Role of Hydrogen in Ethylene-Based Synthesis of Single-Walled Carbon Nanotubes
Abstract
:1. Introduction
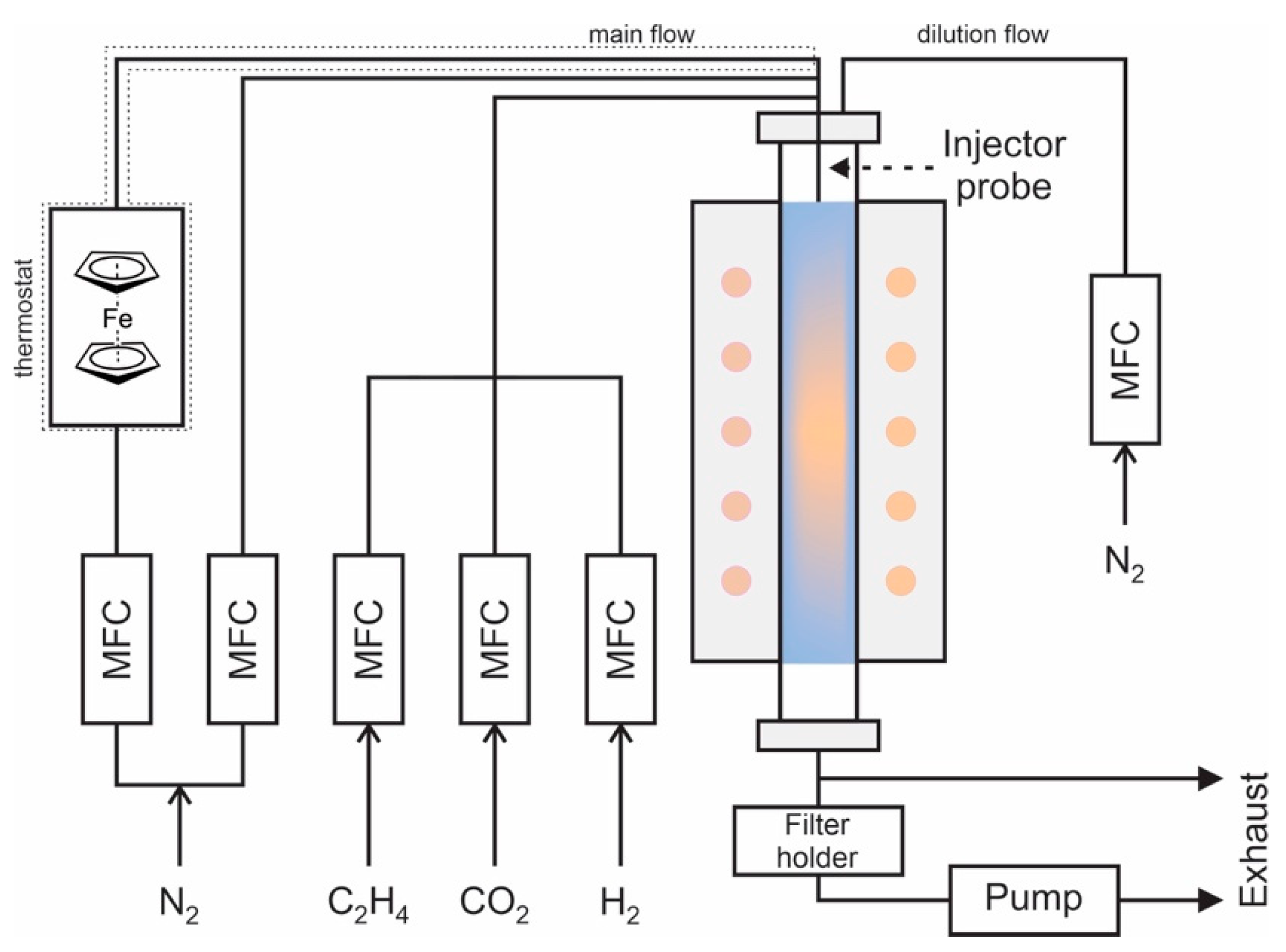
2. Materials and Methods
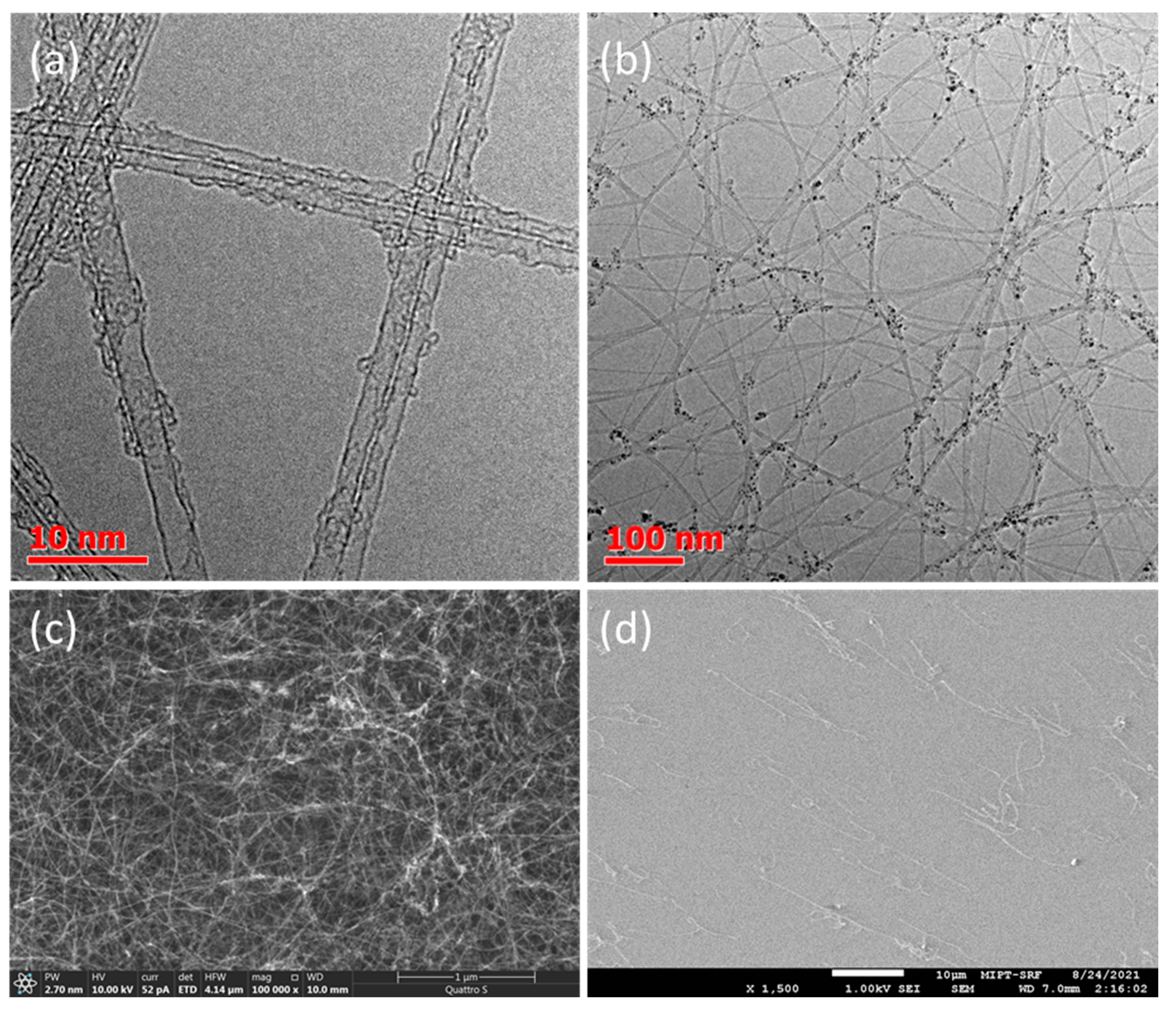
3. Results
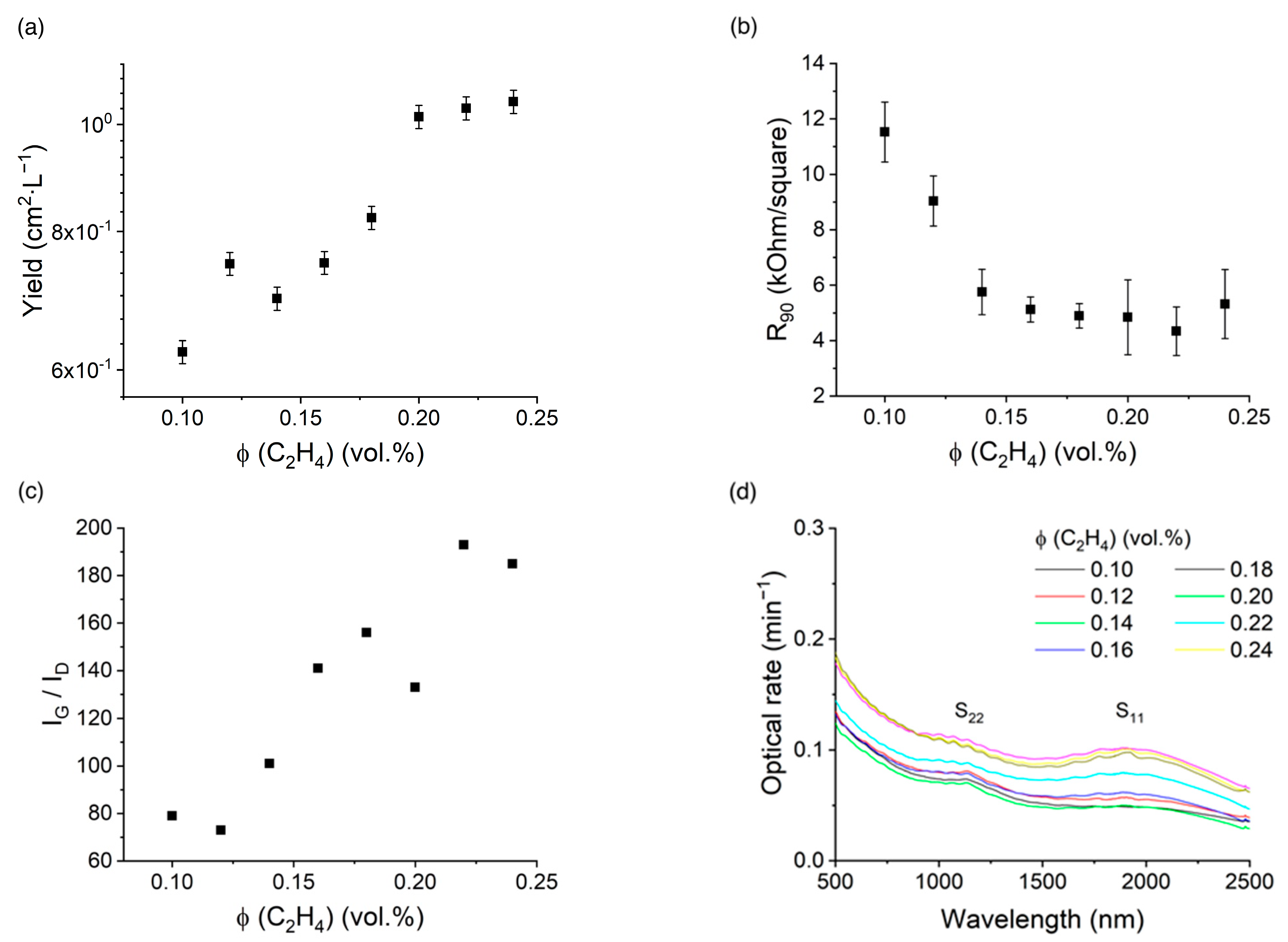
3.1. Role of Ethyelene and Ferrocene
3.2. Role of Hydrogen
- The surface cleaning/activation promotion (II regime) is the most optimal for the SWCNT synthesis (ϕ (H2) = 0.0–3.6 vol.% at 900 °C and ϕ (H2) = 4.6–23.0 vol.% at 1000 °C) and the catalyst surface cleaning resulting in increased SWCNT activation via the stable length (Figure 6d), defectiveness (Figure 6c), higher yield (Figure 6a), and slightly lower equivalent sheet resistance (Figure 6b).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monthioux, M.; Kuznetsov, V.L. Who Should Be given the Credit for the Discovery of Carbon Nanotubes? Carbon 2006, 44, 1621–1623. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Charlier, J.C.; Hernandez, E. Electronic, Thermal and Mechanical Properties of Carbon Nanotubes. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2065–2098. [Google Scholar] [CrossRef]
- Fujisawa, K.; Kim, H.J.; Go, S.H.; Muramatsu, H.; Hayashi, T.; Endo, M.; Hirschmann, T.C.; Dresselhaus, M.S.; Kim, Y.A.; Araujo, P.T. A Review of Double-Walled and Triple-Walled Carbon Nanotube Synthesis and Applications. Appl. Sci. 2016, 6, 109. [Google Scholar] [CrossRef]
- Cheng, H.M.; Li, F.; Su, G.; Pan, H.Y.; He, L.L.; Sun, X.; Dresselhaus, M.S. Large-Scale and Low-Cost Synthesis of Single-Walled Carbon Nanotubes by the Catalytic Pyrolysis of Hydrocarbons. Appl. Phys. Lett. 1998, 72, 3282–3284. [Google Scholar] [CrossRef]
- Endo, M.; Hayashi, T.; Kim, Y.A.; Terrones, M.; Dresselhaus, M.S. Applications of Carbon Nanotubes in the Twenty-First Century. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 2223–2238. [Google Scholar] [CrossRef]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; De Heer, W.A. Carbon Nanotubes—The Route Toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Novikov, I.V.; Krasnikov, D.V.; Vorobei, A.M.; Zuev, Y.I.; Butt, H.A.; Fedorov, F.S.; Gusev, S.A.; Safonov, A.A.; Shulga, E.V.; Konev, S.D.; et al. Multifunctional Elastic Nanocomposites with Extremely Low Concentrations of Single-Walled Carbon Nanotubes. ACS Appl. Mater. Interfaces 2022, 14, 18866–18876. [Google Scholar] [CrossRef]
- Butt, H.A.; Novikov, I.V.; Krasnikov, D.V.; Sulimov, A.V.; Pal, A.K.; Evlashin, S.A.; Vorobei, A.M.; Zuev, Y.I.; Ostrizhiniy, D.; Dzhurinskiy, D.; et al. Binder-Free, Pre-Consolidated Single-Walled Carbon Nanotubes for Manufacturing Thermoset Nanocomposites. Carbon 2023, 202, 450–463. [Google Scholar] [CrossRef]
- Predtechenskiy, M.R.; Khasin, A.A.; Smirnov, S.N.; Bezrodny, A.E.; Bobrenok, O.F.; Dubov, D.Y.; Kosolapov, A.G.; Lyamysheva, E.G.; Muradyan, V.E.; Saik, V.O.; et al. New Perspectives in SWCNT Applications: Tuball SWCNTs. Part 2. New Composite Materials through Augmentation with Tuball. Carbon Trends 2022, 8, 100176. [Google Scholar] [CrossRef]
- Predtechenskiy, M.R.; Khasin, A.A.; Bezrodny, A.E.; Bobrenok, O.F.; Dubov, D.Y.; Muradyan, V.E.; Saik, V.O.; Smirnov, S.N. New Perspectives in SWCNT Applications: Tuball SWCNTs.Part 1. Tuball by Itself—All You Need to Know about It. Carbon Trends 2022, 8, 100175. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-Shell Carbon Nanotubes of 1-Nm Diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Li, Y.; Maruyama, S. Single-Walled Carbon Nanotubes; Li, Y., Maruyama, S., Eds.; Topics in Current Chemistry Collections; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-12699-5. [Google Scholar]
- Hirsch, A.; Backes, C. Carbon Nanotube Science. In Synthesis, Properties and Applications; Harris, P.J.F., Ed.; Cambridge University Press: Cambridge, UK, 2010; Volume 49, ISBN 9781139478151. [Google Scholar]
- Ilatovskii, D.A.; Gilshtein, E.P.; Glukhova, O.E.; Nasibulin, A.G. Transparent Conducting Films Based on Carbon Nanotubes: Rational Design toward the Theoretical Limit. Adv. Sci. 2022, 9, 2201673. [Google Scholar] [CrossRef]
- Li, Z.; Kandel, H.R.; Dervishi, E.; Saini, V.; Biris, A.S.; Biris, A.R.; Lupu, D. Does the Wall Number of Carbon Nanotubes Matter as Conductive Transparent Material? Appl. Phys. Lett. 2007, 91, 053115. [Google Scholar] [CrossRef]
- Yang, F.; Wang, M.; Zhang, D.; Yang, J.; Zheng, M.; Li, Y. Chirality Pure Carbon Nanotubes: Growth, Sorting, and Characterization. Chem. Rev. 2020, 120, 2693–2758. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, N.; Laiho, P.; Kauppinen, E.I. Recent Developments in Single-Walled Carbon Nanotube Thin Films Fabricated by Dry Floating Catalyst Chemical Vapor Deposition. Top Curr. Chem. 2017, 375, 90. [Google Scholar] [CrossRef]
- Khabushev, E.M.; Krasnikov, D.V.; Zaremba, O.T.; Tsapenko, A.P.; Goldt, A.E.; Nasibulin, A.G. Machine Learning for Tailoring Optoelectronic Properties of Single-Walled Carbon Nanotube Films. J. Phys. Chem. Lett. 2019, 10, 56. [Google Scholar] [CrossRef]
- Weller, L.; Smail, F.R.; Elliott, J.A.; Windle, A.H.; Boies, A.M.; Hochgreb, S. Mapping the Parameter Space for Direct-Spun Carbon Nanotube Aerogels. Carbon 2019, 146, 789–812. [Google Scholar] [CrossRef]
- Khabushev, E.M.; Krasnikov, D.V.; Kolodiazhnaia, J.V.; Bubis, A.V.; Nasibulin, A.G. Structure-Dependent Performance of Single-Walled Carbon Nanotube Films in Transparent and Conductive Applications. Carbon 2020, 161, 712–717. [Google Scholar] [CrossRef]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef]
- Yuan, Y.; Wei, L.; Jiang, W.; Goh, K.; Jiang, R.; Lau, R.; Chen, Y. Sulfur-Induced Chirality Changes in Single-Walled Carbon Nanotube Synthesis by Ethanol Chemical Vapor Deposition on a Co/SiO2 Catalyst. J. Mater. Chem. A Mater. 2015, 3, 3310–3319. [Google Scholar] [CrossRef]
- Balogh, Z.; Halasi, G.; Korbély, B.; Hernadi, K. CVD-Synthesis of Multiwall Carbon Nanotubes over Potassium-Doped Supported Catalysts. Appl. Catal. A Gen. 2008, 344, 191–197. [Google Scholar] [CrossRef]
- Nasibulin, A.G.; Brown, D.P.; Queipo, P.; Gonzalez, D.; Jiang, H.; Kauppinen, E.I. An Essential Role of CO2 and H2O during Single-Walled CNT Synthesis from Carbon Monoxide. Chem. Phys. Lett. 2006, 417, 179–184. [Google Scholar] [CrossRef]
- Guellati, O.; Janowska, I.; Bégin, D.; Guerioune, M.; Mekhalif, Z.; Delhalle, J.; Moldovan, S.; Ersen, O.; Pham-Huu, C. Influence of Ethanol in the Presence of H 2 on the Catalytic Growth of Vertically Aligned Carbon Nanotubes. Appl. Catal. A Gen. 2012, 423–424, 7–14. [Google Scholar] [CrossRef]
- Kuo, D.H.; Su, M.Y. The Effects of Hydrogen and Temperature on the Growth and Microstructure of Carbon Nanotubes Obtained by the Fe(CO)5 Gas-Phase-Catalytic Chemical Vapor Deposition. Surf. Coat Technol. 2007, 201, 9172–9178. [Google Scholar] [CrossRef]
- Zhang, G.; Mann, D.; Zhang, L.; Javey, A.; Li, Y.; Yenilmez, E.; Wang, Q.; Mcvittie, J.P.; Nishi, Y.; Gibbons, J.; et al. Ultra-High-Yield Growth of Vertical Single-Walled Carbon Nanotubes: Hidden Roles of Hydrogen and Oxygen. Proc. Natl. Acad. Sci. USA 2005, 102, 16141–16145. [Google Scholar] [CrossRef]
- Franklin, N.R.; Li, Y.; Chen, R.J.; Javey, A.; Dai, H. Patterned Growth of Single-Walled Carbon Nanotubes on Full 4-Inch Wafers. Appl. Phys. Lett. 2001, 79, 4571–4573. [Google Scholar] [CrossRef]
- Rao, F.B.; Li, T.; Wang, Y.L. Effect of Hydrogen on the Growth of Single-Walled Carbon Nanotubes by Thermal Chemical Vapor Deposition. Phys. E Low Dimens. Syst. Nanostruct. 2008, 40, 779–784. [Google Scholar] [CrossRef]
- Chung, U.C.; Lee, D.B.; Jeong, Y.U.; Ha, M.J.; Chung, W.S. Effect of H2 Gas on Carbon Nanotubes Synthesis. Mater. Sci. Forum 2005, 475–479, 3559–3562. [Google Scholar] [CrossRef]
- Li, Y.; Ji, K.; Duan, Y.; Meng, G.; Dai, Z. Effect of Hydrogen Concentration on the Growth of Carbon Nanotube Arrays for Gecko-Inspired Adhesive Applications. Coatings 2017, 7, 221. [Google Scholar] [CrossRef]
- Ma, Y.; Dichiara, A.B.; He, D.; Zimmer, L.; Bai, J. Control of Product Nature and Morphology by Adjusting the Hydrogen Content in a Continuous Chemical Vapor Deposition Process for Carbon Nanotube Synthesis. Carbon 2016, 107, 171–179. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, G.; Wang, Z.; Yang, Y.; Shi, Z.; Gu, Z. Influence of Ethylene and Hydrogen Flow Rates on the Wall Number, Crystallinity, and Length of Millimeter-Long Carbon Nanotube Array. J. Phys. Chem. C 2008, 112, 12706–12709. [Google Scholar] [CrossRef]
- Behr, M.J.; Gaulding, E.A.; Mkhoyan, K.A.; Aydil, E.S. Effect of Hydrogen on Catalyst Nanoparticles in Carbon Nanotube Growth. J. Appl. Phys. 2010, 108, 053303. [Google Scholar] [CrossRef]
- Bokros, J.C.; Thrower, P.A. Chemistry and Physics of Carbon, 5th ed.; CRC Press: Boca Raton, FL, USA, 1969. [Google Scholar]
- Tesner, P.A.; Rafalkes, I.S. Dokl. Akad. Nauk. SSSR 1952, 87, 821.
- Arefieva, E.F.; Snegireva, T.D. Khimiya Tverd. Topl. 1978, 41.
- Arefieva, E.F.; Snegireva, T.D. Zhurnal Fiz. Khimii 1978, 52, 1839.
- Tesner, P.A.; Gorodetskii, A.E.; Snegireva, T.D.; Arefieva, E.F. Dokl. Akad. Nauk. SSSR 1978, 239, 901.
- Nasibulin, A.G.; Moisala, A.; Brown, D.P.; Jiang, H.; Kauppinen, E.I. A Novel Aerosol Method for Single Walled Carbon Nanotube Synthesis. Chem. Phys. Lett. 2005, 402, 227–232. [Google Scholar] [CrossRef]
- Kaskela, A.; Nasibulin, A.G.; Timmermans, M.Y.; Aitchison, B.; Papadimitratos, A.; Tian, Y.; Zhu, Z.; Jiang, H.; Brown, D.P.; Zakhidov, A.; et al. Aerosol-Synthesized SWCNT Networks with Tunable Conductivity and Transparency by a Dry Transfer Technique. Nano Lett. 2010, 10, 4349–4355. [Google Scholar] [CrossRef]
- Anoshkin, I.V.; Nasibulin, A.G.; Tian, Y.; Liu, B.; Jiang, H.; Kauppinen, E.I. Hybrid Carbon Source for Single-Walled Carbon Nanotube Synthesis by Aerosol CVD Method. Carbon 2014, 78, 130–136. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.Q.; Zhao, M.Q.; Qian, W.Z.; Wei, F. Carbon Nanotube Mass Production: Principles and Processes. ChemSusChem 2011, 4, 864–889. [Google Scholar] [CrossRef] [PubMed]
- Khabushev, E.M.; Krasnikov, D.V.; Goldt, A.E.; Fedorovskaya, E.O.; Tsapenko, A.P.; Zhang, Q.; Kauppinen, E.I.; Kallio, T.; Nasibulin, A.G. Joint Effect of Ethylene and Toluene on Carbon Nanotube Growth. Carbon 2022, 189, 474–483. [Google Scholar] [CrossRef]
- Ermolaev, G.A.; Tsapenko, A.P.; Volkov, V.S.; Anisimov, A.S.; Gladush, Y.G.; Nasibulin, A.G. Express Determination of Thickness and Dielectric Function of Single-Walled Carbon Nanotube Films. Appl. Phys. Lett. 2020, 116, 231103. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman Spectroscopy of Carbon Nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Khabushev, E.M.; Kolodiazhnaia, J.V.; Krasnikov, D.V.; Nasibulin, A.G. Activation of Catalyst Particles for Single-Walled Carbon Nanotube Synthesis. Chem. Eng. J. 2021, 413, 127475. [Google Scholar] [CrossRef]
- Hecht, D.; Hu, L.; Grüner, G. Conductivity Scaling with Bundle Length and Diameter in Single Walled Carbon Nanotube Networks. Appl. Phys. Lett. 2006, 89, 133112. [Google Scholar] [CrossRef]
- Krasnikov, D.V.; Kuznetsov, V.L.; Romanenko, A.I.; Shmakov, A.N. Side Reaction in Catalytic CVD Growth of Carbon Nanotubes: Surface Pyrolysis of a Hydrocarbon Precursor with the Formation of Lateral Carbon Deposits. Carbon 2018, 139, 105–117. [Google Scholar] [CrossRef]
- Novikov, I.V.; Khabushev, E.M.; Krasnikov, D.V.; Bubis, A.V.; Goldt, A.E.; Shandakov, S.D.; Nasibulin, A.G. Residence time effect on single-walled carbon nanotube synthesis in an aerosol CVD reactor. Chem. Eng. J. 2021, 420, 129869. [Google Scholar] [CrossRef]
- Topinka, M.A.; Rowell, M.W.; Goldhaber-Gordon, D.; McGehee, M.D.; Hecht, D.S.; Gruner, G. Charge Transport in Interpenetrating Networks of Semiconducting and Metallic Carbon Nanotubes. Nano Lett. 2009, 9, 1866–1871. [Google Scholar] [CrossRef]
- Liao, Y.; Hussain, A.; Laiho, P.; Zhang, Q.; Tian, Y.; Wei, N.; Ding, E.-X.; Khan, S.A.; Nguyen, N.N.; Ahmad, S.; et al. Tuning Geometry of SWCNTs by CO2 in Floating Catalyst CVD for High-Performance Transparent Conductive Films. Adv. Mater. Interfaces 2018, 5, 1801209. [Google Scholar] [CrossRef]
- Saito, T.; Ohshima, S.; Okazaki, T.; Ohmori, S.; Yumura, M.; Iijima, S. Selective Diameter Control of Single-Walled Carbon Nanotubes in the Gas-Phase Synthesis. J. Nanosci. Nanotechnol. 2008, 8, 6153–6157. [Google Scholar] [CrossRef] [PubMed]
- Krasnikov, D.V.; Bokova-Sirosh, S.N.; Tsendsuren, T.-O.; Romanenko, A.I.; Obraztsova, E.D.; Volodin, V.A.; Kuznetsov, V.L. Influence of the Growth Temperature on the Defective Structure of the Multi-Walled Carbon Nanotubes. Phys. Status Solidi B 2017, 1700255, 1700255. [Google Scholar] [CrossRef]
- Nasibulin, A.G.; Queipo, P.; Shandakov, S.D.; Brown, D.P.; Jiang, H.; Pikhitsa, P.V.; Tolochko, O.V.; Kauppinen, E.I. Studies on Mechanism of Single-Walled Carbon Nanotube Formation. J. Nanosci. Nanotechnol. 2006, 6, 1233–1246. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current Understanding of the Growth of Carbon Nanotubes in Catalytic Chemical Vapour Deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef]
- MacKenzie, K.J.; Dunens, O.M.; Harris, A.T. An Updated Review of Synthesis Parameters and Growth Mechanisms for Carbon Nanotubes in Fluidized Beds. Ind. Eng. Chem. Res. 2010, 49, 5323–5338. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanova, A.R.; Krasnikov, D.V.; Khabushev, E.M.; Ramirez B., J.A.; Matyushkin, Y.E.; Nasibulin, A.G. Role of Hydrogen in Ethylene-Based Synthesis of Single-Walled Carbon Nanotubes. Nanomaterials 2023, 13, 1504. https://doi.org/10.3390/nano13091504
Bogdanova AR, Krasnikov DV, Khabushev EM, Ramirez B. JA, Matyushkin YE, Nasibulin AG. Role of Hydrogen in Ethylene-Based Synthesis of Single-Walled Carbon Nanotubes. Nanomaterials. 2023; 13(9):1504. https://doi.org/10.3390/nano13091504
Chicago/Turabian StyleBogdanova, Alisa R., Dmitry V. Krasnikov, Eldar M. Khabushev, Javier A. Ramirez B., Yakov E. Matyushkin, and Albert G. Nasibulin. 2023. "Role of Hydrogen in Ethylene-Based Synthesis of Single-Walled Carbon Nanotubes" Nanomaterials 13, no. 9: 1504. https://doi.org/10.3390/nano13091504




