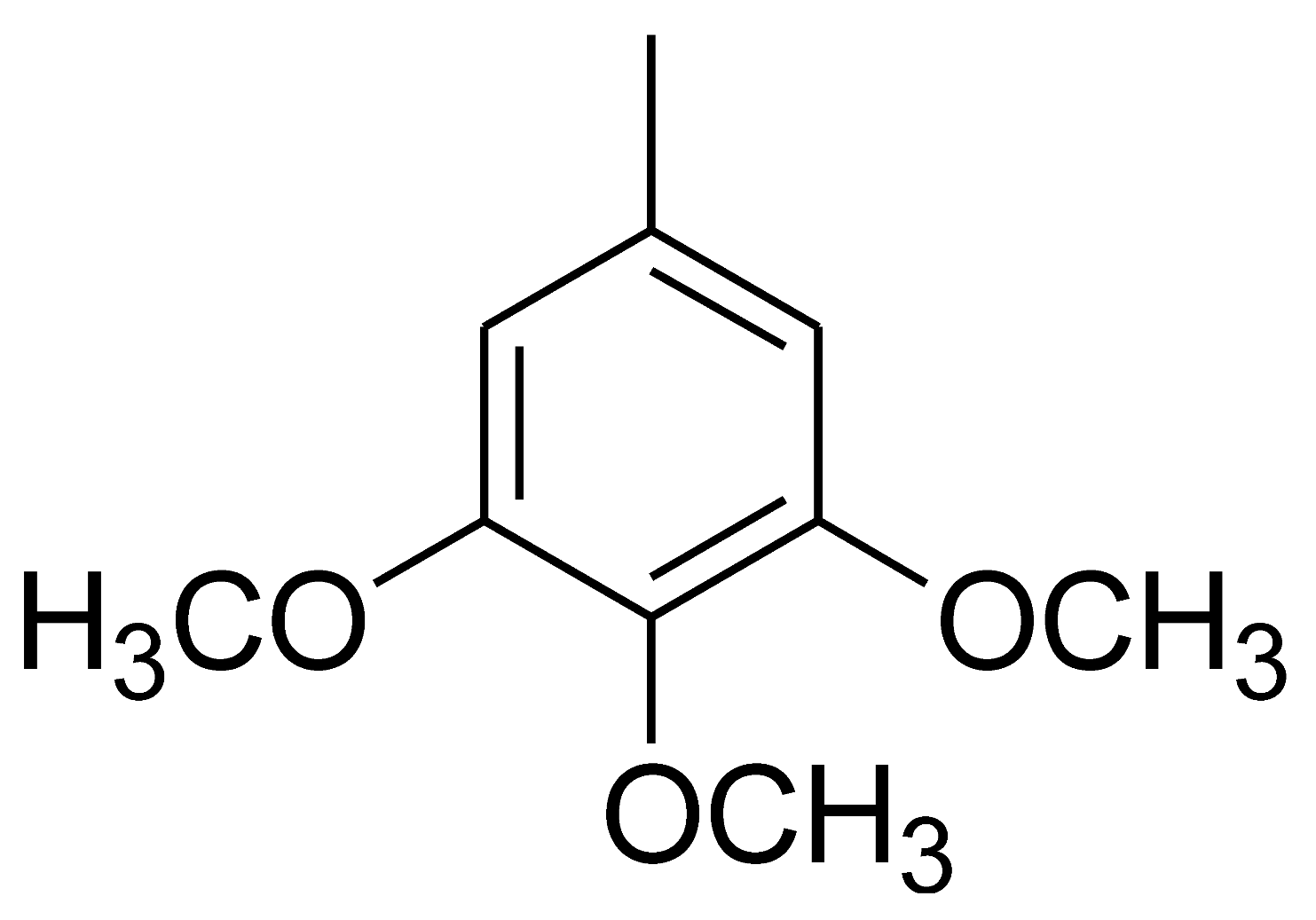 | 4d | 35 | 94 | 279–281 | - | [53] |
| 5 | 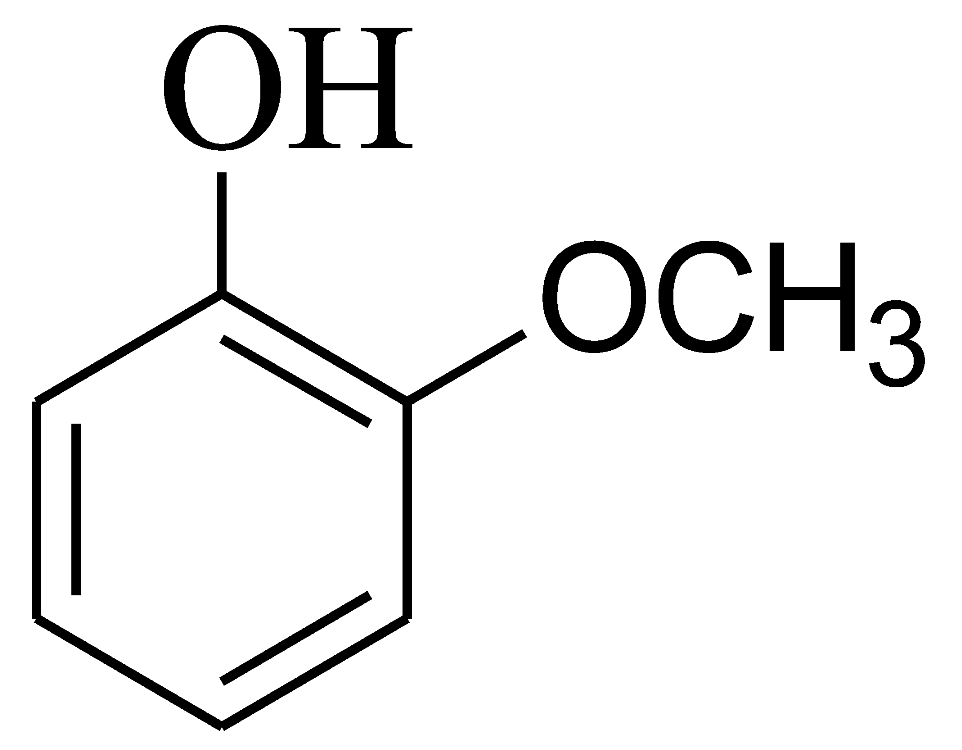 | 4e | 40 | 93 | 222–225 | - | - |
| 6 |  | 4f | 45 | 90 | 235–237 | 236–237 | [52] |
| 7 | 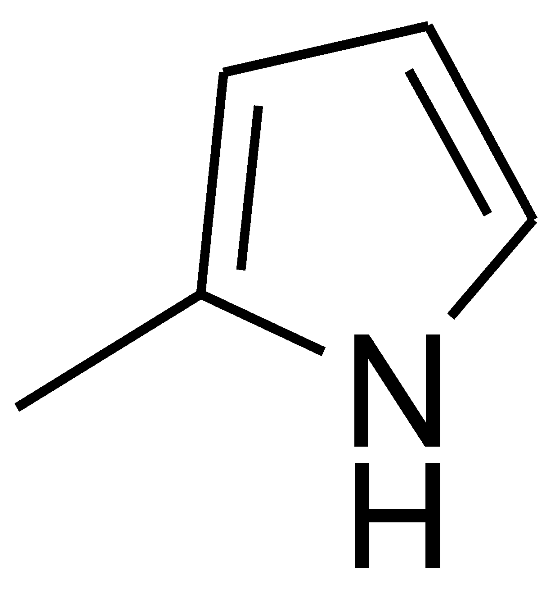 | 4g | 60 | 89 | 215–216 | 217–218 | [52] |
| 8 |  | 4h | 55 | 88 | 218–220 | 219–220 | [52] |
| Run | Time (min) | Yield (%) | Catalyst Amount (mg) |
|---|---|---|---|
| 1 | 30 | 96 | 10 |
| 2 | 30 | 95 | 9 |
| 3 | 30 | 93 | 8 |
| 4 | 30 | 92 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, M.A.; Alharthi, A.I.; Qahtan, T.F.; Alotibi, S.; Alansi, A.M.; Bakht, M.A. Photocatalytic Synthesis of Coumarin Derivatives Using Visible-Light-Responsive Strawberry Dye-Sensitized Titanium Dioxide Nanoparticles. Nanomaterials 2023, 13, 3001. https://doi.org/10.3390/nano13233001
Alotaibi MA, Alharthi AI, Qahtan TF, Alotibi S, Alansi AM, Bakht MA. Photocatalytic Synthesis of Coumarin Derivatives Using Visible-Light-Responsive Strawberry Dye-Sensitized Titanium Dioxide Nanoparticles. Nanomaterials. 2023; 13(23):3001. https://doi.org/10.3390/nano13233001
Chicago/Turabian StyleAlotaibi, Mshari A., Abdulrahman I. Alharthi, Talal F. Qahtan, Satam Alotibi, Amani M. Alansi, and Md. Afroz Bakht. 2023. "Photocatalytic Synthesis of Coumarin Derivatives Using Visible-Light-Responsive Strawberry Dye-Sensitized Titanium Dioxide Nanoparticles" Nanomaterials 13, no. 23: 3001. https://doi.org/10.3390/nano13233001






