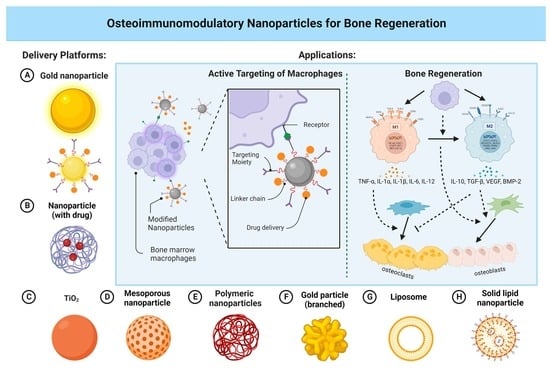
Osteoimmunomodulatory Nanoparticles for Bone Regeneration
Abstract
:1. Introduction
2. Bone Regeneration Process
3. Osteoimmunology in Bone Regeneration
4. Bioapplication of Nanoparticles
5. Application of NPs in Bone Regeneration
6. Applications of NPs in Osteoimmunomodulation
7. Conclusions and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, L.; Ma, Y.; Crawford, R.; Mendhi, J.; Zhang, Y.; Lu, H.; Zhao, Q.; Cao, J.; Wu, C.; Wang, X.; et al. The Interplay between Hemostasis and Immune Response in Biomaterial Development for Osteogenesis. Mater. Today 2022, 54, 202–224. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of Critical-Sized Bone Defects: Clinical and Tissue Engineering Perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Tal, H. (Ed.) Bone Regeneration; InTech: Rijeka, Croatia, 2012; ISBN 978-953-51-0487-2. [Google Scholar]
- Bhatt, R.A.; Rozental, T.D. Bone Graft Substitutes. Hand. Clin. 2012, 28, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Ball, A.N.; Donahue, S.W.; Wojda, S.J.; McIlwraith, C.W.; Kawcak, C.E.; Ehrhart, N.; Goodrich, L.R. The Challenges of Promoting Osteogenesis in Segmental Bone Defects and Osteoporosis: CHALLENGES OF PROMOTING OSTEOGENESIS. J. Orthop. Res. 2018, 36, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Lobb, D.C.; DeGeorge, B.R.; Chhabra, A.B. Bone Graft Substitutes: Current Concepts and Future Expectations. J. Hand Surg. 2019, 44, 497–505.e2. [Google Scholar] [CrossRef]
- Gillman, C.E.; Jayasuriya, A.C. FDA-Approved Bone Grafts and Bone Graft Substitute Devices in Bone Regeneration. Mater. Sci. Eng. C 2021, 130, 112466. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Schwartz, N.G.; Hernandez-Romieu, A.C.; Annambhotla, P.; Filardo, T.D.; Althomsons, S.P.; Free, R.J.; Li, R.; Wyatt Wilson, W.; Deutsch-Feldman, M.; Drees, M.; et al. Nationwide Tuberculosis Outbreak in the USA Linked to a Bone Graft Product: An Outbreak Report. Lancet Infect. Dis. 2022, 22, 1617–1625. [Google Scholar] [CrossRef]
- Imerb, N.; Thonusin, C.; Chattipakorn, N.; Chattipakorn, S.C. Aging, Obese-Insulin Resistance, and Bone Remodeling. Mech. Ageing Dev. 2020, 191, 111335. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the Nano/Microscale Structure of Biomaterial Scaffolds on Bone Regeneration. Int. J. Oral. Sci. 2020, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 922. [Google Scholar] [CrossRef] [PubMed]
- Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gonzalez, M.C.; Gallarate, M. Bone Diseases: Current Approach and Future Perspectives in Drug Delivery Systems for Bone Targeted Therapeutics. Nanomaterials 2020, 10, 875. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-H.; You, S.; Taghizadeh, A.; Taghizadeh, M.; Kim, H.S. Cell Membrane-Cloaked Nanotherapeutics for Targeted Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2223. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bachhuka, A.; Wei, F.; Wang, X.; Liu, G.; Vasilev, K.; Xiao, Y. Nanotopography-Based Strategy for the Precise Manipulation of Osteoimmunomodulation in Bone Regeneration. Nanoscale 2017, 9, 18129–18152. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture Healing under Healthy and Inflammatory Conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.R.; Garla, V.V. Fracture Healing Overview. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current Concepts of Molecular Aspects of Bone Healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone Morphogenetic Proteins in Tissue Engineering: The Road from Laboratory to Clinic, Part II (BMP Delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef]
- Carreira, A.C.; Lojudice, F.H.; Halcsik, E.; Navarro, R.D.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins: Facts, Challenges, and Future Perspectives. J. Dent. Res. 2014, 93, 335–345. [Google Scholar] [CrossRef]
- Osteoimmunology: Interactions of the Bone and Immune System|Endocrine Reviews|; Oxford Academic: Oxford, UK, 2008; Available online: https://academic.oup.com/edrv/article/29/4/403/2354973?login=false (accessed on 25 January 2023).
- Walsh, M.C.; Takegahara, N.; Kim, H.; Choi, Y. Updating Osteoimmunology: Regulation of Bone Cells by Innate and Adaptive Immunity. Nat. Rev. Rheumatol. 2018, 14, 146–156. [Google Scholar] [CrossRef]
- Crockett, J.C.; Mellis, D.J.; Scott, D.I.; Helfrich, M.H. New Knowledge on Critical Osteoclast Formation and Activation Pathways from Study of Rare Genetic Diseases of Osteoclasts: Focus on the RANK/RANKL Axis. Osteoporos. Int. 2011, 22, 1–20. [Google Scholar] [CrossRef]
- Zaheer, S.; LeBoff, M.; Lewiecki, E.M. Denosumab for the Treatment of Osteoporosis. Expert. Opin. Drug Metab. Toxicol. 2015, 11, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Könnecke, I.; Serra, A.; El Khassawna, T.; Schlundt, C.; Schell, H.; Hauser, A.; Ellinghaus, A.; Volk, H.-D.; Radbruch, A.; Duda, G.N.; et al. T and B Cells Participate in Bone Repair by Infiltrating the Fracture Callus in a Two-Wave Fashion. Bone 2014, 64, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Ohkura, N.; Sakaguchi, S. Molecular Determinants of Regulatory T Cell Development: The Essential Roles of Epigenetic Changes. Front. Immunol. 2013, 4, 106. [Google Scholar] [CrossRef]
- Monocyte and Macrophage Biology: An Overview-ClinicalKey. Available online: https://www.clinicalkey.com.au/#!/content/playContent/1-s2.0-S0270929510000525 (accessed on 25 January 2023).
- Wilson, H.M.; Barker, R.N.; Erwig, L.-P. Macrophages: Promising Targets for the Treatment of Atherosclerosis. Curr. Vasc. Pharmacol. 2009, 7, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Macrophage Polarization and Bone Formation: A Review; SpringerLink: Berlin, Germany, 2016; Available online: https://link.springer.com/article/10.1007/s12016-015-8519-2 (accessed on 25 January 2023).
- Guihard, P.; Danger, Y.; Brounais, B.; David, E.; Brion, R.; Delecrin, J.; Richards, C.D.; Chevalier, S.; Rédini, F.; Heymann, D. Induction of Osteogenesis in Mesenchymal Stem Cells by Activated Monocytes/Macrophages Depends on Oncostatin M Signaling. Stem. Cells 2012, 30, 762–772. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. Expanded Applications, Shifting Paradigms and an Improved Understanding of Host–Biomaterial Interactions. Acta Biomater. 2013, 9, 4948–4955. [Google Scholar] [CrossRef]
- Mokarram, N.; Bellamkonda, R.V. A Perspective on Immunomodulation and Tissue Repair. Ann. Biomed. Eng. 2014, 42, 338–351. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Awojoodu, A.O.; Ogle, M.E.; Sefcik, L.S.; Bowers, D.T.; Martin, K.; Brayman, K.L.; Lynch, K.R.; Peirce-Cottler, S.M.; Botchwey, E. Sphingosine 1-Phosphate Receptor 3 Regulates Recruitment of Anti-Inflammatory Monocytes to Microvessels during Implant Arteriogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 13785–13790. [Google Scholar] [CrossRef]
- Zhou, A.; Wu, B.; Yu, H.; Tang, Y.; Liu, J.; Jia, Y.; Yang, X.; Xiang, L. Current Understanding of Osteoimmunology in Certain Osteoimmune Diseases. Front. Cell Dev. Biol. 2021, 9, 698068. [Google Scholar] [CrossRef]
- Bains, P.S.; Sidhu, S.S.; Bahraminasab, M.; Prakash, C. (Eds.) Biomaterials in Orthopaedics and Bone Regeneration: Design and Synthesis; Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2019; ISBN 9789811399770. [Google Scholar]
- Mieszawska, A.J.; Kaplan, D.L. Smart Biomaterials-Regulating Cell Behavior through Signaling Molecules. BMC Biol. 2010, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Ahmed, W. Emerging Nanotechnologies in Dentistry: Materials, Processes, and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-812292-1. [Google Scholar]
- Čitaković, N.M. Physical Properties of Nanomaterials. Encycl. Nanosci. Nanotechnol. 2019, 67, 159–171. [Google Scholar] [CrossRef]
- Patil, S.P.; Burungale, V.V. 2-Physical and Chemical Properties of Nanomaterials. In Nanomedicines for Breast Cancer Theranostics; Thorat, N.D., Bauer, J., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–31. ISBN 978-0-12-820016-2. [Google Scholar]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Nanoparticles for Bone Tissue Engineering-Vieira-2017-Biotechnology Progress-Wiley Online Library. Available online: https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/btpr.2469?casa_token=gdFiIaTgL7gAAAAA%3AIf2hUMWXZC4FIyGCY-RrsVVjhoeEzfx4r22oy8Z1S-TkhTPN7S7PSRFBTUTKf0I3p13NUcOZsyijjLO2 (accessed on 1 February 2023).
- Crush, J.; Hussain, A.; Seah, K.T.M.; Khan, W.S. Bioactive Glass: Methods for Assessing Angiogenesis and Osteogenesis. Front. Cell Dev. Biol. 2021, 9, 643781. [Google Scholar] [CrossRef]
- Materials|Free Full-Text|Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Available online: https://www.mdpi.com/1996-1944/3/7/3867 (accessed on 1 February 2023).
- Nanostructured Hybrid Materials for Bone Tissue Regeneration: Ingenta Connect. Available online: https://www.ingentaconnect.com/content/ben/cnano/2006/00000002/00000003/art00003 (accessed on 1 February 2023).
- Eivazzadeh-Keihan, R.; Bahojb Noruzi, E.; Khanmohammadi Chenab, K.; Jafari, A.; Radinekiyan, F.; Hashemi, S.M.; Ahmadpour, F.; Behboudi, A.; Mosafer, J.; Mokhtarzadeh, A.; et al. Metal-based Nanoparticles for Bone Tissue Engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1687–1714. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tian, Y.; Ouyang, J.; Shen, Y.; Wang, X.; Luan, J. Carbon Nanomaterials for Drug Delivery and Tissue Engineering. Front. Chem. 2022, 10, 1106. [Google Scholar] [CrossRef]
- Baldelli, A.; Ou, J.; Li, W.; Amirfazli, A. Spray-On Nanocomposite Coatings: Wettability and Conductivity. Langmuir 2020, 36, 11393–11410. [Google Scholar] [CrossRef]
- Murthy, S.K. Nanoparticles in Modern Medicine: State of the Art and Future Challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Laschke, M.W.; Strohe, A.; Menger, M.D.; Alini, M.; Eglin, D. In Vitro and in Vivo Evaluation of a Novel Nanosize Hydroxyapatite Particles/Poly(Ester-Urethane) Composite Scaffold for Bone Tissue Engineering. Acta Biomater. 2010, 6, 2020–2027. [Google Scholar] [CrossRef]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent Advancements in Liposome Technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef]
- Liu, Y.; Castro Bravo, K.M.; Liu, J. Targeted Liposomal Drug Delivery: A Nanoscience and Biophysical Perspective. Nanoscale Horiz. 2021, 6, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, W.T.; Kostarelos, K. Liposomes: From a Clinically Established Drug Delivery System to a Nanoparticle Platform for Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Thamake, S.I.; Raut, S.L.; Gryczynski, Z.; Ranjan, A.P.; Vishwanatha, J.K. Alendronate Coated Poly-Lactic-Co-Glycolic Acid (PLGA) Nanoparticles for Active Targeting of Metastatic Breast Cancer. Biomaterials 2012, 33, 7164–7173. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhao, Y.; Wang, X.; Lin, T. Layer-by-Layer Assembly of Silica Nanoparticles on 3D Fibrous Scaffolds: Enhancement of Osteoblast Cell Adhesion, Proliferation, and Differentiation. J. Biomed. Mater. Res. A 2014, 102, 3803–3812. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous Silica Nanoparticles in Drug Delivery and Biomedical Applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Gounani, Z.; Asadollahi, M.A.; Pedersen, J.N.; Lyngsø, J.; Skov Pedersen, J.; Arpanaei, A.; Meyer, R.L. Mesoporous Silica Nanoparticles Carrying Multiple Antibiotics Provide Enhanced Synergistic Effect and Improved Biocompatibility. Colloids Surf. B Biointerfaces 2019, 175, 498–508. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, J.-H.; Kim, H.-W. Osteoinductive Fibrous Scaffolds of Biopolymer/Mesoporous Bioactive Glass Nanocarriers with Excellent Bioactivity and Long-Term Delivery of Osteogenic Drug. ACS Appl. Mater. Interfaces 2015, 7, 1140–1152. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Wu, Y.; Chapman, S.; Ding, Y. Synthesis, Structure Evolution, and Optical Properties of Gold Nanobones. Res. Chem. Intermed 2019, 45, 3973–3983. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Chen, C. Remote Control and Modulation of Cellular Events by Plasmonic Gold Nanoparticles: Implications and Opportunities for Biomedical Applications. ACS Nano 2017, 11, 2403–2409. [Google Scholar] [CrossRef]
- Kairdolf, B.A.; Qian, X.; Nie, S. Bioconjugated Nanoparticles for Biosensing, in Vivo Imaging, and Medical Diagnostics. Anal. Chem. 2017, 89, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and Bone Regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef] [PubMed]
- Dyondi, D.; Webster, T.J.; Banerjee, R. A Nanoparticulate Injectable Hydrogel as a Tissue Engineering Scaffold for Multiple Growth Factor Delivery for Bone Regeneration. Int. J. Nanomed. 2013, 8, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Weir, M.D.; Simon, C.G. Injectable and Strong Nano-Apatite Scaffolds for Cell/Growth Factor Delivery and Bone Regeneration. Dent. Mater. 2008, 24, 1212–1222. [Google Scholar] [CrossRef]
- Cai, L.; Guinn, A.S.; Wang, S. Exposed Hydroxyapatite Particles on the Surface of Photo-Crosslinked Nanocomposites for Promoting MC3T3 Cell Proliferation and Differentiation. Acta Biomater. 2011, 7, 2185–2199. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, Y.; Cui, W.; Li, Y.; Xiao, D.; Zhou, R. Nanomaterials-Based Cell Osteogenic Differentiation and Bone Regeneration. Curr. Stem Cell Res. Ther. 2021, 16, 36–47. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, W.; Wen, X.; He, B.; Zeng, X.; Wang, G.; Gu, Z. A Novel Calcium Phosphate Ceramic–Magnetic Nanoparticle Composite as a Potential Bone Substitute. Biomed. Mater. 2010, 5, 015001. [Google Scholar] [CrossRef]
- Lv, L.; Liu, Y.; Zhang, P.; Zhang, X.; Liu, J.; Chen, T.; Su, P.; Li, H.; Zhou, Y. The Nanoscale Geometry of TiO2 Nanotubes Influences the Osteogenic Differentiation of Human Adipose-Derived Stem Cells by Modulating H3K4 Trimethylation. Biomaterials 2015, 39, 193–205. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Mihaila, S.M.; Swami, A.; Patel, A.; Sant, S.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Bioactive Silicate Nanoplatelets for Osteogenic Differentiation of Human Mesenchymal Stem Cells. Adv. Mater. 2013, 25, 3329–3336. [Google Scholar] [CrossRef]
- Kim, B.-S.; Yang, S.-S.; Kim, C.S. Incorporation of BMP-2 Nanoparticles on the Surface of a 3D-Printed Hydroxyapatite Scaffold Using an ε-Polycaprolactone Polymer Emulsion Coating Method for Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2018, 170, 421–429. [Google Scholar] [CrossRef]
- Wang, L.; Xu, W.; Chen, Y.; Wang, J. Alveolar Bone Repair of Rhesus Monkeys by Using BMP-2 Gene and Mesenchymal Stem Cells Loaded Three-Dimensional Printed Bioglass Scaffold. Sci. Rep. 2019, 9, 18175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poth, N.; Seiffart, V.; Gross, G.; Menzel, H.; Dempwolf, W. Biodegradable Chitosan Nanoparticle Coatings on Titanium for the Delivery of BMP-2. Biomolecules 2015, 5, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Moradikhah, F.; Doosti-Telgerd, M.; Shabani, I.; Soheili, S.; Dolatyar, B.; Seyedjafari, E. Microfluidic Fabrication of Alendronate-Loaded Chitosan Nanoparticles for Enhanced Osteogenic Differentiation of Stem Cells. Life Sci. 2020, 254, 117768. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, Y.; Kuang, Y.; Xu, S.; Lu, Q.; Diao, J.; Zhao, N. Hierarchically Porous Calcium–Silicon Nanosphere-Enabled Co-Delivery of MicroRNA-210 and Simvastatin for Bone Regeneration. J. Mater. Chem. B 2021, 9, 3573–3583. [Google Scholar] [CrossRef] [PubMed]
- Seddighian, A.; Ganji, F.; Baghaban-Eslaminejad, M.; Bagheri, F. Electrospun PCL Scaffold Modified with Chitosan Nanoparticles for Enhanced Bone Regeneration. Prog. Biomater. 2021, 10, 65–76. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Chandran, S.V.; Arumugam, B.; Saravanan, S.; Venkatasubbu, G.D.; Selvamurugan, N. Chitosan/Nano-Hydroxyapatite/Nano-Zirconium Dioxide Scaffolds with MiR-590-5p for Bone Regeneration. Int. J. Biol. Macromol. 2018, 111, 953–958. [Google Scholar] [CrossRef]
- Bu, W.; Xu, X.; Wang, Z.; Jin, N.; Liu, L.; Liu, J.; Zhu, S.; Zhang, K.; Jelinek, R.; Zhou, D. Ascorbic Acid-PEI Carbon Dots with Osteogenic Effects as MiR-2861 Carriers to Effectively Enhance Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 50287–50302. [Google Scholar] [CrossRef]
- Cenni, E.; Granchi, D.; Avnet, S.; Fotia, C.; Salerno, M.; Micieli, D.; Sarpietro, M.G.; Pignatello, R.; Castelli, F.; Baldini, N. Biocompatibility of Poly(d,l-Lactide-Co-Glycolide) Nanoparticles Conjugated with Alendronate. Biomaterials 2008, 29, 1400–1411. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Lee, J.-S.; Ryu, T.-K.; Kang, R.-H.; Jeong, K.-Y.; Jun, D.-R.; Koh, J.-M.; Kim, S.-E.; Choi, S.-W. Alendronate-Modified Hydroxyapatite Nanoparticles for Bone-Specific Dual Delivery of Drug and Bone Mineral. Macromol. Res. 2016, 24, 623–628. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Y.; Chen, X.; Zeng, C.; Hu, Q.; Yin, W.; Li, W.; Xie, H.; Zhang, B.; Huang, X.; et al. Nanoparticle-Modified Chitosan-Agarose-Gelatin Scaffold for Sustained Release of SDF-1 and BMP-2. Int. J. Nanomed. 2018, 13, 7395–7408. [Google Scholar] [CrossRef]
- Heparin-Regulated Release of Growth Factors in Vitro and Angiogenic Response in Vivo to Implanted Hyaluronan Hydrogels Containing VEGF and BFGF-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S014296120600456X (accessed on 24 September 2022).
- Kang, W.; Yun, Y.-R.; Lee, D.-S.; Kim, T.-H.; Kim, J.-H.; Kim, H.-W.; Jang, J.-H. Fluorescence-Based Retention Assays Reveals Sustained Release of Vascular Endothelial Growth Factor from Bone Grafts. J. Biomed. Mater. Res. Part A 2016, 104, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Geuze, R.E.; Theyse, L.F.; Kempen, D.H.; Hazewinkel, H.A.; Kraak, H.Y.; Öner, F.C.; Dhert, W.J.; Alblas, J. A Differential Effect of Bone Morphogenetic Protein-2 and Vascular Endothelial Growth Factor Release Timing on Osteogenesis at Ectopic and Orthotopic Sites in a Large-Animal Model. Tissue Eng. Part A 2012, 18, 2052–2062. [Google Scholar] [CrossRef]
- Cao, L.; Kong, X.; Lin, S.; Zhang, S.; Wang, J.; Liu, C.; Jiang, X. Synergistic Effects of Dual Growth Factor Delivery from Composite Hydrogels Incorporating 2-N, 6-O-Sulphated Chitosan on Bone Regeneration. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1–S17. [Google Scholar] [CrossRef]
- Talavera-Adame, D.; Wu, G.; He, Y.; Ng, T.T.; Gupta, A.; Kurtovic, S.; Hwang, J.Y.; Farkas, D.L.; Dafoe, D.C. Endothelial Cells in Co-Culture Enhance Embryonic Stem Cell Differentiation to Pancreatic Progenitors and Insulin-Producing Cells through BMP Signaling. Stem Cell Rev. Rep. 2011, 7, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Young Park, J.; Shim, J.-H.; Choi, S.-A.; Jang, J.; Kim, M.; Hwa Lee, S.; Cho, D.-W. 3D Printing Technology to Control BMP-2 and VEGF Delivery Spatially and Temporally to Promote Large-Volume Bone Regeneration. J. Mater. Chem. B 2015, 3, 5415–5425. [Google Scholar] [CrossRef]
- Kempen, D.H.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J. Effect of Local Sequential VEGF and BMP-2 Delivery on Ectopic and Orthotopic Bone Regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.D.; Zhou, G.; Liu, H.W.; Zhang, J.; Liu, M.L.; Xiao, X.F.; Fei, J.J.; Guan, X.L.; Fan, Y.B. Sequential Releasing of VEGF and BMP-2 in Hydroxyapatite Collagen Scaffolds for Bone Tissue Engineering: Design and Characterization. Int. J. Biol. Macromol. 2019, 123, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Kupikowska-Stobba, B.; Kasprzak, M. Fabrication of Nanoparticles for Bone Regeneration: New Insight into Applications of Nanoemulsion Technology. J. Mater. Chem. B 2021, 9, 5221–5244. [Google Scholar] [CrossRef]
- Nirmala, R.; Sheikh, F.A.; Kanjwal, M.A.; Lee, J.H.; Park, S.-J.; Navamathavan, R.; Kim, H.Y. Synthesis and Characterization of Bovine Femur Bone Hydroxyapatite Containing Silver Nanoparticles for the Biomedical Applications. J. Nanopart. Res. 2011, 13, 1917–1927. [Google Scholar] [CrossRef]
- Baba Ismail, Y.M.; Ferreira, A.M.; Bretcanu, O.; Dalgarno, K.; El Haj, A.J. Polyelectrolyte Multi-Layers Assembly of SiCHA Nanopowders and Collagen Type I on Aminolysed PLA Films to Enhance Cell-Material Interactions. Colloids Surf. B Biointerfaces 2017, 159, 445–453. [Google Scholar] [CrossRef]
- Narciso, A.M.; da Rosa, C.G.; Nunes, M.R.; Sganzerla, W.G.; Hansen, C.M.; de Melo, A.P.Z.; Paes, J.V.; Bertoldi, F.C.; Barreto, P.L.M.; Masiero, A.V. Antimicrobial Green Silver Nanoparticles in Bone Grafts Functionalization for Biomedical Applications. Biocatal. Agric. Biotechnol. 2021, 35, 102074. [Google Scholar] [CrossRef]
- Cheng, H.; Chawla, A.; Yang, Y.; Li, Y.; Zhang, J.; Jang, H.L.; Khademhosseini, A. Development of Nanomaterials for Bone-Targeted Drug Delivery. Drug Discov. Today 2017, 22, 1336–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cornel, E.J.; He, S.; Du, J. Recent Advances in Bone-Targeting Nanoparticles for Biomedical Applications. Mater. Chem. Front. 2021, 5, 6735–6759. [Google Scholar] [CrossRef]
- Ossipov, D.A. Bisphosphonate-Modified Biomaterials for Drug Delivery and Bone Tissue Engineering. Expert Opin. Drug Deliv. 2015, 12, 1443–1458. [Google Scholar] [CrossRef]
- Leu, C.-T.; Luegmayr, E.; Freedman, L.P.; Rodan, G.A.; Reszka, A.A. Relative Binding Affinities of Bisphosphonates for Human Bone and Relationship to Antiresorptive Efficacy. Bone 2006, 38, 628–636. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Deng, T.; Yao, P.; Song, H.; Zhou, S.; Yan, W. Development of Drug Loaded Nanoparticles Binding to Hydroxyapatite Based on a Bisphosphonate Modified Nonionic Surfactant. J. Nanomater. 2015, 16, 145. Available online: https://dl.acm.org/doi/abs/10.1155/2015/393968 (accessed on 24 September 2022). [CrossRef]
- de Miguel, L.; Noiray, M.; Surpateanu, G.; Iorga, B.I.; Ponchel, G. Poly(γ-Benzyl-l-Glutamate)-PEG-Alendronate Multivalent Nanoparticles for Bone Targeting. Int. J. Pharm. 2014, 460, 73–82. [Google Scholar] [CrossRef]
- Lee, M.-S.; Su, C.-M.; Yeh, J.-C.; Wu, P.-R.; Tsai, T.-Y.; Lou, S.-L. Synthesis of Composite Magnetic Nanoparticles Fe3O4 with Alendronate for Osteoporosis Treatment. Int. J. Nanomed. 2016, 11, 4583–4594. [Google Scholar] [CrossRef]
- Piao, H.; Kim, M.H.; Cui, M.; Choi, G.; Choy, J.-H. Alendronate-Anionic Clay Nanohybrid for Enhanced Osteogenic Proliferation and Differentiation. J. Korean Med. Sci. 2019, 34, e37. [Google Scholar] [CrossRef]
- Sadeghi, L.; Tanwir, F.; Yousefi Babadi, V. Antioxidant Effects of Alfalfa Can Improve Iron Oxide Nanoparticle Damage: Invivo and Invitro Studies. Regul. Toxicol. Pharmacol. 2016, 81, 39–46. [Google Scholar] [CrossRef]
- Malachowski, T.; Hassel, A. Engineering Nanoparticles to Overcome Immunological Barriers for Enhanced Drug Delivery. Eng. Regen. 2020, 1, 35–50. [Google Scholar] [CrossRef]
- Park, M.V.D.Z.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The Effect of Particle Size on the Cytotoxicity, Inflammation, Developmental Toxicity and Genotoxicity of Silver Nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef] [PubMed]
- Cao, H. Silver Nanoparticles for Antibacterial Devices: Biocompatibility and Toxicity; Taylor & Francis Group: London, UK, 2017; ISBN 978-1-315-35347-0. [Google Scholar]
- Chen, Z.; Klein, T.; Murray, R.Z.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. Osteoimmunomodulation for the Development of Advanced Bone Biomaterials. Mater. Today 2016, 19, 304–321. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, C.; Feng, Y.; Li, D.; Ai, T.; Huang, Y.; Chen, X.; Huang, L.; Tan, J. Osteoimmunomodulatory Effects of Biomaterial Modification Strategies on Macrophage Polarization and Bone Regeneration. Regen. Biomater. 2020, 7, 233–245. [Google Scholar] [CrossRef]
- Song, G.; SPetschauer, J.; JMadden, A.; CZamboni, W. Nanoparticles and the Mononuclear Phagocyte System: Pharmacokinetics and Applications for Inflammatory Diseases. Curr. Rheumatol. Rev. 2014, 10, 22–34. [Google Scholar] [CrossRef]
- Liu, Y.; Hardie, J.; Zhang, X.; Rotello, V.M. Effects of Engineered Nanoparticles on the Innate Immune System. Semin. Immunol. 2017, 34, 25–32. [Google Scholar] [CrossRef]
- Zhao, Y.-J.; Gao, Z.-C.; He, X.-J.; Li, J. The Let-7f-5p–Nme4 Pathway Mediates Tumor Necrosis Factor α-Induced Impairment in Osteogenesis of Bone Marrow-Derived Mesenchymal Stem Cells. Biochem. Cell Biol. 2021, 99, 488–498. [Google Scholar] [CrossRef]
- Ono, T.; Takayanagi, H. Osteoimmunology in Bone Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 367–375. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Sun, L.; Gao, W.; Xiong, Y.; Ma, A.; Liu, X.; Shen, L.; Li, Q.; Yang, H. Manipulation of Macrophage Polarization by Peptide-Coated Gold Nanoparticles and Its Protective Effects on Acute Lung Injury. J. Nanobiotechnol. 2020, 18, 38. [Google Scholar] [CrossRef]
- Rifas, L. T-Cell Cytokine Induction of BMP-2 Regulates Human Mesenchymal Stromal Cell Differentiation and Mineralization. J. Cell. Biochem. 2006, 98, 706–714. [Google Scholar] [CrossRef]
- Freytes, D.O.; Wan, L.Q.; Vunjak-Novakovic, G. Geometry and Force Control of Cell Function. J. Cell. Biochem. 2009, 108, 1047–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schett, G. Osteoimmunology in Rheumatic Diseases. Arthritis Res. Ther. 2009, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yu, Y.; Ma, P.; Luo, Z.; Hu, Y.; Li, M.; He, Y.; Zhang, Y.; Peng, Z.; Song, G. Titania Nanotubes Promote Osteogenesis via Mediating Crosstalk between Macrophages and MSCs under Oxidative Stress. Colloids Surf. B Biointerfaces 2019, 180, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Kim, Y.-J.; Jang, J.-H.; Park, J.-W. Modulating Macrophage Polarization with Divalent Cations in Nanostructured Titanium Implant Surfaces. Nanotechnology 2016, 27, 085101. [Google Scholar] [CrossRef] [PubMed]
- Chigurupati, S.; Mughal, M.R.; Okun, E.; Das, S.; Kumar, A.; McCaffery, M.; Seal, S.; Mattson, M.P. Effects of Cerium Oxide Nanoparticles on the Growth of Keratinocytes, Fibroblasts and Vascular Endothelial Cells in Cutaneous Wound Healing. Biomaterials 2013, 34, 2194–2201. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, S.; Han, S.; Crawford, R.; Lu, S.; Wei, F.; Chang, J.; Wu, C.; Xiao, Y. Nanoporous Microstructures Mediate Osteogenesis by Modulating the Osteo-Immune Response of Macrophages. Nanoscale 2017, 9, 706–718. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, C.; Wang, X.; Shi, M.; Zhu, Y.; Jing, L.; Wu, C.; Chang, J. Stimulation of Osteogenesis and Angiogenesis by Micro/Nano Hierarchical Hydroxyapatite via Macrophage Immunomodulation. Nanoscale 2019, 11, 17699–17708. [Google Scholar] [CrossRef]
- Almatroudi, A. Silver Nanoparticles: Synthesis, Characterisation and Biomedical Applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium Surface Characteristics, Including Topography and Wettability, Alter Macrophage Activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef]
- Cai, D.; Gao, W.; Li, Z.; Zhang, Y.; Xiao, L.; Xiao, Y. Current Development of Nano-Drug Delivery to Target Macrophages. Biomedicines 2022, 10, 1203. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, F.; Huang, D.; Fu, X.; Li, X.; Chen, X. Strontium-Substituted Submicrometer Bioactive Glasses Modulate Macrophage Responses for Improved Bone Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 30747–30758. [Google Scholar] [CrossRef]
- Lin, R.; Deng, C.; Li, X.; Liu, Y.; Zhang, M.; Qin, C.; Yao, Q.; Wang, L.; Wu, C. Copper-Incorporated Bioactive Glass-Ceramics Inducing Anti-Inflammatory Phenotype and Regeneration of Cartilage/Bone Interface. Theranostics 2019, 9, 6300–6313. [Google Scholar] [CrossRef] [PubMed]
- Bartneck, M.; Ritz, T.; Keul, H.A.; Wambach, M.; Bornemann, J.; Gbureck, U.; Ehling, J.; Lammers, T.; Heymann, F.; Gassler, N. Peptide-Functionalized Gold Nanorods Increase Liver Injury in Hepatitis. Acs Nano 2012, 6, 8767–8777. [Google Scholar] [CrossRef]
- Raimondo, T.M.; Mooney, D.J. Functional Muscle Recovery with Nanoparticle-Directed M2 Macrophage Polarization in Mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10648–10653. [Google Scholar] [CrossRef]
- Li, K.; Shen, Q.; Xie, Y.; You, M.; Huang, L.; Zheng, X. Incorporation of Cerium Oxide into Hydroxyapatite Coating Regulates Osteogenic Activity of Mesenchymal Stem Cell and Macrophage Polarization. J. Biomater. Appl. 2017, 31, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Nanostructured Surface Modification to Bone Implants for Bone Reg...: Ingenta Connect. Available online: https://www.ingentaconnect.com/contentone/asp/jbn/2018/00000014/00000004/art00002 (accessed on 1 February 2023).
- Zhang, W.; Huang, D.; Zhao, F.; Gao, W.; Sun, L.; Li, X.; Chen, X. Synergistic Effect of Strontium and Silicon in Strontium-Substituted Sub-Micron Bioactive Glass for Enhanced Osteogenesis. Mater. Sci. Eng. C 2018, 89, 245–255. [Google Scholar] [CrossRef]
- IL-4 Administration Exerts Preventive Effects via Suppression of Underlying Inflammation and TNF-α-Induced Apoptosis in Steroid-Induced Osteonecrosis; SpringerLink: Berlin, Germany, 2016; Available online: https://link.springer.com/article/10.1007/s00198-015-3474-6 (accessed on 24 September 2022).
- He, X.-T.; Li, X.; Xia, Y.; Yin, Y.; Wu, R.-X.; Sun, H.-H.; Chen, F.-M. Building Capacity for Macrophage Modulation and Stem Cell Recruitment in High-Stiffness Hydrogels for Complex Periodontal Regeneration: Experimental Studies in Vitro and in Rats. Acta Biomater. 2019, 88, 162–180. [Google Scholar] [CrossRef]
- Kwon, D.; Cha, B.G.; Cho, Y.; Min, J.; Park, E.-B.; Kang, S.-J.; Kim, J. Extra-Large Pore Mesoporous Silica Nanoparticles for Directing in Vivo M2 Macrophage Polarization by Delivering IL-4. Nano Lett. 2017, 17, 2747–2756. [Google Scholar] [CrossRef]
- Hughes, J.E.; Srinivasan, S.R.; Hedrick, C.C. Sphingosine-1-Phosphate Induces an Anti-Inflammatory Phenotype in Macrophages During Inflammation. FASEB J. 2017, 21, A772. Available online: https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fasebj.21.6.A772-b (accessed on 24 September 2022).
- Das, A.; Segar, C.E.; Hughley, B.B.; Bowers, D.T.; Botchwey, E.A. The Promotion of Mandibular Defect Healing by the Targeting of S1P Receptors and the Recruitment of Alternatively Activated Macrophages. Biomaterials 2013, 34, 9853–9862. [Google Scholar] [CrossRef]
- Etzerodt, A.; Moestrup, S.K. CD163 and Inflammation: Biological, Diagnostic, and Therapeutic Aspects. Antioxid. Redox Signal. 2013, 18, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Vazquez, P.A.; Bernal, L.; Paige, C.A.; Grosick, R.L.; Vilrriales, C.M.; Ferreira, D.W.; Ulecia-Morón, C.; Romero-Sandoval, E.A. Macrophage-Specific Nanotechnology-Driven CD163 Overexpression in Human Macrophages Results in an M2 Phenotype under Inflammatory Conditions. Immunobiology 2017, 222, 900–912. [Google Scholar] [CrossRef]
- Saleh, B.; Dhaliwal, H.K.; Portillo-Lara, R.; Shirzaei Sani, E.; Abdi, R.; Amiji, M.M.; Annabi, N. Local Immunomodulation Using an Adhesive Hydrogel Loaded with MiRNA-Laden Nanoparticles Promotes Wound Healing. Small 2019, 15, 1902232. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Zhao, Q.; Li, W.; Zhao, Z.; Wang, J.; Deng, T.; Zhang, P.; Shen, K.; Li, Z.; Zhang, Y. Biomimetic Anti-Inflammatory Nano-Capsule Serves as a Cytokine Blocker and M2 Polarization Inducer for Bone Tissue Repair. Acta Biomater. 2020, 102, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiao, L.; Prasadam, I.; Crawford, R.; Zhou, Y.; Xiao, Y. Inflammatory Macrophages Interrupt Osteocyte Maturation and Mineralization via Regulating the Notch Signaling Pathway. Mol. Med. 2022, 28, 102. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Xie, H.; Fang, J.; Shen, J.; Li, W.; Shen, D.; Wu, J.; Wu, S.; Liu, X.; Zheng, Y. Sequential Activation of Heterogeneous Macrophage Phenotypes Is Essential for Biomaterials-Induced Bone Regeneration. Biomaterials 2021, 276, 121038. [Google Scholar] [CrossRef]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential Delivery of Immunomodulatory Cytokines to Facilitate the M1-to-M2 Transition of Macrophages and Enhance Vascularization of Bone Scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef]
- Cai, X.; Yin, Y.; Li, N.; Zhu, D.; Zhang, J.; Zhang, C.-Y.; Zen, K. Re-Polarization of Tumor-Associated Macrophages to pro-Inflammatory M1 Macrophages by MicroRNA-155. J. Mol. Cell Biol. 2012, 4, 341–343. [Google Scholar] [CrossRef]
- Jablonski, K.A.; Gaudet, A.D.; Amici, S.A.; Popovich, P.G.; Guerau-de-Arellano, M. Control of the Inflammatory Macrophage Transcriptional Signature by MiR-155. PloS ONE 2016, 11, e0159724. [Google Scholar] [CrossRef]
- MicroRNA-155 Inhibits Polarization of Macrophages to M2-Type and Suppresses Choroidal Neovascularization; SpringerLink: Berlin, Germany, 2018; Available online: https://link.springer.com/article/10.1007/s10753-017-0672-8 (accessed on 25 September 2022).
- Li, X.; Xue, S.; Zhan, Q.; Sun, X.; Chen, N.; Li, S.; Zhao, J.; Hou, X.; Yuan, X. Sequential Delivery of Different MicroRNA Nanocarriers Facilitates the M1-to-M2 Transition of Macrophages. ACS Omega 2022, 7, 8174–8183. [Google Scholar] [CrossRef]
- Gao, H.; Dai, W.; Zhao, L.; Min, J.; Wang, F. The Role of Zinc and Zinc Homeostasis in Macrophage Function. J. Immunol. Res. 2018, 2018, e6872621. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-J.; Bao, S.; Gálvez-Peralta, M.; Pyle, C.J.; Rudawsky, A.C.; Pavlovicz, R.E.; Killilea, D.W.; Li, C.; Nebert, D.W.; Wewers, M.D.; et al. ZIP8 Regulates Host Defense through Zinc-Mediated Inhibition of NF-ΚB. Cell Rep. 2013, 3, 386–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Liu, W.; Xu, L.; Ye, Q.; Zhou, H.; Berg, C.; Yuan, H.; Li, J.; Xia, W. Sequential Macrophage Transition Facilitates Endogenous Bone Regeneration Induced by Zn-Doped Porous Microcrystalline Bioactive Glass. J. Mater. Chem. B 2021, 9, 2885–2898. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, J.; Yu, K.; Yang, S.; Sun, T.; Ji, Y.; Xiong, Z.; Guo, X. Surface Modified Small Intestinal Submucosa Membrane Manipulates Sequential Immunomodulation Coupled with Enhanced Angio-and Osteogenesis towards Ameliorative Guided Bone Regeneration. Mater. Sci. Eng. C 2021, 119, 111641. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. Surface Engineering of Nanomaterials with Polymers, Biomolecules, and Small Ligands for Nanomedicine. Materials 2022, 15, 3251. [Google Scholar] [CrossRef]






| In Vitro and In Vivo Assays | Physical and Chemical Characterization | Biocompatibility Evaluation and Biomechanical Analysis |
|---|---|---|
| Cell culture-based assays (osteoblast, osteoclast, macrophage) Enzyme-linked immunosorbent assays (ELISA) Alkaline phosphatase activity assays Mineralization assays | X-ray diffraction (XRD) Transmission electron microscopy (TEM) Scanning electron microscopy (SEM) Dynamic light scattering (DLS) | Cytotoxicity assays (MTT, LDH) Hemocompatibility assays (hemolysis, platelet activation) Inflammation assays (IL-1β, TNF-α, IL-6) |
| Implantation studies in animal models (rats, mice, rabbits) Histological analysis (bone formation and resorption) Micro-computed tomography (μCT) Bone density measurements | Fourier transform infrared spectroscopy (FTIR) Energy-dispersive X-ray spectroscopy (EDS) X-ray fluorescence (XRF) Nuclear magnetic resonance spectroscopy (NMR) | Contact angle measurements Zeta potential measurements Surface roughness analysis Compression tests Tensile tests Indentation tests |
| Strategies for Regulating Macrophage Polarization | Applications of NPs in Osteoimunomodulation | References |
|---|---|---|
| Intrinsic properties | Gold, TiO2, and cerium oxide (CeO2) NPs can enhance M2 polarization. | [116,117,118] |
| Nanopore structure and pore size | NPs with pores of larger size (100 and 200 nm) were highly anti-inflammatory and inhibited M1 polarization. | [119] |
| The nanoneedle structure induced M2 polarization. The micropattern sizes of 12 μm and 36 μm in the micro/nano hierarchy enhanced M2 polarization. | [120] | |
| Surface roughness | Ti with smooth surface stimulated M1 activation. Ti with rough surface enhanced M2 polarization. | [122] |
| Composition | Gold NPs fused hexapeptides Cys-Leu-Pro-Phe-Phe-Asp, peptide arginine-glycine-aspartic acid (RGD), and IL-4 could stimulate M2 polarization. | [112,126,127] |
| CeO2 NPs with hydroxyapatite could enhance M2 polarization. | [128] | |
| Strontium (Sr)- or copper (Cu)-doped bioactive glass particles promoted M2 polarization and enhanced osteogenesis. | [124,125] | |
| Drug delivery | Various nanocarriers have delivered IL-4 (anti-inflammatory cytokine) to induce M2 polarization. | [131,132,133] |
| NPs can deliver S1P synthetic analog to direct macrophage polarization toward M2. | [134] | |
| CD163 gene has been encapsulated into polyethyleneimine NPs decorated with a mannose ligand to induce CD163 expression and macrophage polarization toward M2. | [137] | |
| miR-223 5p mimic was delivered to induce macrophage polarization to M2. | [138] | |
| Resolvin D1-loaded gold nanocages (AuNC) were coated with M1-like macrophage membranes to enhance M2 polarization. | [139] | |
| A sequential release of therapeutics induces the M1-to-M2 phenotype switch during tissue regeneration. | Spillar et al. designed a scaffold that achieved a sequential release of first IFN-γ and then IL-4 to modulate macrophage polarization from early stage M1 to later-stage M2. | [142] |
| NPs carry both miRNA-155 and miRNA-21 to sequentially stimulate macrophage polarization first toward M1 and then the M2 phenotype. | [146] | |
| Microcrystalline bioactive glass scaffolds with different doses of ZnO orchestrate the sequential M1-to-M2 macrophage polarization. | [149] | |
| Sr-substituted nanohydroxyapatite (nano-SrHA) coatings and IFN-γ to the surface of native SIS membrane control a sequential M1-M2 macrophage transition. | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Cai, D.; Gao, W.; He, R.; Li, Y.; Zhou, Y.; Klein, T.; Xiao, L.; Xiao, Y. Osteoimmunomodulatory Nanoparticles for Bone Regeneration. Nanomaterials 2023, 13, 692. https://doi.org/10.3390/nano13040692
Wen J, Cai D, Gao W, He R, Li Y, Zhou Y, Klein T, Xiao L, Xiao Y. Osteoimmunomodulatory Nanoparticles for Bone Regeneration. Nanomaterials. 2023; 13(4):692. https://doi.org/10.3390/nano13040692
Chicago/Turabian StyleWen, Jingyi, Donglin Cai, Wendong Gao, Ruiying He, Yulin Li, Yinghong Zhou, Travis Klein, Lan Xiao, and Yin Xiao. 2023. "Osteoimmunomodulatory Nanoparticles for Bone Regeneration" Nanomaterials 13, no. 4: 692. https://doi.org/10.3390/nano13040692