Experimental and Theoretical Investigation of Gadolinium Oxyhydride (GdHO) Thin Films: Optical, Photocatalytic, and Electronic Properties
Abstract
:1. Introduction
2. Methods and Computational Description
2.1. Experimental Description
2.2. Computational Details, Methods, and Modeling
3. Results and Discussion
3.1. Experimental Results and Discussions
3.2. Theoretical Insights into the GdHO
3.3. Computation of Work Function
3.4. Chemical Bonding
3.5. Electronic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mongstad, T.; Platzer-Björkman, C.; Maehlen, J.P.; Mooij, L.P.A.; Pivak, Y.; Dam, B.; Marstein, E.S.; Hauback, B.C.; Karazhanov, S.Z. A new thin film photochromic material: Oxygen-containing yttrium hydride. Sol. Energy Mater. Sol. Cells 2011, 95, 3596–3599. [Google Scholar] [CrossRef]
- Nafezarefi, F.; Schreuders, H.; Dam, B.; Cornelius, S. Photochromism of rare-earth metal-oxy-hydrides. Appl. Phys. Lett. 2017, 111, 103903. [Google Scholar] [CrossRef]
- Baba, E.M.; Montero, J.; Strugovshchikov, E.; Zayim, E.Ö.; Karazhanov, S. Light-induced breathing in photochromic yttrium oxyhydrides. Phys. Rev. Mater. 2020, 4, 025201. [Google Scholar] [CrossRef]
- You, C.C.; Mongstad, T.; Maehlen, J.P.; Karazhanov, S. Engineering of the band gap and optical properties of thin films of yttrium hydride. Appl. Phys. Lett. 2014, 105, 031910. [Google Scholar] [CrossRef]
- Moldarev, D.; Moro, M.V.; You, C.C.; Baba, E.M.; Karazhanov, S.Z.; Wolff, M.; Primetzhofer, D. Yttrium oxyhydrides for photochromic applications: Correlating composition and optical response. Phys. Rev. Mater. 2018, 2, 115203. [Google Scholar] [CrossRef]
- Andronic, L.; Moldarev, D.; Deribew, D.; Moons, E.; Karazhanov, S.Z. Photocatalytic self-cleaning properties of thin films of photochromic yttrium oxyhydride. J. Solid State Chem. 2022, 316, 123599. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Semiconductor Photocatalysis—Past, Present, and Future Outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Benjamin, S.; Ameta, S.C. Chapter 6—Photocatalysis. In Advanced Oxidation Processes for Waste Water Treatment; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 135–175. ISBN 978-0-12-810499-6. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Gómez-Avilés, A.; Peñas-Garzón, M.; Rodriguez, J.J. Chapter 22—Semiconductor Photocatalysis for Water Purification. In Nanoscale Materials in Water Purification; Thomas, S., Pasquini, D., Leu, S.-Y., Gopakumar, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 581–651. ISBN 978-0-12-813926-4. [Google Scholar]
- Tucci, F.G. and P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini-Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef]
- Acosta-Esparza, M.A.; Rivera, L.P.; Pérez-Centeno, A.; Zamudio-Ojeda, A.; González, D.R.; Chávez-Chávez, A.; Santana-Aranda, M.A.; Santos-Cruz, J.; Quiñones-Galván, J.G. UV and Visible light photodegradation of methylene blue with graphene decorated titanium dioxide. Mater. Res. Express 2020, 7, 35504. [Google Scholar] [CrossRef]
- Tachikawa, T.; Fujitsuka, M.; Majima, T. Mechanistic Insight into the TiO2 Photocatalytic Reactions: Design of New Photocatalysts. J. Phys. Chem. C 2007, 111, 5259–5275. [Google Scholar] [CrossRef]
- Weber, A.S.; Grady, A.M.; Koodali, R.T. Lanthanide modified semiconductor photocatalysts. Catal. Sci. Technol. 2012, 2, 683–693. [Google Scholar] [CrossRef]
- Che Ramli, Z.A.; Asim, N.; Isahak, W.N.R.W.; Emdadi, Z.; Ahmad-Ludin, N.; Yarmo, M.A.; Sopian, K. Photocatalytic Degradation of Methylene Blue under UV Light Irradiation on Prepared Carbonaceous TiO2. Sci. World J. 2014, 2014, 415136. [Google Scholar] [CrossRef] [PubMed]
- Mongstad, T.; Thøgersen, A.; Subrahmanyam, A.; Karazhanov, S. The electronic state of thin films of yttrium, yttrium hydrides and yttrium oxide. Sol. Energy Mater. Sol. Cells 2014, 128, 270–274. [Google Scholar] [CrossRef]
- Pishtshev, A.; Karazhanov, S.Z. Role of oxygen in materials properties of yttrium trihydride. Solid State Commun. 2014, 194, 39–42. [Google Scholar] [CrossRef]
- Pishtshev, A.; Strugovshchikov, E.; Karazhanov, S. Conceptual Design of Yttrium Oxyhydrides: Phase Diagram, Structure, and Properties. Cryst. Growth Des. 2019, 19, 2574–2582. [Google Scholar] [CrossRef]
- Strugovshchikov, E.; Pishtshev, A.; Karazhanov, S. Orthogonal chemistry in the design of rare-earth metal oxyhydrides. Pure Appl. Chem. 2021, 93, 1293–1299. [Google Scholar] [CrossRef]
- Strugovshchikov, E.; Pishtshev, A.; Karazhanov, S. Theoretical Design of Effective Multilayer Optical Coatings Using Oxyhydride Thin Films. Phys. Status Solidi 2021, 258, 2100179. [Google Scholar] [CrossRef]
- Maehlen, J.P.; Mongstad, T.T.; You, C.C.; Karazhanov, S. Lattice contraction in photochromic yttrium hydride. J. Alloys Compd. 2013, 580, S119–S121. [Google Scholar] [CrossRef]
- Chandran, C.V.; Schreuders, H.; Dam, B.; Janssen, J.W.G.; Bart, J.; Kentgens, A.P.M.; van Bentum, P.J.M. Solid-State NMR Studies of the Photochromic Effects of Thin Films of Oxygen-Containing Yttrium Hydride. J. Phys. Chem. C 2014, 118, 22935–22942. [Google Scholar] [CrossRef]
- Andronic, L.; Perniu, D.; Duta, A. Synergistic effect between TiO2 sol–gel and Degussa P25 in dye photodegradation. J. Sol-Gel Sci. Technol. 2013, 66, 472–480. [Google Scholar] [CrossRef]
- Dolgonos, A.; Mason, T.O.; Poeppelmeier, K.R. Direct optical band gap measurement in polycrystalline semiconductors: A critical look at the Tauc method. J. Solid State Chem. 2016, 240, 43–48. [Google Scholar] [CrossRef]
- Fernández Garrillo, P.A.; Grévin, B.; Chevalier, N.; Borowik, Ł. Calibrated work function mapping by Kelvin probe force microscopy. Rev. Sci. Instrum. 2018, 89, 43702. [Google Scholar] [CrossRef] [PubMed]
- Asenjo, N.G.; Santamaría, R.; Blanco, C.; Granda, M.; Álvarez, P.; Menéndez, R. Correct use of the Langmuir-Hinshelwood equation for proving the absence of a synergy effect in the photocatalytic degradation of phenol on a suspended mixture of titania and activated carbon. Carbon 2013, 55, 62–69. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes forab initiototal-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Yu, M.; Trinkle, D.R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 2011, 134, 64111. [Google Scholar] [CrossRef]
- Goerigk, L. A Comprehensive Overview of the DFT-D3 London-Dispersion Correction. In Non-Covalent Interactions in Quantum Chemistry and Physics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 195–219. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Neugebauer, J.; Scheffler, M. Adsorbate-substrate and adsorbate-adsorbate interactions of Na and K adlayers on Al(111). Phys. Rev. B 1992, 46, 16067–16080. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, L. Dipole correction for surface supercell calculations. Phys. Rev. B 1999, 59, 12301–12304. [Google Scholar] [CrossRef]
- Wu, P.; Huang, M.; Yin, N.; Li, P. The Modulation Effect of MoS2 Monolayers on the Nucleation and Growth of Pd Clusters: First-Principles Study. Nanomaterials 2019, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qi, Y.; Jia, C.-J.; Jin, Z.; Fan, W. Enhanced visible-light photocatalytic activity of g-C3N4/Zn2GeO4 heterojunctions with effective interfaces based on band match. Nanoscale 2014, 6, 2649. [Google Scholar] [CrossRef] [PubMed]
- De Santis, L.; Resta, R. Electron localization at metal surfaces. Surf. Sci. 2000, 450, 126–132. [Google Scholar] [CrossRef]
- Karazhanov, S.Z.; Kroll, P.; Marstein, E.S.; Holt, A. Doping-induced modulation of electrical and optical properties of silicon nitride. Thin Solid Films 2010, 518, 4918–4922. [Google Scholar] [CrossRef]
- Aðalsteinsson, S.M.M.; Moro, M.V.V.; Moldarev, D.; Droulias, S.; Wolff, M.; Primetzhofer, D. Correlating chemical composition and optical properties of photochromic rare-earth oxyhydrides using ion beam analysis. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2020, 485, 36–40. [Google Scholar] [CrossRef]
- Moldarev, D.; Primetzhofer, D.; You, C.C.; Karazhanov, S.Z.; Montero, J.; Martinsen, F.; Mongstad, T.; Marstein, E.S.; Wolff, M. Composition of photochromic oxygen-containing yttrium hydride films. Sol. Energy Mater. Sol. Cells 2018, 177, 66–69. [Google Scholar] [CrossRef]
- Calzolari, A.; Catellani, A. Controlling the TiN Electrode Work Function at the Atomistic Level: A First Principles Investigation. IEEE Access 2020, 8, 156308–156313. [Google Scholar] [CrossRef]
- Baba, E.M.; Montero, J.; Moldarev, D.; Moro, M.V.; Wolff, M.; Primetzhofer, D.; Sartori, S.; Zayim, E.; Karazhanov, S. Preferential Orientation of Photochromic Gadolinium Oxyhydride Films. Molecules 2020, 25, 3181. [Google Scholar] [CrossRef] [PubMed]
- Moldarev, D.; Wolff, M.; Baba, E.M.; Moro, M.V.; You, C.C.; Primetzhofer, D.; Karazhanov, S.Z. Photochromic properties of yttrium oxyhydride thin films: Surface versus bulk effect. Materialia 2020, 11, 100706. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Colombi, G.; Cornelius, S.; Longo, A.; Dam, B. Structure Model for Anion-Disordered Photochromic Gadolinium Oxyhydride Thin Films. J. Phys. Chem. C 2020, 124, 13541–13549. [Google Scholar] [CrossRef]
- Molkenova, A.; Shin, Y.C.; Kang, M.S.; Mulikova, T.; Han, D.-W.; Atabaev, T.S. Gd2O3 Nanoparticles Coated with a Fluorescent Carbon Layer for Potential T 1 -Weighted Magnetic Resonance and Cells Imaging. Nanosci. Nanotechnol. Lett. 2019, 11, 813–817. [Google Scholar] [CrossRef]
- Guisbiers, G.; Abudukelimu, G.; Hourlier, D. Size-dependent catalytic and melting properties of platinum-palladium nanoparticles. Nanoscale Res. Lett. 2011, 6, 396. [Google Scholar] [CrossRef]
- Savin, A.; Becke, A.D.; Flad, J.; Nesper, R.; Preuss, H.; von Schnering, H.G. A New Look at Electron Localization. Angew. Chem. Int. Ed. Engl. 1991, 30, 409–412. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 84204. [Google Scholar] [CrossRef]
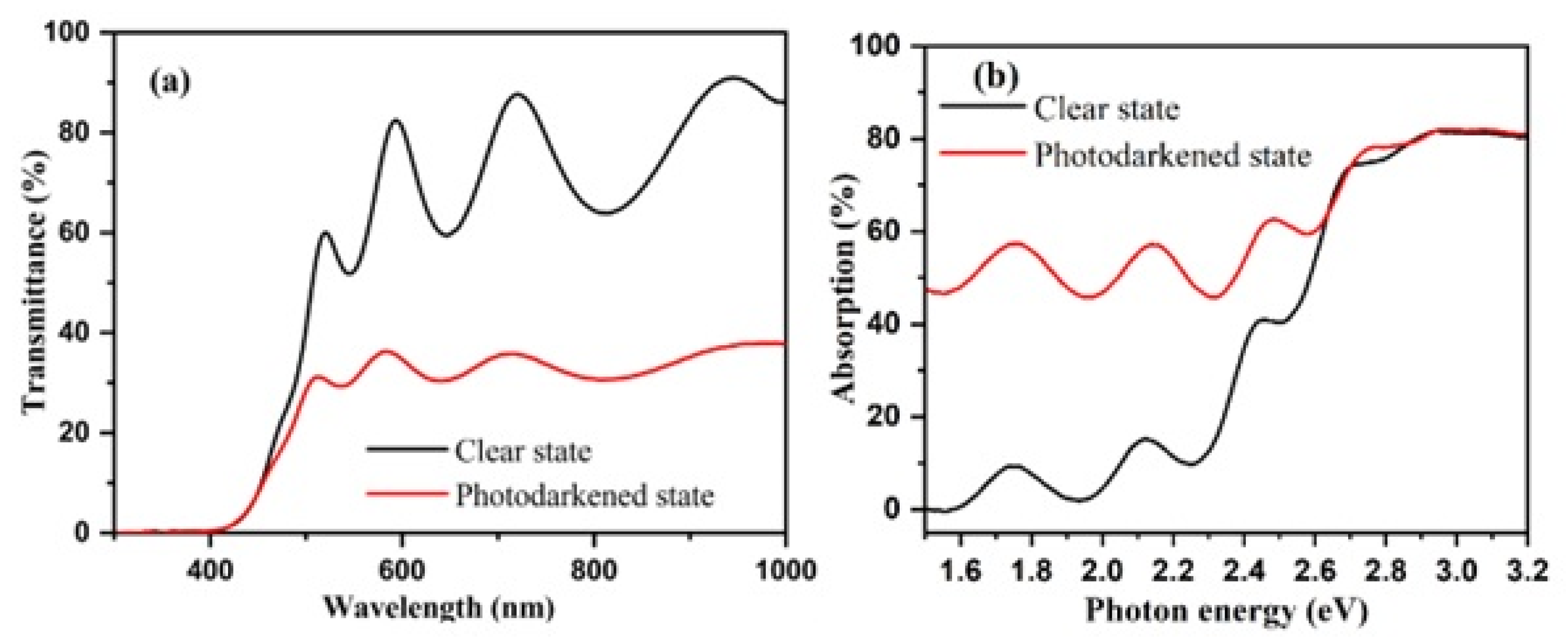




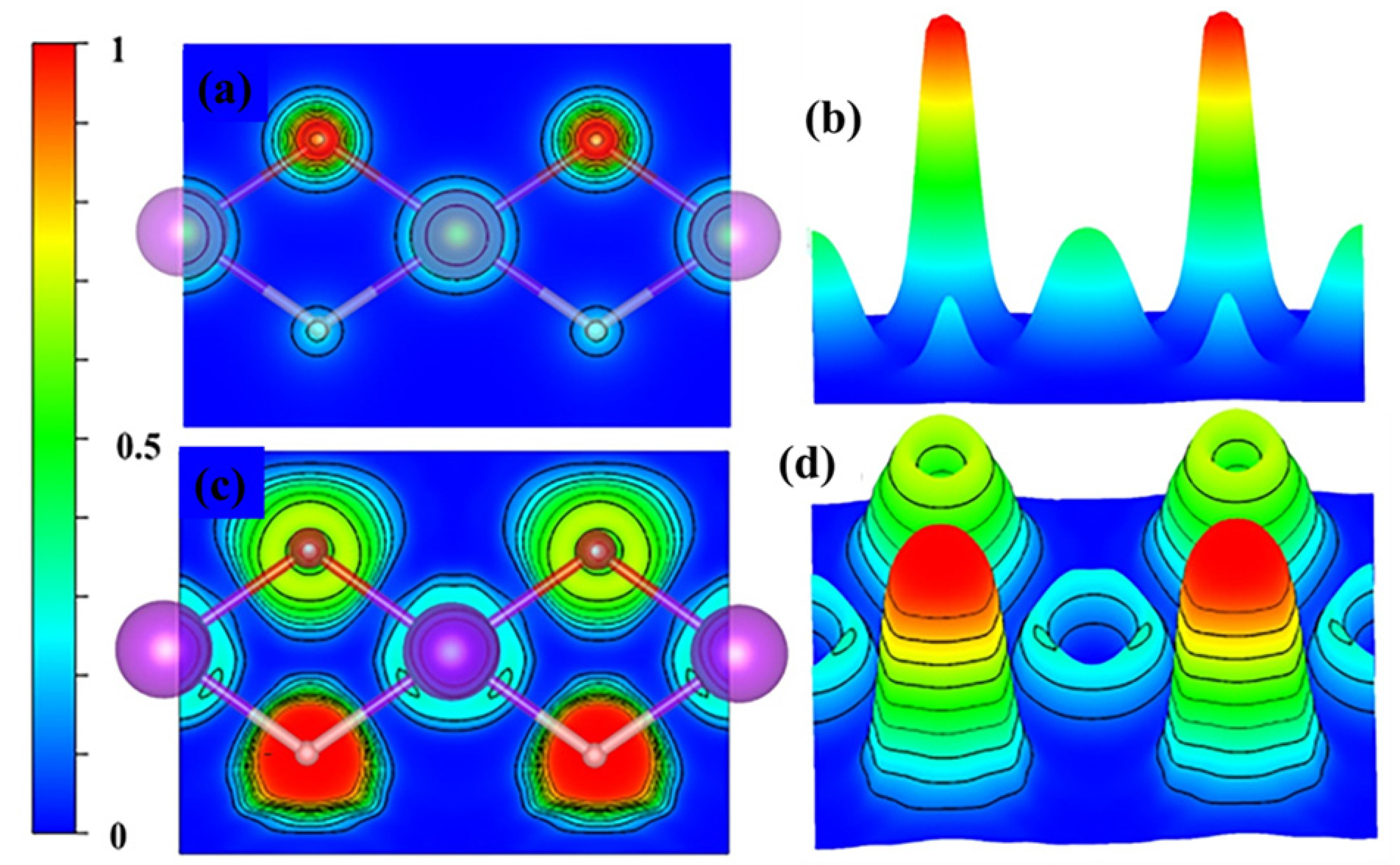
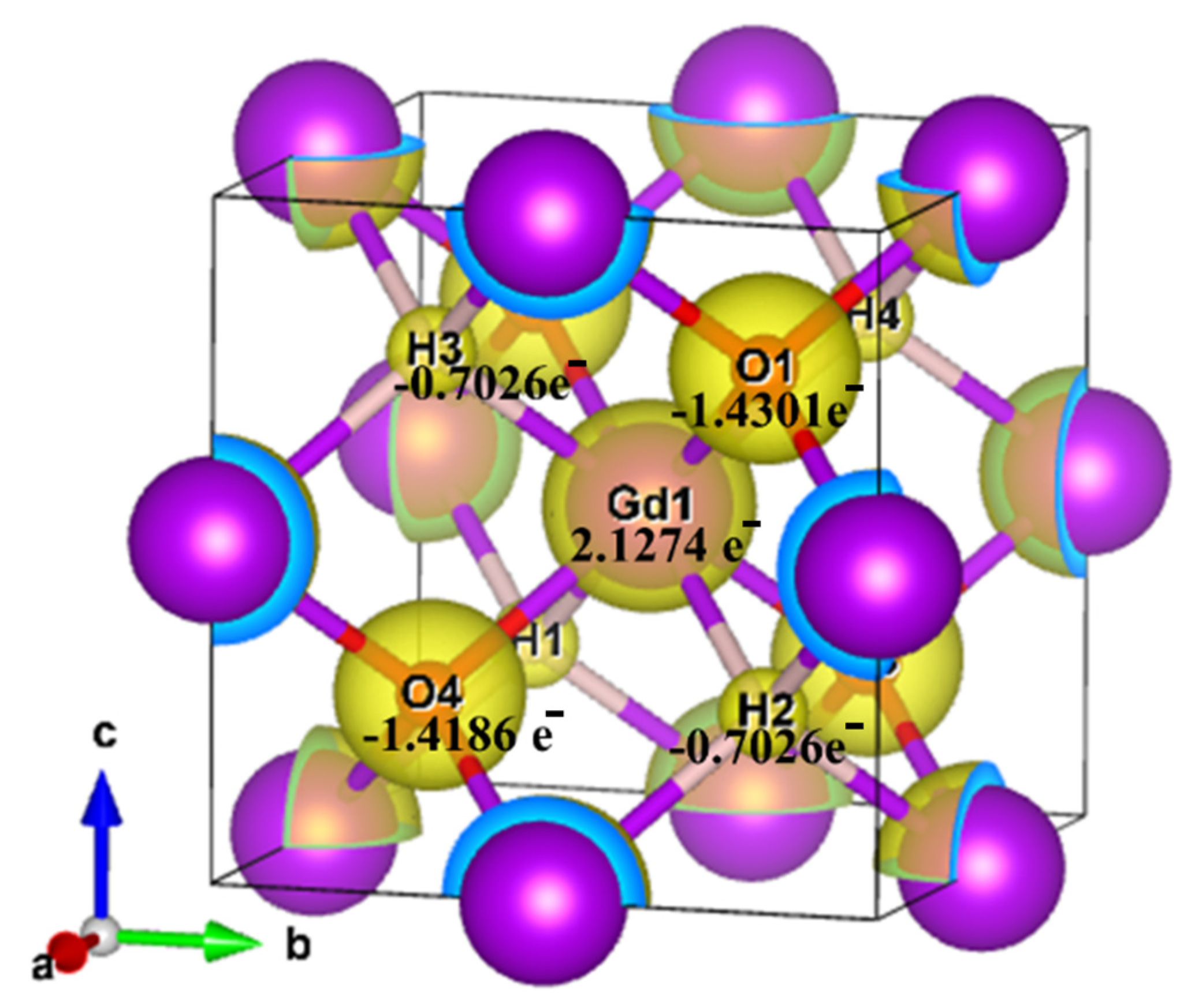
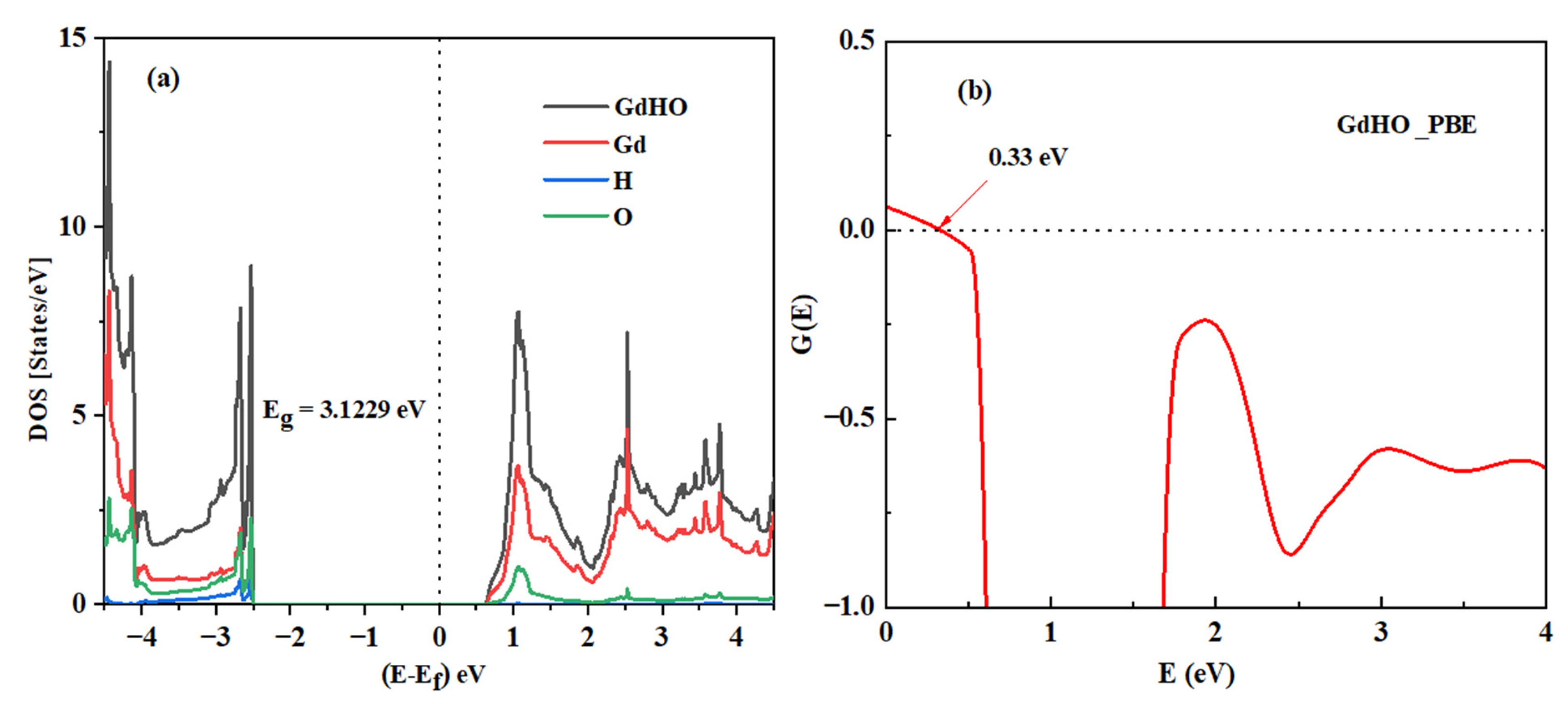
| Sample | Deposition Pressure (Pa) | H2:Ar (sccm) | Number of Passes | Eg (eV) | Photochromic Response (%) |
|---|---|---|---|---|---|
| G1 | 1.0 | 35:160 | 200 | -- | 0 |
| G2 | 1.5 | 35:160 | 200 | 2.8 | 39.37 |
| G3 | 2.0 | 25:160 | 200 | 3.0 | 25.13 |
| G4 | 2.0 | 35:160 | 200 | 3.0 | 24.15 |
| G5 | 2.8 | 35:160 | 200 | 3.1 | 13.68 |
| Chemical Formula | a/b/c (Å) | ρ (g/cm3) | Atom | Wyckoff Position | x | y | z |
|---|---|---|---|---|---|---|---|
| GdH2 | 5.2145 | 7.46 | Gd | 4a | 0 | 0 | 0 |
| H | 8c | ¾ | ¾ | ¾ | |||
| GdHO | 5.3658 | 7.49 | Gd | 4b | ½ | ½ | ½ |
| O | 4d | ¾ | ¾ | ¾ | |||
| H | 4c | ¼ | ¼ | ¼ | |||
| Gd2O3 | 8.7948 | 9.19 | Gd1 | 24d | ½ | ¾ | 0.4676 |
| Gd2 | 8b | ¾ | ¼ | ¼ | |||
| O | 48e | 0.8468 | 0.1220 | 0.3942 |
| Atom | Bader Charge (e−) | Charge Transfer (e−) |
|---|---|---|
| Gd1 | 6.87255 | 2.1274 |
| O1 | 7.430157 | −1.4301 |
| H1 | 1.702618 | −0.7026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, K.V.; Andronic, L.; Baba, E.M.; Deribew, D.; Mayandi, J.; Moons, E.; Karazhanov, S.Z. Experimental and Theoretical Investigation of Gadolinium Oxyhydride (GdHO) Thin Films: Optical, Photocatalytic, and Electronic Properties. Nanomaterials 2023, 13, 3093. https://doi.org/10.3390/nano13243093
Kumar KV, Andronic L, Baba EM, Deribew D, Mayandi J, Moons E, Karazhanov SZ. Experimental and Theoretical Investigation of Gadolinium Oxyhydride (GdHO) Thin Films: Optical, Photocatalytic, and Electronic Properties. Nanomaterials. 2023; 13(24):3093. https://doi.org/10.3390/nano13243093
Chicago/Turabian StyleKumar, Kasi Vinoth, Luminita Andronic, Elbruz Murat Baba, Dargie Deribew, Jeyanthinath Mayandi, Ellen Moons, and Smagul Zh. Karazhanov. 2023. "Experimental and Theoretical Investigation of Gadolinium Oxyhydride (GdHO) Thin Films: Optical, Photocatalytic, and Electronic Properties" Nanomaterials 13, no. 24: 3093. https://doi.org/10.3390/nano13243093







