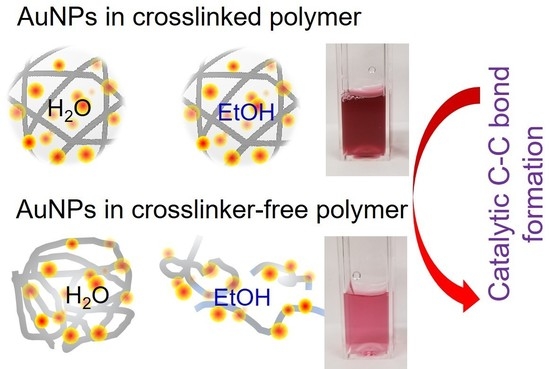
Integration of Gold Nanoparticles into Crosslinker-Free Polymer Particles and Their Colloidal Catalytic Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Crosslinker-Free and Crosslinked PNIPAM Particles and AuNP-Integrated Composite Particles
2.3. Homocoupling of Phenylboronic Acid in the Presence of Composite Particles
2.4. Characterization
3. Results
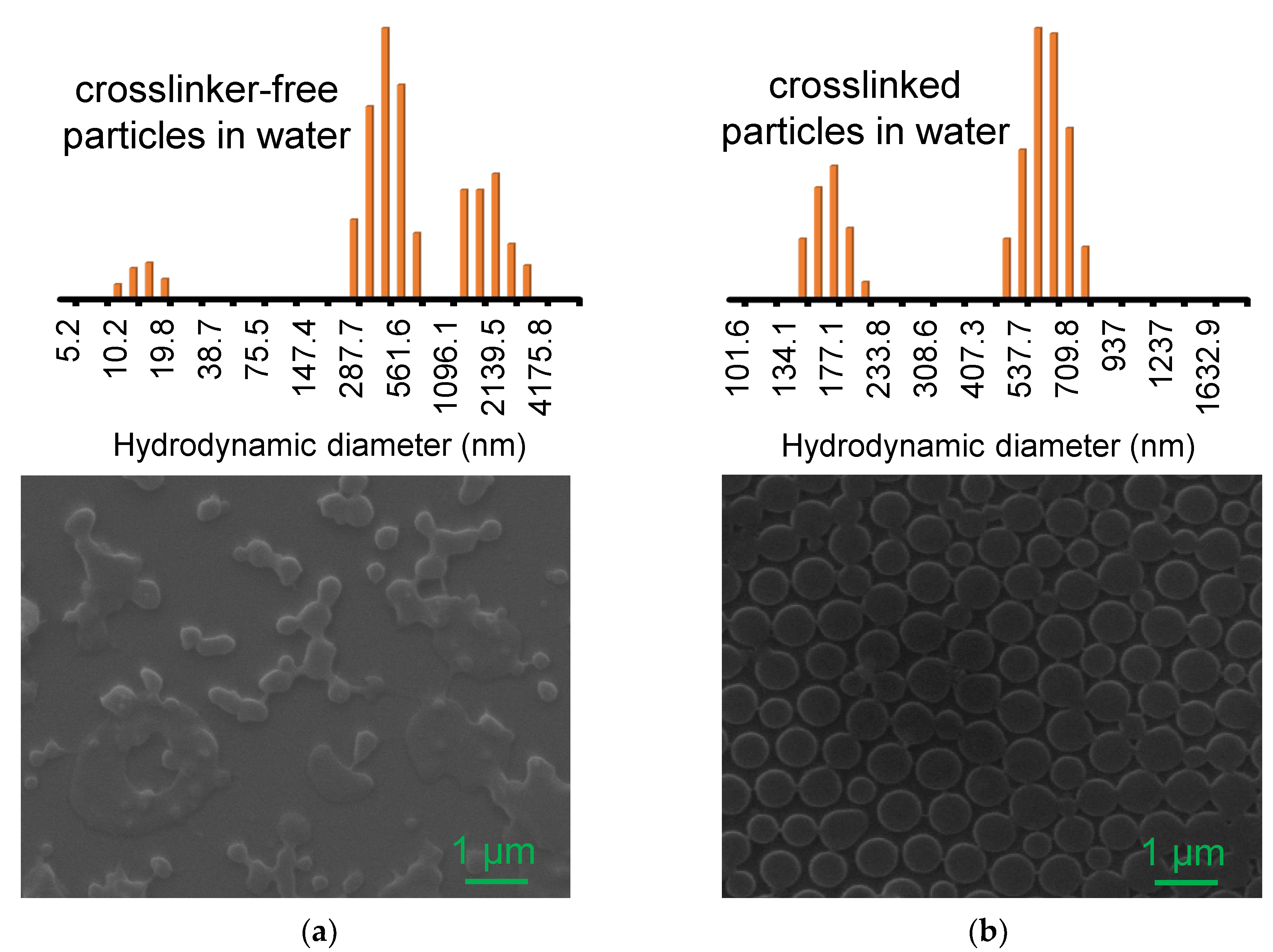
3.1. Formation of Crosslinker-Free and Crosslinked PNIPAM Particles
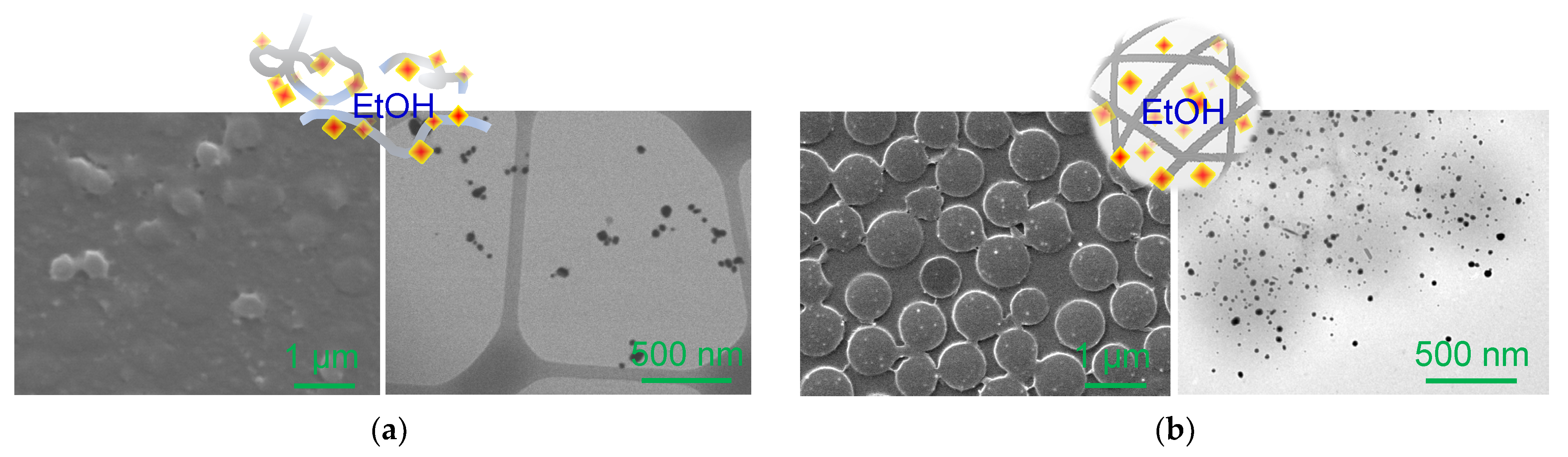
3.2. Incorporation of AuNPs into Crosslinker-Free and Crosslinked PNIPAM Particles
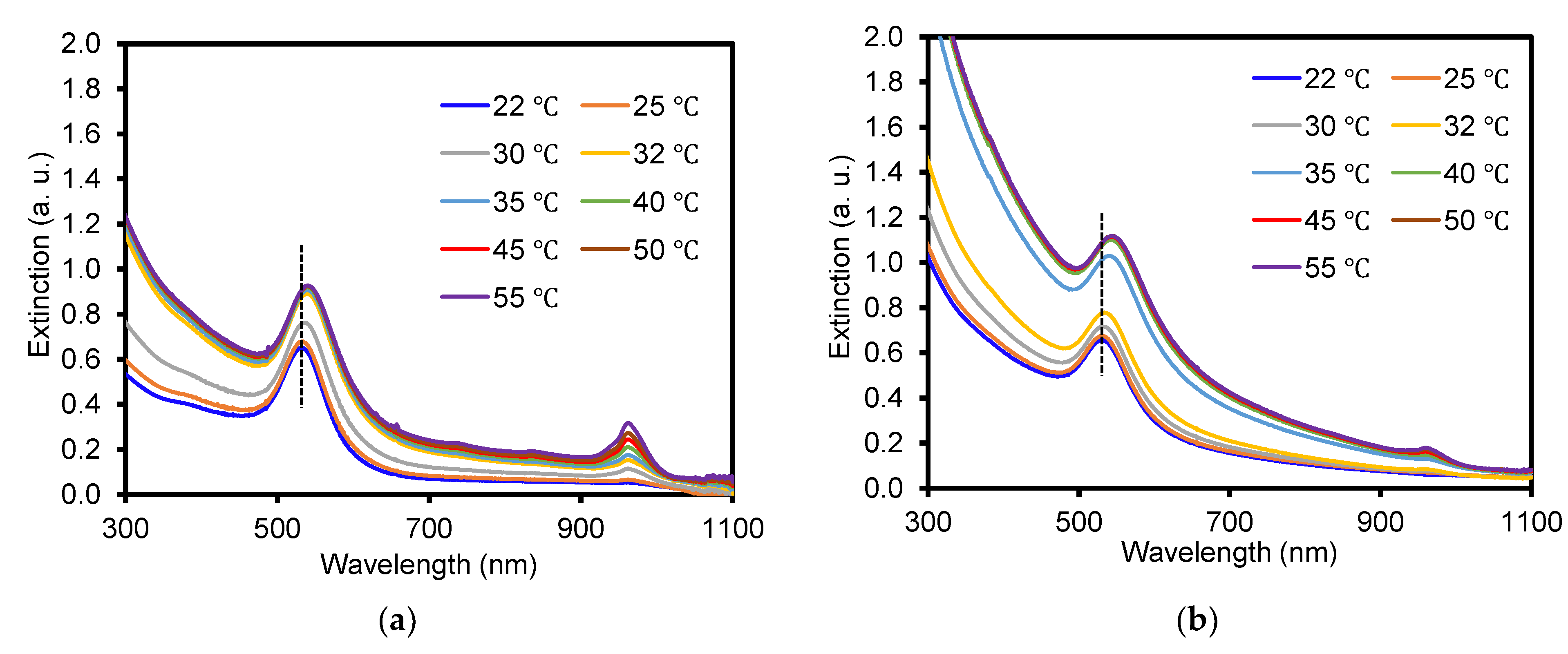
3.3. Physical Properties of Composite Particles Prepared from Crosslinker-Free and Crosslinked PNIPAM Particles
3.4. Catalytic Properties of Crosslinker-Free and Crosslinked Composite Particles in the Homocoupling Reaction of Phenylboronic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhol, P.; Mohanty, P.S. Smart microgel-metal hybrid particles of PNIPAM-co-PAA@AgAu: Synthesis, characterizations and modulated catalytic activity. J. Phys. Condens. Matter 2021, 33, 084002. [Google Scholar] [CrossRef] [PubMed]
- Buonerba, A.; Grassi, A. Trends in sustainable synthesis of organics by gold nanoparticles embedded in polymer matrices. Catalysts 2021, 11, 714. [Google Scholar] [CrossRef]
- Lu, J.; Yang, Y.; Gao, J.; Duan, H.; Lu, C. Thermoresponsive amphiphilic block copolymer-stablilized gold nanoparticles: Synthesis and high catalytic properties. Langmuir 2018, 34, 8205–8214. [Google Scholar] [CrossRef] [PubMed]
- Mohammadparast, F.; Dadgar, A.P.; Tirumala, R.T.A.; Mohammad, S.; Topal, C.O.; Kalkan, A.K.; Andiappan, M. C−C coupling reactions catalyzed by gold nanoparticles: Evidence for substrate-mediated leaching of surface atoms using localized surface plasmon resonance spectroscopy. J. Phys. Chem. C 2019, 123, 11539–11545. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Matveeva, V.G.; Bronstein, L.M. Role of polymer structures in catalysis by transition metal and metal oxide nanoparticle composites. Chem. Rev. 2020, 120, 1350–1396. [Google Scholar] [CrossRef]
- Hutchings, G.J. Heterogeneous gold catalysis. ACS Cent. Sci. 2018, 4, 1095–1101. [Google Scholar] [CrossRef] [Green Version]
- Fenger, R.; Fertitta, E.; Kirmse, H.; Thunemann, A.F.; Rademann, K. Size dependent catalysis with CTAB-stabilized gold nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9343–9349. [Google Scholar] [CrossRef]
- Guczi, L.; Beck, A.; Paszti, Z. Gold catalysis: Effect of particle size on reactivity towards various substrates. Catal. Today 2012, 181, 26–32. [Google Scholar] [CrossRef]
- Panigrahi, S.; Basu, S.; Praharaj, S.; Pande, S.; Jana, S.; Pal, A.; Ghosh, S.K.; Pal, T. Synthesis and size-selective catalysis by supported gold nanoparticles: Study on heterogeneous and homogeneous catalytic process. J. Phys. Chem. C 2007, 111, 4596–4605. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, Z.; Xu, H.; Li, G. Heterogeneous cross-coupling over gold nanoclusters. Nanomaterials 2019, 9, 838. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, J.; Polzer, F.; Drechsler, M.; Preussner, J. In situ growth of catalytic active Au−Pt bimetallic nanorods in thermoresponsive core−shell microgels. ACS Nano 2010, 4, 7078–7086. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Suwannarat, K.; Thongthai, K.; Ananta, S.; Srisombat, L. Synthesis of hollow trimettallic Ag/Au/Pd nanoparticles for reduction of 4-nitrophenol. Coll. Surf. A 2018, 540, 73–80. [Google Scholar] [CrossRef]
- Wan, J.; Fan, B.; Thang, S.H. Sonochemical preparation of polymer–metal nanocomposites with catalytic and plasmonic properties. Nanoscale Adv. 2021, 3, 3306–3315. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, C.M.; Sekine, K.; Koshikawa, T.; Tanaka, K.; Hashmi, A.S.K. Homogeneous and heterogeneous gold catalysis for materials science. Chem. Rev. 2021, 121, 9113–9163. [Google Scholar] [CrossRef]
- Reddy, K.P.; Murugadoss, A. Microcrystalline cellulose-supported gold nanoparticle catalysts for homocoupling of phenylboronic acids. Langmuir 2022, 38, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the support in gold-containing nanoparticles as heterogeneous catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef] [Green Version]
- Haleem, A.; Chen, J.; Guo, X.-X.; Wang, J.-Y.; Li, H.-J.; Li, P.-Y.; Chen, S.-Q.; He, W.-D. Hybrid cryogels composed of P(NIPAM-co-AMPS) and metal nanoparticles for rapid reduction of p-nitrophenol. Polymer 2020, 193, 122352. [Google Scholar] [CrossRef]
- Haleem, A.; Chen, S.-Q.; Ullah, M.; Siddiq, M.; He, W.-D. Highly porous cryogels loaded with bimetallic nanoparticles as an efficient antimicrobial agent and catalyst for rapid reduction of water-soluble organic contaminants. J. Environ. Chem. Eng. 2021, 9, 106510. [Google Scholar] [CrossRef]
- Carregal-Romero, S.; Buurma, N.J.; Pérez-Juste, J.; Liz-Marzán, L.M.; Hervés, P. Catalysis by Au@pNIPAM nanocomposites: Effect of the cross-linking density. Chem. Mater. 2010, 22, 3051–3059. [Google Scholar] [CrossRef]
- Harrison, A.; Vuong, T.T.; Zeevi, M.; Hittel, B.; Wi, S.; Tang, C. Rapid self-assembly of metal/polymer nanocomposite particles as nanoreactors and their kinetic characterization. Nanomaterials 2019, 9, 318. [Google Scholar] [CrossRef] [Green Version]
- Jang, W.; Taylor, R., IV; Eyimegwu, P.N.; Byun, H.; Kim, J.-H. In situ formation of gold nanoparticles within a polymer particle and their catalytic activities in various chemical reactions. ChemPhysChem 2019, 20, 70–77. [Google Scholar] [CrossRef]
- Arif, M.; Farooqi, Z.H.; Irfan, A.; Begum, R. Gold nanoparticles and polymer microgels: Last five years of their happy and successful marriage. J. Mol. Liq. 2021, 336, 116270. [Google Scholar] [CrossRef]
- Jang, W.; Byun, H.; Kim, J.-H. Encapsulated gold nanoparticles as a reactive quasi-homogeneous catalyst in base-free aerobic homocoupling reactions. ChemCatChem 2020, 12, 705–709. [Google Scholar] [CrossRef]
- Sepúlveda, A.; Picard-Lafond, A.; Marette, A.; Boudreau, D. Nucleation points: The forgotten parameter in the synthesis of hydrogel-coated gold nanoparticles. Polymers 2021, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Wongmanee, K.; Khuanamkam, S.; Chairam, S. Gold nanoparticles stabilized by starch polymer and their use as catalyst in homocoupling of phenylboronic acid. J. King Saud Univ. Sci. 2017, 29, 547–552. [Google Scholar] [CrossRef]
- Shah, L.A.; Haleem, A.; Sayed, M.; Siddiq, M. Synthesis of sensitive hybrid polymer microgels for catalytic reduction of organic pollutants. J. Environ. Chem. Eng. 2016, 4, 3492–3497. [Google Scholar] [CrossRef]
- Kim, J.-H.; Boote, B.W.; Pham, J.A.; Hu, J.; Byun, H. Thermally tunable catalytic and optical properties of gold–hydrogel nanocomposites. Nanotechnology 2012, 23, 275606. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Schurings, M.P.; van Rijn, P.; Pich, A. Formation of catalytically active gold–polymer microgel hybrids via a controlled in situ reductive process. J. Mater. Chem. A 2013, 1, 13244–13251. [Google Scholar] [CrossRef]
- Egemole, F.; Eyimegwu, F.M.; Yun, J.; Jang, W.; Byun, H.; Hou, J.; Kim, J.-H. Effects of crosslinking density on the in situ formation of gold-polymer composite particles and their catalytic properties. Coll. Surf. A 2022, 640, 128409. [Google Scholar] [CrossRef]
- Ilunga, A.K.; Meijboom, R. Catalytic oxidation of methylene blue by dendrimer encapsulated silver and gold nanoparticles. J. Mol. Catal. A Chem. 2016, 411, 48–60. [Google Scholar] [CrossRef]
- Eyimegwu, P.N.; Kim, J.-H. Atypical catalytic function of embedded gold nanoparticles by controlling structural features of polymer particle in alcohol-rich solvents. Nanotechnology 2019, 30, 285704. [Google Scholar] [CrossRef] [PubMed]
- Eyimegwu, P.N.; Lartey, J.A.; Kim, J.-H. Gold-nanoparticle-embedded poly(N-isopropylacrylamide) microparticles for selective quasi-homogeneous catalytic homocoupling reactions. ACS Appl. Nano Mater. 2019, 2, 6057–6066. [Google Scholar] [CrossRef]
- Jang, W.; Yun, J.; Eyimegwu, P.N.; Hou, J.; Byun, H.; Kim, J.-H. Controlling the formation of encapsulated gold nanoparticles for highly reactive catalysts in the homocoupling of phenylboronic acid. Catal. Today 2022, 388–389, 109–116. [Google Scholar] [CrossRef]
- Hu, J.; Brackemyer, C.A.; Byun, H.; Kim, J.-H. Enhanced stability of anisotropic gold nanoparticles by poly(N-isopropylacrylamide). J. Mater. Sci. Technol. 2014, 30, 441–448. [Google Scholar] [CrossRef]
- Murugadoss, A.; Khan, A. Stabilizer specific interaction of gold nanoparticles with a thermosensitive polymer hydrogel. J. Nanopart. Res. 2010, 12, 1331–1348. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lavin, B.W.; Burnett, R.D.; Boote, B.W. Controlled synthesis of gold nanoparticles by fluorescent light irradiation. Nanotechnology 2021, 22, 285602. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ryu, J.; Choi, W. Preparation of gold and platinum nanoparticles using visible light activated FeIII-complex. Chem. Lett. 2007, 36, 176–177. [Google Scholar] [CrossRef]
- Korir, D.K.; Gwalani, B.; Joseph, A.; Kamras, B.; Arvapally, R.K.; Omary, M.A.; Marpu, S.B. Facile photochemical syntheses of conjoined nanotwin gold-silver particles within a biologically-benign chitosan polymer. Nanomaterials 2019, 9, 596. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.-L.; Teow, S.-Y.; Pushpamalar, J. Application of metal nanoparticle–hydrogel composites in tissue regeneration. Bioengineering 2019, 6, 17. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–hydrogel composites: Concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Frisken, B.J. Cross-linker-free N-isopropylacrylamide gel nanospheres. Langmuir 2003, 19, 5212–5216. [Google Scholar] [CrossRef]
- Virtanen, O.L.J.; Mourran, A.; Pinard, P.T.; Richtering, W. Persulfate initiated ultra-low cross-linked poly(N-isopropylacrylamide) microgels possess an unusual inverted cross-linking structure. Soft Matter 2016, 12, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Xiangli, Q.; Zhenjia, Z.; Side, Y. Preparation of initiator and cross-linker-free poly(N-isopropylacrylamide) nanogels by photopolymerization. J. Photochem. Photobiol. A 2006, 177, 191–196. [Google Scholar] [CrossRef]
- Islam, M.R.; Tumbarello, M.; Lyon, L.A. Deswelling induced morphological changes in dual pH and temperature responsive ultra-low crosslinked poly(N-isopropyl acrylamide)-co-acrylic acid microgels. Colloid Polym. Sci. 2019, 297, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.H.; Cerussi, A.E.; Merritt, S.I.; Ruth, J.; Tromberg, B.J. Non-invasive tissue temperature measurements based on quantitative diffuse optical spectroscopy (DOS) of water. Phys. Med. Biol. 2010, 55, 3753–3765. [Google Scholar] [CrossRef]
- McCabe, W.C.; Fisher, H.F. Near-infrared spectroscopic method for investigating the hydration of a solute in aqueous solution. J. Phys. Chem. 1970, 74, 2990–2998. [Google Scholar] [CrossRef]
- Carrettin, S.; Guzman, J.; Corma, A. Supported gold catalyzes the homocoupling of phenylboronic acid with high conversion and selectivity. Angew. Chem. Int. Ed. 2005, 44, 2242–2245. [Google Scholar] [CrossRef]
- Parmentier, T.E.; Dawson, S.R.; Malta, G.; Lu, L.; Davies, T.E.; Kondrat, S.A.; Freakley, S.J.; Kiely, C.J.; Hutchings, G.J. Homocoupling of phenylboronic acid using atomically dispersed gold on carbon catalysts: Catalyst evolution before reaction. ChemCatChem 2018, 10, 1853–1859. [Google Scholar] [CrossRef]
- Prati, L.; Villa, A. Gold colloids: From quasi-homogeneous to heterogeneous catalytic systems. Acc. Chem. Res. 2013, 47, 855–863. [Google Scholar] [CrossRef]
- Tsunoyama, H.; Sakurai, H.; Ichikuni, N.; Negishi, Y.; Tsukuda, T. Colloidal gold nanoparticles as catalyst for carbon−carbon bond formation: Application to aerobic homocoupling of phenylboronic acid in water. Langmuir 2004, 20, 11293–11296. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, W.; Su, D.S.; Meng, X.; Xiao, F.-S. Supported Au nanoparticles as efficient catalysts for aerobic homocoupling of phenylboronic acid. Chem. Commun. 2012, 48, 5476–5478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willis, N.G.; Guzman, J. Influence of the support during homocoupling of phenylboronic acid catalyzed by supported gold. Appl. Catal. A 2008, 339, 68–75. [Google Scholar] [CrossRef]
- Hore, M.J.A.; Hammouda, B. Co-nonsolvency of poly(n-isopropylacrylamide) in deuterated water/ethanol mixtures. Macromolecules 2013, 46, 7894–7901. [Google Scholar] [CrossRef]
- Tavagnacco, L.; Zaccarelli, E.; Chiessi, E. Molecular description of the coil-to-globule transition of Poly(N-isopropylacrylamide) in water/ethanol mixture at low alcohol concentration. J. Mol. Liq. 2020, 297, 111928. [Google Scholar] [CrossRef]
- Perez-Ramirez, H.A.; Haro-Perez, C.; Odriozola, G. Effect of temperature on the cononsolvency of poly(N-isopropylacrylamide) (PNIPAM) in aqueous 1-propanol. ACS Appl. Polym. Mater. 2019, 1, 2961–2972. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Li, B.; Jang, W.; Yun, J.; Eyimegwu, F.M.; Kim, J.-H. Integration of Gold Nanoparticles into Crosslinker-Free Polymer Particles and Their Colloidal Catalytic Property. Nanomaterials 2023, 13, 416. https://doi.org/10.3390/nano13030416
Hou J, Li B, Jang W, Yun J, Eyimegwu FM, Kim J-H. Integration of Gold Nanoparticles into Crosslinker-Free Polymer Particles and Their Colloidal Catalytic Property. Nanomaterials. 2023; 13(3):416. https://doi.org/10.3390/nano13030416
Chicago/Turabian StyleHou, Jian, Bin Li, Wongi Jang, Jaehan Yun, Faith M. Eyimegwu, and Jun-Hyun Kim. 2023. "Integration of Gold Nanoparticles into Crosslinker-Free Polymer Particles and Their Colloidal Catalytic Property" Nanomaterials 13, no. 3: 416. https://doi.org/10.3390/nano13030416