Synthesis of ZnFe2O4 Nanospheres with Tunable Morphology for Lithium Storage †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnFe2O4
2.2. Materials Characterization
2.3. Electrochemical Tests
3. Results and Discussion
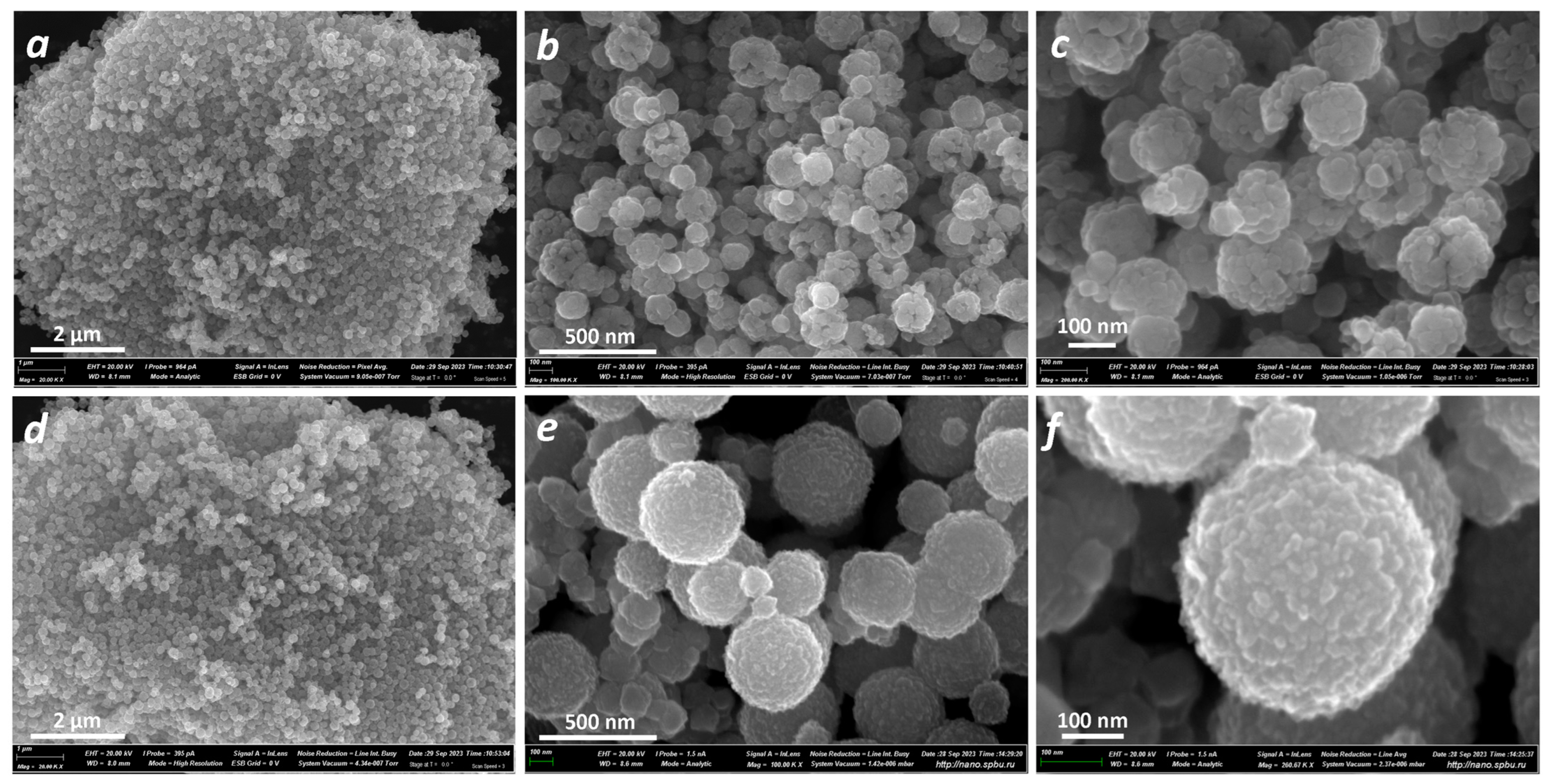
3.1. Physical Characterization
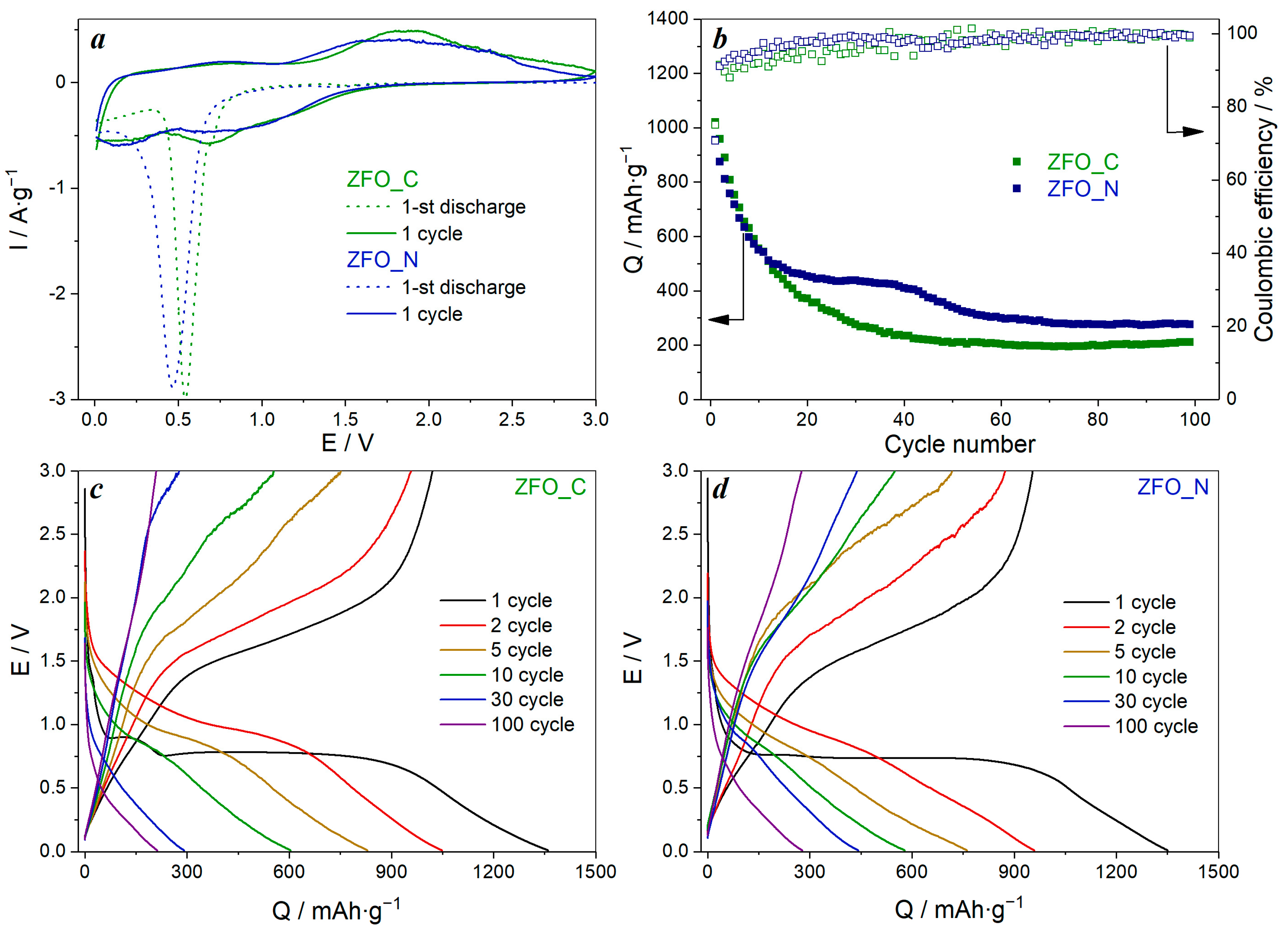
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, H.; He, Y.; Xu, P.; Huang, D.; Wang, Z.; Wang, H.; Wang, Z.; Zhao, Y.; Tian, Q.; Wang, C. Spinel Ferrites (MFe2O4): Synthesis, Improvement and Catalytic Application in Environment and Energy Field. Adv. Colloid Interface Sci. 2021, 294, 102486. [Google Scholar] [CrossRef] [PubMed]
- Šutka, A.; Gross, K.A. Spinel Ferrite Oxide Semiconductor Gas Sensors. Sens. Actuators B Chem. 2016, 222, 95–105. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic Application of Spinel Ferrite Nanoparticles and Nanocomposites in Wastewater Treatment: Review. Sustain. Mater. Technol. 2020, 23, e00140. [Google Scholar] [CrossRef]
- Zhang, Y.; Pelliccione, C.J.; Brady, A.B.; Guo, H.; Smith, P.F.; Liu, P.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Probing the Li Insertion Mechanism of ZnFe2O4 in Li-Ion Batteries: A Combined X-ray Diffraction, Extended X-ray Absorption Fine Structure, and Density Functional Theory Study. Chem. Mater. 2017, 29, 4282–4292. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, L.; Kim, S.J.; Yang, H.; Jeon, I.; Kim, J.P.; Jeong, S.Y.; Lee, H.W.; Cho, C.R. Enhanced Lithium Storage by ZnFe2O4 Nanofibers as Anode Materials for Lithium-Ion Battery. Electrochim. Acta 2019, 296, 565–574. [Google Scholar] [CrossRef]
- Guo, X.; Lu, X.; Fang, X.; Mao, Y.; Wang, Z.; Chen, L.; Xu, X.; Yang, H.; Liu, Y. Lithium Storage in Hollow Spherical ZnFe2O4 as Anode Materials for Lithium Ion Batteries. Electrochem. Commun. 2010, 12, 847–850. [Google Scholar] [CrossRef]
- Hwang, H.; Shin, H.; Lee, W.-J. Effects of Calcination Temperature for Rate Capability of Triple-Shelled ZnFe2O4 Hollow Microspheres for Lithium Ion Battery Anodes. Sci. Rep. 2017, 7, 46378. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhu, S.; Yang, C.; Bao, R.; Tong, L.; Hou, L.; Zhang, X.; Yuan, C. Metal-Organic-Framework-Derived Two-Dimensional Ultrathin Mesoporous Hetero-ZnFe2O4/ZnO Nanosheets with Enhanced Lithium Storage Properties for Li-Ion Batteries. Nanotechnology 2016, 27, 3–7. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Nan, Z. Facile Synthesis of Stoichiometric Zinc Ferrite Nanocrystal Clusters with Superparamagnetism and High Magnetization. Mater. Res. Bull. 2014, 60, 270–278. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, P.; Liu, M.; Xu, J.; Duan, M.; Luo, D. Synthesis and Characterization of Magnetic ZnFe2O4/Bi0-Bi2MoO6 with Z-Scheme Heterojunction for Antibiotics Degradation under Visible Light. Sep. Purif. Technol. 2021, 277, 119339. [Google Scholar] [CrossRef]
- Xu, H.; Chen, X.; Chen, L.; Li, L.; Xu, L.; Yang, J.; Qian, Y. A Comparative Study of Nanoparticles and Nanospheres ZnFe2O4 as Anode Material for Lithium Ion Batteries. Int. J. Electrochem. Sci. 2012, 7, 7976–7983. [Google Scholar] [CrossRef]
- Yu, M.; Huang, Y.; Wang, K.; Han, X.; Wang, M.; Zhu, Y.; Liu, L. Complete Hollow ZnFe2O4 Nanospheres with Huge Internal Space Synthesized by a Simple Solvothermal Method as Anode for Lithium Ion Batteries. Appl. Surf. Sci. 2018, 462, 955–962. [Google Scholar] [CrossRef]
- Spada, D.; Ambrosetti, M.; Mozzati, M.C.; Albini, B.; Galinetto, P.; Cini, A.; Fittipaldi, M.; Bini, M. Understanding the Electrochemical Features of ZnFe2O4, Anode for LIBs, by Deepening Its Physico-Chemical Properties. Mater. Res. Bull. 2023, 160, 112132. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, Y.; Shao, H. High Capacity ZnFe2O4 Anode Material for Lithium Ion Batteries. Electrochim. Acta 2011, 56, 9433–9438. [Google Scholar] [CrossRef]
- Wang, M.; Huang, Y.; Chen, X.; Wang, K.; Wu, H.; Zhang, N.; Fu, H. Synthesis of Nitrogen and Sulfur Co-Doped Graphene Supported Hollow ZnFe2O4 Nanosphere Composites for Application in Lithium-Ion Batteries. J. Alloys Compd. 2017, 691, 407–415. [Google Scholar] [CrossRef]
- Dong, Y.; Xia, Y.; Chui, Y.-S.; Cao, C.; Zapien, J.A. Self-Assembled Three-Dimensional Mesoporous ZnFe2O4-Graphene Composites for Lithium Ion Batteries with Significantly Enhanced Rate Capability and Cycling Stability. J. Power Sources 2015, 275, 769–776. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, L.; Qi, H.; Yue, H.; Zhang, T.; Zhao, X.; Chen, G.; Wei, Y.; Wang, C.; Zhang, D. Nanosheet Assembled Hollow ZnFe2O4 Microsphere as Anode for Lithium-Ion Batteries. J. Alloys Compd. 2018, 762, 480–487. [Google Scholar] [CrossRef]
- Su, Q.; Wang, S.; Yao, L.; Li, H.; Du, G.; Ye, H.; Fang, Y. Study on the Electrochemical Reaction Mechanism of ZnFe2O4 by In Situ Transmission Electron Microscopy. Sci. Rep. 2016, 6, 28197. [Google Scholar] [CrossRef]
- Yue, H.; Chen, S.; Tie, W.; Wu, L.; Xie, W.; Li, T.; Li, W.; Li, H. Facile Synthesis of Hierarchical ZnFe2O4 Hollow Microspheres as High-Performance Anode for Lithium-Ion Batteries. Ionics 2021, 27, 2835–2845. [Google Scholar] [CrossRef]
- Rezvani, S.J.; Nobili, F.; Gunnella, R.; Ali, M.; Tossici, R.; Passerini, S.; Di Cicco, A. SEI Dynamics in Metal Oxide Conversion Electrodes of Li-Ion Batteries. J. Phys. Chem. C 2017, 121, 26379–26388. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, H.J. Confinement of Zn-Mg-Al-Layered Double Hydroxide and α-Fe2O3 Nanorods on Hollow Porous Carbon Nanofibers: A Free-Standing Electrode for Solid-State Symmetric Supercapacitors. Chem. Eng. J. 2022, 429, 132345. [Google Scholar] [CrossRef]
- Rabin, N.N.; Morshed, J.; Akhter, H.; Islam, S.; Hossain, A.; Alam, M.; Karim, M.R.; Hasnat, M.A. Surface Modification of the ZnO Nanoparticles with γ -Aminopropyltriethoxysilane and Study of Their Photocatalytic Activity, Optical Properties and Antibacterial Activities. Int. J. Chem. React. Eng. 2016, 14, 785–794. [Google Scholar] [CrossRef]
- Dai, L.; Strelow, C.; Kipp, T.; Mews, A.; Benkenstein, I.; Ei, D.; Vuong, T.H.; Rabeah, J.; Mcgettrick, J.; Lesyuk, R.; et al. Colloidal Manganese-Doped ZnS Nanoplatelets and Their Optical Properties. Chem. Mater. 2021, 33, 275–284. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, Y.J.; Kim, J.H.; Jang, J.W.; Choi, S.H.; Lee, J.S. Defective ZnFe2O4 Nanorods with Oxygen Vacancy for Photoelectrochemical Water Splitting. Nanoscale 2015, 7, 19144–19151. [Google Scholar] [CrossRef]
- Al-Najar, B.; Younis, A.; Hazeem, L.; Sehar, S.; Rashdan, S.; Shaikh, M.N.; Albuflasa, H.; Hankins, N.P. Thermally Induced Oxygen Related Defects in Eco-Friendly ZnFe2O4 Nanoparticles for Enhanced Wastewater Treatment Efficiencies. Chemosphere 2022, 288, 132525. [Google Scholar] [CrossRef] [PubMed]
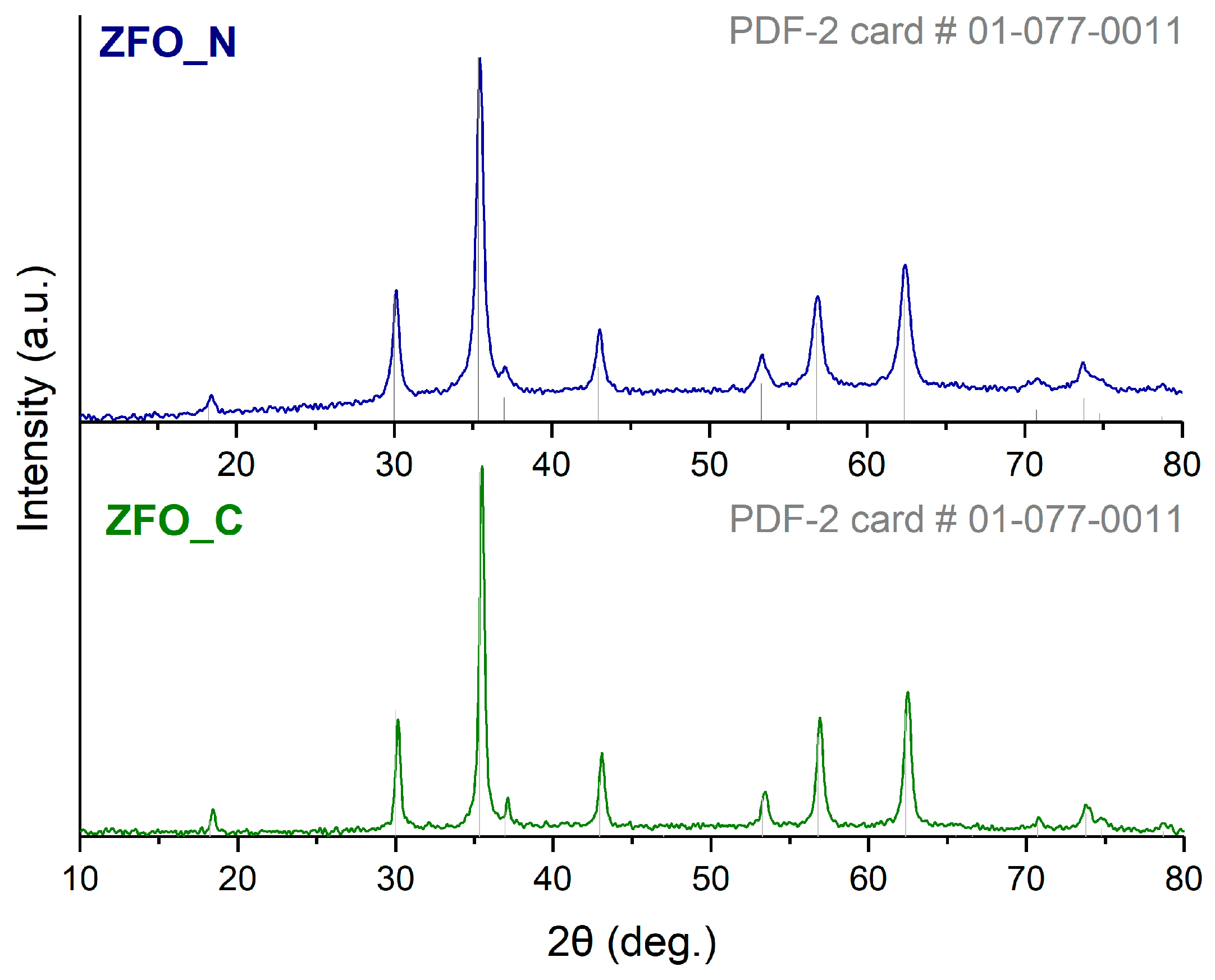


| Sample | a (Å) | dXRD (nm) | Fe Atomic% | Zn Atomic% | Fe:Zn Ratio |
|---|---|---|---|---|---|
| ZFO_C | 8.426 (2) | 19.8 (6) | 24.84 | 9.93 | 2.37 |
| ZFO_N | 8.433 (2) | 12.1 (4) | 17.35 | 8.15 | 2.01 |
| Sample | Precursor | Shape | Size, nm | Structure | Stoichiometry | Nanoplates |
|---|---|---|---|---|---|---|
| ZFO_C | Chloride | Sphere | 100–200 | Loose | No | Large |
| ZFO_N | Nitrate | Sphere | 300–500 | Dense | Yes | Small |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkov, F.S.; Kamenskii, M.A.; Tolstopjatova, E.G.; Voskanyan, L.A.; Bobrysheva, N.P.; Osmolovskaya, O.M.; Eliseeva, S.N. Synthesis of ZnFe2O4 Nanospheres with Tunable Morphology for Lithium Storage. Nanomaterials 2023, 13, 3126. https://doi.org/10.3390/nano13243126
Volkov FS, Kamenskii MA, Tolstopjatova EG, Voskanyan LA, Bobrysheva NP, Osmolovskaya OM, Eliseeva SN. Synthesis of ZnFe2O4 Nanospheres with Tunable Morphology for Lithium Storage. Nanomaterials. 2023; 13(24):3126. https://doi.org/10.3390/nano13243126
Chicago/Turabian StyleVolkov, Filipp S., Mikhail A. Kamenskii, Elena G. Tolstopjatova, Lusine A. Voskanyan, Natalia P. Bobrysheva, Olga M. Osmolovskaya, and Svetlana N. Eliseeva. 2023. "Synthesis of ZnFe2O4 Nanospheres with Tunable Morphology for Lithium Storage" Nanomaterials 13, no. 24: 3126. https://doi.org/10.3390/nano13243126





