Recent Uses of Lipid Nanoparticles, Cell-Penetrating and Bioactive Peptides for the Development of Brain-Targeted Nanomedicines against Neurodegenerative Disorders
Abstract
:1. Introduction
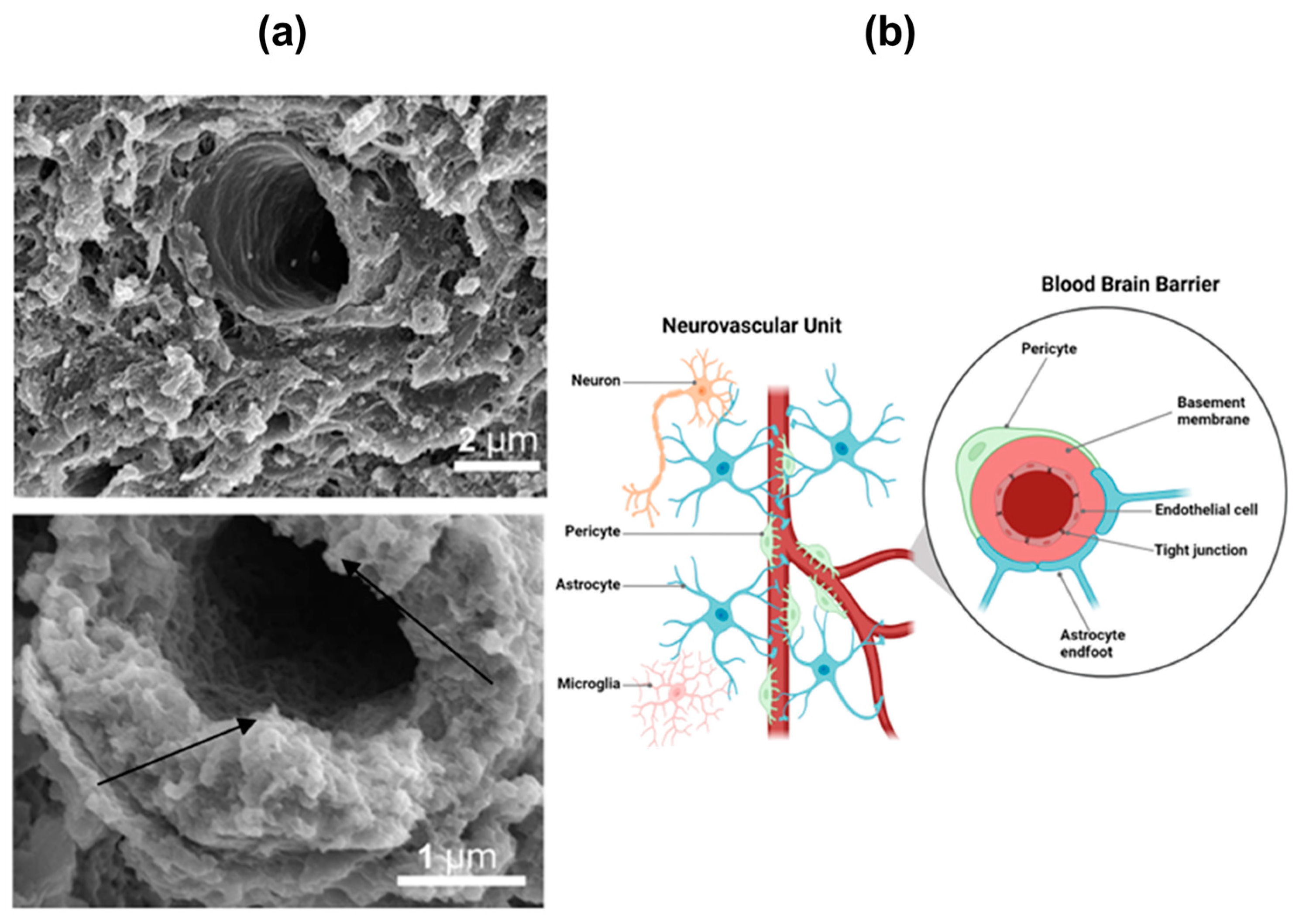
1.1. Overview of the Blood–Brain Barrier Organization Controlling Drug Transport to the Brain
1.2. Strategies Using Cell-Penetrating Peptides and Lipid-Based Nanocarriers to Overcome the Challenges Imposed by the BBB
2. Cell-Penetrating and Blood–Brain Barrier Shuttle Peptides in Therapeutic Delivery to CNS
2.1. CPP Types
2.2. Cellular Uptake Enhanced by CPPs
| Peptide Name/Type | Amino Acid Sequence | Reference |
|---|---|---|
| TAT (Trans-Activating Transcriptor) | CGRKKRRQRRRK | [28,29,91] |
| PACAP-38 | HSDGIFTDSYSRYRKQMAVKKYLAAVLGKRYKQRVKNK | [115,116] |
| PepNeg | SGTQEEY | [25] |
| HR9 | CH-HHHHRRRRRRRRRHHHHHC | [62] |
| Angiopep-2 | TFFYGGSRGKRNNFKTEEY | [109] |
| Penetratin | RQIKIWFQNRRMKWKK | [110] |
| HAI | H-HAIYPRH-NH2 | [117] |
| R8 peptide | YARAAARQARA | [111] |
| Peptide-22 | NH2–NH2–CGGGPKKKRKVGG–COOH | [112] |
| MPG | GALFLGFLGAAGSTMGAWSQPKKKRKV | [113] |
| C105Y | CSIPPEVKFNKPFVYLI | [114] |
2.3. Blood–Brain Barrier Shuttle Peptides
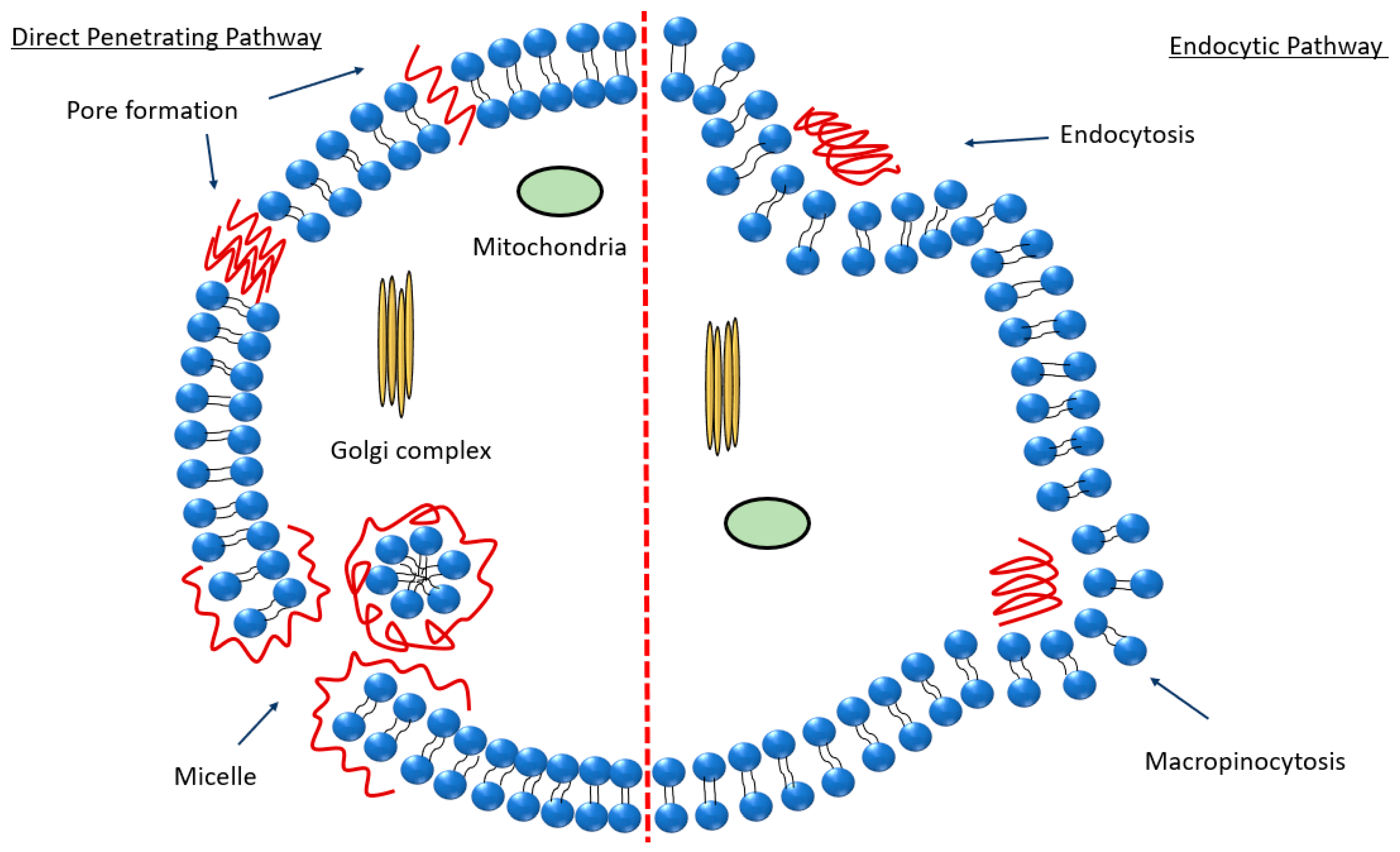
2.4. Peptide Internalization Mechanisms in Relation to the Peptide-Mediated Passage of the BBB
3. Nanoparticle-Mediated Drug Delivery
3.1. Lipid Nanoparticle Types
3.2. Targeting and Internalization Capacities of Nanocarriers
4. Examples of Nanomedicine Development for Neurodegenerative Diseases
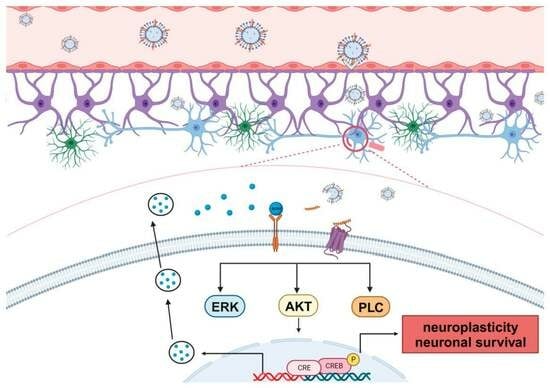
4.1. Bioactive Peptides for Neuroprotection and Neurorepair
4.2. Lipid Nanoparticles for Neuroprotection and Neurorepair
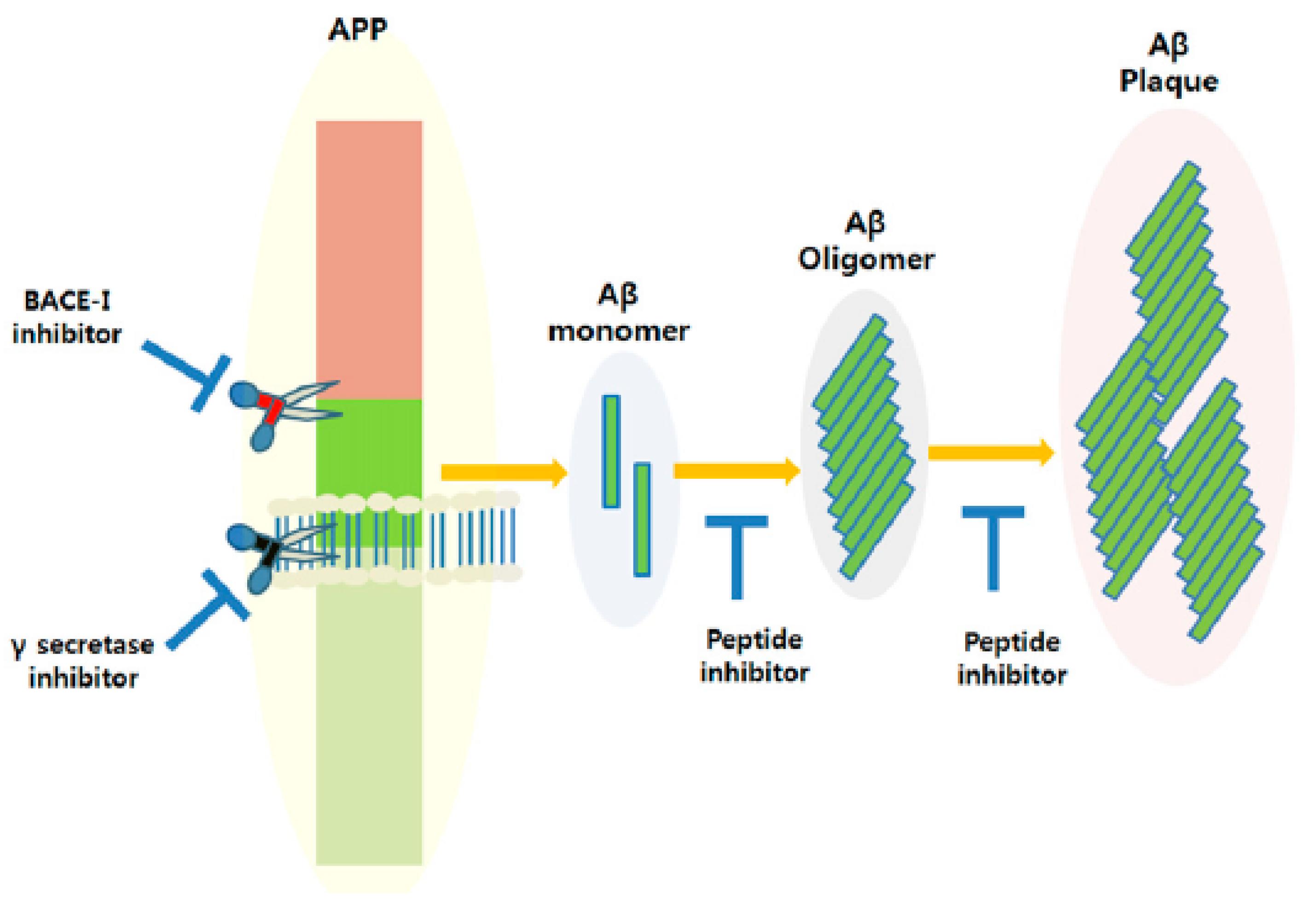
4.3. Neurotherapeutic Delivery for the Treatment of Alzheimer’s Disease
4.3.1. Peptide-Based Strategies
4.3.2. Protein-Based Strategies
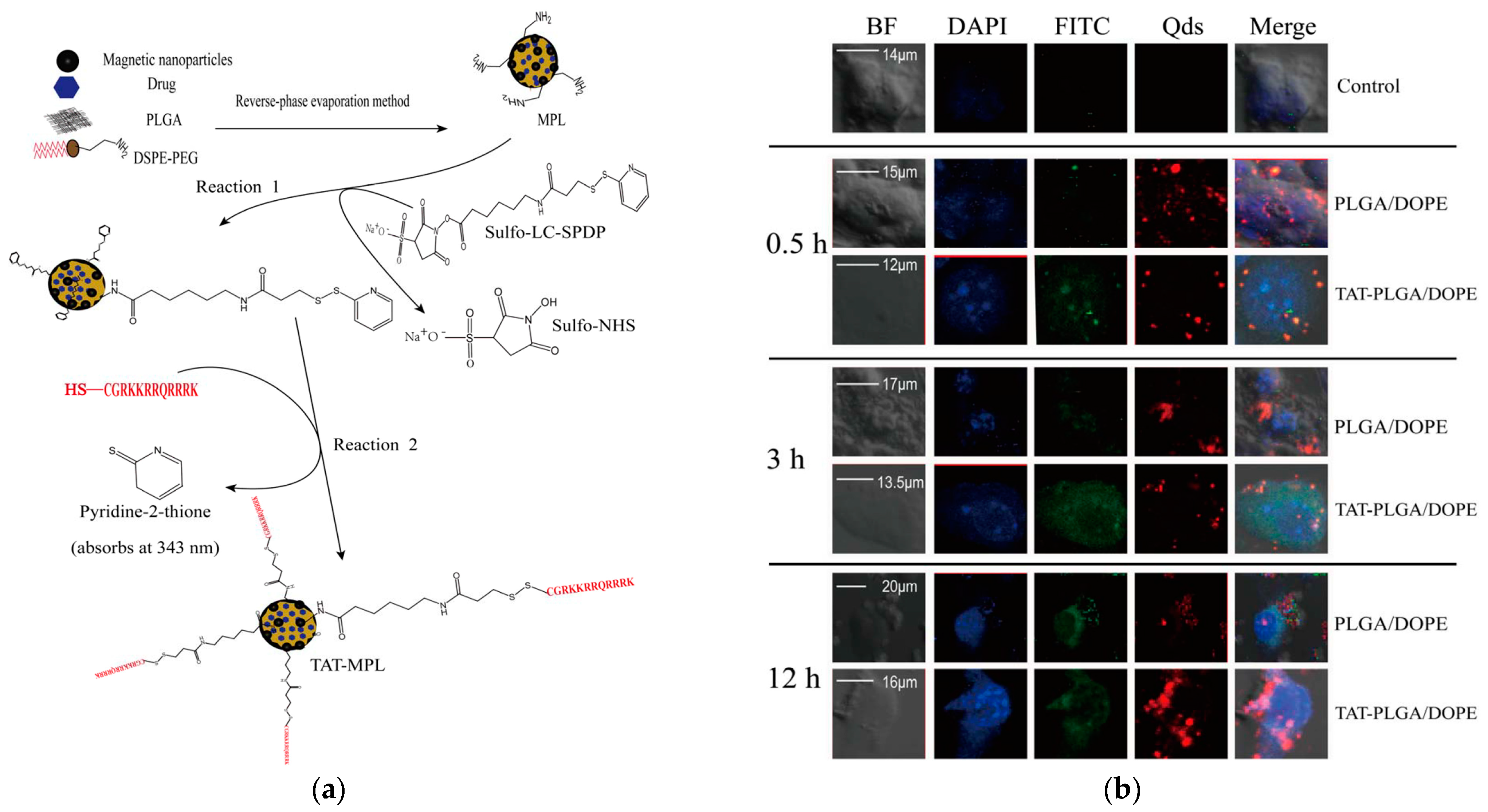
4.4. Combination of Cell-Penetrating Peptides with Nanoparticles for the Treatment of Neurodegenerative Diseases
5. Perspective Applications of the Nose-to-Brain CPP-Mediated Delivery of Bioactive Molecules in Nanomedicine
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Sriramoju, B.; Kanwar, R.K. Neurological Disorders and Therapeutics Targeted to Surmount the Blood–Brain Barrier. Int. J. Nanomed. 2012, 7, 3259–3278. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Collodel, A.; Hasbun, R.; Morales, R. An Overview of the Blood-Brain Barrier. Blood-Brain Barrier 2019, 142, 1–8. [Google Scholar] [CrossRef]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.-P.; Fenart, L. Modelling of the Blood–Brain Barrier in Drug Discovery and Development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Okada, H.; Takemura, G.; Suzuki, K.; Takada, C.; Tomita, H.; Zaikokuji, R.; Hotta, Y.; Miyazaki, N.; Yano, H.; et al. Brain-Specific Ultrastructure of Capillary Endothelial Glycocalyx and Its Possible Contribution for Blood Brain Barrier. Sci. Rep. 2018, 8, 17523. [Google Scholar] [CrossRef]
- Wong, K.; Riaz, M.; Xie, Y.; Zhang, X.; Liu, Q.; Chen, H.; Bian, Z.; Chen, X.; Lu, A.; Yang, Z. Review of Current Strategies for Delivering Alzheimer’s Disease Drugs across the Blood-Brain Barrier. Int. J. Mol. Sci. 2019, 20, 381. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The Role of Brain Vasculature in Neurodegenerative Disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Egleton, R.D.; Davis, T.P. Development of Neuropeptide Drugs That Cross the Blood-Brain Barrier. NeuroRx 2005, 2, 44–53. [Google Scholar] [CrossRef]
- Brasnjevic, I.; Steinbusch, H.W.M.; Schmitz, C.; Martinez-Martinez, P. Delivery of Peptide and Protein Drugs over the Blood–Brain Barrier. Prog. Neurobiol. 2009, 87, 212–251. [Google Scholar] [CrossRef]
- Ali, I.U.; Chen, X. Penetrating the Blood–Brain Barrier: Promise of Novel Nanoplatforms and Delivery Vehicles. ACS Nano 2015, 9, 9470–9474. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Receptor-Mediated Peptide Transport through the Blood-Brain Barrier. Endocr. Rev. 1986, 7, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Friden, P.M.; Walus, L.R.; Musso, G.F.; Taylor, M.A.; Malfroy, B.; Starzyk, R.M. Anti-Transferrin Receptor Antibody and Antibody-Drug Conjugates Cross the Blood-Brain Barrier. Proc. Natl. Acad. Sci. USA 1991, 88, 4771–4775. [Google Scholar] [CrossRef] [PubMed]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The Neurovascular Unit—Concept Review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef]
- Yu, X.; Ji, C.; Shao, A. Neurovascular Unit Dysfunction and Neurodegenerative Disorders. Front. Neurosci. 2020, 14, 334. [Google Scholar] [CrossRef]
- Alexander, A.; Agrawal, M.; Uddin, A.; Siddique, S.; Shehata, A.M.; Shaker, M.A.; Ata Ur Rahman, S.; Abdul, M.I.M.; Shaker, M.A. Recent Expansions of Novel Strategies towards the Drug Targeting into the Brain. Int. J. Nanomed. 2019, 14, 5895–5909. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Drechsler, M.; Lesieur, S. Neurotrophin Delivery Using Nanotechnology. Drug Discov. Today 2013, 18, 1263–1271. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Darłak, P.; Markiewicz, A.; Sikora, J.; Kumar Adla, S.; Bagina, S.; Huttunen, K.M. Current Approaches to Facilitate Improved Drug Delivery to the Central Nervous System. Eur. J. Pharm. Biopharm. 2022, 181, 249–262. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Li, M. Overcoming the Cellular Barriers and beyond: Recent Progress on Cell Penetrating Peptide Modified Nanomedicine in Combating Physiological and Pathological Barriers. Asian J. Pharm. Sci. 2022, 17, 523–543. [Google Scholar] [CrossRef]
- Gonzalez-Carter, D.; Liu, X.; Tockary, T.A.; Dirisala, A.; Toh, K.; Anraku, Y.; Kataoka, K. Targeting Nanoparticles to the Brain by Exploiting the Blood–Brain Barrier Impermeability to Selectively Label the Brain Endothelium. Proc. Natl. Acad. Sci. USA 2020, 117, 19141–19150. [Google Scholar] [CrossRef]
- Zou, Y.; Sun, X.; Yang, Q.; Zheng, M.; Shimoni, O.; Ruan, W.; Wang, Y.; Zhang, D.; Yin, J.; Huang, X.; et al. Blood-brain barrier-penetrating single CRISPR-Cas9 nanocapsules for effective and safe glioblastoma gene therapy. Sci. Adv. 2022, 8, eabm8011. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting Transferrin Receptors at the Blood-Brain Barrier Improves the Uptake of Immunoliposomes and Subsequent Cargo Transport into the Brain Parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef] [PubMed]
- Desale, K.; Kuche, K.; Jain, S. Cell-Penetrating Peptides (CPPs): An Overview of Applications for Improving the Potential of Nanotherapeutics. Biomater. Sci. 2021, 9, 1153–1188. [Google Scholar] [CrossRef] [PubMed]
- Langel, Ü. Kinetics of CPPs Cellular Uptake. In CPP, Cell-Penetrating Peptides; Springer: Singapore, 2019; pp. 325–337. ISBN 9789811387470. [Google Scholar] [CrossRef]
- Neves-Coelho, S.; Eleutério, R.P.; Enguita, F.J.; Neves, V.; Castanho, M.A.R.B. A New Noncanonical Anionic Peptide That Translocates a Cellular Blood–Brain Barrier Model. Molecules 2017, 22, 1753. [Google Scholar] [CrossRef]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Keighron, C.N.; Avazzadeh, S.; Goljanek-Whysall, K.; McDonagh, B.; Howard, L.; Ritter, T.; Quinlan, L.R. Extracellular Vesicles, Cell-Penetrating Peptides and miRNAs as Future Novel Therapeutic Interventions for Parkinson’s and Alzheimer’s Disease. Biomedicines 2023, 11, 728. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Jafari, S. Cell Penetrating Peptides: A Concise Review with Emphasis on Biomedical Applications. Biomed. Pharmacother. 2018, 108, 1090–1096. [Google Scholar] [CrossRef]
- Duchardt, F.; Fotin-Mleczek, M.; Schwarz, H.; Fischer, R.; Brock, R. A Comprehensive Model for the Cellular Uptake of Cationic Cell-Penetrating Peptides. Traffic 2007, 8, 848–866. [Google Scholar] [CrossRef]
- Futaki, S. Membrane-Permeable Arginine-Rich Peptides and the Translocation Mechanisms. Adv. Drug Deliv. Rev. 2005, 57, 547–558. [Google Scholar] [CrossRef]
- Amin, F.M.; Schytz, H.W. Transport of the Pituitary Adenylate Cyclase-Activating Polypeptide across the Blood-Brain Barrier: Implications for Migraine. J. Headache Pain 2018, 19, 35. [Google Scholar] [CrossRef]
- Arimura, A. Pituitary Adenylate Cyclase Activating Polypeptide (PACAP): Discovery and Current Status of Research. Regul. Pept. 1992, 37, 285–303. [Google Scholar] [CrossRef]
- Deguil, J.; Chavant, F.; Lafay-Chebassier, C.; Pérault-Pochat, M.-C.; Fauconneau, B.; Pain, S. Neuroprotective Effect of PACAP on Translational Control Alteration and Cognitive Decline in MPTP Parkinsonian Mice. Neurotox. Res. 2010, 17, 142–155. [Google Scholar] [CrossRef]
- Lamine, A.; Létourneau, M.; Doan, N.D.; Maucotel, J.; Couvineau, A.; Vaudry, H.; Chatenet, D.; Vaudry, D.; Fournier, A. Characterizations of a Synthetic Pituitary Adenylate Cyclase-Activating Polypeptide Analog Displaying Potent Neuroprotective Activity and Reduced in Vivo Cardiovascular Side Effects in a Parkinson’s Disease Model. Neuropharmacology 2016, 108, 440–450. [Google Scholar] [CrossRef]
- Matsumoto, M.; Nakamachi, T.; Watanabe, J.; Sugiyama, K.; Ohtaki, H.; Murai, N.; Sasaki, S.; Xu, Z.; Hashimoto, H.; Seki, T.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Is Involved in Adult Mouse Hippocampal Neurogenesis After Stroke. J. Mol. Neurosci. 2016, 59, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Tsumuraya, T.; Ohtaki, H.; Dohi, K.; Satoh, K.; Xu, Z.; Tanaka, S.; Murai, N.; Watanabe, J.; Sugiyama, K.; et al. PACAP38 Suppresses Cortical Damage in Mice with Traumatic Brain Injury by Enhancing Antioxidant Activity. J. Mol. Neurosci. 2014, 54, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Solés-Tarrés, I.; Cabezas-Llobet, N.; Vaudry, D.; Xifró, X. Protective Effects of Pituitary Adenylate Cyclase-Activating Polypeptide and Vasoactive Intestinal Peptide Against Cognitive Decline in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 221. [Google Scholar] [CrossRef]
- Figueiredo, C.A.; Düsedau, H.P.; Steffen, J.; Ehrentraut, S.; Dunay, M.P.; Toth, G.; Reglödi, D.; Heimesaat, M.M.; Dunay, I.R. The neuropeptide PACAP alleviates T. gondii infection-induced neuroinflammation and neuronal impairment. J. Neuroinflamm. 2022, 19, 274. [Google Scholar] [CrossRef]
- Rivnyak, A.; Kiss, P.; Tamas, A.; Balogh, D.; Reglodi, D. Review on PACAP-Induced Transcriptomic and Proteomic Changes in Neuronal Development and Repair. Int. J. Mol. Sci. 2018, 19, 1020. [Google Scholar] [CrossRef]
- Ohtaki, H.; Nakamachi, T.; Dohi, K.; Aizawa, Y.; Takaki, A.; Hodoyama, K.; Yofu, S.; Hashimoto, H.; Shintani, N.; Baba, A.; et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc. Natl. Acad. Sci. USA 2006, 103, 7488–7493. [Google Scholar] [CrossRef]
- Nakamachi, T.; Tanigawa, A.; Konno, N.; Shioda, S.; Matsuda, K. Expression Patterns of PACAP and PAC1R Genes and Anorexigenic Action of PACAP1 and PACAP2 in Zebrafish. Front. Endocrinol. 2019, 10, 227. [Google Scholar] [CrossRef]
- Cherait, A.; Maucotel, J.; Lefranc, B.; Leprince, J.; Vaudry, D. Intranasal Administration of PACAP Is an Efficient Delivery Route to Reduce Infarct Volume and Promote Functional Recovery after Transient and Permanent Middle Cerebral Artery Occlusion. Front. Endocrinol. 2021, 11, 585082. [Google Scholar] [CrossRef]
- Prades, R.; Oller-Salvia, B.; Schwarzmaier, S.M.; Selva, J.; Moros, M.; Balbi, M.; Grazú, V.; de La Fuente, J.M.; Egea, G.; Plesnila, N.; et al. Applying the Retro-Enantio Approach To Obtain a Peptide Capable of Overcoming the Blood-Brain Barrier. Angew. Chem. Int. Ed. 2015, 54, 3967–3972. [Google Scholar] [CrossRef]
- Wei, X.; Zhan, C.; Shen, Q.; Fu, W.; Xie, C.; Gao, J.; Peng, C.; Zheng, P.; Lu, W. A D-Peptide Ligand of Nicotine Acetylcholine Receptors for Brain-Targeted Drug Delivery. Angew. Chem. 2015, 127, 3066–3070. [Google Scholar] [CrossRef]
- Drappatz, J.; Brenner, A.; Wong, E.T.; Eichler, A.; Schiff, D.; Groves, M.D.; Mikkelsen, T.; Rosenfeld, S.; Sarantopoulos, J.; Meyers, C.A.; et al. Phase I Study of GRN1005 in Recurrent Malignant Glioma. Clin. Cancer Res. 2013, 19, 1567–1576. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, P.; Ma, Z.; Lu, P.; Kebebe, D.; Liu, Z. Combination of Cell-Penetrating Peptides with Nanomaterials for the Potential Therapeutics of Central Nervous System Disorders: A Review. J. Nanobiotechnol. 2021, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Suga, T.; Umino, M.; Yamayoshi, A.; Mukai, H.; Kawakami, S. Investigation of Enhanced Intracellular Delivery of Nanomaterials Modified with Novel Cell-Penetrating Zwitterionic Peptide-Lipid Derivatives. Drug Deliv. 2023, 30, 2191891. [Google Scholar] [CrossRef]
- Nong, J.; Glassman, P.M.; Myerson, J.W.; Zuluaga-Ramirez, V.; Rodriguez-Garcia, A.; Mukalel, A.; Omo-Lamai, S.; Walsh, L.R.; Zamora, M.E.; Gong, X.; et al. Targeted Nanocarriers Co-Opting Pulmonary Intravascular Leukocytes for Drug Delivery to the Injured Brain. ACS Nano 2023, 17, 13121–13136. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Pawar, A.; Mahadik, K.R.; Gajbhiye, V. PEGylated Nanocarriers: A Promising Tool for Targeted Delivery to the Brain. Colloids Surf. B Biointerfaces 2020, 187, 110770. [Google Scholar] [CrossRef]
- Fukuta, T.; Ishii, T.; Asai, T.; Oku, N. Applications of Liposomal Drug Delivery Systems to Develop Neuroprotective Agents for the Treatment of Ischemic Stroke. Biol. Pharm. Bull. 2019, 42, 319–326. [Google Scholar] [CrossRef]
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A Comprehensive Status Report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef]
- Khare, P.; Edgecomb, S.X.; Hamadani, C.M.; Tanner, E.E.L.; Manickam, D.S. Lipid Nanoparticle-Mediated Drug Delivery to the Brain. Adv. Drug Deliv. Rev. 2023, 197, 114861. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Angelov, B.; Drechsler, M.; Bizien, T.; Gorshkova, Y.E.; Deng, Y. Plasmalogen-Based Liquid Crystalline Multiphase Structures Involving Docosapentaenoyl Derivatives Inspired by Biological Cubic Membranes. Front. Cell Dev. Biol. 2021, 9, 617984. [Google Scholar] [CrossRef] [PubMed]
- Shabani, L.; Abbasi, M.; Azarnew, Z.; Amani, A.M.; Vaez, A. Neuro-nanotechnology: Diagnostic and therapeutic nano-based strategies in applied neuroscience. BioMed. Eng. OnLine 2023, 22, 1. [Google Scholar] [CrossRef]
- Palanki, R.; Bose, S.K.; Dave, A.; White, B.M.; Berkowitz, C.; Luks, V.; Yaqoob, F.; Han, E.; Swingle, K.L.; Menon, P.; et al. Ionizable Lipid Nanoparticles for Therapeutic Base Editing of Congenital Brain Disease. ACS Nano 2023, 17, 13594–13610. [Google Scholar] [CrossRef]
- Pardridge, W.M. Brain Gene Therapy with Trojan Horse Lipid Nanoparticles. Trends Mol. Med. 2023, 29, 343–353. [Google Scholar] [CrossRef]
- Torres, J.; Costa, I.; Peixoto, A.F.; Silva, R.; Sousa Lobo, J.M.; Silva, A.C. Intranasal Lipid Nanoparticles Containing Bioactive Compounds Obtained from Marine Sources to Manage Neurodegenerative Diseases. Pharmaceuticals 2023, 16, 311. [Google Scholar] [CrossRef]
- Akanchise, T.; Angelova, A. Potential of Nano-Antioxidants and Nanomedicine for Recovery from Neurological Disorders Linked to Long COVID Syndrome. Antioxidants 2023, 12, 393. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Hamano, N.; Li, S.-D.; Chougule, M.; Shoyele, S.A.; Gupta, U.; Ajazuddin; et al. Recent Advancements in the Field of Nanotechnology for the Delivery of Anti-Alzheimer Drug in the Brain Region. Expert Opin. Drug Deliv. 2018, 15, 589–617. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarisoa, M.; Angelova, A. Amphiphilic Nanocarrier Systems for Curcumin Delivery in Neurodegenerative Disorders. In The Road from Nanomedicine to Precision Medicine; Jenny Stanford Publishing: Singapore, 2020; p. 39. ISBN 978-0-429-29501-0. [Google Scholar]
- Huang, L.; Hu, J.; Huang, S.; Wang, B.; Siaw-Debrah, F.; Nyanzu, M.; Zhang, Y.; Zhuge, Q. Nanomaterial Applications for Neurological Diseases and Central Nervous System Injury. Prog. Neurobiol. 2017, 157, 29–48. [Google Scholar] [CrossRef]
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Pep-Lipid Cubosomes and Vesicles Compartmentalized by Micelles from Self-Assembly of Multiple Neuroprotective Building Blocks Including a Large Peptide Hormone PACAP-DHA. ChemNanoMat 2019, 5, 1381–1389. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B. Dual and Multi-Drug Delivery Nanoparticles towards Neuronal Survival and Synaptic Repair. Neural Regen. Res. 2017, 12, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Xu, S.; Yang, P.; Wang, P.; Lu, S.; Sheng, D.; Qian, K.; Cao, J.; Lu, W.; Zhang, Q. A Dual-Ligand Fusion Peptide Improves the Brain-Neuron Targeting of Nanocarriers in Alzheimer’s Disease Mice. J. Control. Release Off. J. Control. Release Soc. 2020, 320, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Kanekiyo, T.; Singh, J. Functionalized Nanoparticles for Brain Targeted BDNF Gene Therapy to Rescue Alzheimer’s Disease Pathology in Transgenic Mouse Model. Int. J. Biol. Macromol. 2022, 208, 901–911. [Google Scholar] [CrossRef]
- Silva, S.; Marto, J.; Gonçalves, L.M.; Duarte, D.; Soares, O.S.G.P.; Vasques-Nóvoa, F.; Almeida, A.J.; Vale, N. New Peptide Functionalized Nanostructured Lipid Carriers with CNS Drugs and Evaluation Anti-Proliferative Activity. Int. J. Mol. Sci. 2022, 23, 7109. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Rodrigues, B.; Lakkadwala, S.; Kanekiyo, T.; Singh, J. Dual-Modified Liposome for Targeted and Enhanced Gene Delivery into Mice Brain. J. Pharmacol. Exp. Ther. 2020, 374, 354–365. [Google Scholar] [CrossRef]
- Fressinaud, C.; Thomas, O.; Umerska, A.M.; Saulnier, P. Lipid Nanoparticles Vectorized with NFL-TBS.40-63 Peptide Target Oligodendrocytes and Promote Neurotrophin-3 Effects after Demyelination In Vitro. Neurochem. Res. 2020, 45, 2732–2748. [Google Scholar] [CrossRef]
- Huang, T.; Sato, Y.; Kuramochi, A.; Ohba, Y.; Sano, M.; Miyagishi, M.; Tateno, H.; Wadhwa, R.; Kawasaki, K.; Uchida, T.; et al. Surface Modulation of Extracellular Vesicles with Cell-Penetrating Peptide-Conjugated Lipids for Improvement of Intracellular Delivery to Endothelial Cells. Regen. Ther. 2023, 22, 90–98. [Google Scholar] [CrossRef]
- Yi Kang, J.; Kim, S.; Kim, J.; Kang, N.-G.; Yang, C.-S.; Min, S.-J.; Woong Kim, J. Cell-Penetrating Peptide-Conjugated Lipid/Polymer Hybrid Nanovesicles for Endoplasmic Reticulum-Targeting Intracellular Delivery. J. Mater. Chem. B 2021, 9, 464–470. [Google Scholar] [CrossRef]
- Benchenane, K.; Berezowski, V.; Ali, C.; Fernández-Monreal, M.; López-Atalaya, J.P.; Brillault, J.; Chuquet, J.; Nouvelot, A.; MacKenzie, E.T.; Bu, G.; et al. Tissue-Type Plasminogen Activator Crosses the Intact Blood-Brain Barrier by Low-Density Lipoprotein Receptor–Related Protein-Mediated Transcytosis. Circulation 2005, 111, 2241–2249. [Google Scholar] [CrossRef]
- Molino, Y.; David, M.; Varini, K.; Jabès, F.; Gaudin, N.; Fortoul, A.; Bakloul, K.; Masse, M.; Bernard, A.; Drobecq, L.; et al. Use of LDL Receptor—Targeting Peptide Vectors for in Vitro and in Vivo Cargo Transport across the Blood-Brain Barrier. FASEB J. 2017, 31, 1807–1827. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Moos, T. Revisiting Nanoparticle Technology for Blood–Brain Barrier Transport: Unfolding at the Endothelial Gate Improves the Fate of Transferrin Receptor-Targeted Liposomes. J. Control. Release 2016, 222, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Sarmento, B.; Ferreira, D.C.; Souto, E.B. Lipid-Based Colloidal Carriers for Peptide and Protein Delivery–Liposomes versus Lipid Nanoparticles. Int. J. Nanomed. 2007, 2, 595–607. [Google Scholar]
- Ghorai, S.M.; Deep, A.; Magoo, D.; Gupta, C.; Gupta, N. Cell-Penetrating and Targeted Peptides Delivery Systems as Potential Pharmaceutical Carriers for Enhanced Delivery across the Blood–Brain Barrier (BBB). Pharmaceutics 2023, 15, 1999. [Google Scholar] [CrossRef] [PubMed]
- Parrasia, S.; Szabò, I.; Zoratti, M.; Biasutto, L. Peptides as Pharmacological Carriers to the Brain: Promises, Shortcomings and Challenges. Mol. Pharm. 2022, 19, 3700–3729. [Google Scholar] [CrossRef]
- Diaz, J.; Pietsch, M.; Davila, M.; Jaimes, G.; Hudson, A.; Pellois, J.-P. Elucidating the Impact of Payload Conjugation on the Cell-Penetrating Efficiency of the Endosomal Escape Peptide dfTAT: Implications for Future Designs for CPP-Based Delivery Systems. Bioconjug. Chem. 2023, 34, 1861–1872. [Google Scholar] [CrossRef]
- Feger, G.; Angelov, B.; Angelova, A. Prediction of Amphiphilic Cell-Penetrating Peptide Building Blocks from Protein-Derived Amino Acid Sequences for Engineering of Drug Delivery Nanoassemblies. J. Phys. Chem. B 2020, 124, 4069–4078. [Google Scholar] [CrossRef]
- Sadeghian, I.; Heidari, R.; Sadeghian, S.; Raee, M.J.; Negahdaripour, M. Potential of cell-penetrating peptides (CPPs) in delivery of antiviral therapeutics and vaccines. Eur. J. Pharm. Sci. 2022, 169, 106094. [Google Scholar] [CrossRef]
- Rice, A.; Liu, Y.; Michaelis, M.L.; Himes, R.H.; Georg, G.I.; Audus, K.L. Chemical Modification of Paclitaxel (Taxol) Reduces P-Glycoprotein Interactions and Increases Permeation across the Blood−Brain Barrier in Vitro and in Situ. J. Med. Chem. 2005, 48, 832–838. [Google Scholar] [CrossRef]
- Kroll, R.A.; Neuwelt, E.A. Outwitting the Blood-Brain Barrier for Therapeutic Purposes: Osmotic Opening and Other Means. Neurosurgery 1998, 42, 1083. [Google Scholar] [CrossRef]
- Gernert, M.; Feja, M. Bypassing the Blood–Brain Barrier: Direct Intracranial Drug Delivery in Epilepsies. Pharmaceutics 2020, 12, 1134. [Google Scholar] [CrossRef]
- Hanson, L.R.; Frey, W.H. Intranasal Delivery Bypasses the Blood-Brain Barrier to Target Therapeutic Agents to the Central Nervous System and Treat Neurodegenerative Disease. BMC Neurosci. 2008, 9, S5. [Google Scholar] [CrossRef]
- Pooga, M.; Langel, Ü. Classes of Cell-Penetrating Peptides. In Cell-Penetrating Peptides: Methods and Protocols; Langel, Ü., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; pp. 3–28. ISBN 978-1-4939-2806-4. [Google Scholar]
- Henning-Knechtel, A.; Kumar, S.; Wallin, C.; Król, S.; Wärmländer, S.K.T.S.; Jarvet, J.; Esposito, G.; Kirmizialtin, S.; Gräslund, A.; Hamilton, A.D.; et al. Designed Cell-Penetrating Peptide Inhibitors of Amyloid-Beta Aggregation and Cytotoxicity. Cell Rep. Phys. Sci. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Dietz, G.P.H.; Bähr, M. Synthesis of Cell-Penetrating Peptides and Their Application in Neurobiology. In Neuroprotection Methods and Protocols; Borsello, T., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2007; pp. 181–198. ISBN 978-1-59745-504-6. [Google Scholar]
- Green, M.; Loewenstein, P.M. Autonomous Functional Domains of Chemically Synthesized Human Immunodeficiency Virus Tat Trans-Activator Protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Jobin, M.-L.; Alves, I.D. On the Importance of Electrostatic Interactions between Cell Penetrating Peptides and Membranes: A Pathway toward Tumor Cell Selectivity? Biochimie 2014, 107, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Khan, A.R.; Fu, M.; Wang, R.; Ji, J.; Zhai, G. Cell-Penetrating Peptide: A Means of Breaking through the Physiological Barriers of Different Tissues and Organs. J. Control. Release 2019, 309, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Binder, H.; Lindblom, G. Charge-Dependent Translocation of the Trojan Peptide Penetratin across Lipid Membranes. Biophys. J. 2003, 85, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, M.; Cui, Y.; Huang, C.; Sun, M. Arginine-Rich Membrane-Permeable Peptides Are Seriously Toxic. Pharmacol. Res. Perspect. 2017, 5, e00334. [Google Scholar] [CrossRef]
- Simeoni, F.; Morris, M.C.; Heitz, F.; Divita, G. Insight into the Mechanism of the Peptide-based Gene Delivery System MPG: Implications for Delivery of siRNA into Mammalian Cells. Nucleic Acids Res. 2003, 31, 2717–2724. [Google Scholar] [CrossRef]
- Christiaens, B.; Grooten, J.; Reusens, M.; Joliot, A.; Goethals, M.; Vandekerckhove, J.; Prochiantz, A.; Rosseneu, M. Membrane Interaction and Cellular Internalization of Penetratin Peptides. Eur. J. Biochem. 2004, 271, 1187–1197. [Google Scholar] [CrossRef]
- Di Pisa, M.; Chassaing, G.; Swiecicki, J.-M. Translocation Mechanism(s) of Cell-Penetrating Peptides: Biophysical Studies Using Artificial Membrane Bilayers. Biochemistry 2015, 54, 194–207. [Google Scholar] [CrossRef]
- Kalafatovic, D.; Giralt, E. Cell-Penetrating Peptides: Design Strategies beyond Primary Structure and Amphipathicity. Molecules 2017, 22, 1929. [Google Scholar] [CrossRef]
- Ochocki, J.D.; Mullen, D.G.; Wattenberg, E.V.; Distefano, M.D. Evaluation of a Cell Penetrating Prenylated Peptide Lacking an Intrinsic Fluorophore via in Situ Click Reaction. Bioorg. Med. Chem. Lett. 2011, 21, 4998–5001. [Google Scholar] [CrossRef]
- O’Callaghan, K.; Kuliopulos, A.; Covic, L. Turning Receptors On and Off with Intracellular Pepducins: New Insights into G-Protein-Coupled Receptor Drug Development. J. Biol. Chem. 2012, 287, 12787–12796. [Google Scholar] [CrossRef]
- Walensky, L.D.; Bird, G.H. Hydrocarbon-Stapled Peptides: Principles, Practice, and Progress. J. Med. Chem. 2014, 57, 6275–6288. [Google Scholar] [CrossRef]
- Schafmeister, C.E.; Po, J.; Verdine, G.L. An All-Hydrocarbon Cross-Linking System for Enhancing the Helicity and Metabolic Stability of Peptides. J. Am. Chem. Soc. 2000, 122, 5891–5892. [Google Scholar] [CrossRef]
- de Mello, L.R.; Porosk, L.; Lourenço, T.C.; Garcia, B.B.M.; Costa, C.A.R.; Han, S.W.; de Souza, J.S.; Langel, Ü.; da Silva, E.R. Amyloid-like Self-Assembly of a Hydrophobic Cell-Penetrating Peptide and Its Use as a Carrier for Nucleic Acids. ACS Appl. Bio Mater. 2021, 4, 6404–6416. [Google Scholar] [CrossRef]
- Sadiq, I.Z.; Muhammad, A.; Mada, S.B.; Ibrahim, B.; Umar, U.A. Biotherapeutic Effect of Cell-Penetrating Peptides against Microbial Agents: A Review. Tissue Barriers 2022, 10, 1995285. [Google Scholar] [CrossRef]
- Hango, C.R.; Backlund, C.M.; Davis, H.C.; Posey, N.D.; Minter, L.M.; Tew, G.N. Non-Covalent Carrier Hydrophobicity as a Universal Predictor of Intracellular Protein Activity. Biomacromolecules 2021, 22, 2850–2863. [Google Scholar] [CrossRef]
- Gayraud, F.; Klußmann, M.; Neundorf, I. Recent Advances and Trends in Chemical CPP–Drug Conjugation Techniques. Molecules 2021, 26, 1591. [Google Scholar] [CrossRef]
- Lee, J.-H.; Zhang, A.; You, S.S.; Lieber, C.M. Spontaneous Internalization of Cell Penetrating Peptide-Modified Nanowires into Primary Neurons. Nano Lett. 2016, 16, 1509–1513. [Google Scholar] [CrossRef]
- Wen, X.; Wang, K.; Zhao, Z.; Zhang, Y.; Sun, T.; Zhang, F.; Wu, J.; Fu, Y.; Du, Y.; Zhang, L.; et al. Brain-Targeted Delivery of Trans-Activating Transcriptor-Conjugated Magnetic PLGA/Lipid Nanoparticles. PLoS ONE 2014, 9, e106652. [Google Scholar] [CrossRef]
- Rao, K.S.; Reddy, M.K.; Horning, J.L.; Labhasetwar, V. TAT-Conjugated Nanoparticles for the CNS Delivery of Anti-HIV Drugs. Biomaterials 2008, 29, 4429–4438. [Google Scholar] [CrossRef]
- Rizzuti, M.; Nizzardo, M.; Zanetta, C.; Ramirez, A.; Corti, S. Therapeutic Applications of the Cell-Penetrating HIV-1 Tat Peptide. Drug Discov. Today 2015, 20, 76–85. [Google Scholar] [CrossRef]
- Ballarin, B.; Tymianski, M. Discovery and Development of NA-1 for the Treatment of Acute Ischemic Stroke. Acta Pharmacol. Sin. 2018, 39, 661–668. [Google Scholar] [CrossRef]
- Ying, X.; Wang, Y.; Liang, J.; Yue, J.; Xu, C.; Lu, L.; Xu, Z.; Gao, J.; Du, Y.; Chen, Z. Angiopep-Conjugated Electro-Responsive Hydrogel Nanoparticles: Therapeutic Potential for Epilepsy. Angew. Chem. Int. Ed. 2014, 126, 12644–12648. [Google Scholar] [CrossRef]
- Alves, I.D.; Carré, M.; Montero, M.-P.; Castano, S.; Lecomte, S.; Marquant, R.; Lecorché, P.; Burlina, F.; Schatz, C.; Sagan, S.; et al. A Proapoptotic Peptide Conjugated to Penetratin Selectively Inhibits Tumor Cell Growth. Biochim. Biophys. Acta BBA—Biomembr. 2014, 1838, 2087–2098. [Google Scholar] [CrossRef]
- Ringhieri, P.; Diaferia, C.; Galdiero, S.; Palumbo, R.; Morelli, G.; Accardo, A. Liposomal Doxorubicin Doubly Functionalized with CCK8 and R8 Peptide Sequences for Selective Intracellular Drug Delivery. J. Pept. Sci. 2015, 21, 415–425. [Google Scholar] [CrossRef]
- Chen, C.; Duan, Z.; Yuan, Y.; Li, R.; Pang, L.; Liang, J.; Xu, X.; Wang, J. Peptide-22 and Cyclic RGD Functionalized Liposomes for Glioma Targeting Drug Delivery Overcoming BBB and BBTB. ACS Appl. Mater. Interfaces 2017, 9, 5864–5873. [Google Scholar] [CrossRef]
- Saleh, T.; Bolhassani, A.; Shojaosadati, S.A.; Aghasadeghi, M.R. MPG-Based Nanoparticle: An Efficient Delivery System for Enhancing the Potency of DNA Vaccine Expressing HPV16E7. Vaccine 2015, 33, 3164–3170. [Google Scholar] [CrossRef]
- Rhee, M.; Davis, P. Mechanism of Uptake of C105Y, a Novel Cell-Penetrating Peptide. J. Biol. Chem. 2006, 281, 1233–1240. [Google Scholar] [CrossRef]
- Cherait, A.; Banks, W.A.; Vaudry, D. The Potential of the Nose-to-Brain Delivery of PACAP for the Treatment of Neuronal Disease. Pharmaceutics 2023, 15, 2032. [Google Scholar] [CrossRef]
- Edvinsson, L.; Tajti, J.; Szalárdy, L.; Vécsei, L. PACAP and Its Role in Primary Headaches. J. Headache Pain 2018, 19, 21. [Google Scholar] [CrossRef]
- Arranz-Gibert, P.; Prades, R.; Guixer, B.; Guerrero, S.; Araya, E.; Ciudad, S.; Kogan, M.J.; Giralt, E.; Teixidó, M. HAI Peptide and Backbone Analogs—Validation and Enhancement of Biostability and Bioactivity of BBB Shuttles. Sci. Rep. 2018, 8, 17932. [Google Scholar] [CrossRef]
- Neves, V.; Aires-da-Silva, F.; Morais, M.; Gano, L.; Ribeiro, E.; Pinto, A.; Aguiar, S.; Gaspar, D.; Fernandes, C.; Correia, J.D.G.; et al. Novel Peptides Derived from Dengue Virus Capsid Protein Translocate Reversibly the Blood–Brain Barrier through a Receptor-Free Mechanism. ACS Chem. Biol. 2017, 12, 1257–1268. [Google Scholar] [CrossRef]
- Wang, L.; Hao, Y.; Li, H.; Zhao, Y.; Meng, D.; Li, D.; Shi, J.; Zhang, H.; Zhang, Z.; Zhang, Y. Co-Delivery of Doxorubicin and siRNA for Glioma Therapy by a Brain Targeting System: Angiopep-2-Modified Poly(Lactic-Co-Glycolic Acid) Nanoparticles. J. Drug Target. 2015, 23, 832–846. [Google Scholar] [CrossRef]
- Eiamphungporn, W.; Yainoy, S.; Prachayasittikul, V. Angiopep-2-Mediated Delivery of Human Manganese Superoxide Dismutase in Brain Endothelial Cells and Its Protective Effect Against Oxidative Stress. Int. J. Pept. Res. Ther. 2015, 21, 63–71. [Google Scholar] [CrossRef]
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor Microenvironment Sensitive Doxorubicin Delivery and Release to Glioma Using Angiopep-2 Decorated Gold Nanoparticles. Biomaterials 2015, 37, 425–435. [Google Scholar] [CrossRef]
- Li, X.; Zheng, L.; Xia, Q.; Liu, L.; Mao, M.; Zhou, H.; Zhao, Y.; Shi, J. A Novel Cell-Penetrating Peptide Protects against Neuron Apoptosis after Cerebral Ischemia by Inhibiting the Nuclear Translocation of Annexin A1. Cell Death Differ. 2019, 26, 260–275. [Google Scholar] [CrossRef]
- Oba, M.; Kunitake, M.; Kato, T.; Ueda, A.; Tanaka, M. Enhanced and Prolonged Cell-Penetrating Abilities of Arginine-Rich Peptides by Introducing Cyclic α,α-Disubstituted α-Amino Acids with Stapling. Bioconjug. Chem. 2017, 28, 1801–1806. [Google Scholar] [CrossRef]
- Madani, F.; Lindberg, S.; Langel, Ü.; Futaki, S.; Gräslund, A. Mechanisms of Cellular Uptake of Cell-Penetrating Peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef]
- Zorko, M.; Langel, Ü. Cell-Penetrating Peptides: Mechanism and Kinetics of Cargo Delivery. Adv. Drug Deliv. Rev. 2005, 57, 529–545. [Google Scholar] [CrossRef]
- Deshayes, S.; Gerbal-Chaloin, S.; Morris, M.C.; Aldrian-Herrada, G.; Charnet, P.; Divita, G.; Heitz, F. On the Mechanism of Non-Endosomial Peptide-Mediated Cellular Delivery of Nucleic Acids. Biochim. Biophys. Acta BBA—Biomembr. 2004, 1667, 141–147. [Google Scholar] [CrossRef]
- Vives, E.; Richard, J.; Rispal, C.; Lebleu, B. TAT Peptide Internalization: Seeking the Mechanism of Entry. Curr. Protein Pept. Sci. 2003, 4, 125–132. [Google Scholar] [CrossRef]
- Ludtke, S.; He, K.; Huang, H. Membrane Thinning Caused by Magainin 2. Biochemistry 1995, 34, 16764–16769. [Google Scholar] [CrossRef]
- Pouny, Y.; Rapaport, D.; Mor, A.; Nicolas, P.; Shai, Y. Interaction of Antimicrobial Dermaseptin and Its Fluorescently Labeled Analogs with Phospholipid Membranes. Biochemistry 1992, 31, 12416–12423. [Google Scholar] [CrossRef]
- Derossi, D.; Chassaing, G.; Prochiantz, A. Trojan Peptides: The Penetratin System for Intracellular Delivery. Trends Cell Biol. 1998, 8, 84–87. [Google Scholar] [CrossRef]
- Ruseska, I.; Zimmer, A. Internalization Mechanisms of Cell-Penetrating Peptides. Beilstein J. Nanotechnol. 2020, 11, 101–123. [Google Scholar] [CrossRef]
- Sánchez-Navarro, M.; Teixidó, M.; Giralt, E. Jumping Hurdles: Peptides Able to Overcome Biological Barriers. Acc. Chem. Res. 2017, 50, 1847–1854. [Google Scholar] [CrossRef]
- Reglodi, D.; Kiss, P.; Szabadfi, K.; Atlasz, T.; Gabriel, R.; Horvath, G.; Szakaly, P.; Sandor, B.; Lubics, A.; Laszlo, E.; et al. PACAP Is an Endogenous Protective Factor—Insights from PACAP-Deficient Mice. J. Mol. Neurosci. 2012, 48, 482–492. [Google Scholar] [CrossRef]
- Tompkins, J.D.; Clason, T.A.; Buttolph, T.R.; Girard, B.M.; Linden, A.K.; Hardwick, J.C.; Merriam, L.A.; May, V.; Parsons, R.L. Src Family Kinase Inhibitors Blunt PACAP-Induced PAC1 Receptor Endocytosis, Phosphorylation of ERK, and the Increase in Cardiac Neuron Excitability. Am. J. Physiol.—Cell Physiol. 2018, 314, C233–C241. [Google Scholar] [CrossRef]
- Doan, N.-D.; Chatenet, D.; Létourneau, M.; Vaudry, H.; Vaudry, D.; Fournier, A. Receptor-Independent Cellular Uptake of Pituitary Adenylate Cyclase-Activating Polypeptide. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2012, 1823, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Tashima, T. Intelligent Substance Delivery into Cells Using Cell-Penetrating Peptides. Bioorg. Med. Chem. Lett. 2017, 27, 121–130. [Google Scholar] [CrossRef] [PubMed]
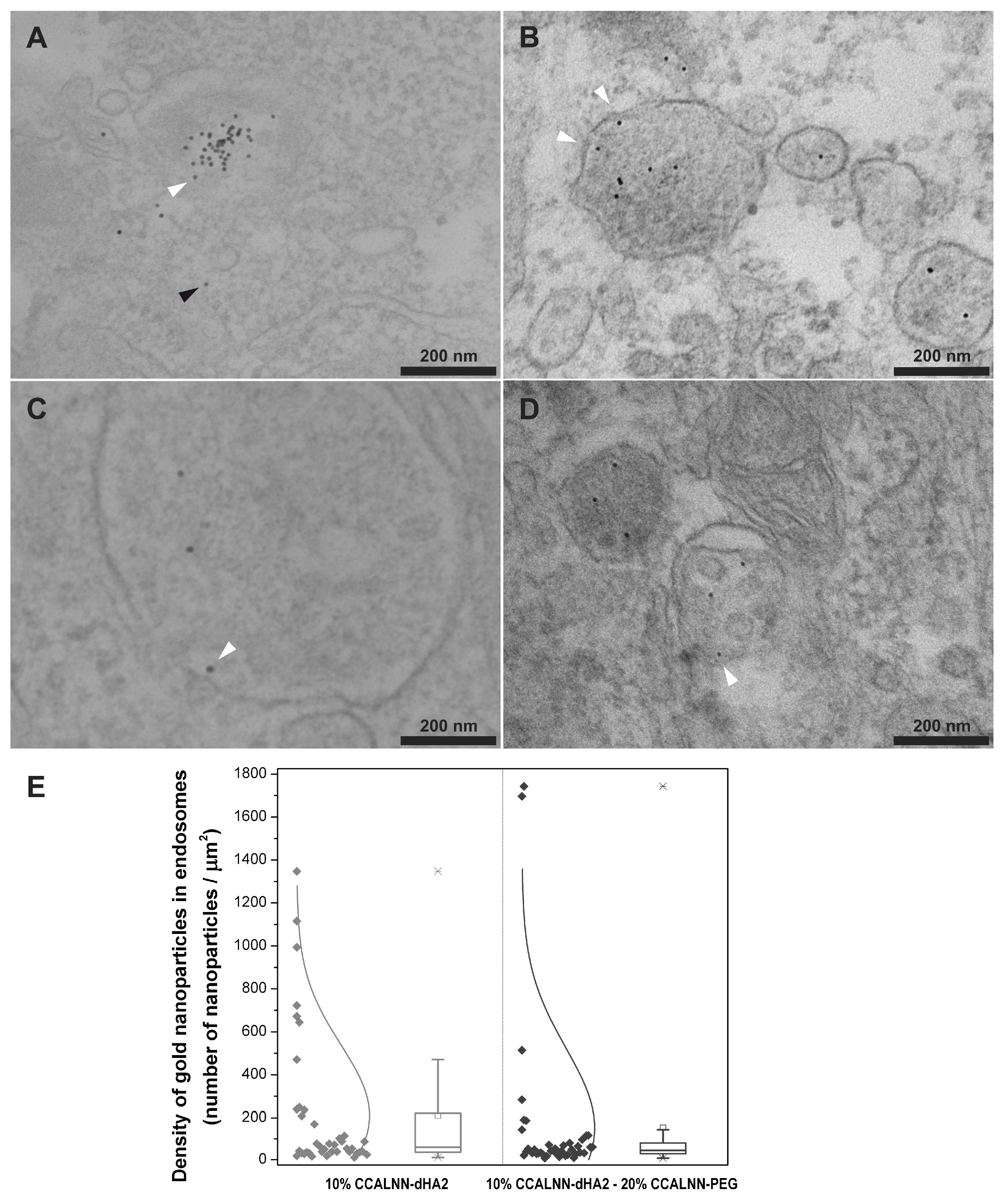
- Cesbron, Y.; Shaheen, U.; Free, P.; Lévy, R. TAT and HA2 Facilitate Cellular Uptake of Gold Nanoparticles but Do Not Lead to Cytosolic Localisation. PLoS ONE 2015, 10, e0121683. [Google Scholar] [CrossRef] [PubMed]
- Masoudi Asil, S.; Ahlawat, J.; Guillama Barroso, G.; Narayan, M. Nanomaterial Based Drug Delivery Systems for the Treatment of Neurodegenerative Diseases. Biomater. Sci. 2020, 8, 4109–4128. [Google Scholar] [CrossRef]
- Liu, R.; Luo, C.; Pang, Z.; Zhang, J.; Ruan, S.; Wu, M.; Wang, L.; Sun, T.; Li, N.; Han, L.; et al. Advances of Nanoparticles as Drug Delivery Systems for Disease Diagnosis and Treatment. Chin. Chem. Lett. 2023, 34, 107518. [Google Scholar] [CrossRef]
- Maity, P.P.; Kapat, K.; Poddar, P.; Bora, H.; Das, C.K.; Das, P.; Ganguly, S.; Das, N.C.; Dhara, D.; Mandal, M.; et al. Capra Cartilage-Derived Peptide Delivery via Carbon Nano-Dots for Cartilage Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1213932. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Design of Magnetic Hydrogels for Hyperthermia and Drug Delivery. Polymers 2021, 13, 4259. [Google Scholar] [CrossRef]
- Dos Santos Rodrigues, B.; Lakkadwala, S.; Kanekiyo, T.; Singh, J. Development and Screening of Brain-Targeted Lipid-Based Nanoparticles with Enhanced Cell Penetration and Gene Delivery Properties. Int. J. Nanomed. 2019, 14, 6497–6517. [Google Scholar] [CrossRef]
- Graverini, G.; Piazzini, V.; Landucci, E.; Pantano, D.; Nardiello, P.; Casamenti, F.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Solid Lipid Nanoparticles for Delivery of Andrographolide across the Blood-Brain Barrier: In Vitro and in Vivo Evaluation. Colloids Surf. B Biointerfaces 2018, 161, 302–313. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the Design of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Targeting Brain Diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured Lipid Carriers: Promising Drug Delivery Systems for Future Clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: A Review Emphasizing on Particle Structure and Drug Release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Karimi, N.; Safaei, M. Application of Various Types of Liposomes in Drug Delivery Systems. Adv. Pharm. Bull. 2017, 7, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Mu, H. Recent Advances in Drug Delivery Applications of Cubosomes, Hexosomes, and Solid Lipid Nanoparticles. Acta Pharm. Sin. B 2021, 11, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as Pharmaceutical Carrier for Dermal and Transdermal Drug Delivery: Formulation Development, Stability Issues, Basic Considerations and Applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, Development and Applications in Drug Delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef]
- Uyama, M.; Handa, T.; Nakano, M. Novel Cubosome System Resistant to Lipid Removal by Serum Albumin. Chem. Pharm. Bull. 2019, 67, 1099–1103. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Angelova, A. Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells. Molecules 2019, 24, 3058. [Google Scholar] [CrossRef]
- Martínez Leo, E.E.; Segura Campos, M.R. Neuroprotective Effect from Salvia Hispanica Peptide Fractions on pro-Inflammatory Modulation of HMC3 Microglial Cells. J. Food Biochem. 2020, 44, e13207. [Google Scholar] [CrossRef]
- Ibáñez, C.F.; Andressoo, J.-O. Biology of GDNF and Its Receptors—Relevance for Disorders of the Central Nervous System. Neurobiol. Dis. 2017, 97, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Dietz, G.P.H.; Valbuena, P.C.; Dietz, B.; Meuer, K.; Müller, P.; Weishaupt, J.H.; Bähr, M. Application of a Blood–Brain-Barrier-Penetrating Form of GDNF in a Mouse Model for Parkinson’s Disease. Brain Res. 2006, 1082, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The Involvement of BDNF, NGF and GDNF in Aging and Alzheimer’s Disease. Aging Dis. 2015, 6, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Tang, J.; Wei, Y.; Sun, Y.; Wang, X.; Wu, L.; Liu, H. Enhanced Bioavailability of Nerve Growth Factor with Phytantriol Lipid-Based Crystalline Nanoparticles in Cochlea. Int. J. Nanomed. 2015, 10, 6879–6889. [Google Scholar] [CrossRef]
- Azhari, H.; Younus, M.; Hook, S.M.; Boyd, B.J.; Rizwan, S.B. Cubosomes Enhance Drug Permeability across the Blood–Brain Barrier in Zebrafish. Int. J. Pharm. 2021, 600, 120411. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Angelov, B.; Deng, Y.; Fujino, T.; Hossain, M.S.; Drechsler, M.; Angelova, A. Sustained CREB Phosphorylation by Lipid-Peptide Liquid Crystalline Nanoassemblies. Commun. Chem. 2023, 6, 241. [Google Scholar] [CrossRef]
- Mwema, A.; Bottemanne, P.; Paquot, A.; Ucakar, B.; Vanvarenberg, K.; Alhouayek, M.; Muccioli, G.G.; des Rieux, A. Lipid Nanocapsules for the Nose-to-Brain Delivery of the Anti-Inflammatory Bioactive Lipid PGD2-G. Nanomed. Nanotechnol. Biol. Med. 2023, 48, 102633. [Google Scholar] [CrossRef]
- Zhu, S.-P.; Wang, Z.-G.; Zhao, Y.-Z.; Wu, J.; Shi, H.-X.; Ye, L.-B.; Wu, F.-Z.; Cheng, Y.; Zhang, H.-Y.; He, S.; et al. Gelatin Nanostructured Lipid Carriers Incorporating Nerve Growth Factor Inhibit Endoplasmic Reticulum Stress-Induced Apoptosis and Improve Recovery in Spinal Cord Injury. Mol. Neurobiol. 2016, 53, 4375–4386. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the Blood-Brain Barrier with Nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Mikitsh, J.L.; Chacko, A.-M. Pathways for Small Molecule Delivery to the Central Nervous System across the Blood-Brain Barrier. Perspect. Med. Chem. 2014, 6, 11–24. [Google Scholar] [CrossRef]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming Blood–Brain Barrier Transport: Advances in Nanoparticle-Based Drug Delivery Strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sui, B.; Sun, J. Size- and Shape-Dependent Effects of Titanium Dioxide Nanoparticles on the Permeabilization of the Blood–Brain Barrier. J. Mater. Chem. B 2017, 5, 9558–9570. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-Mediated Brain Drug Delivery: Overcoming Blood–Brain Barrier to Treat Neurodegenerative Diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, P.; Shalviri, A.; Henderson, J.T.; He, C.; Foltz, W.D.; Prasad, P.; Brodersen, P.M.; Chen, Y.; DaCosta, R.; et al. A Multifunctional Polymeric Nanotheranostic System Delivers Doxorubicin and Imaging Agents across the Blood–Brain Barrier Targeting Brain Metastases of Breast Cancer. ACS Nano 2014, 8, 9925–9940. [Google Scholar] [CrossRef]
- Householder, K.T.; Dharmaraj, S.; Sandberg, D.I.; Wechsler-Reya, R.J.; Sirianni, R.W. Fate of Nanoparticles in the Central Nervous System after Intrathecal Injection in Healthy Mice. Sci. Rep. 2019, 9, 12587. [Google Scholar] [CrossRef]
- Yemisci, M.; Caban, S.; Gursoy-Ozdemir, Y.; Lule, S.; Novoa-Carballal, R.; Riguera, R.; Fernandez-Megia, E.; Andrieux, K.; Couvreur, P.; Capan, Y.; et al. Systemically Administered Brain-Targeted Nanoparticles Transport Peptides across the Blood—Brain Barrier and Provide Neuroprotection. J. Cereb. Blood Flow Metab. 2015, 35, 469–475. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Drechsler, M.; Nicolas, V.; Bizien, T.; Gorshkova, Y.E.; Deng, Y.; Angelova, A. Liquid Crystalline Lipid Nanoparticles for Combined Delivery of Curcumin, Fish Oil and BDNF: In Vitro Neuroprotective Potential in a Cellular Model of Tunicamycin-Induced Endoplasmic Reticulum Stress. Smart Mater. Med. 2022, 3, 274–288. [Google Scholar] [CrossRef]
- Dudhipala, N.; Gorre, T. Neuroprotective Effect of Ropinirole Lipid Nanoparticles Enriched Hydrogel for Parkinson’s Disease: In Vitro, Ex Vivo, Pharmacokinetic and Pharmacodynamic Evaluation. Pharmaceutics 2020, 12, 448. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Granja, A.; Loureiro, J.A.; Pereira, M.C.; Pinheiro, M.; Neves, A.R.; Reis, S. Quercetin Lipid Nanoparticles Functionalized with Transferrin for Alzheimer’s Disease. Eur. J. Pharm. Sci. 2020, 148, 105314. [Google Scholar] [CrossRef]
- Blasi, P.; Giovagnoli, S.; Schoubben, A.; Ricci, M.; Rossi, C. Solid Lipid Nanoparticles for Targeted Brain Drug Delivery. Adv. Drug Deliv. Rev. 2007, 59, 454–477. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Dai, Y.; Zhao, Y.; Qi, S.; Liu, L.; Lu, L.; Luo, Q.; Zhang, Z. Melittin-Lipid Nanoparticles Target to Lymph Nodes and Elicit a Systemic Anti-Tumor Immune Response. Nat. Commun. 2020, 11, 1110. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; May, J.P.; Li, S.-D. Immune Responses of Therapeutic Lipid Nanoparticles. Nanotechnol. Rev. 2013, 2, 201–213. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Manecka, D.-L.; Boukhzar, L.; Falluel-Morel, A.; Lihrmann, I.; Anouar, Y. PACAP Signaling in Neuroprotection. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Current Topics in Neurotoxicity; Springer International Publishing: Cham, Switzerland, 2016; pp. 549–561. ISBN 978-3-319-35135-3. [Google Scholar]
- Han, P.; Caselli, R.J.; Baxter, L.; Serrano, G.; Yin, J.; Beach, T.G.; Reiman, E.M.; Shi, J. Association of Pituitary Adenylate Cyclase–Activating Polypeptide With Cognitive Decline in Mild Cognitive Impairment Due to Alzheimer Disease. JAMA Neurol. 2015, 72, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Rat, D.; Schmitt, U.; Tippmann, F.; Dewachter, I.; Theunis, C.; Wieczerzak, E.; Postina, R.; van Leuven, F.; Fahrenholz, F.; Kojro, E. Neuropeptide Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Slows down Alzheimer’s Disease-like Pathology in Amyloid Precursor Protein-Transgenic Mice. FASEB J. 2011, 25, 3208–3218. [Google Scholar] [CrossRef]
- Chen, Y.; Samal, B.; Hamelink, C.R.; Xiang, C.C.; Chen, Y.; Chen, M.; Vaudry, D.; Brownstein, M.J.; Hallenbeck, J.M.; Eiden, L.E. Neuroprotection by Endogenous and Exogenous PACAP Following Stroke. Regul. Pept. 2006, 137, 4–19. [Google Scholar] [CrossRef]
- Watanabe, T.; Masuo, Y.; Matsumoto, H.; Suzuki, N.; Ohtaki, T.; Masuda, Y.; Kitada, C.; Tsuda, M.; Fujino, M. Pituitary Adenylate Cyclase Activating Polypeptide Provokes Cultured Rat Chromaffin Cells to Secrete Adrenaline. Biochem. Biophys. Res. Commun. 1992, 182, 403–411. [Google Scholar] [CrossRef]
- Jungling, A.; Reglodi, D.; Maasz, G.; Zrinyi, Z.; Schmidt, J.; Rivnyak, A.; Horvath, G.; Pirger, Z.; Tamas, A. Alterations of Nigral Dopamine Levels in Parkinson’s Disease after Environmental Enrichment and PACAP Treatment in Aging Rats. Life 2021, 11, 35. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, G.; Zhao, T.; Wang, S.; Sun, B.; Zheng, L.; Zhao, M. The Memory Improving Effects of Round Scad (Decapterus maruadsi) Hydrolysates on Sleep Deprivation-Induced Memory Deficits in Rats via Antioxidant and Neurotrophic Pathways. Food Funct. 2019, 10, 7733–7744. [Google Scholar] [CrossRef]
- Findeis, M.A. Peptide Inhibitors of Beta Amyloid Aggregation. Curr. Top. Med. Chem. 2002, 2, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.H.; Ahmad, K.; Rabbani, G.; Choi, I. Use of Peptides for the Management of Alzheimer’s Disease: Diagnosis and Inhibition. Front. Aging Neurosci. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Keum, T.; Noh, G.; Seo, J.-E.; Bashyal, S.; Sohn, D.H.; Lee, S. Examination of Effective Buccal Absorption of Salmon Calcitonin Using Cell-Penetrating Peptide-Conjugated Liposomal Drug Delivery System. Int. J. Nanomed. 2022, 17, 697–710. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wang, N.; Hasan, M.M.; Pang, H.-B. Co-Administration of Transportan Peptide Enhances the Cellular Entry of Liposomes in the Bystander Manner Both In Vitro and In Vivo. Mol. Pharm. 2022, 19, 4123–4134. [Google Scholar] [CrossRef]
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, T.; Ishii, T.; Asai, T.; Nakamura, G.; Takeuchi, Y.; Sato, A.; Agato, Y.; Shimizu, K.; Akai, S.; Fukumoto, D.; et al. Real-Time Trafficking of PEGylated Liposomes in the Rodent Focal Brain Ischemia Analyzed by Positron Emission Tomography. Artif. Organs 2014, 38, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Deng, Y.; Akbar, D.; Da, K.; Létourneau, M.; Morshead, C.M.; Chatenet, D.; Shoichet, M.S. Tunable Surface Charge Enables the Electrostatic Adsorption-Controlled Release of Neuroprotective Peptides from a Hydrogel–Nanoparticle Drug Delivery System. ACS Appl. Mater. Interfaces 2023, 15, 91–105. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Barichello De Quevedo, C.E.; Petronilho, F. Microbiota-Gut-Brain Axis in the Alzheimer’s Disease Pathology—An Overview. Neurosci. Res. 2022, 181, 17–21. [Google Scholar] [CrossRef]
- Scheltens, P.; Strooper, B.D.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; Flier, W.M. van der Alzheimer’s Disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Association, A. 2019 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Yamada, A.; Sakurai, T.; Ochi, D.; Mitsuyama, E.; Yamauchi, K.; Abe, F. Novel Angiotensin I-Converting Enzyme Inhibitory Peptide Derived from Bovine Casein. Food Chem. 2013, 141, 3781–3789. [Google Scholar] [CrossRef] [PubMed]
- Min, L.-J.; Kobayashi, Y.; Mogi, M.; Tsukuda, K.; Yamada, A.; Yamauchi, K.; Abe, F.; Iwanami, J.; Xiao, J.-Z.; Horiuchi, M. Administration of Bovine Casein-Derived Peptide Prevents Cognitive Decline in Alzheimer Disease Model Mice. PLoS ONE 2017, 12, e0171515. [Google Scholar] [CrossRef] [PubMed]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Almeida, A.J.; Vale, N. Combination of Cell-Penetrating Peptides with Nanoparticles for Therapeutic Application: A Review. Biomolecules 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Ahlschwede, K.M.; Curran, G.L.; Rosenberg, J.T.; Grant, S.C.; Sarkar, G.; Jenkins, R.B.; Ramakrishnan, S.; Poduslo, J.F.; Kandimalla, K.K. Cationic Carrier Peptide Enhances Cerebrovascular Targeting of Nanoparticles in Alzheimer’s Disease Brain. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 258–266. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, Z.; Shen, L.; Liu, X.; Lin, H. A Dual Targeting Drug Delivery System for Penetrating Blood-Brain Barrier and Selectively Delivering siRNA to Neurons for Alzheimer’s Disease Treatment. Curr. Pharm. Biotechnol. 2017, 18, 1124–1131. [Google Scholar] [CrossRef]
- Akel, H.; Ismail, R.; Csóka, I. Progress and Perspectives of Brain-Targeting Lipid-Based Nanosystems via the Nasal Route in Alzheimer’s Disease. Eur. J. Pharm. Biopharm. 2020, 148, 38–53. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Direct Nose to Brain Drug Delivery via Integrated Nerve Pathways Bypassing the Blood–Brain Barrier: An Excellent Platform for Brain Targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef]
- Borrajo, M.L.; Alonso, M.J. Using Nanotechnology to Deliver Biomolecules from Nose to Brain—Peptides, Proteins, Monoclonal Antibodies and RNA. Drug Deliv. Transl. Res. 2022, 12, 862–880. [Google Scholar] [CrossRef]
- Boche, M.; Pokharkar, V. Quetiapine Nanoemulsion for Intranasal Drug Delivery: Evaluation of Brain-Targeting Efficiency. AAPS PharmSciTech 2017, 18, 686–696. [Google Scholar] [CrossRef]
- Brown, V.; Liu, F. Intranasal Delivery of a Peptide with Antidepressant-Like Effect. Neuropsychopharmacology 2014, 39, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.P.; Smyth, H.D.C.; Cui, Z. Strategies to Facilitate or Block Nose-to-Brain Drug Delivery. Int. J. Pharm. 2019, 570, 118635. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Kathuria, H.; Pandey, M.M. Intranasal Nanotherapeutics for Brain Targeting and Clinical Studies in Parkinson’s Disease. J. Control. Release 2023, 358, 293–318. [Google Scholar] [CrossRef] [PubMed]

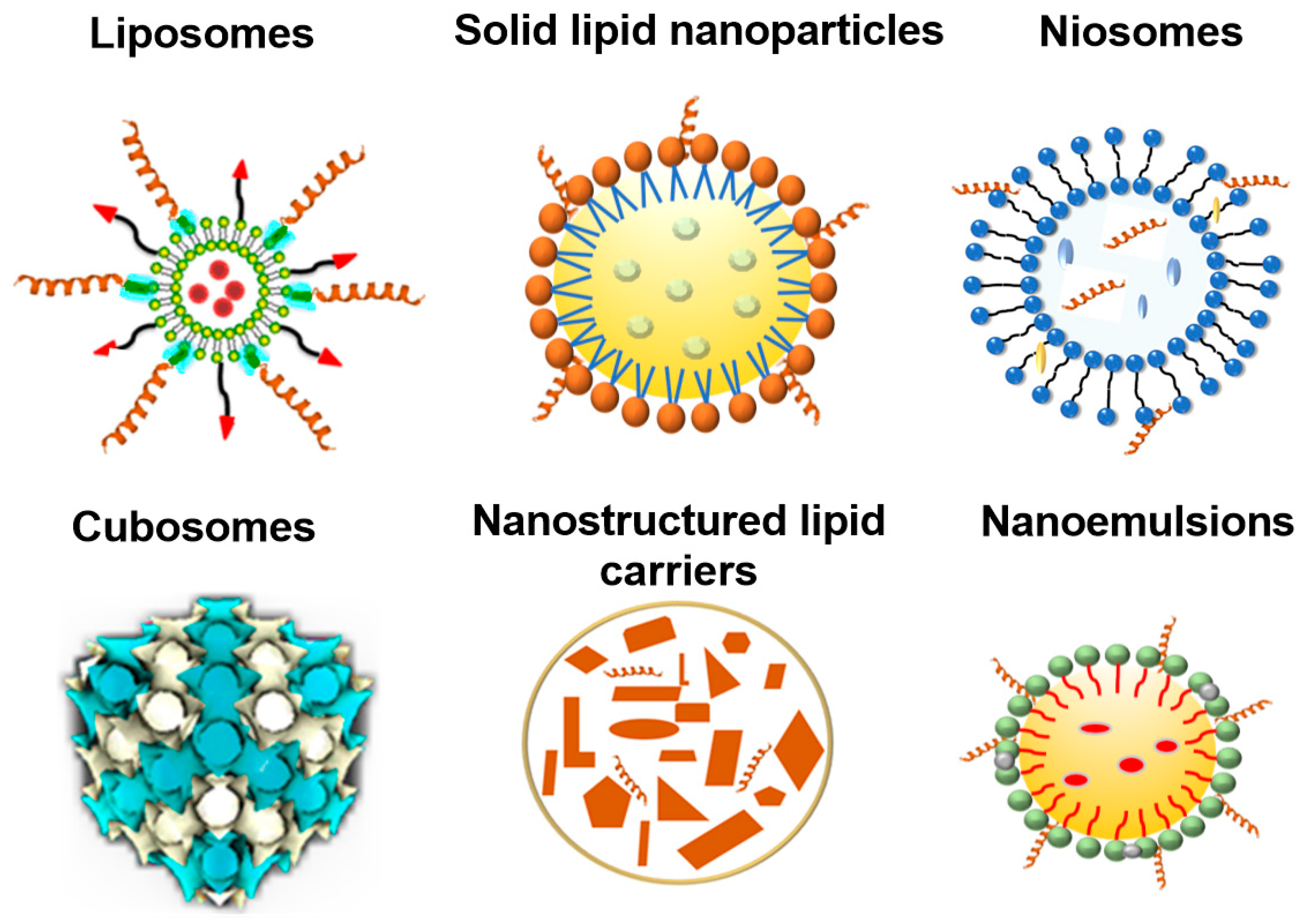



| Lipid-Based System | Active Compound | Delivery Approach | Outcome | Ref. |
|---|---|---|---|---|
| Lipid liquid crystalline nanoparticles (Cubosomes) | Nerve growth factor (NGF) | Round window membrane administration (Guinea pigs) | Lipid cubosomes with encapsulated NGF enabled the overcoming of the barrier of the round window membrane (RWM) and enhanced the bioavailability of the NGF protein in the inner ear, with a promising potential for treating sensorineural hearing loss. | [158] |
| Lipid liquid crystalline nanoparticles (Cubosomes) | Model drug lissamine rhodamine (RhoB), a P-gp substrate and a molecule with poor BBB permeability | In vivo microinjection (Zebrafish larvae) | Cubosomes coated with Tween 80, Pluronic F127, or Pluronic F68 surfactants enabled the BBB targeting of the nanocarriers and enhanced the in vivo uptake of RhoB in Zebrafish. | [159] |
| Lipid liquid crystalline nanoparticles (Hexosomes) | Plasmalogen | In vitro (neuronal cell culture) | Lipid cubosomes and hexosomes, encapsulating plasmalogen, can significantly prolong the CREB activation up to 24 h. | [160] |
| TfR-targeting liposomes functionalized with different CPPs (TAT, pVec, QL) | Plasmid DNA | In vitro BBB model and intravenous administration (mice) | Biodistribution analysis revealed the enhanced targeting delivery of TAT-Tf liposomes in the brains of mice, with significantly increased fluorescent intensity. | [142] |
| TAT Lipid nanocapsules | D2-Glycerol ester (PGD2-G) | Intranasal administration (mice) | TAT-lipid nanocapsules were able to cross the olfactory monolayer and reach the CNS after nasal administration. TAT increased the portion of lipid-nanocapsules that reached the brain. | [161] |
| Gelatin nanostructured lipid carriers | Nerve growth factor (NGF) | Intravenous administration (rat) | NGF-gelatin nanostructured lipid carriers enhanced neuronal survival and contributed to improved functional recovery in a rat model of acute spinal cord injury. | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Angelova, A. Recent Uses of Lipid Nanoparticles, Cell-Penetrating and Bioactive Peptides for the Development of Brain-Targeted Nanomedicines against Neurodegenerative Disorders. Nanomaterials 2023, 13, 3004. https://doi.org/10.3390/nano13233004
Wu Y, Angelova A. Recent Uses of Lipid Nanoparticles, Cell-Penetrating and Bioactive Peptides for the Development of Brain-Targeted Nanomedicines against Neurodegenerative Disorders. Nanomaterials. 2023; 13(23):3004. https://doi.org/10.3390/nano13233004
Chicago/Turabian StyleWu, Yu, and Angelina Angelova. 2023. "Recent Uses of Lipid Nanoparticles, Cell-Penetrating and Bioactive Peptides for the Development of Brain-Targeted Nanomedicines against Neurodegenerative Disorders" Nanomaterials 13, no. 23: 3004. https://doi.org/10.3390/nano13233004