Nanofluids for Direct-Absorption Solar Collectors—DASCs: A Review on Recent Progress and Future Perspectives
Abstract
:1. Introduction
2. Nanoparticles Used for DASC Nanofluids
2.1. A Classification of Nanoparticles Used for Nanofluids
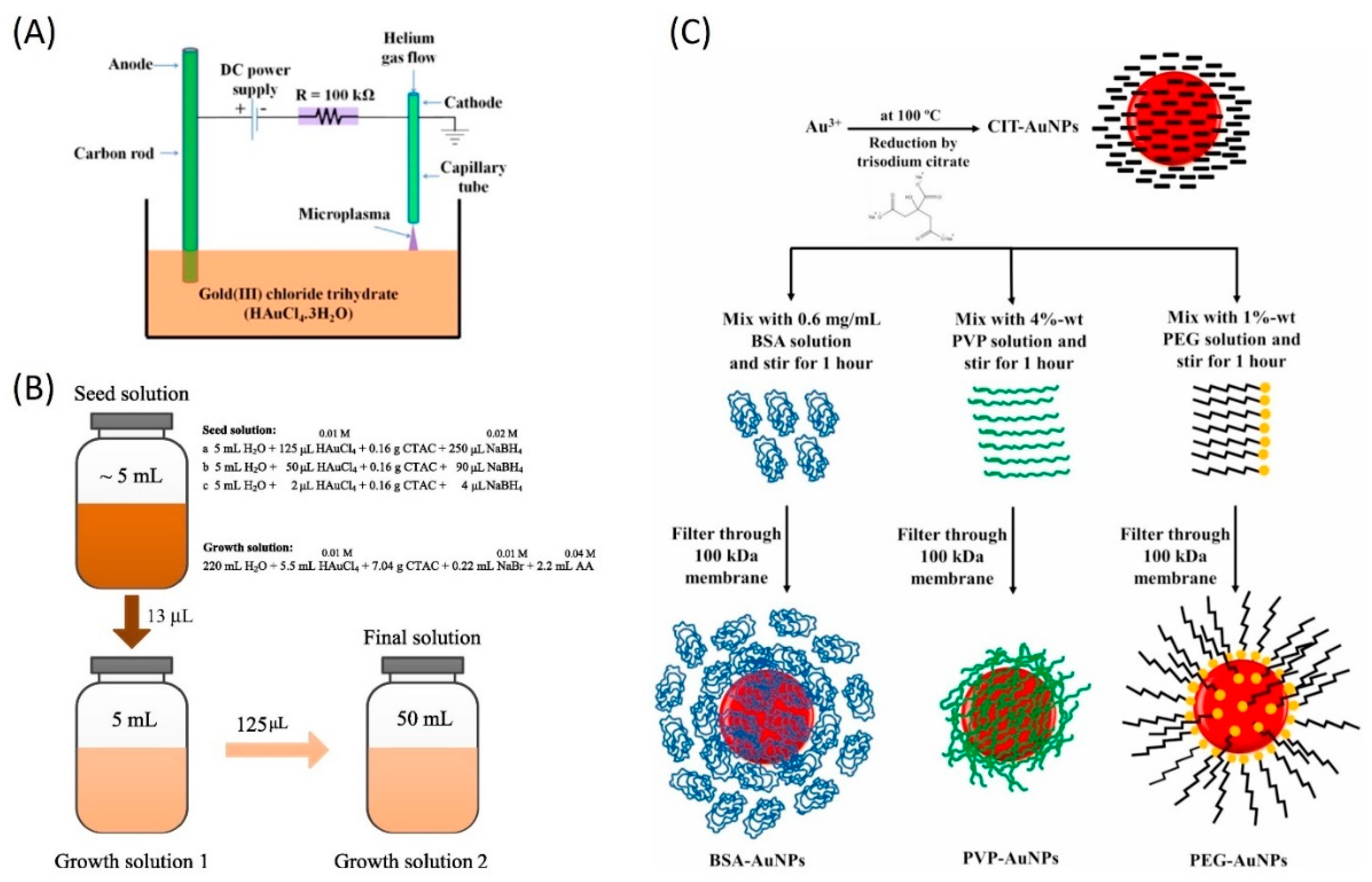
2.2. Metallic Nanoparticles
2.3. Carbon-Based Nanomaterials
2.4. Metal Oxide Nanoparticles
| Nanofluid | NP Synthesis Method | NP Morphology | NP Loading | Stabilizer | NF Preparation | Measured Optical Property | Stability Tests | [Ref] Year |
|---|---|---|---|---|---|---|---|---|
| Plasma-induced nonequilibrium electrochemistry (PiNE) synthesis at atmospheric pressure |
|
| Electrostatic stabilization | Heat drying of the as-prepared samples followed by sonication in EG | Absorption and scattering | 11 weeks of ambient storage | [61] 2020 |
| Au NPs/water | Seed-mediated synthesis method | Average sizes of 25 nm, 33 nm, and 40 nm | 0.07 mg/L, 0.18 mg/L, and 0.39 mg/L | CTAC surfactant and ultrasonication (30 min at 20 °C) | Ultrasonication for 30 min at 20 °C | Absorbance | 16 h of ambient storage and heating at 90 °C | [58] 2016 |
| Au NPs/water | Citrate, PEG, PVP, and BSA coatings | - | 0.1611 mg/mL | PEG, PVP, and BSA dispersants | The excessive polymer was filtered, and samples were diluted | - | 3 years of ambient storage, continuous irradiation, and cyclic irradiation | [60] 2021 |
| TiN/EG | Commercial | 30 nm diameter | 0.01, 0.001, 0.003, 0.005, and 0.007 wt.% | - | 30 min sonication and dilution | - | - | [43] 2021 |
| ATO–Ag/water | Chemical reduction | Nonspherical of an average size <40 nm | 0.01–0.2 wt% | - | Dispersion in distilled water by adding SDS surfactant | Extinction | - | [76] 2020 |
| A reducing–oxidizing method with adding citrate followed by Coating Ag NPs with SiO2 using TEOS |
| 6 mL |
|
| - | 3 weeks of ambient storage and solar radiation (simulated and real) | [62] 2020 |
|
| - | 0.05–1 vol%. | - | Direct dispersion and centrifuge | Extinction | - | [73] 2016 |
| Commercial | 60 nm (110 nm after heating) | 0.5–2 wt% | - | Mechanical stirring and sonication for 30 min at 130 W | - | - | [74] 2022 |
| Commercial | 20–50 nm diameter | 0.001, 0.01, and 0.02 vol% | SDBS dispersant | Ultrasonication for 20–60 min at 300 W | - | - | [75] 2022 |
|
| Less than 10 nm dimeter | - | PEG-200 | Stirring of 22 mL (∼500 rpm) | - | One month of ambient storage | [68] 2022 |
|
|
|
| - |
| - | - | [69] 2022 |
| Each material was chemically functionalized by acid and potassium persulfate (KPS) | - | - |
|
| - | Working temperature change (80–250 °C) | [70] 2016 |
|
| Near-spherical particles of 10 nm diameter |
|
| The as-made samples were diluted after post-synthesis purification |
| 16 months of ambient storage and heating for 12 h | [78] 2019 |
| Commercial | 5–10 nm and 20–30 nm inner and outer diameters and 10–30 μm length
| 10, 20, and 30 ppm | CTAB surfactant | Magnetic stirring at 800 r/min for 30 min
| - | One week of ambient storage | [79] 2022 |
| Commercial |
| - | TPABr surfactant and sulfuric acid treatment | - | - | - | [80] 2016 |
| SiC–MWCNTs/EG | - |
| 0.01–1 wt% | PVP–K30 and hexane dispersant and ultrasonication for 1 h | - | - | One month of ambient storage, mass fraction, temperature, and thermal cycling | [81] 2020 |
| Commercial | Nanoplates of 2 nm thickness and 2 µm diameter | 0.00025–0.005 wt% | - | - | Extinction | - | [82] 2016 |
| - |
|
| - | - | Absorbance | - | [73] 2016 |
| - |
| 40 ppm | - | - | Extinction | - | [80] 2016 |
| - |
| - | TEOS shell, Na2SiO3 shell, and citrate coating | - | - | Cyclic heating and cyclic UV radiation | [83] 2018 |
| - |
| 0.1 and 0.3 vol% | - | - | Absorption and scattering | - | [84] 2014 |
| - |
|
| SHMP surfactant, pH control, and ultrasonication | - | Extinction | Two weeks of ambient storage | [85] 2016 |
| ZrC/water | - | 40 nm diameter | - | Ultrasonication for 10 min) | - | - | - | [86] 2019 |
| - | GO: nanosheets of 0.55–1.2 nm thickness and 0.5–3 µm size | - | PVP | - | - | Two months of ambient storage | [87] 2017 |
| - | - | - |
| - | - | Two months of ambient storage | [88] 2019 |
| - | 20 nm diameter and 1–25 µm length | - | SDBS, CTAB, SDS, and Triton X-100 | - | - |
| [89] 2018 |
| - | - |
| - | - | - | - | [90] 2021 |
|
|
|
|
|
|
|
| [29] 2023 |
|
|
|
|
| - |
|
| [59] 2023 |
|
|
|
| - | - | - |
| [66] 2023 |
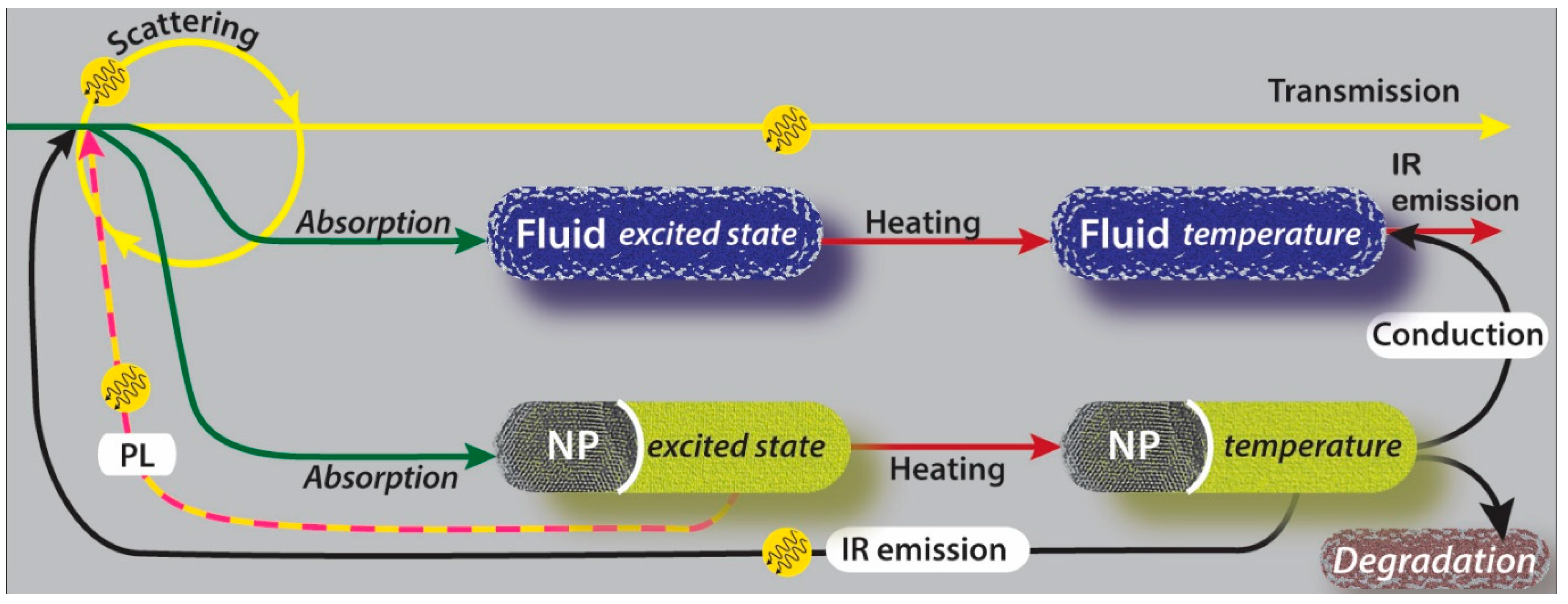
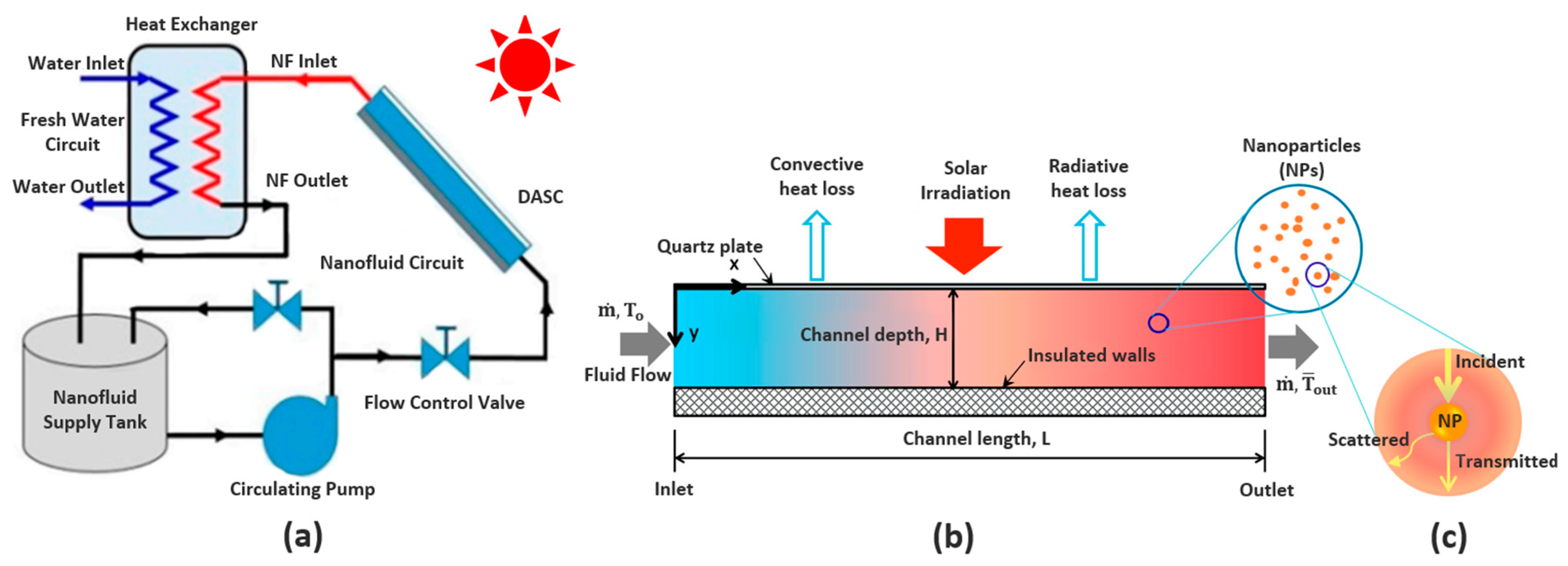
3. Energy Flow in Solar Thermal Nanofluids Used in DASCs
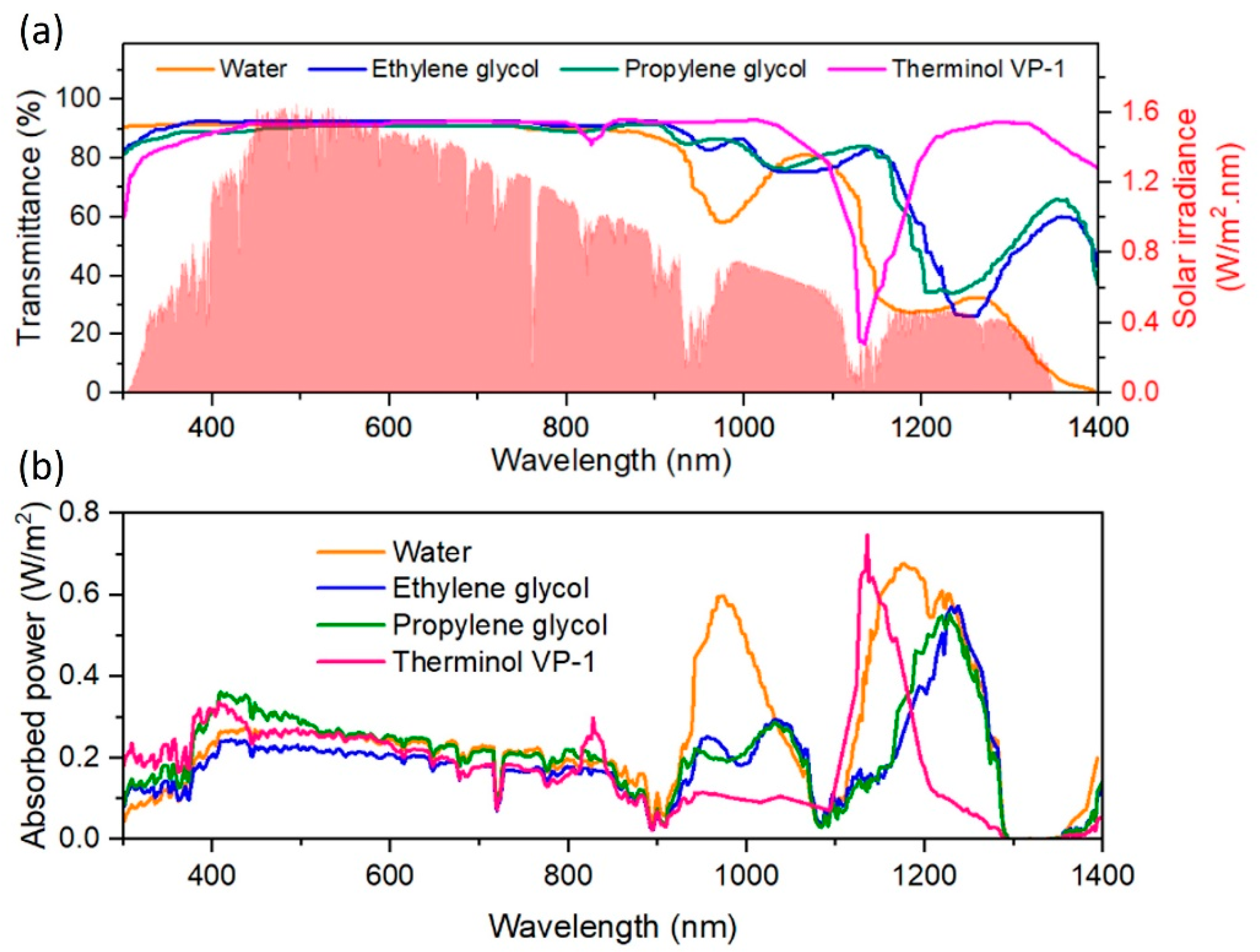
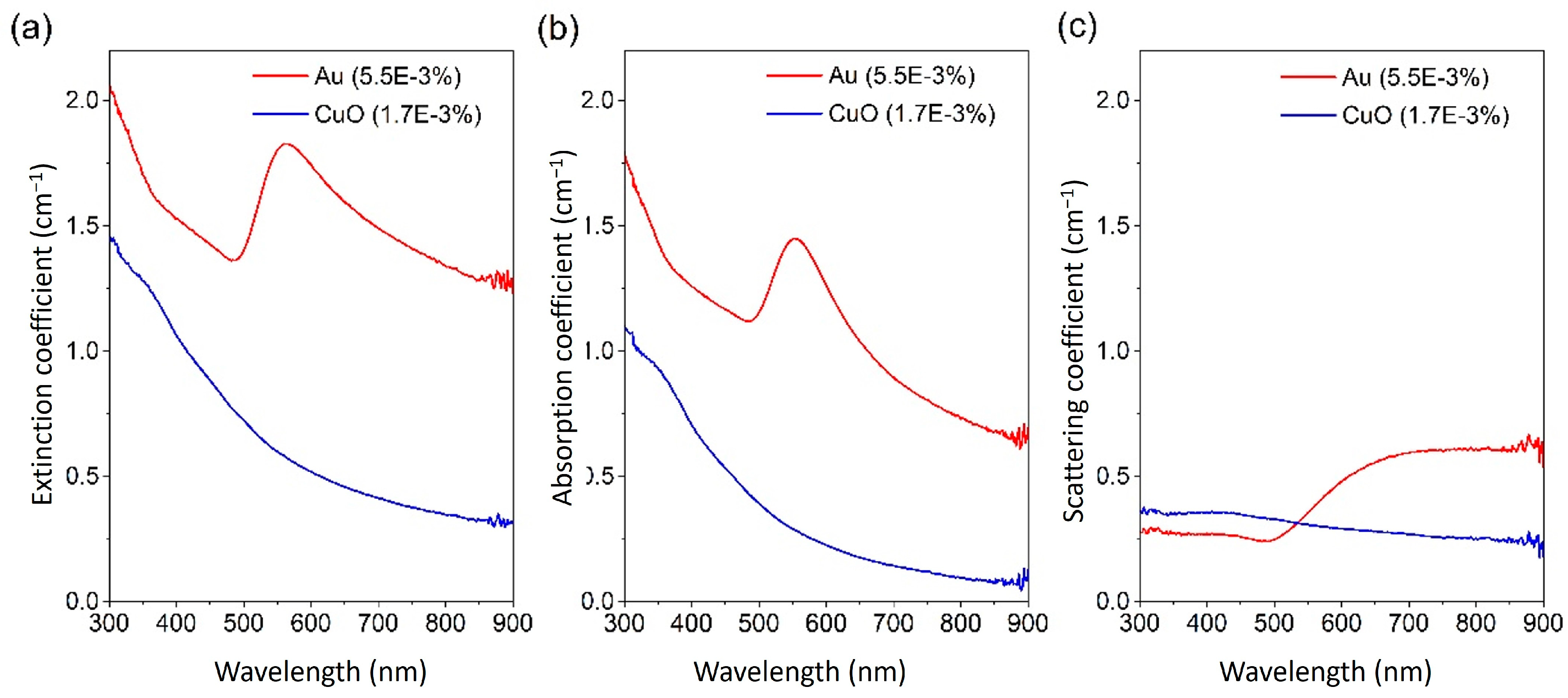
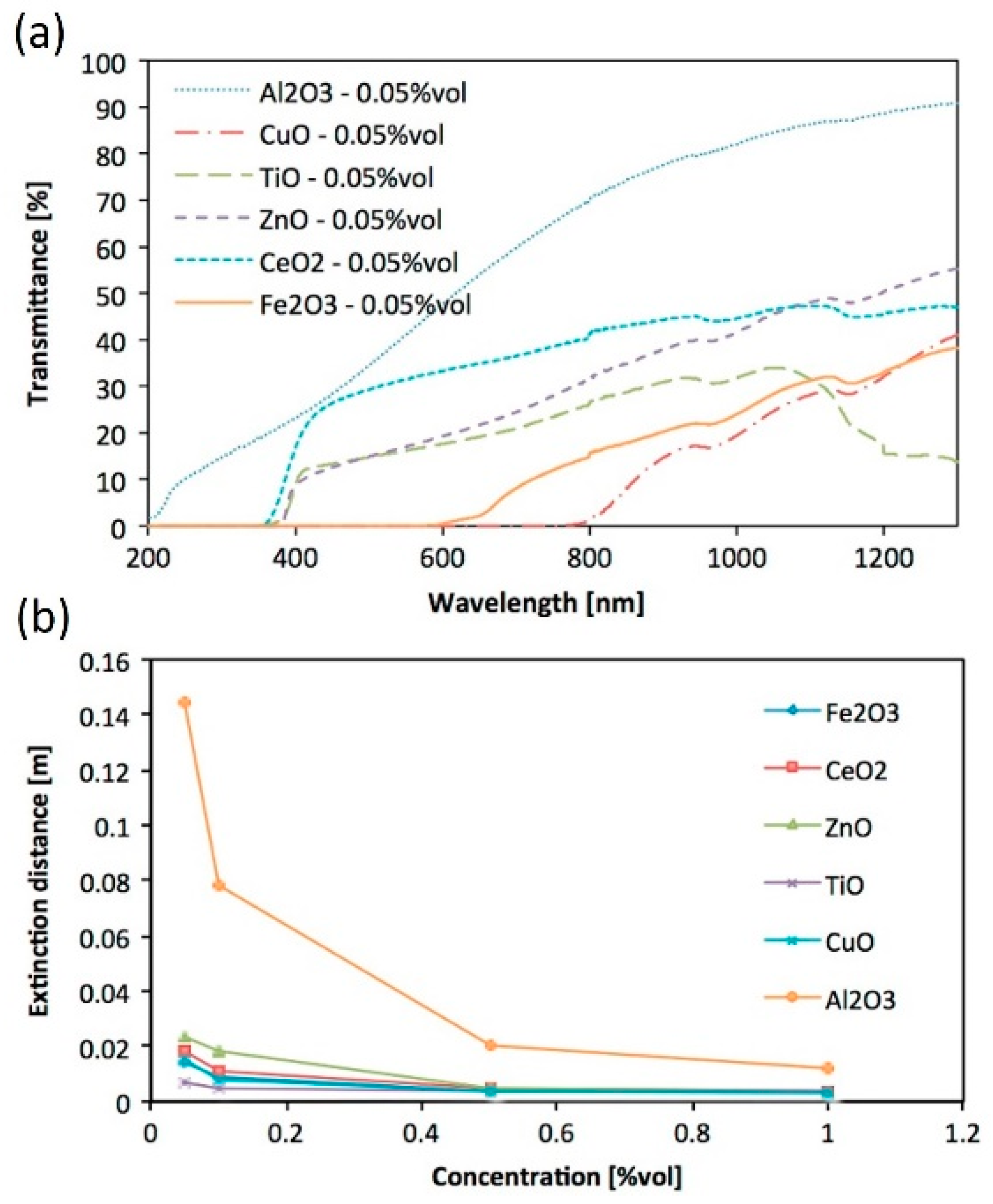
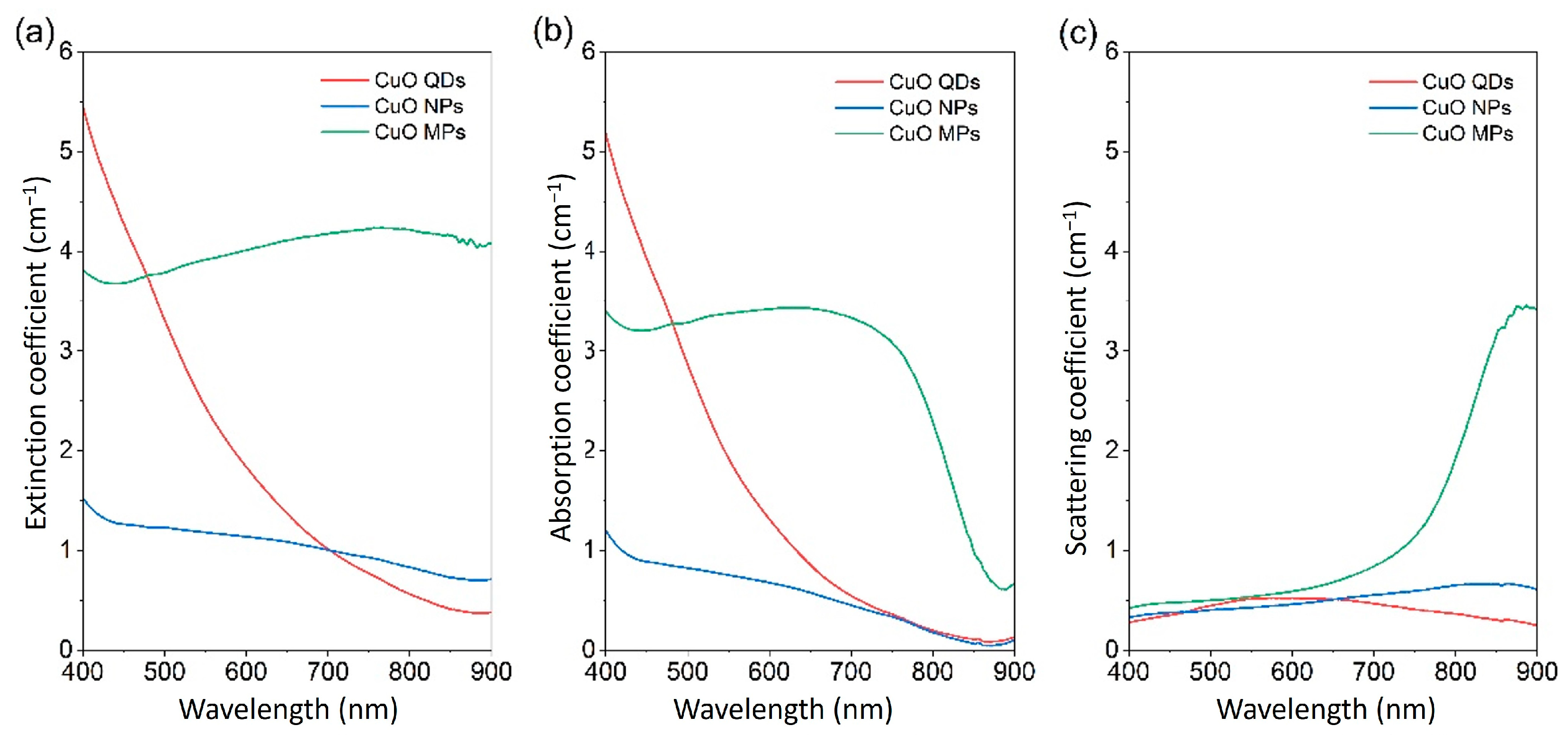
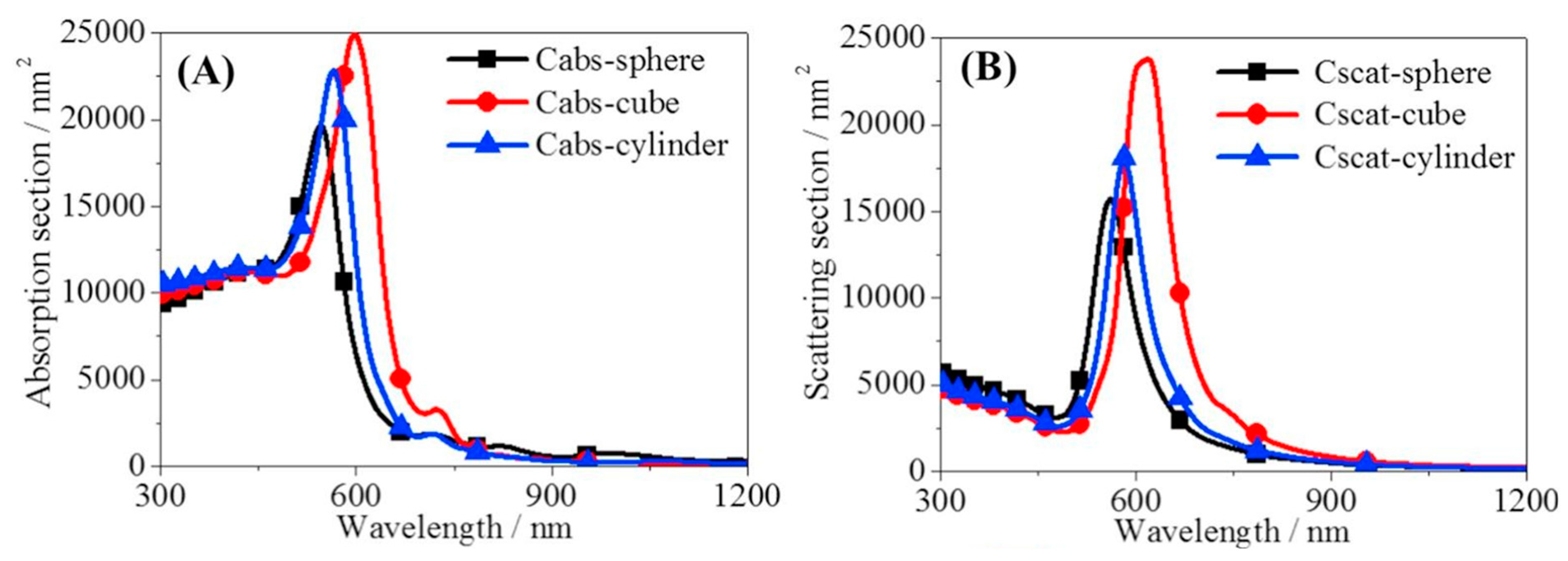
4. Fundamental Optical Characterisation of Nanofluids for DASCs
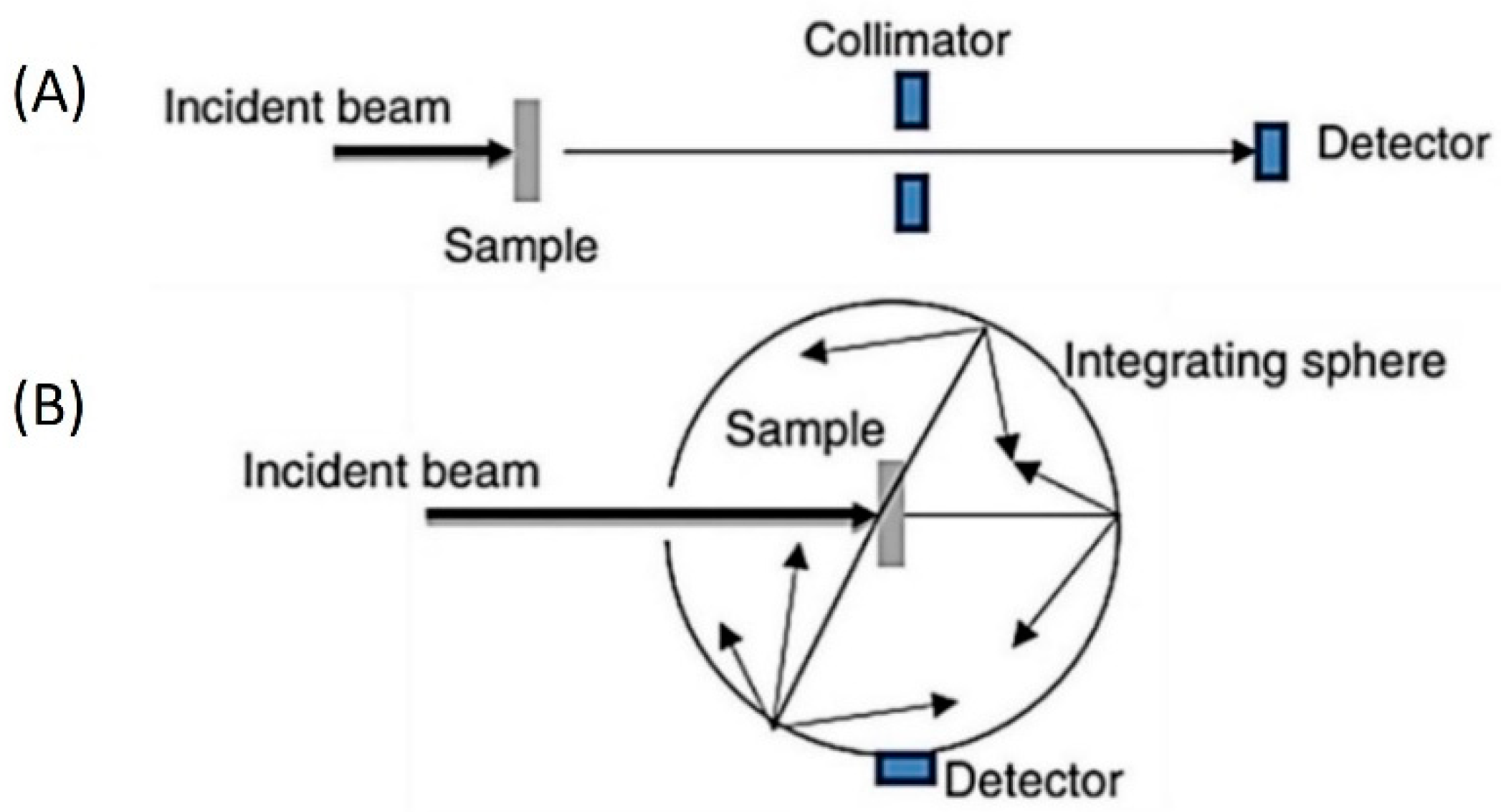
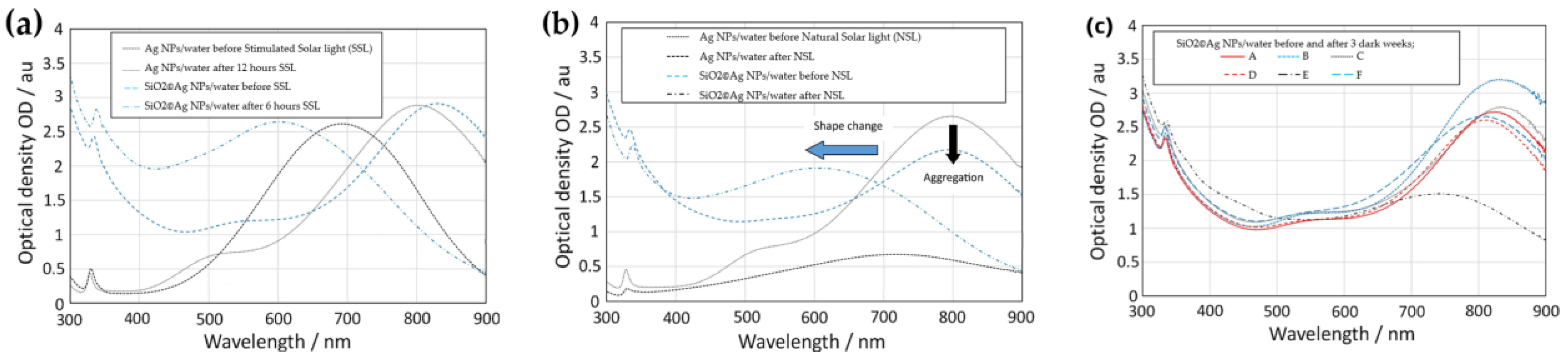
5. Characterization of the Stability of Nanofluids for DASC Applications
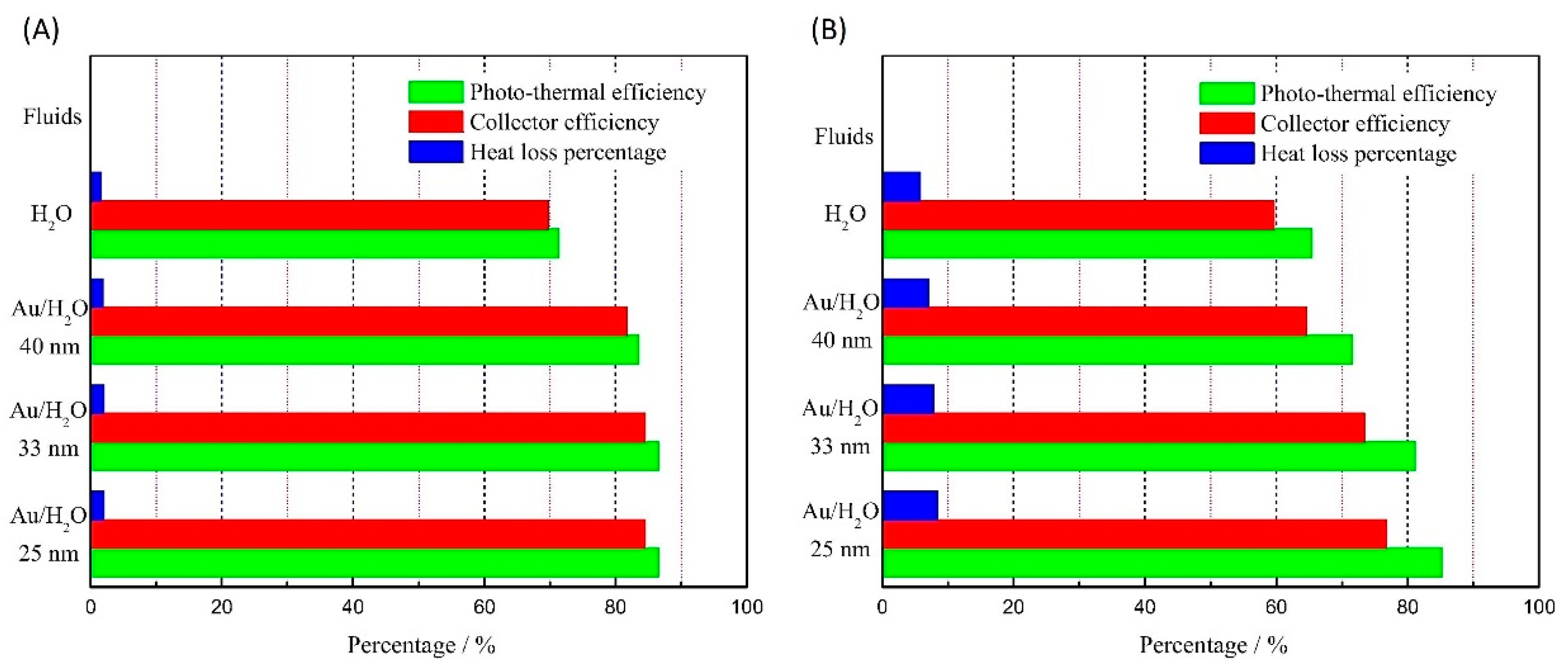
Performance of Nanofluid-Based DASCs
6. Conclusions: Challenges, Research Gaps and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alper, A.; Oguz, O. The role of renewable energy consumption in economic growth: Evidence from asymmetric causality. Renew. Sustain. Energy Rev. 2016, 60, 953–959. [Google Scholar] [CrossRef]
- Ohler, A.; Fetters, I. The causal relationship between renewable electricity generation and GDP growth: A study of energy sources. Energy Econ. 2014, 43, 125–139. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2017; IEA: Paris, France, 2017. [Google Scholar]
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.A.; Kim, K.-H. Solar energy: Potential and future prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900. [Google Scholar] [CrossRef]
- Conti, J.; Holtberg, P.; Diefenderfer, J.; LaRose, A.; Turnure, J.T.; Westfall, L. International Energy Outlook 2016 with Projections to 2040; USDOE Energy Information Administration (EIA): Washington, DC, USA, 2016.
- Kalogirou, S.A. Solar thermal collectors and applications. Prog. Energy Combust. Sci. 2004, 30, 231–295. [Google Scholar] [CrossRef]
- Birol, F. World Energy Outlook; International Energy Agency: Paris, France, 2010; Volume 1. [Google Scholar]
- Mallah, A.R.; Zubir, M.N.M.; Alawi, O.A.; Newaz, K.M.S.; Badry, A.B.M. Plasmonic nanofluids for high photothermal conversion efficiency in direct absorption solar collectors: Fundamentals and applications. Sol. Energy Mater. Sol. Cells 2019, 201, 110084. [Google Scholar] [CrossRef]
- Huide, F.; Xuxin, Z.; Lei, M.; Tao, Z.; Qixing, W.; Hongyuan, S. A comparative study on three types of solar utilization technologies for buildings: Photovoltaic, solar thermal and hybrid photovoltaic/thermal systems. Energy Convers. Manag. 2017, 140, 1–13. [Google Scholar] [CrossRef]
- Nwaji, G.N.; Okoronkwo, C.A.; Ogueke, N.V.; Anyanwu, E.E. Hybrid solar water heating/nocturnal radiation cooling system I: A review of the progress, prospects and challenges. Energy Build. 2019, 198, 412–430. [Google Scholar] [CrossRef]
- Pérez-Aparicio, E.; Lillo-Bravo, I.; Moreno-Tejera, S.; Silva-Pérez, M. Economical and environmental analysis of thermal and photovoltaic solar energy as source of heat for industrial processes. In AIP Conference Proceedings; AIP Publishing: Long Island, NY, USA, 2017; Volume 1850, p. 180005. [Google Scholar]
- Goel, N.; Taylor, R.A.; Otanicar, T.J.R.E. A review of nanofluid-based direct absorption solar collectors: Design considerations and experiments with hybrid PV/Thermal and direct steam generation collectors. Renew. Energy 2020, 145, 903–913. [Google Scholar] [CrossRef]
- Bhalla, V.; Tyagi, H. Parameters influencing the performance of nanoparticles-laden fluid-based solar thermal collectors: A review on optical properties. Renew. Sustain. Energy Rev. 2018, 84, 12–42. [Google Scholar] [CrossRef]
- Gorji, T.B.; Ranjbar, A.A.J.R.; Reviews, S.E. A review on optical properties and application of nanofluids in direct absorption solar collectors (DASCs). Renew. Sustain. Energy Rev. 2017, 72, 10–32. [Google Scholar] [CrossRef]
- Phelan, P.; Otanicar, T.; Taylor, R.; Tyagi, H. Trends and opportunities in direct-absorption solar thermal collectors. J. Therm. Sci. Eng. Appl. 2013, 5, 021003. [Google Scholar] [CrossRef]
- Minardi, J.E.; Chuang, H.N. Performance of a “black” liquid flat-plate solar collector. Sol. Energy 1975, 17, 179–183. [Google Scholar] [CrossRef]
- Huang, B.-J.J.; Wung, T.-Y.Y.; Nieh, S. Thermal analysis of black liquid cylindrical parabolic collector. Sol. Energy 1979, 22, 221–224. [Google Scholar] [CrossRef]
- Arai, N.; Itaya, Y.; Hasatani, M. Development of a “volume heat-trap” type solar collector using a fine-particle semitransparent liquid suspension (FPSS) as a heat vehicle and heat storage medium Unsteady, one-dimensional heat transfer in a horizontal FPSS layer heated by thermal radiatio. Sol. Energy 1984, 32, 49–56. [Google Scholar] [CrossRef]
- DeWinter, F. Solar Collectors, Energy Storage, and Materials; MIT Press: Cambridge, MA, USA, 1990; Volume 5. [Google Scholar]
- Otanicar, T.P.; Phelan, P.E.; Golden, J.S. Optical properties of liquids for direct absorption solar thermal energy systems. Sol. Energy 2009, 83, 969–977. [Google Scholar] [CrossRef]
- Das, S.K.; Choi, S.U.S.; Patel, H.E.J.H.t.e. Heat transfer in nanofluids—A review. Heat Transf. Eng. 2006, 27, 3–19. [Google Scholar] [CrossRef]
- Mercatelli, L.; Sani, E.; Fontani, D.; Zaccanti, G.; Martelli, F.; Di Ninni, P. Scattering and absorption properties of carbon nanohorn-based nanofluids for solar energy applications. J. Eur. Opt. Soc.-Rapid Publ. 2011, 6, 11025. [Google Scholar] [CrossRef] [Green Version]
- Duncan, A.B.; Peterson, G.P. Review of microscale heat transfer. Appl. Mech. Rev. 1994, 47, 397–428. [Google Scholar] [CrossRef]
- Chamsa-Ard, W.; Brundavanam, S.; Fung, C.C.; Fawcett, D.; Poinern, G. Nanofluid types, their synthesis, properties and incorporation in direct solar thermal collectors: A review. Nanomaterials 2017, 7, 131. [Google Scholar] [CrossRef]
- Masuda, H.; Ebata, A.; Teramae, K. Alteration of thermal conductivity and viscosity of liquid by dispersing ultra-fine particles. Dispersion of Al2O3, SiO2 and TiO2 ultra-fine particles. Netsu Bussei 1993, 7, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.U.S.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; Argonne National Lab: Lemont, IL, USA, 1995.
- Mahian, O.; Kianifar, A.; Sahin, A.Z.; Wongwises, S. Performance analysis of a minichannel-based solar collector using different nanofluids. Energy Convers. Manag. 2014, 88, 129–138. [Google Scholar] [CrossRef]
- Raj, P.; Subudhi, S. A review of studies using nanofluids in flat-plate and direct absorption solar collectors. Renew. Sustain. Energy Rev. 2018, 84, 54–74. [Google Scholar] [CrossRef]
- Moghaieb, H.S.; Padmanaban, D.B.; Kumar, P.; Haq, A.U.; Maddi, C.; McGlynn, R.; Arredondo, M.; Singh, H.; Maguire, P.; Mariotti, D. Efficient solar-thermal energy conversion with surfactant-free Cu-oxide nanofluids. Nano Energy 2023, 108, 108112. [Google Scholar] [CrossRef]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef] [Green Version]
- Bordiwala, R.V. Green Synthesis and Applications of Metal Nanoparticles—A Review article. Results Chem. 2023, 5, 100832. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Luo, B.; Wei, K.; Zeng, G. The MXene/water nanofluids with high stability and photo-thermal conversion for direct absorption solar collectors: A comparative study. Energy 2021, 227, 120483. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Hristovski, K.; Crittenden, J.C. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008, 42, 2204–2212. [Google Scholar] [CrossRef]
- Liu, M.-S.; Lin, M.C.-C.; Tsai, C.Y.; Wang, C.-C. Enhancement of thermal conductivity with Cu for nanofluids using chemical reduction method. Int. J. Heat Mass Transf. 2006, 49, 3028–3033. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, S.U.S.; Li, S.; Yu, W.; Thompson, L.J. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A review on nanofluids: Preparation, stability mechanisms, and applications. J. Nanomater. 2012, 2012, 435873. [Google Scholar] [CrossRef] [Green Version]
- Alaghmandfard, A.; Ghandi, K. A comprehensive review of graphitic carbon nitride (g-C3N4)–metal oxide-based nanocomposites: Potential for photocatalysis and sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Kakavelakis, G.; Petridis, K.; Kymakis, E. Recent advances in plasmonic metal and rare-earth-element upconversion nanoparticle doped perovskite solar cells. J. Mater. Chem. A 2017, 5, 21604–21624. [Google Scholar] [CrossRef]
- Fu, Y.; Zeng, G.; Lai, C.; Huang, D.; Qin, L.; Yi, H.; Liu, X.; Zhang, M.; Li, B.; Liu, S. Hybrid architectures based on noble metals and carbon-based dots nanomaterials: A review of recent progress in synthesis and applications. Chem. Eng. J. 2020, 399, 125743. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Carbon-based functional nanomaterials: Preparation, properties and applications. Compos. Sci. Technol. 2019, 179, 10–40. [Google Scholar] [CrossRef]
- Wang, L.; Hasanzadeh Kafshgari, M.; Meunier, M. Optical properties and applications of plasmonic-metal nanoparticles. Adv. Funct. Mater. 2020, 30, 2005400. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Concina, I.; Vomiero, A. Metal oxide semiconductors for dye-and quantum-dot-sensitized solar cells. Small 2015, 11, 1744–1774. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Swihart, M.T. Heavily-doped colloidal semiconductor and metal oxide nanocrystals: An emerging new class of plasmonic nanomaterials. Chem. Soc. Rev. 2014, 43, 3908–3920. [Google Scholar] [CrossRef]
- Runnerstrom, E.L.; Bergerud, A.; Agrawal, A.; Johns, R.W.; Dahlman, C.J.; Singh, A.; Selbach, S.M.; Milliron, D.J. Defect engineering in plasmonic metal oxide nanocrystals. Nano Lett. 2016, 16, 3390–3398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urmi, W.T.; Rahman, M.; Kadirgama, K.; Ramasamy, D.; Maleque, M. An overview on synthesis, stability, opportunities and challenges of nanofluids. Mater. Today Proc. 2021, 41, 30–37. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, T. Fine Particles: Synthesis, Characterization, and Mechanisms of Growth; CRC Press: Boca Raton, FL, USA, 2000; Volume 92. [Google Scholar]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Speranza, G. Carbon nanomaterials: Synthesis, functionalization and sensing applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, J. Nanofluids research: Key issues. Nanoscale Res. Lett. 2010, 5, 1241–1252. [Google Scholar] [CrossRef] [Green Version]
- Mittal, A.; Brajpuriya, R.; Gupta, R. Solar Steam Generation Using Hybrid Nanomaterials to Address Global Environmental Water Pollution and Shortage Crisis. Mater. Today Sustain. 2023, 21, 100319. [Google Scholar] [CrossRef]
- Sreekumar, S.; Shah, N.; Mondol, J.D.; Hewitt, N.; Chakrabarti, S. Broadband absorbing mono, blended and hybrid nanofluids for direct absorption solar collector: A comprehensive review. Nano Futur. 2022, 6, 022002. [Google Scholar] [CrossRef]
- Wang, M.; Ye, M.; Iocozzia, J.; Lin, C.; Lin, Z. Plasmon—mediated solar energy conversion via photocatalysis in noble metal/semiconductor composites. Adv. Sci. 2016, 3, 1600024. [Google Scholar] [CrossRef] [Green Version]
- Paściak, A.; Pilch-Wróbel, A.; Marciniak, Ł.; Schuck, P.J.; Bednarkiewicz, A. Standardization of methodology of light-to-heat conversion efficiency determination for colloidal nanoheaters. ACS Appl. Mater. Interfaces 2021, 13, 44556–44567. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cheng, X.; Zhang, H.; Bai, X.; Ai, R.; Shao, L.; Wang, J. Gold nanorods: The most versatile plasmonic nanoparticles. Chem. Rev. 2021, 121, 13342–13453. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Zhu, J.; Kim, D.R.J.E.C.; Management. Enhancement of photo-thermal conversion using gold nanofluids with different particle sizes. Energy Convers. Manag. 2016, 112, 21–30. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, S.; Kukreja, R.; Chander, N. Experimental thermal performance investigation of a direct absorption solar collector using hybrid nanofluid of gold nanoparticles with natural extract of Azadirachta Indica leaves. Renew. Energy 2023, 202, 1021–1031. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Rizk, N.; Munro, C.J.; Joshi, C.P.; Anjum, D.H.; Abu-Nada, E.; Martin, M.N.; Alazzam, A. Radiation stability and photothermal performance of surface-functionalized plasmonic nanofluids for direct-absorption solar applications. Sol. Energy Mater. Sol. Cells 2021, 227, 111115. [Google Scholar] [CrossRef]
- McGlynn, R.; Chakrabarti, S.; Alessi, B.; Moghaieb, H.S.; Maguire, P.; Singh, H.; Mariotti, D. Plasma-induced non-equilibrium electrochemistry synthesis of nanoparticles for solar thermal energy harvesting. Sol. Energy 2020, 203, 37–45. [Google Scholar] [CrossRef]
- Kimpton, H.; Cristaldi, D.A.; Stulz, E.; Zhang, X. Thermal performance and physicochemical stability of silver nanoprism-based nanofluids for direct solar absorption. Sol. Energy 2020, 199, 366–376. [Google Scholar] [CrossRef]
- Kumar, S.; Chander, N.; Gupta, V.K.; Kukreja, R. Progress, challenges and future prospects of plasmonic nanofluid based direct absorption solar collectors—A state-of-the-art review. Sol. Energy 2021, 227, 365–425. [Google Scholar] [CrossRef]
- Borode, A.; Ahmed, N.; Olubambi, P. A review of solar collectors using carbon-based nanofluids. J. Clean. Prod. 2019, 241, 118311. [Google Scholar] [CrossRef]
- Trong Tam, N.; Viet Phuong, N.; Hong Khoi, P.; Ngoc Minh, P.; Afrand, M.; Van Trinh, P.; Hung Thang, B.; Żyła, G.; Estellé, P. Carbon nanomaterial-based nanofluids for direct thermal solar absorption. Nanomaterials 2020, 10, 1199. [Google Scholar] [CrossRef]
- Yu, S.-P.; Teng, T.-P.; Huang, C.-C.; Hsieh, H.-K.; Wei, Y.-J. Performance Evaluation of Carbon-Based Nanofluids for Direct Absorption Solar Collector. Energies 2023, 16, 1157. [Google Scholar] [CrossRef]
- McGlynn, R.J.; Moghaieb, H.S.; Brunet, P.; Chakrabarti, S.; Maguire, P.; Mariotti, D. Hybrid Plasma–Liquid Functionalisation for the Enhanced Stability of CNT Nanofluids for Application in Solar Energy Conversion. Nanomaterials 2022, 12, 2705. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, Z.; Chen, M.; Zhou, P. Ultra-stable carbon quantum dot nanofluids for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2022, 240, 111720. [Google Scholar] [CrossRef]
- Guo, J.; Wang, F.; Li, S.; Wang, Y.; Hu, X.; Zu, D.; Shen, Y.; Li, C. Enhanced optical properties and light-to-heat conversion performance of Ti3C2/[BMIM]BF4 nanofluids based direct absorption solar collector. Sol. Energy Mater. Sol. Cells 2022, 237, 111558. [Google Scholar] [CrossRef]
- Mesgari, S.; Taylor, R.A.; Hjerrild, N.E.; Crisostomo, F.; Li, Q.; Scott, J. An investigation of thermal stability of carbon nanofluids for solar thermal applications. Sol. Energy Mater. Sol. Cells 2016, 157, 652–659. [Google Scholar] [CrossRef]
- Toroker, M.C.; Carter, E.A. Transition metal oxide alloys as potential solar energy conversion materials. J. Mater. Chem. A 2013, 1, 2474–2484. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Qiu, J.; Jin, X.; Umair, M.M.; Lu, R.; Zhang, S.; Tang, B. Ag-graphene/PEG composite phase change materials for enhancing solar-thermal energy conversion and storage capacity. Appl. Energy 2019, 237, 83–90. [Google Scholar] [CrossRef]
- Milanese, M.; Colangelo, G.; Cretì, A.; Lomascolo, M.; Iacobazzi, F.; De Risi, A.J.S.E.M.; Cells, S. Optical absorption measurements of oxide nanoparticles for application as nanofluid in direct absorption solar power systems–Part I: Water-based nanofluids behavior. Sol. Energy Mater. Sol. Cells 2016, 147, 315–320. [Google Scholar] [CrossRef]
- Balakin, B.V.; Stava, M.; Kosinska, A. Photothermal convection of a magnetic nanofluid in a direct absorption solar collector. Sol. Energy 2022, 239, 33–39. [Google Scholar] [CrossRef]
- Hosseini, S.M.S.; Dehaj, M.S. The comparison of colloidal, optical, and solar collection characteristics between Fe2O3 and Fe3O4 nanofluids operated in an evacuated tubular volumetric absorption solar collector. J. Taiwan Inst. Chem. Eng. 2022, 135, 104381. [Google Scholar] [CrossRef]
- Sreekumar, S.; Joseph, A.; Sujith Kumar, C.S.; Thomas, S. Investigation on influence of antimony tin oxide/silver nanofluid on direct absorption parabolic solar collector. J. Clean. Prod. 2020, 249, 119378. [Google Scholar] [CrossRef]
- Wang, K.; He, Y.; Zheng, Z.; Gao, J.; Kan, A.; Xie, H.; Yu, W. Experimental optimization of nanofluids based direct absorption solar collector by optical boundary conditions. Appl. Therm. Eng. 2021, 182, 116076. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Rizk, N.; Joshi, C.P.; Abi Jaoudé, M.; Al-Khateeb, A.N.; Kyritsis, D.C.; Abu-Nada, E.; Martin, M.N. Ultrastable plasmonic nanofluids in optimized direct absorption solar collectors. Energy Convers. Manag. 2019, 199, 112010. [Google Scholar] [CrossRef]
- Zheng, N.; Yan, F.; Wang, L.; Sun, Z. Photo-thermal conversion performance of mono MWCNT and hybrid MWCNT-TiN nanofluids in direct absorption solar collectors. Int. J. Energy Res. 2022, 46, 8313–8327. [Google Scholar] [CrossRef]
- Gorji, T.B.; Ranjbar, A.A.J.S.E. A numerical and experimental investigation on the performance of a low-flux direct absorption solar collector (DASC) using graphite, magnetite and silver nanofluids. Sol. Energy 2016, 135, 493–505. [Google Scholar] [CrossRef]
- Li, X.; Zeng, G.; Lei, X. The stability, optical properties and solar-thermal conversion performance of SiC-MWCNTs hybrid nanofluids for the direct absorption solar collector (DASC) application. Sol. Energy Mater. Sol. Cells 2020, 206, 110323. [Google Scholar] [CrossRef]
- Vakili, M.; Hosseinalipour, S.M.; Delfani, S.; Khosrojerdi, S.J.S.E.M.; Cells, S. Photothermal properties of graphene nanoplatelets nanofluid for low-temperature direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2016, 152, 187–191. [Google Scholar] [CrossRef]
- Taylor, R.A.; Hjerrild, N.; Duhaini, N.; Pickford, M.; Mesgari, S. Stability testing of silver nanodisc suspensions for solar applications. Appl. Surf. Sci. 2018, 455, 465–475. [Google Scholar] [CrossRef]
- Said, Z.; Saidur, R.; Rahim, N.A. Optical properties of metal oxides based nanofluids. Int. Commun. Heat Mass Transf. 2014, 59, 46–54. [Google Scholar] [CrossRef]
- Menbari, A.; Alemrajabi, A.A.; Ghayeb, Y.J.E.C.; Management. Experimental investigation of stability and extinction coefficient of Al2O3–CuO binary nanoparticles dispersed in ethylene glycol–water mixture for low-temperature direct absorption solar collectors. Energy Convers. Manag. 2016, 108, 501–510. [Google Scholar] [CrossRef]
- Wang, K.; He, Y.; Kan, A.; Yu, W.; Wang, D.; Zhang, L.; Zhu, G.; Xie, H.; She, X. Significant photothermal conversion enhancement of nanofluids induced by Rayleigh-Bénard convection for direct absorption solar collectors. Appl. Energy 2019, 254, 113706. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Fang, X.; Zhang, Z. Reduced graphene oxide dispersed nanofluids with improved photo-thermal conversion performance for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2017, 163, 125–133. [Google Scholar] [CrossRef]
- Xu, X.; Xu, C.; Liu, J.; Fang, X.; Zhang, Z. A direct absorption solar collector based on a water-ethylene glycol based nanofluid with anti-freeze property and excellent dispersion stability. Renew. Energy 2019, 133, 760–769. [Google Scholar] [CrossRef]
- Choi, T.J.; Jang, S.P.; Kedzierski, M.A. Effect of surfactants on the stability and solar thermal absorption characteristics of water-based nanofluids with multi-walled carbon nanotubes. Int. J. Heat Mass Transf. 2018, 122, 483–490. [Google Scholar] [CrossRef]
- Hazra, S.K.; Michael, M.; Nandi, T.K. Investigations on optical and photo-thermal conversion characteristics of BN-EG and BN/CB-EG hybrid nanofluids for applications in direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2021, 230, 111245. [Google Scholar] [CrossRef]
- Cao, S.; Jiang, Q.; Wu, X.; Ghim, D.; Derami, H.G.; Chou, P.-I.; Jun, Y.-S.; Singamaneni, S. Advances in solar evaporator materials for freshwater generation. J. Mater. Chem. A 2019, 7, 24092–24123. [Google Scholar] [CrossRef]
- Joseph, A.; Thomas, S. Energy, exergy and corrosion analysis of direct absorption solar collector employed with ultra-high stable carbon quantum dot nanofluid. Renew. Energy 2022, 181, 725–737. [Google Scholar] [CrossRef]
- Heberlein, J.; Mentel, J.; Pfender, E. The anode region of electric arcs: A survey. J. Phys. D Appl. Phys. 2009, 43, 23001. [Google Scholar] [CrossRef]
- Krishna, K.S.; Biswas, S.; Navin, C.V.; Yamane, D.G.; Miller, J.T.; Kumar, C.S.S.R.J.J. Millifluidics for chemical synthesis and time-resolved mechanistic studies. JoVE 2013, 81, e50711. [Google Scholar]
- Martinsson, E.; Shahjamali, M.M.; Large, N.; Zaraee, N.; Zhou, Y.; Schatz, G.C.; Mirkin, C.A.; Aili, D.J.S. Influence of surfactant bilayers on the refractive index sensitivity and catalytic properties of anisotropic gold nanoparticles. Small 2016, 12, 330–342. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Saavedra, J.; Asenjo-Garcia, A.; García de Abajo, F.J. Hot-electron dynamics and thermalization in small metallic nanoparticles. Acs Photonics 2016, 3, 1637–1646. [Google Scholar] [CrossRef]
- Jain, P.K.; Qian, W.; El-Sayed, M.A. Ultrafast electron relaxation dynamics in coupled metal nanoparticles in aggregates. J. Phy. Chem. B 2006, 110, 136–142. [Google Scholar] [CrossRef]
- Mehrali, M.; Ghatkesar, M.K.; Pecnik, R. Full-spectrum volumetric solar thermal conversion via graphene/silver hybrid plasmonic nanofluids. Appl. Energy 2018, 224, 103–115. [Google Scholar] [CrossRef]
- Bhanvase, B.; Barai, D. 5-Electrical, optical, and tribological properties of the nanofluids. In Nanofluids for Heat and Mass Transfer; Bharat Bhanvase, D.B., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 167–190. [Google Scholar] [CrossRef]
- Khlebtsov, N.G.; Trachuk, L.A.; Mel’nikov, A.G. The effect of the size, shape, and structure of metal nanoparticles on the dependence of their optical properties on the refractive index of a disperse medium. Opt. Spectrosc. 2005, 98, 77–83. [Google Scholar] [CrossRef]
- SATM-G173; Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37 Tilted Surface. American Society for Testing Materials: West Conshocken PA, USA, 2007; Volume 14.
- Fan, X.; Zheng, W.; Singh, D.J. Light scattering and surface plasmons on small spherical particles. Light Sci. Appl. 2014, 3, e179. [Google Scholar] [CrossRef] [Green Version]
- Said, Z.; Sajid, M.; Saidur, R.; Mahdiraji, G.; Rahim, N. Evaluating the optical properties of TiO2 nanofluid for a direct absorption solar collector. Numer. Heat Transf. Part A Appl. 2015, 67, 1010–1027. [Google Scholar] [CrossRef] [Green Version]
- Wriedt, T. Mie theory: A review. In The Mie Theory; Springer: Berlin/Heidelberg, Germany, 2012; pp. 53–71. [Google Scholar]
- Madsen, S.J.; Wilson, B.C. Optical properties of brain tissue. In Optical Methods and Instrumentation in Brain Imaging and Therapy; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–22. [Google Scholar]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Li, K. Temperature effect on the aggregation kinetics of CeO2 nanoparticles in monovalent and divalent electrolytes. J. Environ. Anal. Toxicol. 2012, 2, 158–162. [Google Scholar]
- Ali, N.; Teixeira, J.A.; Addali, A. A review on nanofluids: Fabrication, stability, and thermophysical properties. J. Nanomater. 2018, 2018, 6978130. [Google Scholar] [CrossRef] [Green Version]
- Hjerrild, N.E.; Scott, J.A.; Amal, R.; Taylor, R.A. Exploring the effects of heat and UV exposure on glycerol-based Ag-SiO2 nanofluids for PV/T applications. Renew. Energy 2018, 120, 266–274. [Google Scholar] [CrossRef]
- Nine, M.J.; Chung, H.; Tanshen, M.R.; Osman, N.A.B.A.; Jeong, H. Is metal nanofluid reliable as heat carrier? J. Hazard. Mater. 2014, 273, 183–191. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Desgranges, F.; Roy, G.; Galanis, N.; Maré, T.; Boucher, e.; Mintsa, H.A. Temperature and particle-size dependent viscosity data for water-based nanofluids–hysteresis phenomenon. Int. J. Heat Fluid Flow 2007, 28, 1492–1506. [Google Scholar] [CrossRef]
- Balamurugan, B.; Mehta, B. Optical and structural properties of nanocrystalline copper oxide thin films prepared by activated reactive evaporation. Thin Solid Film. 2001, 396, 90–96. [Google Scholar] [CrossRef]
- Addala, S.; Bouhdjer, L.; Chala, A.; Bouhdjar, A.; Halimi, O.; Boudine, B.; Sebais, M. Structural and optical properties of a NaCl single crystal doped with CuO nanocrystals. Chin. Phys. B 2013, 22, 098103. [Google Scholar] [CrossRef]
- Piri, F.; Afarani, M.S.; Arabi, A.M. Synthesis of copper oxide quantum dots: Effect of surface modifiers. Mater. Res. Express 2019, 6, 125006. [Google Scholar] [CrossRef]
- Padmanaban, D.B.; McGlynn, R.; Byrne, E.; Velusamy, T.; Swadźba-Kwaśny, M.; Maguire, P.; Mariotti, D. Understanding plasma–ethanol non-equilibrium electrochemistry during the synthesis of metal oxide quantum dots. Green Chem. 2021, 23, 3983–3995. [Google Scholar] [CrossRef]
- Velusamy, T.; Liguori, A.; Macias-Montero, M.; Padmanaban, D.B.; Carolan, D.; Gherardi, M.; Colombo, V.; Maguire, P.; Svrcek, V.; Mariotti, D. Ultra-small CuO nanoparticles with tailored energy-band diagram synthesized by a hybrid plasma-liquid process. Plasma Proc. Poly. 2017, 14, 1600224. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Kang, K.; Lee, I.; Lee, B.J. Optimization of a direct absorption solar collector with blended plasmonic nanofluids. Sol. Energy 2017, 150, 512–520. [Google Scholar] [CrossRef]
- Maheshwary, P.B.; Handa, C.C.; Nemade, K.R.; Chaudhary, S.R. Role of nanoparticle shape in enhancing the thermal conductivity of nanofluids. Mater. Today Proc. 2020, 28, 873–878. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Wang, X.; Hu, Y. Numerically investigating the optical properties of plasmonic metallic nanoparticles for effective solar absorption and heating. Sol. Energy 2018, 161, 17–24. [Google Scholar] [CrossRef]
- Wang, Z.; Quan, X.; Zhang, Z.; Cheng, P. Optical absorption of carbon-gold core-shell nanoparticles. J. Quant. Spectrosc. Radiat. Transf. 2018, 205, 291–298. [Google Scholar] [CrossRef]
- Duan, H.; Zheng, Y.; Xu, C.; Shang, Y.; Ding, F. Experimental investigation on the plasmonic blended nanofluid for efficient solar absorption. Appl. Therm. Eng. 2019, 161, 114192. [Google Scholar] [CrossRef]
- Elsheikh, A.H.; Sharshir, S.W.; Mostafa, M.E.; Essa, F.A.; Ahmed Ali, M.K. Applications of nanofluids in solar energy: A review of recent advances. Renew. Sustain. Energy Rev. 2018, 82, 3483–3502. [Google Scholar] [CrossRef]
- Razi, P.; Akhavan-Behabadi, M.A.; Saeedinia, M. Pressure drop and thermal characteristics of CuO–base oil nanofluid laminar flow in flattened tubes under constant heat flux. Int. Commun. Heat Mass Transf. 2011, 38, 964–971. [Google Scholar] [CrossRef]
- Ahmadlouydarab, M.; Ebadolahzadeh, M.; Ali, H.M. Effects of utilizing nanofluid as working fluid in a lab-scale designed FPSC to improve thermal absorption and efficiency. Phys. A Stat. Mech. Its Appl. 2020, 540, 123109. [Google Scholar] [CrossRef]
- Celata, G.P.; D’Annibale, F.; Mariani, A.; Sau, S.; Serra, E.; Bubbico, R.; Menale, C.; Poth, H. Experimental results of nanofluids flow effects on metal surfaces. Chem. Eng. Res. Des. 2014, 92, 1616–1628. [Google Scholar] [CrossRef]
- Radwan, A.; Ahmed, M.; Ookawara, S. Performance enhancement of concentrated photovoltaic systems using a microchannel heat sink with nanofluids. Energy Convers. Manag. 2016, 119, 289–303. [Google Scholar] [CrossRef]
- Hussein, A.K. Applications of nanotechnology to improve the performance of solar collectors–Recent advances and overview. Renew. Sustain. Energy Rev. 2016, 62, 767–792. [Google Scholar] [CrossRef]
- Sabiha, M.A.; Saidur, R.; Hassani, S.; Said, Z.; Mekhilef, S. Energy performance of an evacuated tube solar collector using single walled carbon nanotubes nanofluids. Energy Convers. Manag. 2015, 105, 1377–1388. [Google Scholar] [CrossRef]
- Elsaid, K.; Olabi, A.G.; Wilberforce, T.; Abdelkareem, M.A.; Sayed, E.T. Environmental impacts of nanofluids: A review. Sci. Total Environ. 2021, 763, 144202. [Google Scholar] [CrossRef]
- Mo, Y.; Gu, A.; Tollerud, D.J.; Zhang, Q. Nanoparticle toxicity and environmental impact. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 117–143. [Google Scholar]
- Bakand, S.; Hayes, A.; Dechsakulthorn, F. Nanoparticles: A review of particle toxicology following inhalation exposure. Inhal. Toxicol. 2012, 24, 125–135. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moghaieb, H.S.; Amendola, V.; Khalil, S.; Chakrabarti, S.; Maguire, P.; Mariotti, D. Nanofluids for Direct-Absorption Solar Collectors—DASCs: A Review on Recent Progress and Future Perspectives. Nanomaterials 2023, 13, 1232. https://doi.org/10.3390/nano13071232
Moghaieb HS, Amendola V, Khalil S, Chakrabarti S, Maguire P, Mariotti D. Nanofluids for Direct-Absorption Solar Collectors—DASCs: A Review on Recent Progress and Future Perspectives. Nanomaterials. 2023; 13(7):1232. https://doi.org/10.3390/nano13071232
Chicago/Turabian StyleMoghaieb, Hussein Sayed, Vincenzo Amendola, Sameh Khalil, Supriya Chakrabarti, Paul Maguire, and Davide Mariotti. 2023. "Nanofluids for Direct-Absorption Solar Collectors—DASCs: A Review on Recent Progress and Future Perspectives" Nanomaterials 13, no. 7: 1232. https://doi.org/10.3390/nano13071232






