Layered Double Hydroxides as an Intercalation System for Hydrophobic Molecules
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Synthesis of MgAl-NO3 LDH
2.3. Intercalation of NR onto LDH
2.4. Determination of the Loading Efficiency of LDH for NR
2.5. Characterizations
3. Results
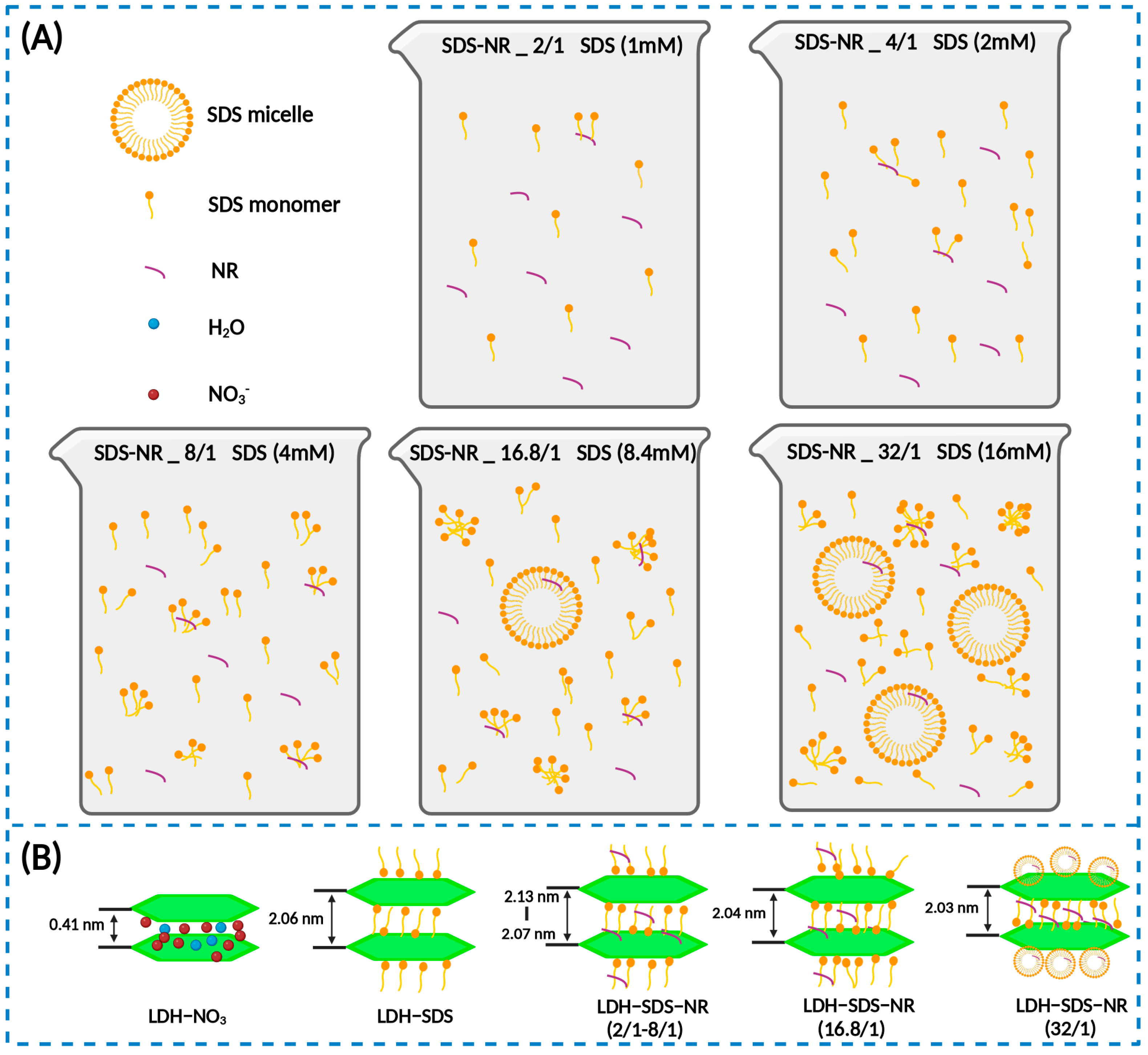
3.1. Preparation of LDH-SDS-NR Complexes
3.2. Characterization of LDH-SDS-NR
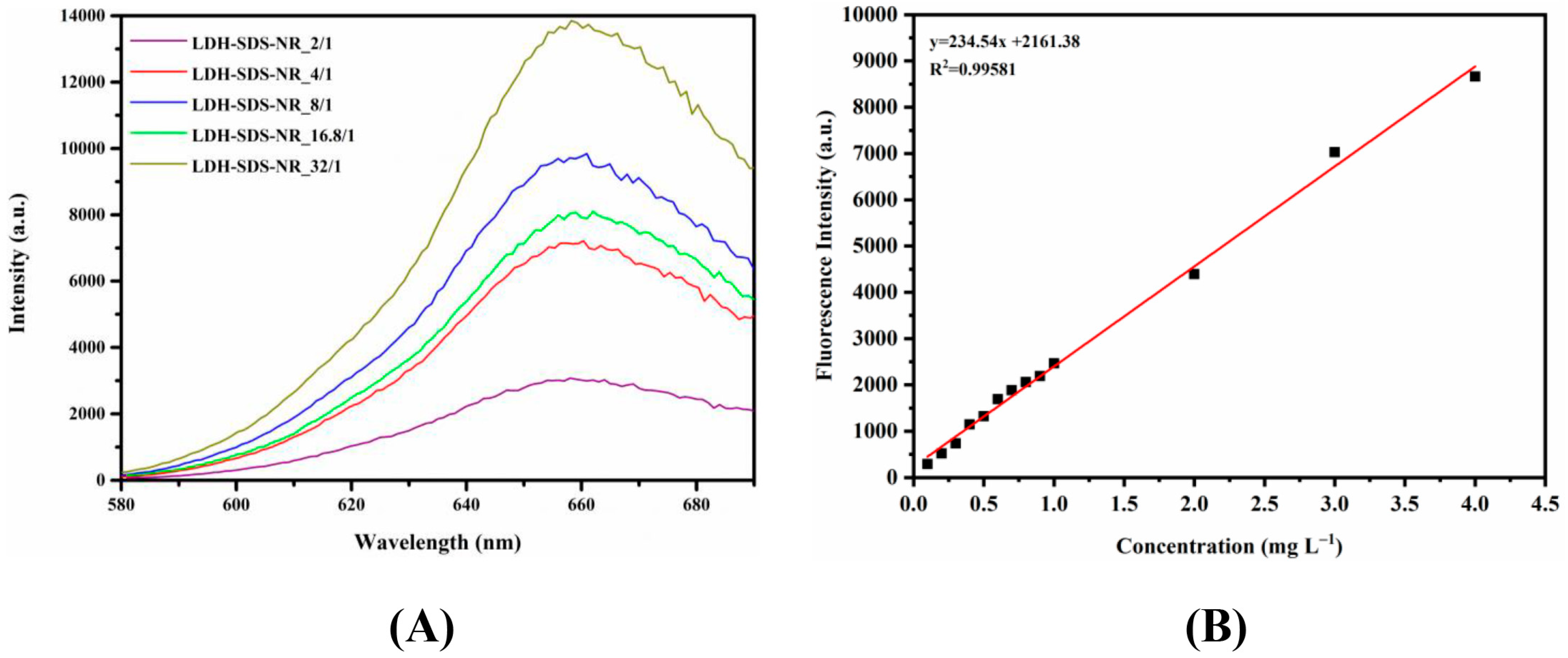
3.3. Drug Loading Efficiency of LDH-SDS-NR
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mao, Y.; Huang, L.; Liu, Z.; He, Y.; Wang, W.; Bao, Y.; Niu, L. Improved Performance of Wrinkled CoNi-LDHs via in Situ Immobilization onto Cotton Gauze for Solid Phase Extraction of Non-Steroidal Anti-Inflammatory Drugs. Microchem. J. 2021, 164, 106053. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, L.; Liang, K.; Cheong, S.; Boyer, C.; Gooding, J.J.; Chen, Y.; Gu, Z. Biodegradable 2D Fe–Al Hydroxide for Nanocatalytic Tumor-Dynamic Therapy with Tumor Specificity. Adv. Sci. 2018, 5, 1801155. [Google Scholar] [CrossRef] [PubMed]
- Cunha, V.R.R.; De Souza, R.B.; Da Fonseca Martins, A.M.C.R.P.; Koh, I.H.J.; Constantino, V.R.L. Accessing the Biocompatibility of Layered Double Hydroxide by Intramuscular Implantation: Histological and Microcirculation Evaluation. Sci. Rep. 2016, 6, 30547. [Google Scholar] [CrossRef] [PubMed]
- Ameena Shirin, V.K.; Sankar, R.; Johnson, A.P.; Gangadharappa, H.V.; Pramod, K. Advanced Drug Delivery Applications of Layered Double Hydroxide. J. Control. Release 2021, 330, 398–426. [Google Scholar] [CrossRef] [PubMed]
- Abniki, M.; Moghimi, A.; Azizinejad, F. Synthesis of Calcium-Layered Double Hydroxide Based Nanohybrid for Controlled Release of an Anti-Inflammatory Drug. J. Chin. Chem. Soc. 2021, 68, 343–352. [Google Scholar] [CrossRef]
- Del Arco, M.; Gutiérrez, S.; Martín, C.; Rives, V.; Rocha, J. Synthesis and Characterization of Layered Double Hydroxides (LDH) Intercalated with Non-Steroidal Anti-Inflammatory Drugs (NSAID). J. Solid State Chem. 2004, 177, 3954–3962. [Google Scholar] [CrossRef]
- Zheng, J.; Li, J.; Zhang, L.; Chen, X.; Yu, Y.; Huang, H. Post-Graphene 2D Materials-Based Antimicrobial Agents: Focus on Fabrication Strategies and Biosafety Assessments. J. Mater. Sci. 2020, 55, 7226–7246. [Google Scholar] [CrossRef]
- Kang, X.; Ye, H. Antimicrobial Alkali-Activated Slag through Self-Intercalation of Benzoate in Layered Double Hydroxides. Cem. Concr. Compos. 2022, 130, 104533. [Google Scholar] [CrossRef]
- Lim, M.; Badruddoza, A.Z.M.; Firdous, J.; Azad, M.; Mannan, A.; Al-Hilal, T.A.; Cho, C.S.; Islam, M.A. Engineered Nanodelivery Systems to Improve DNA Vaccine Technologies. Pharmaceutics 2020, 12, 30. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, R.; Gao, B.; Wu, B.; Li, K.; Sun, X.; Liu, H.; Wang, S. The Enhanced Immune Response of Hepatitis B Virus DNA Vaccine Using SiO2@LDH Nanoparticles as an Adjuvant. Biomaterials 2014, 35, 466–478. [Google Scholar] [CrossRef]
- Chen, B.-Q.; Xia, H.-Y.; Mende, K.; Lee, C.-H.; Wang, S.-B.; Chen, A.-Z.; Xu, Z.-P.; Kankala, R.K. Trends in Layered Double Hydroxides-Based Advanced Nanocomposites: Recent Progress and Latest Advancements. Adv. Mater. Interfaces 2022, 9, 2200373. [Google Scholar] [CrossRef]
- Nava Andrade, K.; Knauth, P.; López, Z.; Hirata, G.A.; Guevara Martinez, S.J.; Carbajal Arízaga, G.G. Assembly of Folate-Carbon Dots in GdDy-Doped Layered Double Hydroxides for Targeted Delivery of Doxorubicin. Appl. Clay Sci. 2020, 192, 105661. [Google Scholar] [CrossRef]
- Ahmadi-Kashani, M.; Dehghani, H.; Zarrabi, A. A Biocompatible Nanoplatform Formed by MgAl-Layered Double Hydroxide Modified Mn3O4/N-Graphene Quantum Dot Conjugated-Polyaniline for PH-Triggered Release of Doxorubicin. Mater. Sci. Eng. C 2020, 114, 111055. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeung, D.G.; Oh, J.M. Boosting the Anticancer Activity of Doxorubicin with a Layered Double Hydroxide Nanocarrier. Appl. Clay Sci. 2021, 203, 106000. [Google Scholar] [CrossRef]
- Wang, L.; Xing, H.; Zhang, S.; Ren, Q.; Pan, L.; Zhang, K.; Bu, W.; Zheng, X.; Zhou, L.; Peng, W.; et al. A Gd-Doped Mg-Al-LDH/Au Nanocomposite for CT/MR Bimodal Imagings and Simultaneous Drug Delivery. Biomaterials 2013, 34, 3390–3401. [Google Scholar] [CrossRef] [PubMed]
- Tyner, K.M.; Schiffman, S.R.; Giannelis, E.P. Nanobiohybrids as Delivery Vehicles for Camptothecin. J. Control. Release 2004, 95, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Bildstein, L.; Dubernet, C.; Couvreur, P. Prodrug-Based Intracellular Delivery of Anticancer Agents. Adv. Drug Deliv. Rev. 2011, 63, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Wasan, K.M.; Brocks, D.R.; Lee, S.D.; Sachs-Barrable, K.; Thornton, S.J. Impact of Lipoproteins on the Biological Activity and Disposition of Hydrophobic Drugs: Implications for Drug Discovery. Nat. Rev. Drug Discov. 2008, 7, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, M.; Kunimura, K.; Suzuki, J.; Kodera, Y. Antimicrobial Properties of Hydrophobic Compounds in Garlic: Allicin, Vinyldithiin, Ajoene and Diallyl Polysulfides (Review). Exp. Ther. Med. 2020, 19, 1550–1553. [Google Scholar] [CrossRef]
- Meng, J.; Agrahari, V.; Youm, I. Advances in Targeted Drug Delivery Approaches for the Central Nervous System Tumors: The Inspiration of Nanobiotechnology. J. Neuroimmune Pharmacol. 2016, 12, 84–98. [Google Scholar] [CrossRef]
- Han, D.; Yang, S.; Yang, J.; Zou, P.; Kong, X.; Yang, L.; Wang, D. Synthesis of Fe3O4 Nanoparticles via Chemical Coprecipitation Method: Modification of Surface with Sodium Dodecyl Sulfate and Biocompatibility Study. Nanosci. Nanotechnol. Lett. 2016, 8, 335–339. [Google Scholar] [CrossRef]
- Terracciano, R.; Zhang, A.; Simeral, M.L.; Demarchi, D.; Hafner, J.H.; Filgueira, C.S. Improvements in Gold Nanorod Biocompatibility with Sodium Dodecyl Sulfate Stabilization. J. Nanotheranostics 2021, 2, 157–173. [Google Scholar] [CrossRef]
- Malaspina, D.C.; Viñas, C.; Teixidor, F.; Faraudo, J. Atomistic Simulations of COSAN: Amphiphiles without a Head-and-Tail Design Display “Head and Tail” Surfactant Behavior. Angew. Chem. Int. Ed. 2020, 59, 3088–3092. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, R. Interaction between Casein and Sodium Dodecyl Sulfate. J. Colloid Interface Sci. 2007, 315, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Manna, U.; Patil, S. Dual Drug Delivery Microcapsules via Layer-by-Layer Self-Assembly. Langmuir 2009, 25, 10515–10522. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.J.; Li, F.; To, S.S.T. Development and Evaluation of a Novel Drug Delivery: Pluronics/SDS Mixed Micelle Loaded with Myricetin In Vitro and In Vivo. J. Pharm. Sci. 2016, 105, 1535–1543. [Google Scholar] [CrossRef]
- Martinez, V.; Henary, M. Nile Red and Nile Blue: Applications and Syntheses of Structural Analogues. Chem. Eur. J. 2016, 22, 13764–13782. [Google Scholar] [CrossRef]
- Débarre, D.; Supatto, W.; Pena, A.M.; Fabre, A.; Tordjmann, T.; Combettes, L.; Schanne-Klein, M.C.; Beaurepaire, E. Imaging Lipid Bodies in Cells and Tissues Using Third-Harmonic Generation Microscopy. Nat. Methods 2005, 3, 47–53. [Google Scholar] [CrossRef]
- Demeule, B.; Gurny, R.; Arvinte, T. Detection and Characterization of Protein Aggregates by Fluorescence Microscopy. Int. J. Pharm. 2007, 329, 37–45. [Google Scholar] [CrossRef]
- Snipstad, S.; Westrøm, S.; Mørch, Y.; Afadzi, M.; Åslund, A.K.O.; de Lange Davies, C. Contact-Mediated Intracellular Delivery of Hydrophobic Drugs from Polymeric Nanoparticles. Cancer Nanotechnol. 2014, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Batheja, P.; Sheihet, L.; Kohn, J.; Singer, A.J.; Michniak-Kohn, B. Topical Drug Delivery by a Polymeric Nanosphere Gel: Formulation Optimization and in Vitro and in Vivo Skin Distribution Studies. J. Control. Release 2011, 149, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Samed, N.; Sharma, V.; Sundaramurthy, A. Hydrogen Bonded Niosomes for Encapsulation and Release of Hydrophilic and Hydrophobic Anti-Diabetic Drugs: An Efficient System for Oral Anti-Diabetic Formulation. Appl. Surf. Sci. 2018, 449, 567–573. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, R.; Xu, P.; Yu, J.; Liu, L.; Fan, Y. Physical Nanochitin/Microemulsion Composite Hydrogels for Hydrophobic Nile Red Release under in Vitro Physiological Conditions. Cellulose 2019, 26, 1221–1230. [Google Scholar] [CrossRef]
- Li, L.; Warszawik, E.; Van Rijn, P.; Li, L.; Warszawik, E.; Van Rijn, P.; Kolff, W.J. PH-Triggered Release and Degradation Mechanism of Layered Double Hydroxides with High Loading Capacity. Adv. Mater. Interfaces 2023, 10, 2202396. [Google Scholar] [CrossRef]
- Dasgupta, S. Controlled Release of Ibuprofen Using Mg Al LDH Nano Carrier. IOP Conf. Ser. Mater. Sci. Eng. 2017, 225, 012005. [Google Scholar] [CrossRef]
- Acharya, H.; Srivastava, S.K.; Bhowmick, A.K. Synthesis of Partially Exfoliated EPDM/LDH Nanocomposites by Solution Intercalation: Structural Characterization and Properties. Compos. Sci. Technol. 2007, 67, 2807–2816. [Google Scholar] [CrossRef]
- Nath, J.; Dolui, S.K. Synthesis of Carboxymethyl Cellulose-g-Poly(Acrylic Acid)/LDH Hydrogel for in Vitro Controlled Release of Vitamin B12. Appl. Clay Sci. 2018, 155, 65–73. [Google Scholar] [CrossRef]
- Arizaga, G.G.C.; Gardolinski, J.E.F.d.C.; Schreiner, W.H.; Wypych, F. Intercalation of an Oxalatooxoniobate Complex into Layered Double Hydroxide and Layered Zinc Hydroxide Nitrate. J. Colloid Interface Sci. 2009, 330, 352–358. [Google Scholar] [CrossRef]
- Wójcik-Bania, M. Influence of the Addition of Organo-Montmorillonite Nanofiller on Cross-Linking of Polysiloxanes—FTIR Studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119491. [Google Scholar] [CrossRef]
- Singh, R.K.; Kunimatsu, K.; Miyatake, K.; Tsuneda, T. Experimental and Theoretical Infrared Spectroscopic Study on Hydrated Nafion Membrane. Macromolecules 2016, 49, 6621–6629. [Google Scholar] [CrossRef]
- Kumru, M.; Küçük, V.; Kocademir, M. Determination of Structural and Vibrational Properties of 6-Quinolinecarboxaldehyde Using FT-IR, FT-Raman and Dispersive-Raman Experimental Techniques and Theoretical HF and DFT (B3LYP) Methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 96, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Karakoti, M.; Dhali, S.; Karki, N.; SanthiBhushan, B.; Tewari, C.; Rana, S.; Srivastava, A.; Melkani, A.B.; Sahoo, N.G. Bulk Synthesis of Graphene Nanosheets from Plastic Waste: An Invincible Method of Solid Waste Management for Better Tomorrow. Waste Manag. 2019, 88, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, I.; Vlamidis, Y.; Mazzei, L.; Musella, E.; Giorgetti, M.; Christian, M.; Morandi, V.; Scavetta, E.; Tonelli, D. Ni/Al Layered Double Hydroxide and Carbon Nanomaterial Composites for Glucose Sensing. ACS Appl. Nano Mater. 2019, 2, 143–155. [Google Scholar] [CrossRef]
- Pope, C.G. X-Ray Diffraction and the Bragg Equation. J. Chem. Educ. 1997, 74, 129–131. [Google Scholar] [CrossRef]
- Zhao, H.; Nagy, K.L. Dodecyl Sulfate–Hydrotalcite Nanocomposites for Trapping Chlorinated Organic Pollutants in Water. J. Colloid Interface Sci. 2004, 274, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Kamada, K.; Ohta, K. Spectroscopic Studies of Nile Red in Organic Solvents and Polymers. J. Photochem. Photobiol. A Chem. 1996, 93, 57–64. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K. Solubilization of Hydrophobic Dyes in Surfactant Solutions. Materials 2013, 6, 580–608. [Google Scholar] [CrossRef]
- Cifuentes, A.; Bernal, J.L.; Diez-Masa, J.C. Determination of Critical Micelle Concentration Values Using Capillary Electrophoresis Instrumentation. Anal. Chem. 1997, 69, 4271–4274. [Google Scholar] [CrossRef]
- Cui, X.; Mao, S.; Liu, M.; Yuan, H.; Du, Y. Mechanism of Surfactant Micelle Formation. Langmuir 2008, 24, 10771–10775. [Google Scholar] [CrossRef]
- Pious, A.; Kamlekar, R.K.; Muthusamy, S.; Jothi, A.; Praneeth, V.K.K.; Ramesh, S.; Veerappan, A. Effectiveness of the Hydrophobic Core of Pyridine Tethered N-Acyl Glycine Micelles in Improving Chromenoquinoline Synthesis in Water. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 664, 131129. [Google Scholar] [CrossRef]
- Hassan, P.A.; Raghavan, S.R.; Kaler, E.W. Microstructural Changes in SDS Micelles Induced by Hydrotropic Salt. Langmuir 2002, 18, 2543–2548. [Google Scholar] [CrossRef]
- Bibette, J.; Roux, D.; Nallet, F. Depletion Interactions and Fluid-Solid Equilibrium in Emulsions. Phys. Rev. Lett. 1990, 65, 2470. [Google Scholar] [CrossRef] [PubMed]
- Subuddhi, U.; Mishra, A.K. Effect of Sodium Deoxycholate and Sodium Cholate on DPPC Vesicles: A Fluorescence Anisotropy Study with Diphenylhexatriene. J. Chem. Sci. 2007, 119, 169–174. [Google Scholar] [CrossRef]
- Trikeriotis, M.; Ghanotakis, D.F. Intercalation of Hydrophilic and Hydrophobic Antibiotics in Layered Double Hydroxides. Int. J. Pharm. 2007, 332, 176–184. [Google Scholar] [CrossRef]


| Band Position (cm−1) | Assignment a |
|---|---|
| 3525 | νa (OH) |
| 2920 | νa (CH2) |
| 2851 | νs (CH2) |
| 1622, 1583, 1493, 1405, 1349 | ν (C-C) |
| 1616 | δ (H2O) |
| 1469 | (CH2) shear |
| 1385 | ν (NO3−) |
| 1310, 1113 | ν (C-O) |
| 1210 | a (S=O) |
| 1063 | s (S=O) |
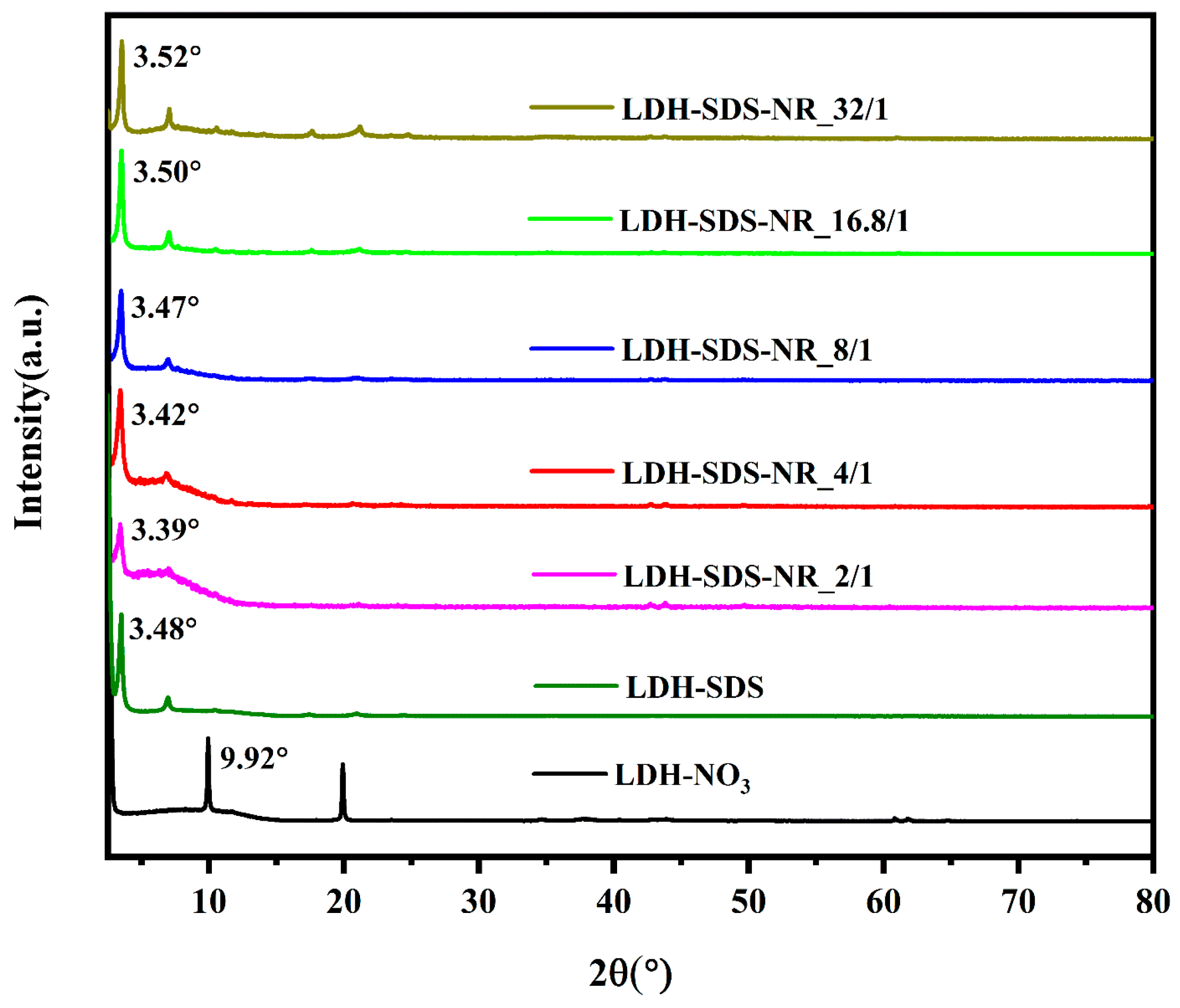
| Samples | 2θ (°) | Interlayer Spacing (nm) |
|---|---|---|
| LDH-NO3 | 9.92 | 0.41 |
| LDH-SDS | 3.48 | 2.06 |
| LDH-SDS-NR_2/1 | 3.39 | 2.13 |
| LDH-SDS-NR_4/1 | 3.42 | 2.10 |
| LDH-SDS-NR_8/1 | 3.47 | 2.07 |
| LDH-SDS-NR_16.8/1 | 3.50 | 2.04 |
| LDH-SDS-NR_32/1 | 3.52 | 2.03 |
| Samples | Loading Efficiency (%) |
|---|---|
| LDH-SDS-NR_2/1 | 1.32 ± 0.10 a |
| LDH-SDS-NR_4/1 | 3.24 ± 0.11 b |
| LDH-SDS-NR_8/1 | 4.46 ± 0.10 d |
| LDH-SDS-NR_16.8/1 | 3.64 ± 0.07 c |
| LDH-SDS-NR_32/1 | 6.31 ± 0.05 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Sevciuc, A.; Rijn, P.v. Layered Double Hydroxides as an Intercalation System for Hydrophobic Molecules. Nanomaterials 2023, 13, 3145. https://doi.org/10.3390/nano13243145
Li L, Sevciuc A, Rijn Pv. Layered Double Hydroxides as an Intercalation System for Hydrophobic Molecules. Nanomaterials. 2023; 13(24):3145. https://doi.org/10.3390/nano13243145
Chicago/Turabian StyleLi, Lei, Anastasia Sevciuc, and Patrick van Rijn. 2023. "Layered Double Hydroxides as an Intercalation System for Hydrophobic Molecules" Nanomaterials 13, no. 24: 3145. https://doi.org/10.3390/nano13243145





