Nanoscale Clustering in an Additively Manufactured Zr-Based Metallic Glass Evaluated by Atom Probe Tomography
Abstract
:1. Introduction
- Visual inspection of the reconstruction and the use of isoconcentration surfaces.
- Binomial analysis: Comparing the local composition in voxels of similar size with the same number of atoms to a binomial distribution and calculation of the Pearson correlation coefficient. For the Pearson correlation coefficient, the chi2 measure is normalized by sample size, and a value of 0 indicates a random distribution.
- Nearest-neighbor analysis.
- Cluster-finding algorithms.
2. Materials and Methods
2.1. Material Selection and Sample Preparation
2.2. Atom Probe Tomography Measurements
2.3. Data Analysis and Simulation
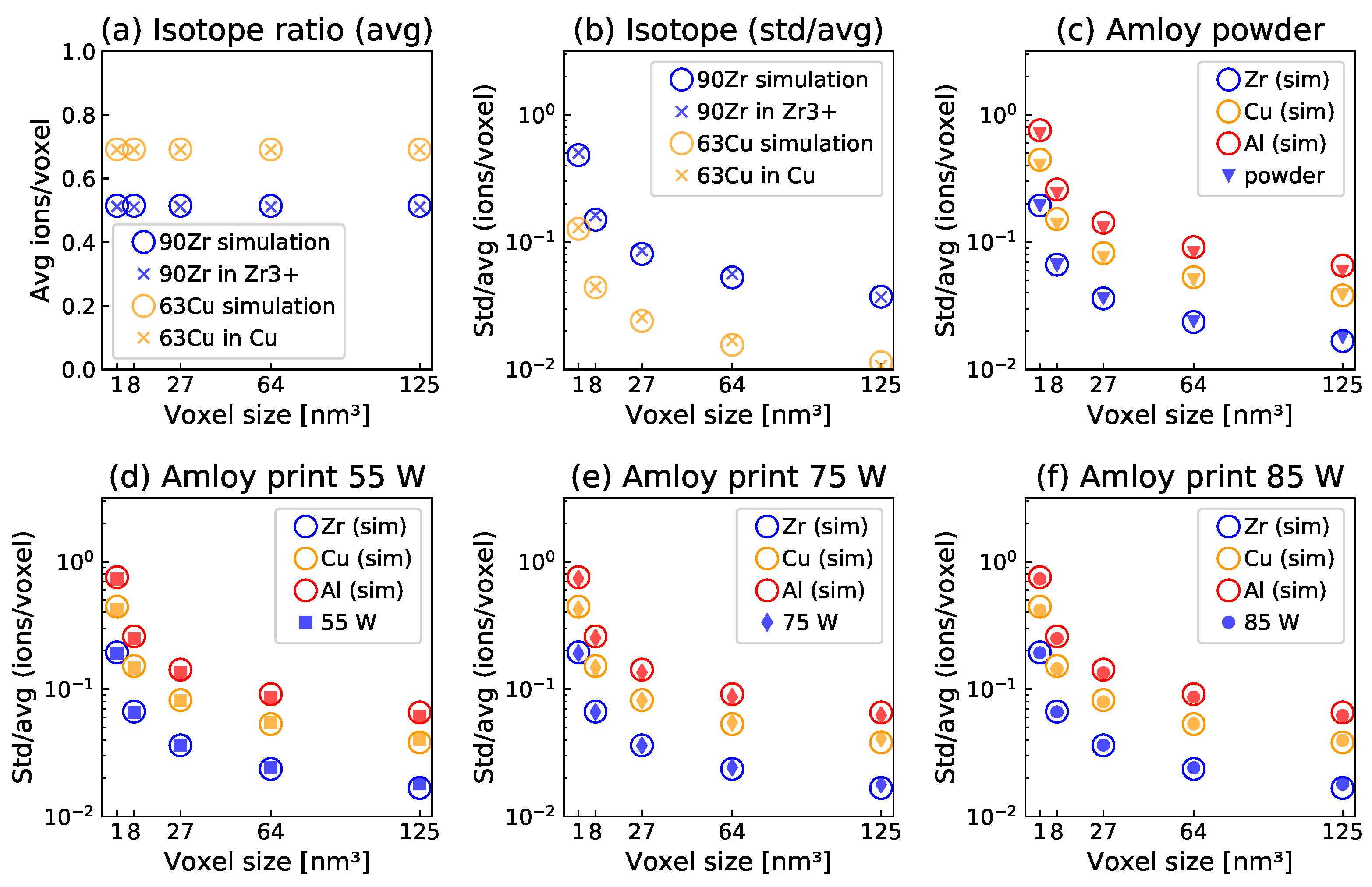
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Local Reconstructed Density

References
- Sohrabi, N.; Jhabvala, J.; Logé, R.E. Additive Manufacturing of Bulk Metallic Glasses—Process, Challenges and Properties: A Review. Metals 2021, 11, 1279. [Google Scholar] [CrossRef]
- Williams, E.; Lavery, N. Laser processing of bulk metallic glass: A review. J. Mater. Process. Technol. 2017, 247, 73–91. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Jiang, Q.; Huo, J.; Zhang, Y.; Yang, W.; Li, X. Crystallization in additive manufacturing of metallic glasses: A review. Addit. Manuf. 2020, 36, 101568. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Y.; Liu, Z.; Wang, G. Laser 3D printing of Zr-based bulk metallic glass. J. Manuf. Process. 2019, 39, 102–105. [Google Scholar] [CrossRef]
- Deng, L.; Wang, S.; Wang, P.; Kühn, U.; Pauly, S. Selective laser melting of a Ti-based bulk metallic glass. Mater. Lett. 2018, 212, 346–349. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Xing, W.; Ouyang, D.; Liu, L. 3D printing of Fe-based bulk metallic glass composites with combined high strength and fracture toughness. Mater. Des. 2018, 143, 285–296. [Google Scholar] [CrossRef]
- Li, X.; Kang, C.; Huang, H.; Sercombe, T. The role of a low-energy–density re-scan in fabricating crack-free Al85Ni5Y6Co2Fe2 bulk metallic glass composites via selective laser melting. Mater. Des. 2014, 63, 407–411. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, V.; Karlsson, D.; Marattukalam, J.J.; Stolpe, M.; Hjörvarsson, B.; Jansson, U.; Sahlberg, M. Thermal stability and crystallization of a Zr-based metallic glass produced by suction casting and selective laser melting. J. Alloys Compd. 2020, 825, 153995. [Google Scholar] [CrossRef]
- Marattukalam, J.J.; Pacheco, V.; Karlsson, D.; Riekehr, L.; Lindwall, J.; Forsberg, F.; Jansson, U.; Sahlberg, M.; Hjörvarsson, B. Development of process parameters for selective laser melting of a Zr-based bulk metallic glass. Addit. Manuf. 2020, 33, 101124. [Google Scholar] [CrossRef]
- Ericsson, A.; Pacheco, V.; Marattukalam, J.J.; Dalgliesh, R.M.; Rennie, A.R.; Fisk, M.; Sahlberg, M. Crystallization of a Zr-based metallic glass produced by laser powder bed fusion and suction casting. J. Non-Cryst. Solids 2021, 571, 120891. [Google Scholar] [CrossRef]
- Barbee, T.W.; Walmsley, R.G.; Marshall, A.F.; Keith, D.L.; Stevenson, D.A. Phase separation in vapor quench synthesized noncrystalline copper zirconium alloys. Appl. Phys. Lett. 1981, 38, 132–135. [Google Scholar] [CrossRef]
- Oh, J.; Ohkubo, T.; Kim, Y.; Fleury, E.; Hono, K. Phase separation in Cu43Zr43Al7Ag7 bulk metallic glass. Scr. Mater. 2005, 53, 165–169. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, S.; Sohrabi, S.; Ma, J. Primary and secondary phase separation in Cu–Zr–Al bulk metallic glass by control of quenching conditions. Intermetallics 2023, 156, 107853. [Google Scholar] [CrossRef]
- Kim, K.B.; Das, J.; Baier, F.; Tang, M.B.; Wang, W.H.; Eckert, J. Heterogeneity of a Cu47.5Zr47.5Al5 bulk metallic glass. Appl. Phys. Lett. 2006, 88, 051911. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Hanada, T.; Ohkubo, T.; Makino, A.; Yoshizawa, Y.; Nieh, T. Nanoscale phase separation in metallic glasses studied by advanced electron microscopy techniques. Intermetallics 2004, 12, 1081–1088. [Google Scholar] [CrossRef]
- Van Steenberge, N.; Concustell, A.; Sort, J.; Das, J.; Mattern, N.; Gebert, A.; Suriñach, S.; Eckert, J.; Baró, M. Microstructural inhomogeneities introduced in a Zr-based bulk metallic glass upon low-temperature annealing. Mater. Sci. Eng. A 2008, 491, 124–130. [Google Scholar] [CrossRef]
- Blavette, D.; Bostel, A.; Sarrau, J.M.; Deconihout, B.; Menand, A. An atom probe for three-dimensional tomography. Nature 1993, 363, 432–435. [Google Scholar] [CrossRef]
- Moody, M.P.; Stephenson, L.T.; Ceguerra, A.V.; Ringer, S.P. Quantitative binomial distribution analyses of nanoscale like-solute atom clustering and segregation in atom probe tomography data. Microsc. Res. Tech. 2008, 71, 542–550. [Google Scholar] [CrossRef]
- Müller, E.W. Field Desorption. Phys. Rev. 1956, 102, 618–624. [Google Scholar] [CrossRef]
- Haydock, R.; Kingham, D.R. Post-Ionization of Field-Evaporated Ions. Phys. Rev. Lett. 1980, 44, 1520–1523. [Google Scholar] [CrossRef]
- Kingham, D.R. The post-ionization of field evaporated ions: A theoretical explanation of multiple charge states. Surf. Sci. 1982, 116, 273–301. [Google Scholar] [CrossRef]
- Hono, K. Atom probe microanalysis and nanoscale microstructures in metallic materials. Acta Mater. 1999, 47, 3127–3145. [Google Scholar] [CrossRef]
- De Geuser, F.; Gault, B. Metrology of small particles and solute clusters by atom probe tomography. Acta Mater. 2020, 188, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Gault, B.; Klaes, B.; Morgado, F.F.; Freysoldt, C.; Li, Y.; De Geuser, F.; Stephenson, L.T.; Vurpillot, F. Reflections on the Spatial Performance of Atom Probe Tomography in the Analysis of Atomic Neighborhoods. Microsc. Microanal. 2022, 28, 1116–1126. [Google Scholar] [CrossRef]
- Stephenson, L.T.; Moody, M.P.; Liddicoat, P.V.; Ringer, S.P. New Techniques for the Analysis of Fine-Scaled Clustering Phenomena within Atom Probe Tomography (APT) Data. Microsc. Microanal. 2007, 13, 448–463. [Google Scholar] [CrossRef]
- Cairney, J.M.; Rajan, K.; Haley, D.; Gault, B.; Bagot, P.A.J.; Choi, P.; Felfer, P.J.; Ringer, S.P.; Marceau, R.K.W.; Moody, M.P. Mining information from atom probe data. Ultramicroscopy 2015, 159, 324–337. [Google Scholar] [CrossRef]
- Gault, B.; Moody, M.P.; De Geuser, F.; La Fontaine, A.; Stephenson, L.T.; Haley, D.; Ringer, S.P. Spatial Resolution in Atom Probe Tomography. Microsc. Microanal. 2010, 16, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Houska, J.; Zeman, P. Role of Al in Cu-Zr-Al thin film metallic glasses: Molecular dynamics and experimental study. Comput. Mater. Sci. 2023, 222, 112104. [Google Scholar] [CrossRef]
- Heinrich, J.; Busch, R.; Nonnenmacher, B. Processing of a bulk metallic glass forming alloy based on industrial grade Zr. Intermetallics 2012, 25, 1–4. [Google Scholar] [CrossRef]
- Thompson, K.; Lawrence, D.; Larson, D.; Olson, J.; Kelly, T.; Gorman, B. In situ site-specific specimen preparation for atom probe tomography. Ultramicroscopy 2007, 107, 131–139. [Google Scholar] [CrossRef]
- Lanford, W.A.; Bedell, S.; Isberg, P.; Hjorvarsson, B.; Lakshmanan, S.K.; Gill, W.N. Low temperature transport of Al into and through copper starting with Cu/Al/SiO2 bilayers. J. Appl. Phys. 1999, 85, 1487–1495. [Google Scholar] [CrossRef]
- Camus, P.P.; Larson, D.J.; Kelly, T.F. A method for reconstructing and locating atoms on the crystal lattice in three-dimensional atom probe data. Appl. Surf. Sci. 1995, 87–88, 305–310. [Google Scholar] [CrossRef]
- Larson, D.J.; Gault, B.; Geiser, B.P.; De Geuser, F.; Vurpillot, F. Atom probe tomography spatial reconstruction: Status and directions. Curr. Opin. Solid State Mater. Sci. 2013, 17, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Vurpillot, F.; Gault, B.; Geiser, B.P.; Larson, D.J. Reconstructing atom probe data: A review. Ultramicroscopy 2013, 132, 19–30. [Google Scholar] [CrossRef] [PubMed]

| Material | Laser Pulse Energy | Laser Pulse Frequency | Base Temperature | Detection Rate |
|---|---|---|---|---|
| [pJ] | [kHz] | [K] | [%] | |
| Zr | 10 | 125 | 30 | 0.5 |
| Cu | 10, 100 | 125 | 30 | 0.5 |
| Amloy | 60 | 200 | 60 | 0.5 |
| Material | Processing | Sample Population [×106 ions] |
|---|---|---|
| Zr | as-received | 1.6 |
| Cu | as-received | 1.6 |
| Amloy | as-received powder | 11.0 |
| Amloy | LPBF—55 W | 10.9 |
| Amloy | LPBF—75 W | 7.9 |
| Amloy | LPBF—85 W | 12.0 |
| Amloy | LPBF—75 W + annealed 1 h | 7.7 |
| Amloy | LPBF—75 W + annealed 5 h | 8.1 |
| Amloy | LPBF—75 W + annealed 10 h | 9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goetz, I.K.; Sälker, J.A.; Hans, M.; Hjörvarsson, B.; Schneider, J.M. Nanoscale Clustering in an Additively Manufactured Zr-Based Metallic Glass Evaluated by Atom Probe Tomography. Nanomaterials 2023, 13, 1341. https://doi.org/10.3390/nano13081341
Goetz IK, Sälker JA, Hans M, Hjörvarsson B, Schneider JM. Nanoscale Clustering in an Additively Manufactured Zr-Based Metallic Glass Evaluated by Atom Probe Tomography. Nanomaterials. 2023; 13(8):1341. https://doi.org/10.3390/nano13081341
Chicago/Turabian StyleGoetz, Inga K., Janis A. Sälker, Marcus Hans, Björgvin Hjörvarsson, and Jochen M. Schneider. 2023. "Nanoscale Clustering in an Additively Manufactured Zr-Based Metallic Glass Evaluated by Atom Probe Tomography" Nanomaterials 13, no. 8: 1341. https://doi.org/10.3390/nano13081341





