Growth and Characterization of Carbon Nanofibers Grown on Vertically Aligned InAs Nanowires via Chemical Vapour Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth of the Indium Arsenide Nanowire Arrays
2.2. Chemical Vapor Deposition of the Carbon Nanofibers
2.3. Surface Characterization
3. Results and Discussion
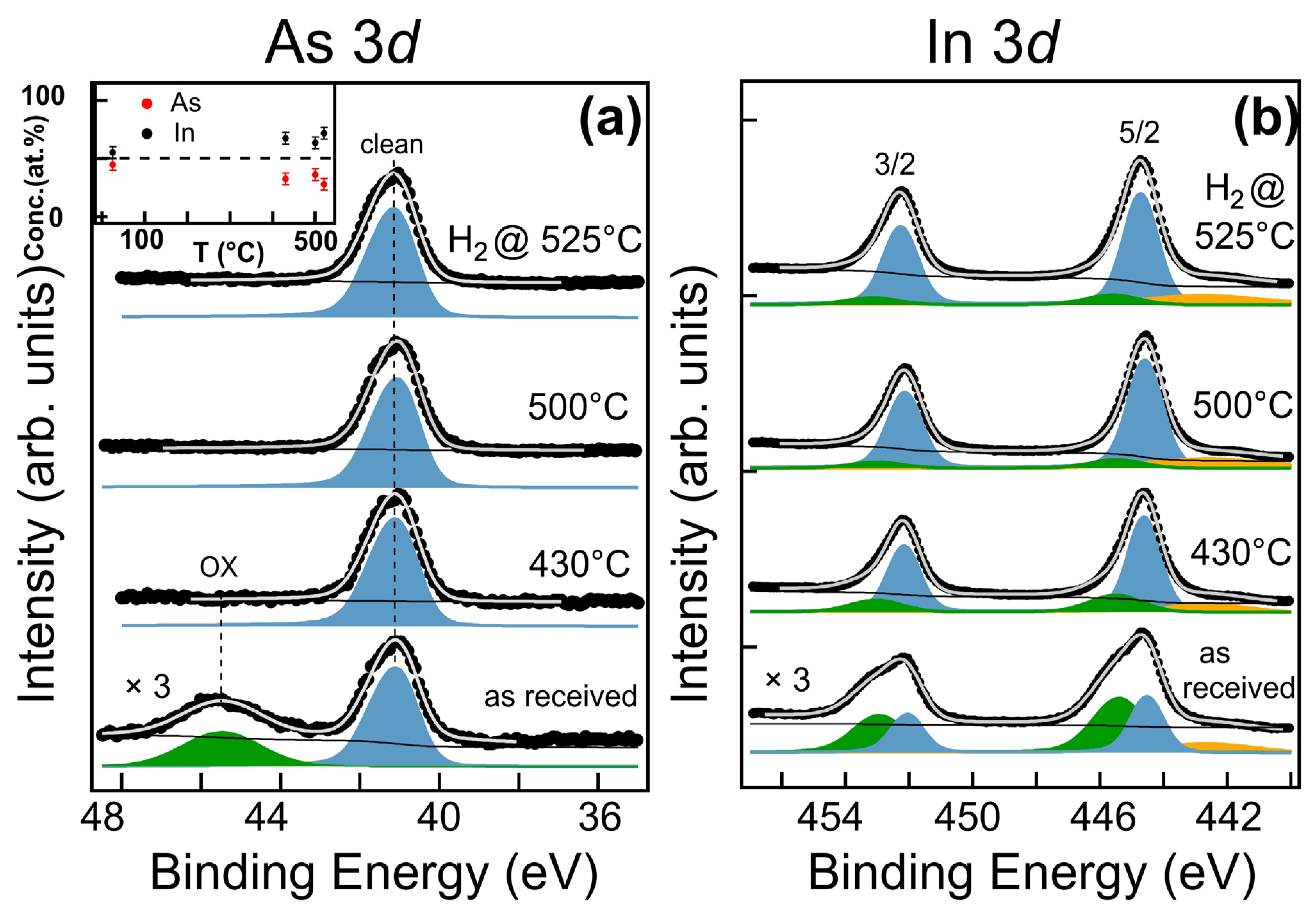
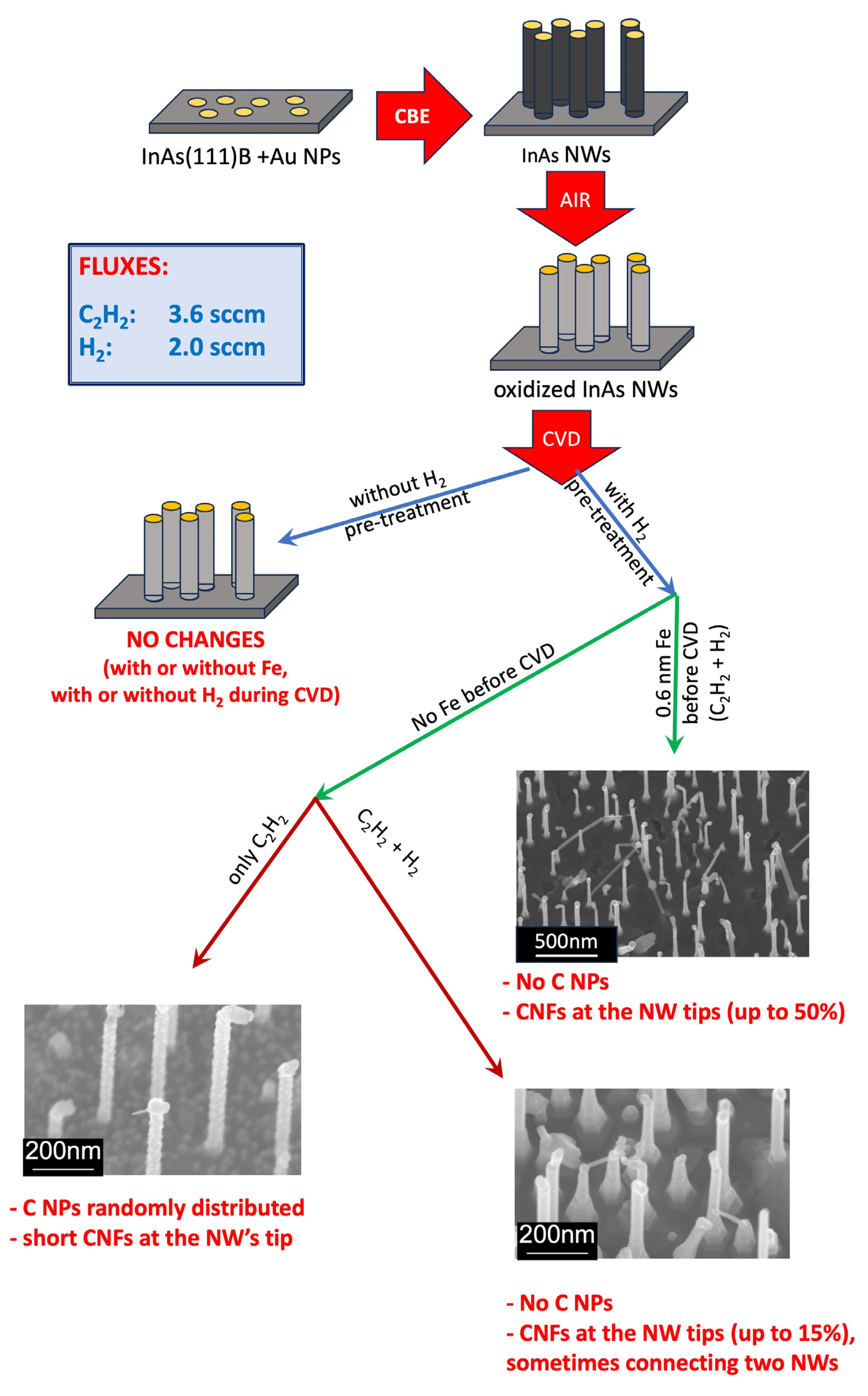
3.1. Substrate Pre-Treatment
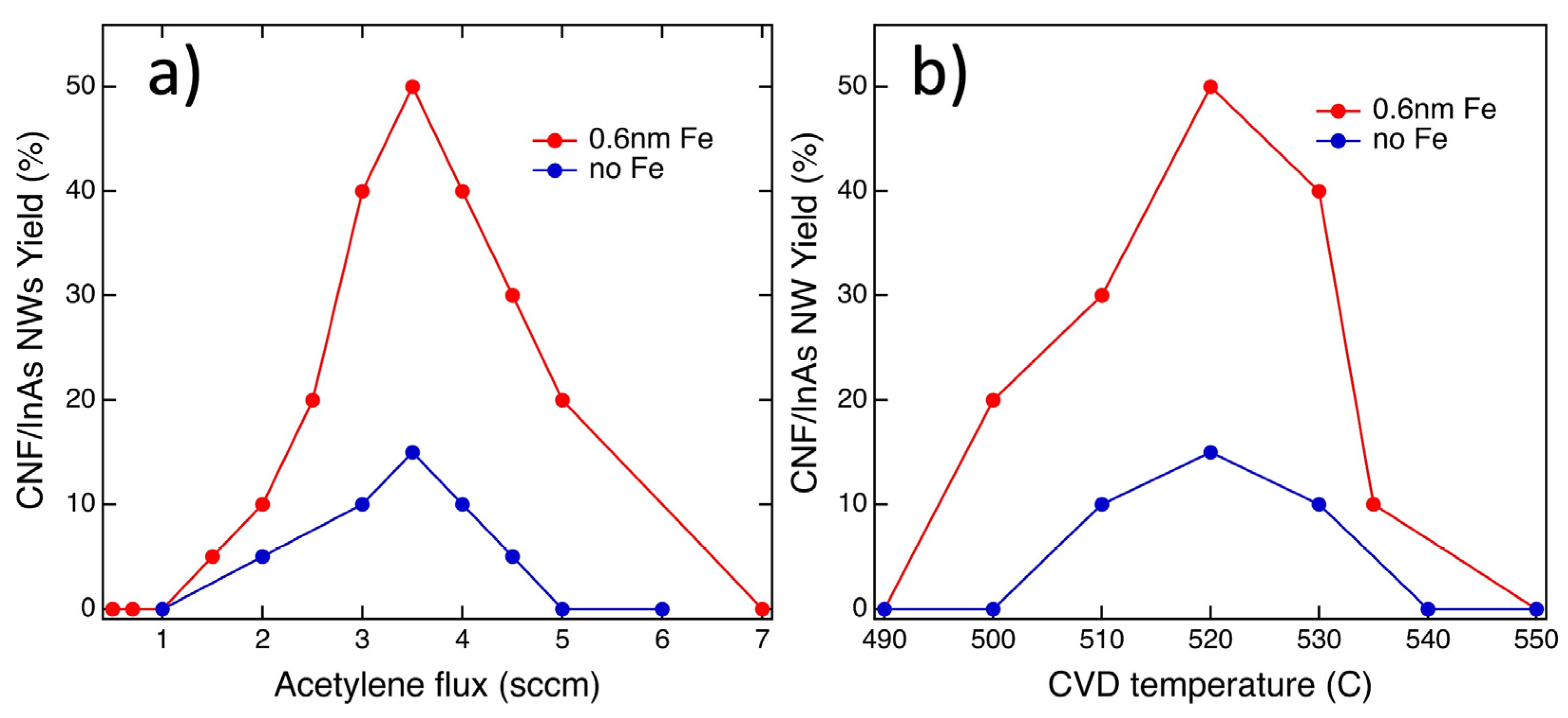
3.2. CVD Synthesis
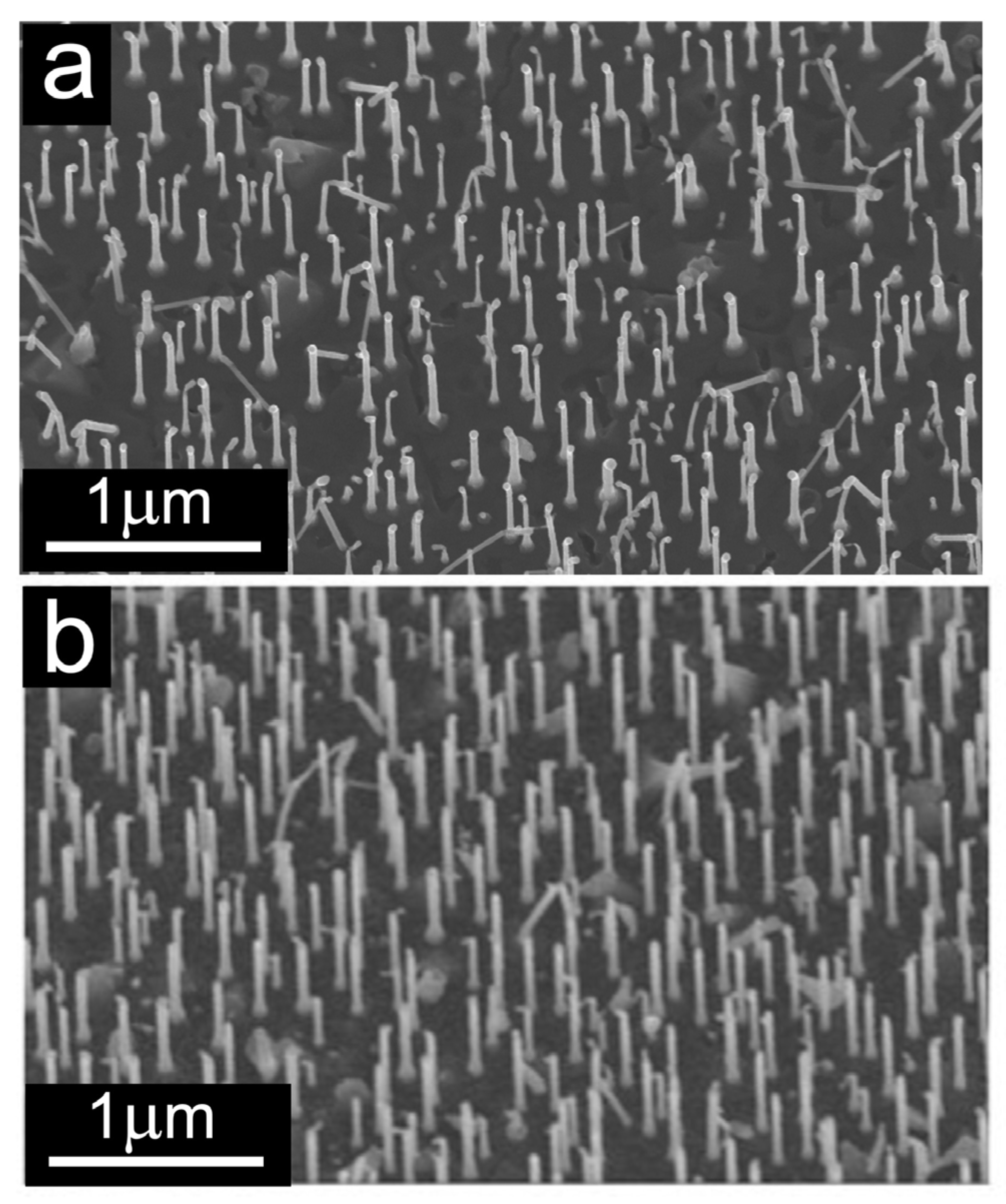
3.2.1. Scanning Electron Microscopy Results
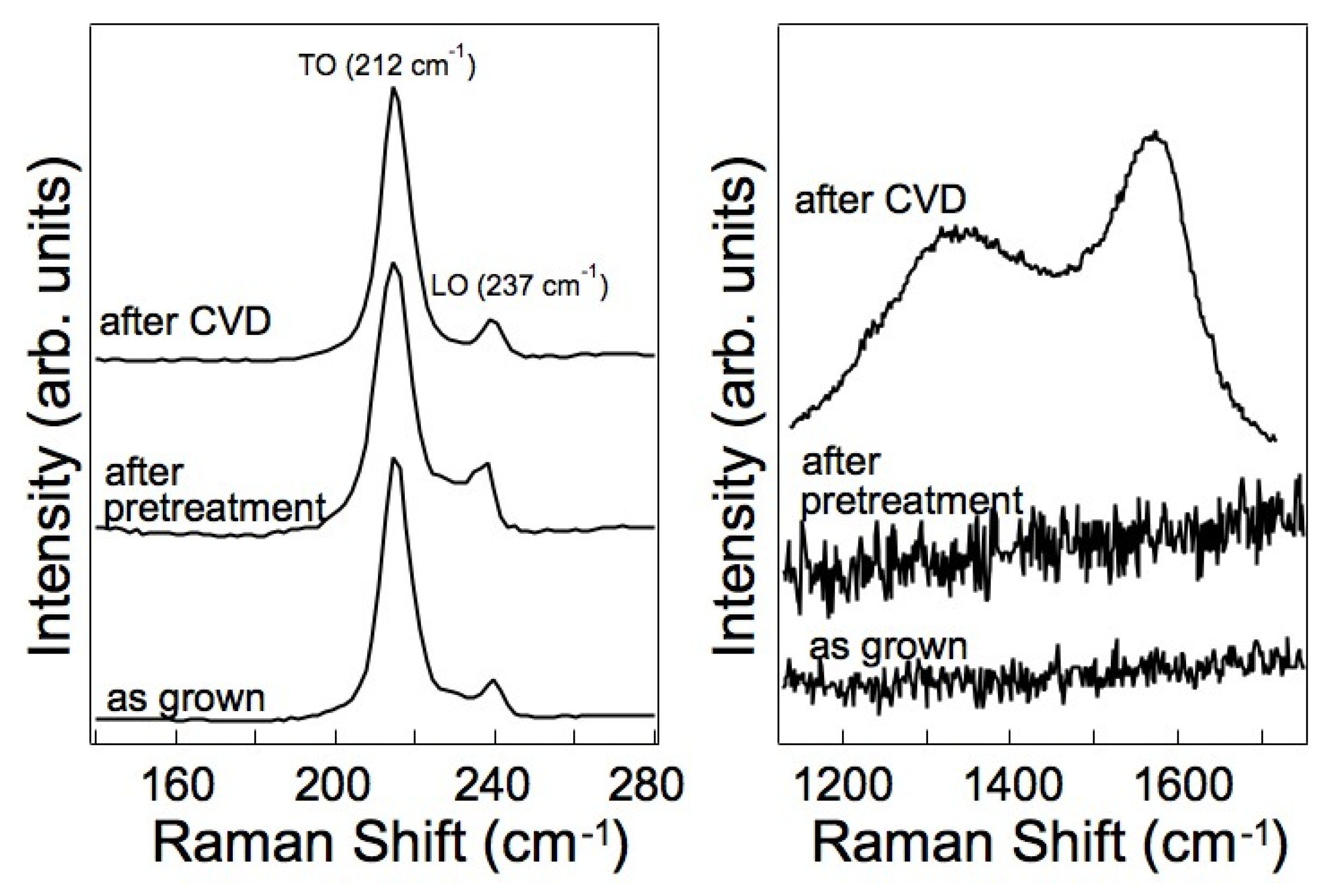
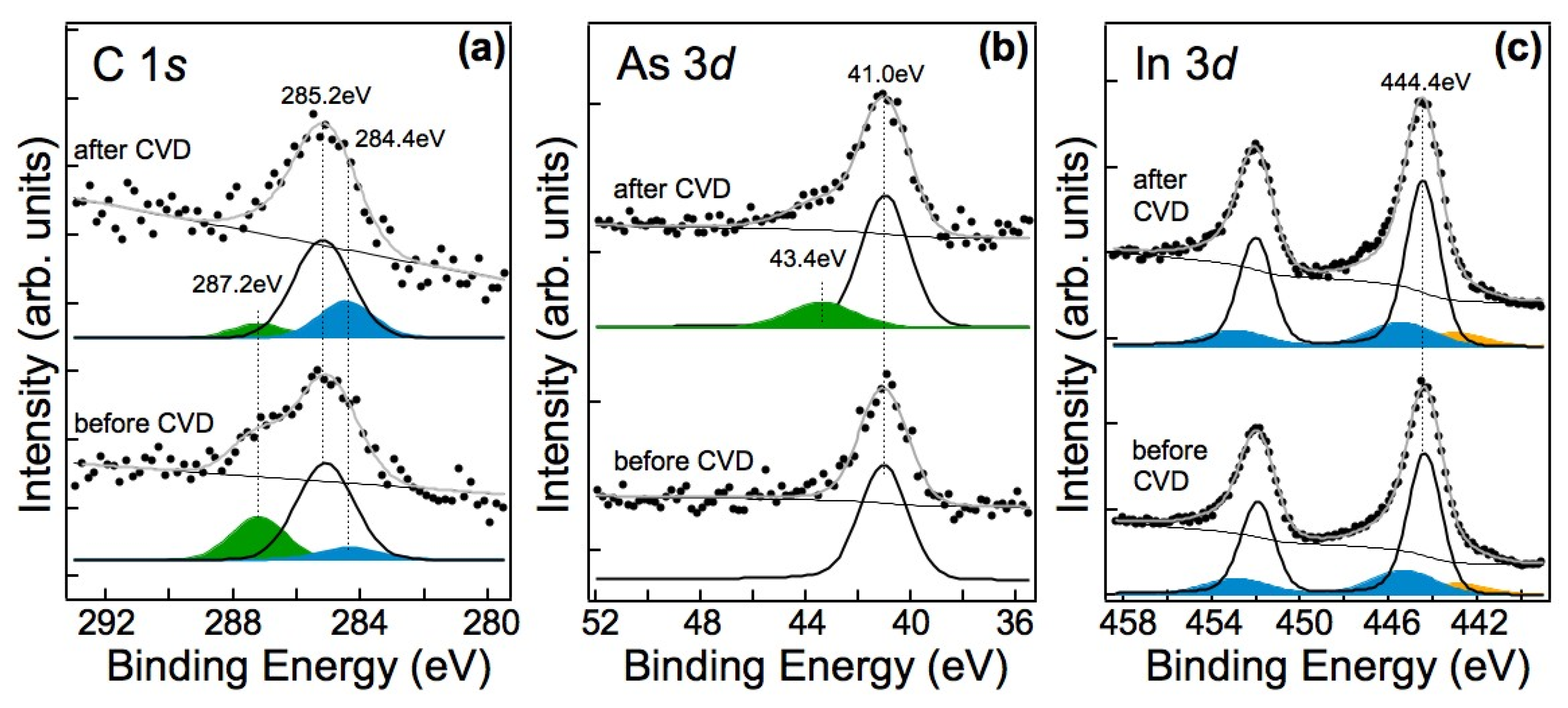
3.2.2. Spectroscopic Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naeemi, A.; Meindl, J.D. Carbon Nanotube Interconnects. Annu. Rev. Mater. Res. 2009, 39, 255–275. [Google Scholar] [CrossRef]
- Zhao, W.-S.; Fu, K.; Wang, D.-W.; Li, M.; Wang, G.; Yin, W.-Y. Mini-Review: Modeling and Performance Analysis of Nanocarbon Interconnects. Appl. Sci. 2019, 9, 2174. [Google Scholar] [CrossRef]
- Ko, H.; Zhang, Z.; Ho, J.C.; Takei, K.; Kapadia, R.; Chueh, Y.L.; Cao, W.; Cruden, B.A.; Javey, A.A. Flexible Carbon-Nanofiber Connectors with Anisotropic Adhesion Properties. Small 2010, 6, 22–26. [Google Scholar] [CrossRef]
- Meng, G.; Han, F.; Zhao, X.; Chen, B.; Yang, D.; Liu, J.; Xu, Q.; Kong, M.; Zhu, X.; Jung, Y.J.; et al. A General Synthetic Approach to Interconnected Nanowire/Nanotube and Nanotube/Nanowire/Nanotube Heterojunctions with Branched Topology. Angew. Chem. Int. Ed. 2009, 48, 7166–7170. [Google Scholar] [CrossRef]
- Liang, H.W.; Liu, S.; Yu, S.H. Controlled Synthesis of One-Dimensional Inorganic Nanostructures Using Pre-Existing One-Dimensional Nanostructures as Templates. Adv. Mater. 2010, 22, 3925–3937. [Google Scholar] [CrossRef]
- Dai, H. Carbon Nanotubes: Synthesis, Integration, and Properties. Acc. Chem. Res. 2002, 35, 1035–1044. [Google Scholar] [CrossRef]
- Vajtai, R.; Wei, B.; Ajayan, P. Controlled growth of carbon nanotubes. Philos. Trans. R. Soc. A 2004, 362, 2143–2160. [Google Scholar] [CrossRef]
- Gomes, U.P.; Ercolani, D.; Zannier, V.; Beltram, F.; Sorba, L. Controlling the Diameter Distribution and Density of InAs Nanowires Grown by Au-assisted Methods. Semicond. Sci. Technol. 2015, 30, 115012. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. Auger and X-ray Photoelectron Spectroscopy, Practical Surface Analysis 2nd ed; John Wiley & Sons Ltd.: New York, NY, USA, 1990; Volume 1. [Google Scholar]
- Milnes, A.G.; Polyakov, A.Y. Indium Arsenide: A Semiconductor for High Speed and Electro-optical Devices. Mater. Sci. Eng. B 1993, 18, 237–259. [Google Scholar] [CrossRef]
- Mönch, W. Semiconductor Surfaces and Interfaces; Springer Series in Surface Sciences; Springer Science &. Business Media: Berlin, Heidelberg, Germany, 2013; Volume 26. [Google Scholar] [CrossRef]
- Teodorescu, C.; Chevrier, F.; Brochier, R.; Richter, C.; Lakovac, V.; Heckmann, O.; De Padova, P.; Hricovini, K. Reactivity and Magnetism of Fe/InAs(100) Interfaces. Eur. Phys. J. B 2002, 28, 305–313. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of Electron Inelastic Mean Free Paths. IX. Data for 41 Elemental Solids over the 50 eV to 30 keV ange. Surf. Interface Anal. 2011, 43, 689–713. [Google Scholar] [CrossRef]
- Hertenberger, S.; Rudolph, D.; Becker, J.; Bichler, M.; Finley, J.J.; Abstreiter, G.; Koblmüller, G. Rate-limiting Mechanisms in High-temperature Growth of Catalyst-free InAs Nanowires with Large Thermal Stability. Nanotechnology 2012, 23, 235602. [Google Scholar] [CrossRef]
- Park, H.; Gaillot, A.-C.; Prokes, S.; Cammarata, R. Observation of Size Dependent Liquidus Depression in the Growth of InAs Nanowires. J. Cryst. Growth 2006, 296, 159–164. [Google Scholar] [CrossRef]
- Begum, N.; Piccin, M.; Jabeen, F.; Bais, G.; Rubini, S.; Martelli, F.; Bhatti, A.S. Structural Characterization of GaAs and InAs Nanowires by Means of Raman Spectroscopy. J. Appl. Phys. 2008, 104, 104311. [Google Scholar] [CrossRef]
- Bachilo, S.M.; Strano, M.S.; Kittrell, C.; Hauge, R.H.; Smalley, R.E.; Weisman, R.B. Structure-Assigned Optical Spectra of Single-Walled Carbon Nanotubes. Science 2002, 298, 2361–2366. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Jorio, A.; Fantini, C.; Dantas, M.S.S.; Pimenta, M.A.; Souza Filho, A.G.; Samsonidze, G.G.; Brar, V.W.; Dresselhaus, G.; Dresselhaus, M.S.; Swan, A.K.; et al. Linewidth of the Raman Features of Individual Single-wall Carbon Nanotubes. Phys. Rev. B 2002, 66, 115411. [Google Scholar] [CrossRef]
- Lazzeri, M.; Piscanec, S.; Mauri, F.; Ferrari, A.C.; Robertson, J. Phonon Linewidths and Electron-phonon Coupling in Graphite and Nanotubes. Phys. Rev. B 2006, 73, 155426. [Google Scholar] [CrossRef]
- Maultzsch, J.; Reich, S.; Schlecht, U.; Thomsen, C. High-Energy Phonon Branches of an Individual Metallic Carbon Nanotube. Phys. Rev. Lett. 2003, 91, 087402. [Google Scholar] [CrossRef]
- Piscanec, S.; Lazzeri, M.; Robertson, J.; Ferrari, A.C.; Mauri, F. Optical Phonons in Carbon Nanotubes: Kohn Anomalies, Peierls Distortions, and Dynamic Effects. Phys. Rev. B 2007, 75, 035427. [Google Scholar] [CrossRef]
- Telg, H.; Maultzsch, J.; Reich, S.; Hennrich, F.; Thomsen, C. Chirality Distribution and Transition Energies of Carbon Nanotubes. Phys. Rev. Lett. 2004, 93, 177401. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.; Roth, S. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Wang, Y.; Serrano, S.; Santiago-Avilés, J.J. Raman Characterization of Carbon Nanofibers Prepared Using Electrospinning. Synth. Met. 2003, 138, 423–427. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, D.; Dong, C.; Li, H.; Xiong, K.; Fei, L.; Qian, Y. Carbon Nanofibers: Synthesis, Characterization, and Electrochemical Properties. Carbon 2006, 44, 828–832. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, C.; Wang, J. Raman Spectra of Carbon Nanotubes and Nanofibers Prepared by Ethanol Flames. J. Mater. Sci. 2004, 39, 1091–1094. [Google Scholar] [CrossRef]
- Bhuvaneswari, M.S.; Bramnik, N.N.; Ensling, D.; Ehrenberg, H.; Jaegermann, W. Synthesis and Characterization of Carbon Nano Fiber/LiFePO4 Composites for Li-ion Batteries. J. Power Sources 2008, 180, 553–560. [Google Scholar] [CrossRef]
- Dick, K.A.; Geretovszky, Z.; Mikkelsen, A.; Karlsson, L.S.; Lundgren, E.; Malm, J.-O.; Andersen, J.N.; Samuelson, L.; Seifert, W.; Wacaser, B.A. Improving InAs Nanotree Growth with Composition-controlled Au–In Nanoparticles. Nanotechnology 2006, 17, 1344. [Google Scholar] [CrossRef]
- Okamoto, H. The Co-In (Cobalt-Indium) System. Bull. Alloy Phase Diagr. 1990, 11, 137–139. [Google Scholar] [CrossRef]
- Okamoto, H. The FeSe (Iron-Selenium) System. JPE 1991, 12, 383–389. [Google Scholar] [CrossRef]
- Snigurenko, D.; Jakiela, R.; Guziewicz, E.; Przezdziecka, E.; Stachowicz, M.; Kopalko, K.; Barcz, A.; Lisowski, W.; Sobczak, J.J.; Krawczyk, M.; et al. XPS Study of Arsenic Doped ZnO Grown by Atomic Layer Deposition. J. Alloys Compd. 2014, 582, 594–597. [Google Scholar] [CrossRef]
- Fukuda, Y.; Suzuki, Y.; Murata, J.; Sanada, N. Adsorption and Decomposition of Triethylindium on the Si(100) Surface Studied by X-ray and Ultra-violet Photoelectron Spectroscopy. Appl. Surf. Sci. 1993, 65, 593–597. [Google Scholar] [CrossRef]
- Meharg, P.F.A.; Ogryzlo, E.A.; Bello, I.; Lau, W.M. Surface Damage and Deposition on Gallium Arsenide Resulting from Low Energy Carbon Ion Bombardment. Surf. Sci. 1992, 271, 468–476. [Google Scholar] [CrossRef]
- Dick, K.A.; Deppert, K.; Karlsson, L.S.; Seifert, W.; Wallenberg, L.R.; Samuelson, L. Position-Controlled Interconnected InAs Nanowire Networks. Nano Lett. 2006, 6, 2842–2847. [Google Scholar] [CrossRef]
- Dick, K.A.; Deppert, K.; Karlsson, L.S.; Wallenberg, L.R.; Samuelson, L.; Seifert, W. A New Understanding of Au-Assisted Growth of III–V Semiconductor Nanowires. Adv. Funct. Mater. 2005, 15, 1603–1610. [Google Scholar] [CrossRef]
- Berchmans, S.; Thomas, P.J.; Rao, C.N.R. Novel Effects of Metal Ion Chelation on the Properties of Lipoic Acid-Capped Ag and Au Nanoparticles. J. Phys. Chem. B 2002, 106, 4647–4651. [Google Scholar] [CrossRef]
- Moriarty, P. Nanostructured Materials. Rep. Prog. Phys. 2001, 64, 297. [Google Scholar] [CrossRef]
- Suemori, K.; Yokoyama, M.; Hiramoto, M. Electrical Shorting of Organic Photovoltaic Films Resulting from Metal Migration. J. Appl. Phys. 2006, 99, 36109. [Google Scholar] [CrossRef]
- Xu, Z.; Hou, Y.; Sun, S. Magnetic Core/Shell Fe3O4/Au and Fe3O4/Au/Ag Nanoparticles with Tunable Plasmonic Properties. J. Am. Chem. Soc. 2007, 129, 8698–8699. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshad, M.; Sorba, L.; Rudolf, P.; Cepek, C. Growth and Characterization of Carbon Nanofibers Grown on Vertically Aligned InAs Nanowires via Chemical Vapour Deposition. Nanomaterials 2023, 13, 3083. https://doi.org/10.3390/nano13243083
Arshad M, Sorba L, Rudolf P, Cepek C. Growth and Characterization of Carbon Nanofibers Grown on Vertically Aligned InAs Nanowires via Chemical Vapour Deposition. Nanomaterials. 2023; 13(24):3083. https://doi.org/10.3390/nano13243083
Chicago/Turabian StyleArshad, Muhammad, Lucia Sorba, Petra Rudolf, and Cinzia Cepek. 2023. "Growth and Characterization of Carbon Nanofibers Grown on Vertically Aligned InAs Nanowires via Chemical Vapour Deposition" Nanomaterials 13, no. 24: 3083. https://doi.org/10.3390/nano13243083




