Growth and Characterization of Sputtered InAlN Nanorods on Sapphire Substrates for Acetone Gas Sensing
Abstract
:1. Introduction
2. Materials and Methods
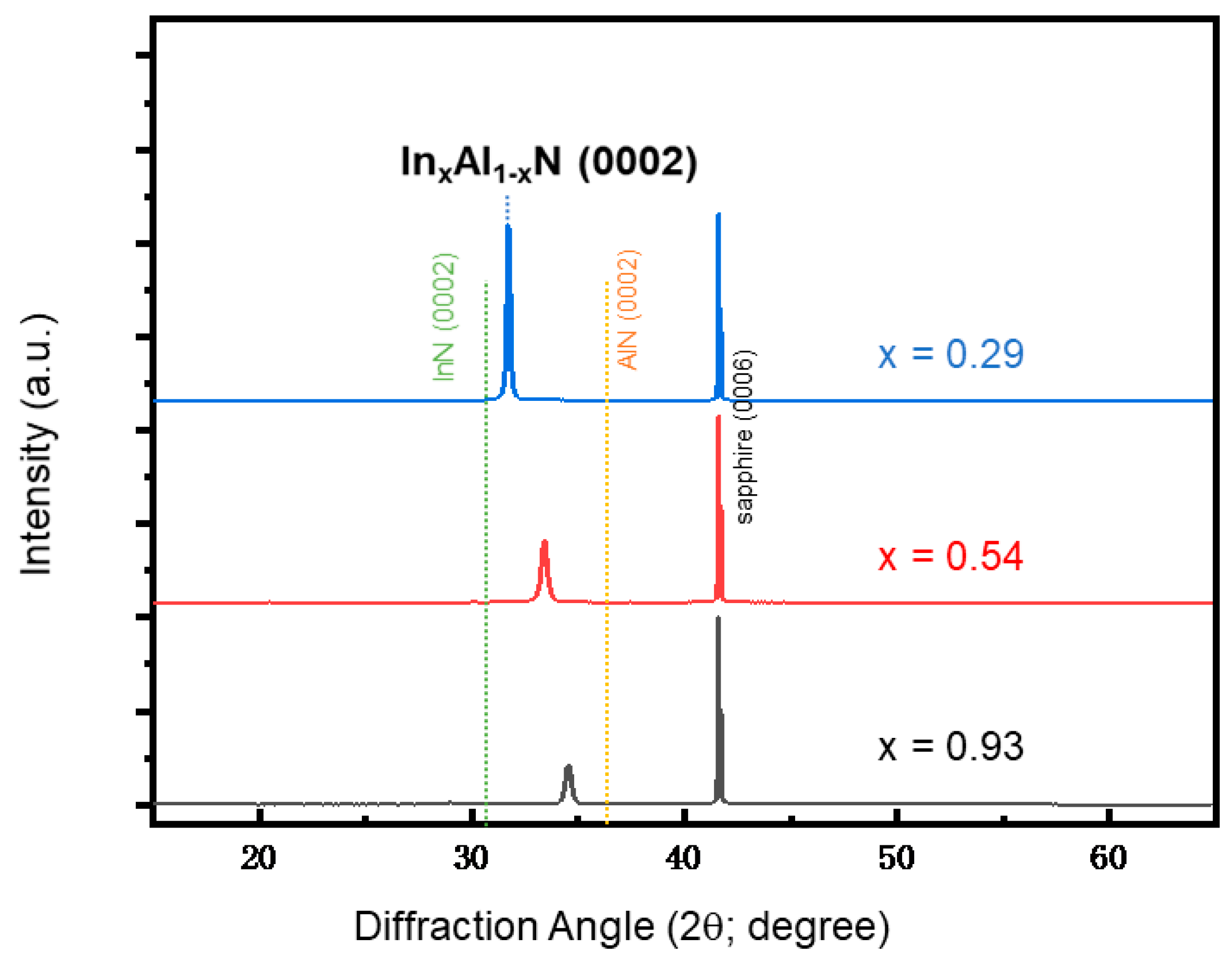
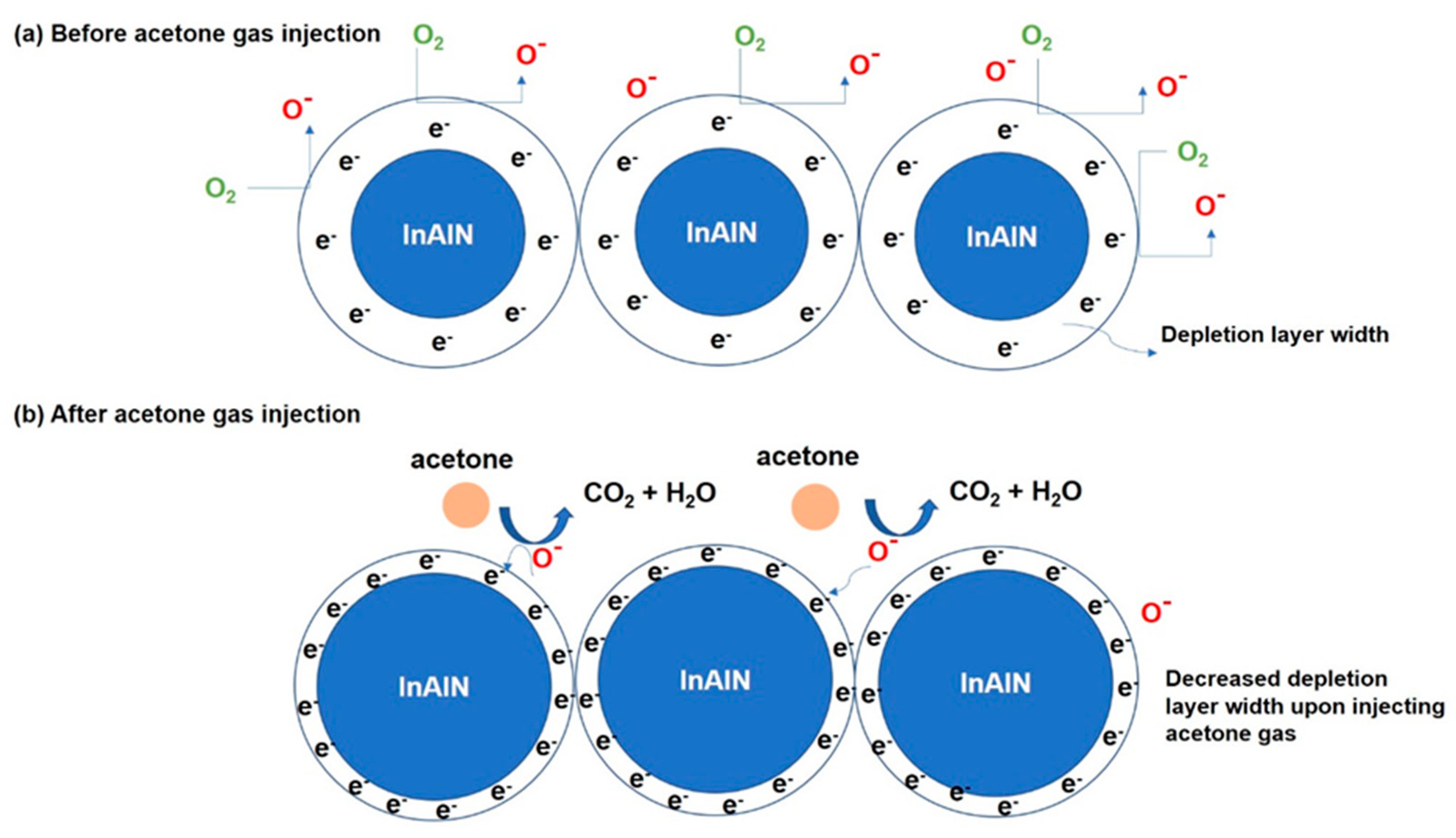
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, N.; Pandey, V.; Gupta, A.; Tan, S.T.; Tripathy, S.; Kumar, M. Recent progress on group III nitride nanostructure-based gas sensors. J. Mater. Chem. C 2022, 10, 12157–12190. [Google Scholar] [CrossRef]
- Tsao, J.Y.; Chowdhury, S.; Hollis, M.A.; Jena, D.; Johnson, N.M.; Jones, K.A.; Kaplar, R.J.; Rajan, S.; Van de Walle, C.G.; Bellotti, E.; et al. Ultrawide-bandgap semiconductors research opportunities and challenges. Adv. Electron. Mater. 2018, 4, 1600501. [Google Scholar] [CrossRef]
- Alaie, Z.; Nejad, S.M.; Yousefi, M.H. Recent advances in ultraviolet photodetectors. Mater. Sci. Semicond. Process. 2015, 29, 16–55. [Google Scholar] [CrossRef]
- Cui, P.; Zeng, Y. Technology of sub-100 nm InAlN/GaN HEMTs on silicon with suppressed leakage current. Solid State Electron. 2021, 185, 108137. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Xu, M.; Zhou, H.P.; Duan, M.Y.; Zhu, W.J.; He, H.L. First-principles calculation of electronic structures and optical properties of wurtzite InxAl1-xN alloys. Phys. B Condens. Matter 2008, 403, 1666–1672. [Google Scholar] [CrossRef]
- Chen, F.; Ji, X.; Lau, S.P. Recent progress in group III-nitride nanostructures: From materials to applications. Mater. Sci. Eng. R Rep. 2020, 142, 100578. [Google Scholar] [CrossRef]
- Wu, J.; Walukiewicz, W. Band gaps of InN and group III nitride alloys. Superlattices Microstruct. 2003, 34, 63–75. [Google Scholar] [CrossRef]
- Afzal, N.; Devarajan, M.; Ibrahim, K. Effects of indium mole fraction on the physical characteristics of magnetron sputtered InxAl1-xN films. J. Alloys Compd. 2015, 652, 407–414. [Google Scholar] [CrossRef]
- Jian, R.S.; Wang, T.Y.; Song, L.Y.; Kuo, C.Y.; Tian, W.C.; Lo, E.W.; Lu, C.J. Field investigations and dynamic measurements of process activity induced VOCs inside a semiconductor cleanroom. Build. Environ. 2015, 94, 287–295. [Google Scholar] [CrossRef]
- Shimoni, O.; Cervenka, J.; Karle, T.J.; Fox, K.; Gibson, B.C.; Tomljenovic-Hanic, S.; Greentree, A.D.; Prawer, S. Development of a templated approach to fabricate diamond patterns on various substrates. ACS Appl. Mater. Interfaces 2014, 6, 8894–8902. [Google Scholar] [CrossRef]
- David, E.; Niculescu, V.C. Volatile organic compounds (Vocs) as environmental pollutants: Occurrence and mitigation using nanomaterials. Int. J. Environ. Res. Public Health 2021, 18, 13147. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Bloemen, K.; Hooyberghs, J.; Desager, K.; Witters, E.; Schoeters, G. Non-invasive bioarker sampling and analysis of the exhaled breath proteome. Proteom. Clin. Appl. 2009, 3, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Riess, U.; Tegtbur, U.; Fauck, C.; Fuhrmann, F.; Markewitz, D.; Salthammer, T. Experimental setup and analytical methods for the non-invasive determination of volatile organic compounds, formaldehyde and NOx in exhaled human breath. Anal. Chim. Acta 2010, 669, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Blaikie, T.P.J.; Edge, J.A.; Hancock, G.; Lunn, D.; Megson, C.; Peverall, R.; Richmond, G.; Ritchie, G.A.D.; Taylor, D. Comparison of breath gases, including acetone, with blood glucose and blood ketones in children and adolescents with type 1 diabetes. J. Breath Res. 2014, 8, 046010. [Google Scholar] [CrossRef] [PubMed]
- Tanda, N.; Hinokio, Y.; Washio, J.; Takahashi, N.; Koseki, T. Analysis of ketone bodies in exhaled breath and blood of ten healthy Japanese at OGTT using a portable gas chromatograph. J. Breath Res. 2014, 8, 046008. [Google Scholar] [CrossRef] [PubMed]
- Ueta, I.; Saito, Y.; Hosoe, M.; Okamoto, M.; Ohkita, H.; Shirai, S.; Tamura, H.; Jinno, K. Breath acetone analysis with miniaturized sample preparation device: In-needle preconcentration and subsequent determination by gas chromatography-mass spectroscopy. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2551–2556. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T.; Deng, J.; Zhang, R.; Lou, Z.; Wang, L. P-type Co3O4 nanomaterials-based gas sensor: Preparation and acetone sensing performance. Sens. Actuators B Chem. 2017, 242, 369–377. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Available online: https://www.cdc.gov/niosh/pel88/67-64.html (accessed on 25 November 2023).
- Bhuiyan, A.G.; Sugita, K.; Hashimoto, A.; Yamamoto, A. InGaN solar cells: Present state of the art and important challenges. IEEE J. Photovolt. 2012, 2, 276–293. [Google Scholar] [CrossRef]
- Chapin, C.A.; Miller, R.A.; Dowling, K.M.; Chen, R.; Senesky, D.G. InAlN/GaN high electron mobility micro-pressure sensors for high-temperature environments. Sens. Actuators A Phys. 2017, 263, 216–223. [Google Scholar] [CrossRef]
- Zhou, Y.; Mi, M.; Han, Y.; Wang, P.; Chen, Y.; Liu, J.; Gong, C.; Yang, M.; Zhang, M.; Zhu, Q.; et al. High Efficiency Over 70% at 3.6-GHz InAlN/GaN HEMT Fabricated by Gate Recess and Oxidation Process for Low-Voltage RF Applications. IEEE Trans. Electron Devices 2023, 70, 43–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Xu, S.; Chen, D.; Bao, W.; Zhang, J.; Zhang, J.; Hao, Y. Studies on the InAlN/InGaN/InAlN/InGaN double channel heterostructures with low sheet resistance. Appl. Phys. Lett. 2017, 111, 222107. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, Y.; Xu, J.; Debliquy, M. Room temperature conductive type metal oxide semiconductor gas sensors for NO2 detection. Sens. Actuators A Phys. 2019, 289, 118–133. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Pang, A.L.; Ardani, M.R.; Pung, S.Y.; Ooi, P.C.; Hamzah, A.A.; Mohd Razip Wee, M.F.; Aniq Shazni Mohammad Haniff, M.; Dee, C.F.; Mahmoudi, E.; et al. Detection of breath acetone by semiconductor metal oxide nanostructures-based gas sensors: A review. Mater. Sci. Semicond. Process. 2022, 149, 106897. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, S.J.; Choi, S.J.; Kim, N.H.; Hakim, M.; Rothschild, A.; Kim, I.D. Thin-walled SnO2 nanotubes functionalized with Pt and Au catalysts via the protein templating route and their selective detection of acetone and hydrogen sulfide molecules. Nanoscale 2015, 7, 16417–16426. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, S.J.; Koo, W.T.; Cho, H.J.; Kim, I.D. Catalyst-loaded porous WO3 nanofibers using catalyst-decorated polystyrene colloid templates for detection of biomarker molecules. Chem. Commun. 2015, 51, 2609–2612. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.; Yoo, R.; Woo, S.P.; Lee, H.S.; Lee, W. Enhanced acetone-sensing properties of Pt-decorated Al-doped ZnO nanoparticles. Sens. Actuators B Chem. 2019, 280, 109–119. [Google Scholar] [CrossRef]
- Liu, H.F.; Tan, C.C.; Dalapati, G.K.; Chi, D.Z. Magnetron-sputter deposition of high-indium-content n- AlInN thin film on p-Si(001) substrate for photovoltaic applications? J. Appl. Phys. 2012, 6, 063114. [Google Scholar] [CrossRef]
- Blasco, R.; Naranjo, F.B.; Valdueza-Felip, S. Design of AlInN on silicon heterojunctions grown by sputtering for solar devices. Curr. Appl. Phys. 2020, 20, 1244–1252. [Google Scholar] [CrossRef]
- Afzal, N.; Devarajan, M.; Ibrahim, K. Influence of substrate temperature on the growth and properties of reactively sputtered In-rich InAlN films. J. Mater. Sci. Mater. Electron. 2016, 27, 4281–4289. [Google Scholar] [CrossRef]
- Han, Q.; Duan, C.; Du, G.; Shi, W.; Ji, L. Magnetron sputter epitaxy and characterization of wurtzite AlInN on Si(111) substrates. J. Electron. Mater. 2010, 39, 489–493. [Google Scholar] [CrossRef]
- Serban, E.A.; Palisaitis, J.; Junaid, M.; Tengdelius, L.; Högberg, H.; Hultman, L.; Persson, P.O.A.; Birch, J.; Hsiao, C.L. Magnetron sputter epitaxy of high-quality GaN nanorods on functional and cost-effective templates/substrates. Energies 2017, 10, 1322. [Google Scholar] [CrossRef]
- Serban, E.A.; Åke Persson, P.O.; Poenaru, I.; Junaid, M.; Hultman, L.; Birch, J.; Hsiao, C.L. Structural and compositional evolutions of InxAl1-xN core-shell nanorods grown on Si(111) substrates by reactive magnetron sputter epitaxy. Nanotechnology 2015, 26, 215602. [Google Scholar] [CrossRef] [PubMed]
- Bangolla, H.K.; Siao, M.D.; Huang, Y.H.; Chen, R.S.; Žukauskaitė, A.; Palisaitis, J.; Persson, P.O.Å.; Hultman, L.; Birch, J.; Hsiao, C.L. Composition-dependent photoconductivities in indium aluminium nitride nanorods grown by magnetron sputter epitaxy. Nanoscale Adv. 2022, 4, 4886–4894. [Google Scholar] [CrossRef]
- Tung, J.C.; Huang, S.W.; Pai, C.A.; Horng, R.H.; Chang, C.C.; Hung, D.R.; Liu, P.L. Ab Initio studies of work function changes of CO adsorption on clean and Pd-doped ZnGa2O4(111) Surfaces for Gas Sensors. Appl. Sci. 2022, 12, 5978. [Google Scholar] [CrossRef]
- Tung, J.C.; Chiang, Y.H.; Wang, D.Y.; Liu, P.L. Adsorption of NO2 and H2S on ZnGa2O4(111) thin films: A first-principles density functional theory study. Appl. Sci. 2020, 10, 8822. [Google Scholar] [CrossRef]
- Tung, J.C.; Wang, D.Y.; Chen, Y.H.; Liu, P.L. Influences of work function changes in NO2 and H2S adsorption on Pd-doped ZnGa2O4(111) thin films: First-principles studies. Appl. Sci. 2021, 11, 5259. [Google Scholar] [CrossRef]
- Wongrat, E.; Chanlek, N.; Chueaiarrom, C.; Thupthimchun, W.; Samransuksamer, B.; Choopun, S. Acetone gas sensors based on ZnO nanostructures decorated with Pt and Nb. Ceram. Int. 2017, 43, S557–S566. [Google Scholar] [CrossRef]
- Hanh, N.H.; Van Duy, L.; Hung, C.M.; Xuan, C.T.; Van Duy, N.; Hoa, N.D. High-performance acetone gas sensor based on Pt–Zn2SnO4 hollow octahedra for diabetic diagnosis. J. Alloys Compd. 2021, 886, 161284. [Google Scholar] [CrossRef]
- Kresse, G.J.F. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 169–186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Yue, W. Accurate and simple density functional for the electronic exchange energy: Generalized gradient approximation. Phys. Rev. B 1986, 33, 8800–8802. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter 1994, 6, 8245–8257. [Google Scholar] [CrossRef]






| Model | EVAC (eV) | EF (eV) | S, gas (eV) | S (eV) | (eV) |
|---|---|---|---|---|---|
| InN | 0.15 | −2.47 | - | 2.62 | - |
| In0.97Al0.03N-1 | 1.67 | −2.62 | - | 4.29 | - |
| In0.97Al0.03N-2 | 0.29 | −2.34 | - | 2.63 | - |
| In0.5Al0.5N | 1.64 | −2.85 | - | 4.49 | - |
| InN-CH3COCH3 | 0.53 | −1.78 | 2.31 | - | −0.31 |
| In0.97Al0.03N-1-CH3COCH3 | 1.03 | −2.07 | 3.10 | - | −1.19 |
| In0.97Al0.03N-2-CH3COCH3 | 0.09 | −1.71 | 1.80 | - | −0.83 |
| In0.5Al0.5N-CH3COCH3 | 0.96 | −2.37 | 3.33 | - | −1.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horng, R.-H.; Cho, P.-H.; Chang, J.-C.; Singh, A.K.; Jhang, S.-Y.; Liu, P.-L.; Wuu, D.-S.; Bairagi, S.; Chen, C.-H.; Järrendahl, K.; et al. Growth and Characterization of Sputtered InAlN Nanorods on Sapphire Substrates for Acetone Gas Sensing. Nanomaterials 2024, 14, 26. https://doi.org/10.3390/nano14010026
Horng R-H, Cho P-H, Chang J-C, Singh AK, Jhang S-Y, Liu P-L, Wuu D-S, Bairagi S, Chen C-H, Järrendahl K, et al. Growth and Characterization of Sputtered InAlN Nanorods on Sapphire Substrates for Acetone Gas Sensing. Nanomaterials. 2024; 14(1):26. https://doi.org/10.3390/nano14010026
Chicago/Turabian StyleHorng, Ray-Hua, Po-Hsiang Cho, Jui-Che Chang, Anoop Kumar Singh, Sheng-Yuan Jhang, Po-Liang Liu, Dong-Sing Wuu, Samiran Bairagi, Cheng-Hsu Chen, Kenneth Järrendahl, and et al. 2024. "Growth and Characterization of Sputtered InAlN Nanorods on Sapphire Substrates for Acetone Gas Sensing" Nanomaterials 14, no. 1: 26. https://doi.org/10.3390/nano14010026







