Enhancing Solid-Phase Extraction of Tamoxifen and Its Metabolites from Human Plasma Using MOF-Integrated Polyacrylonitrile Composites: A Study on CuBTC and ZIF-8 Efficacy
Abstract
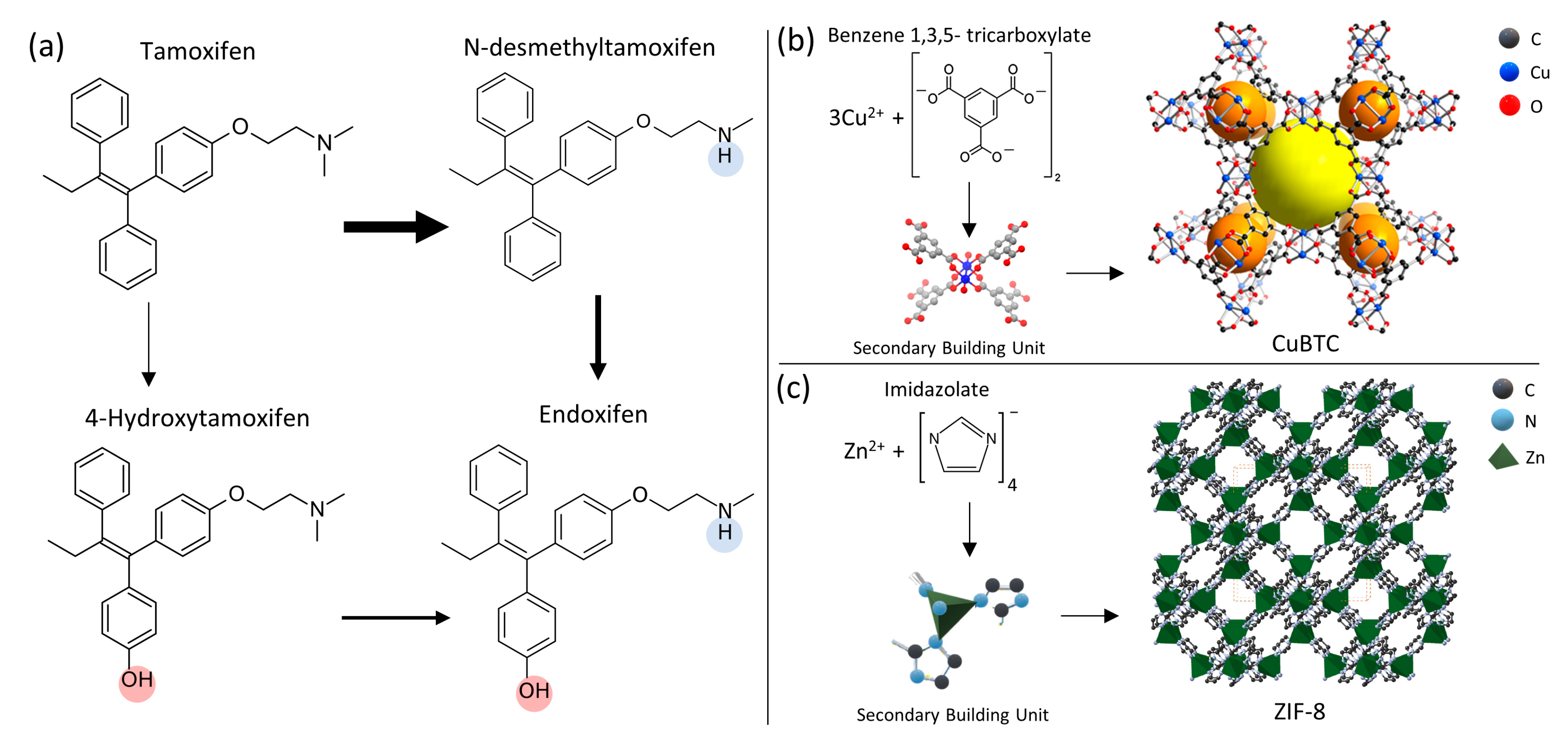
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Preparation of MOF/PAN Electrospun Composites
3. Results and Discussion
3.1. SEM Characterization
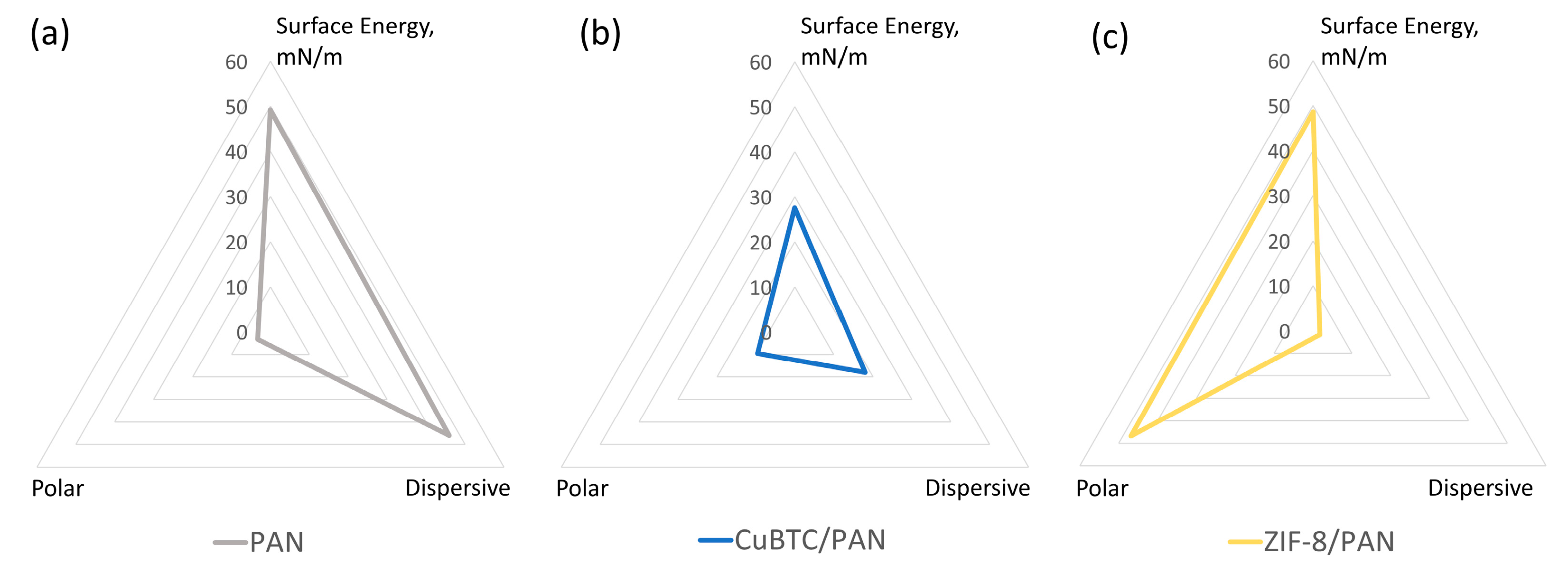
3.2. Surface Energy Characterization
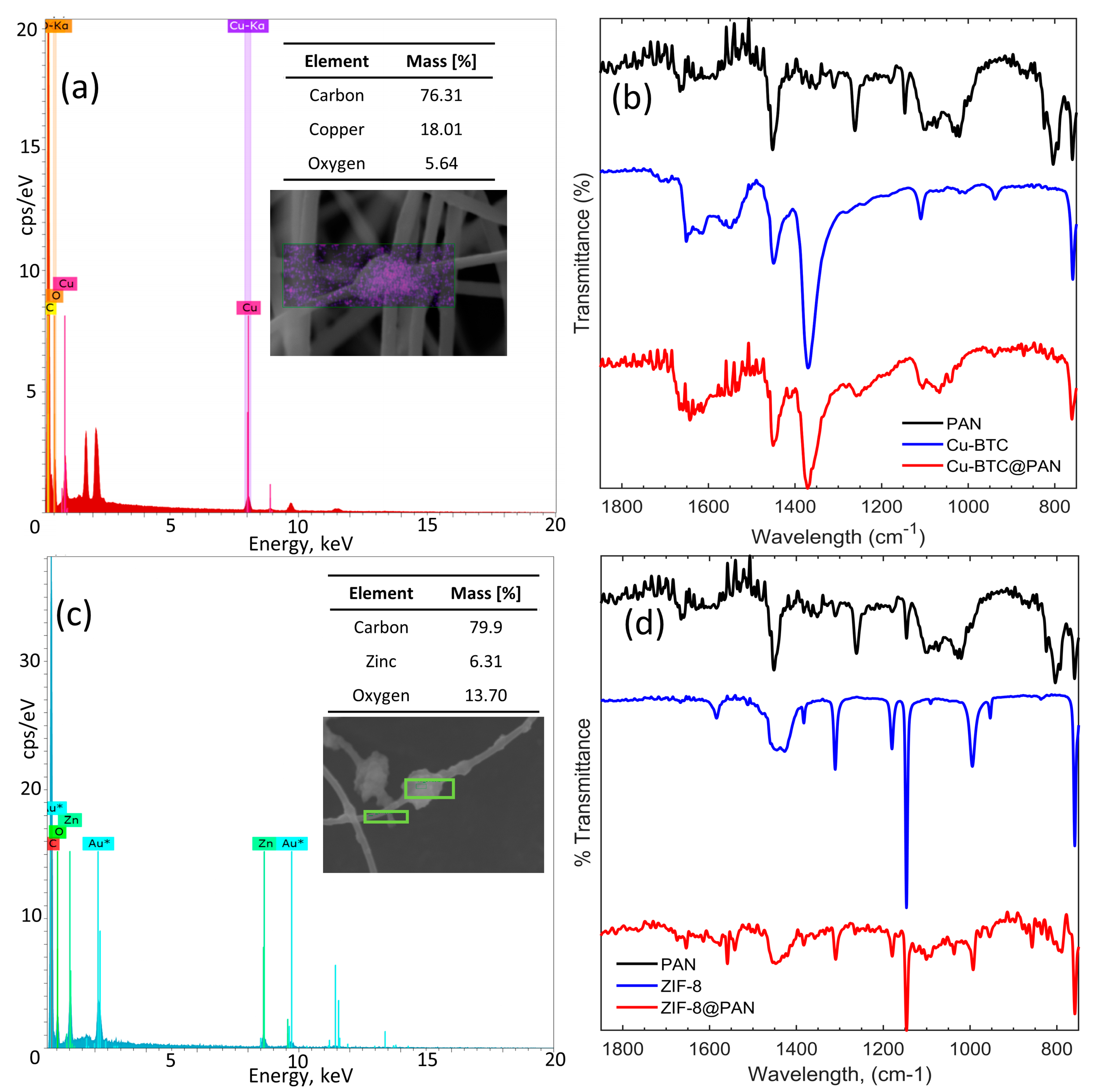
3.3. EDS and FTIR Characterization
3.4. XRD
3.5. UPLC-Ms/Ms
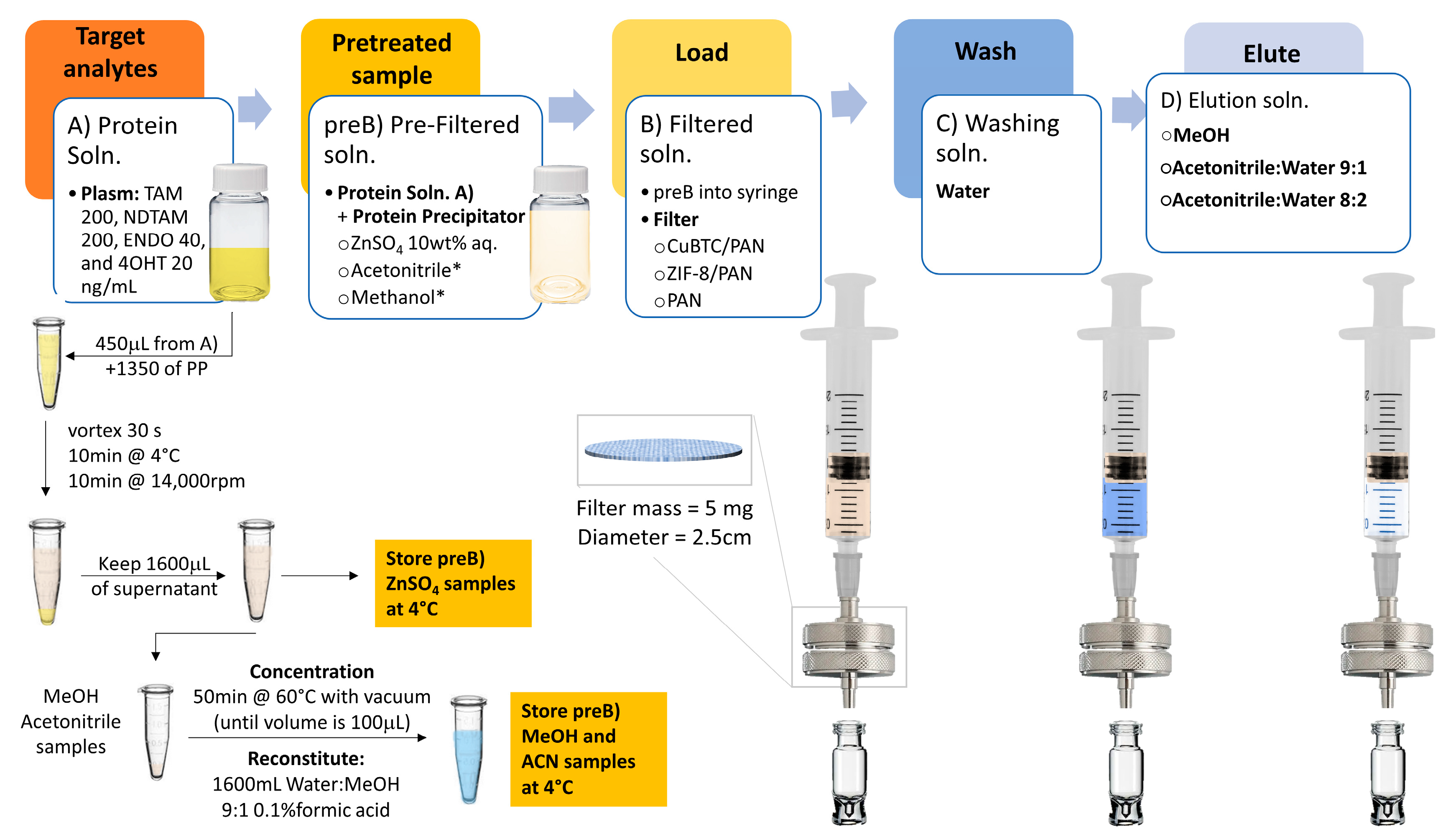
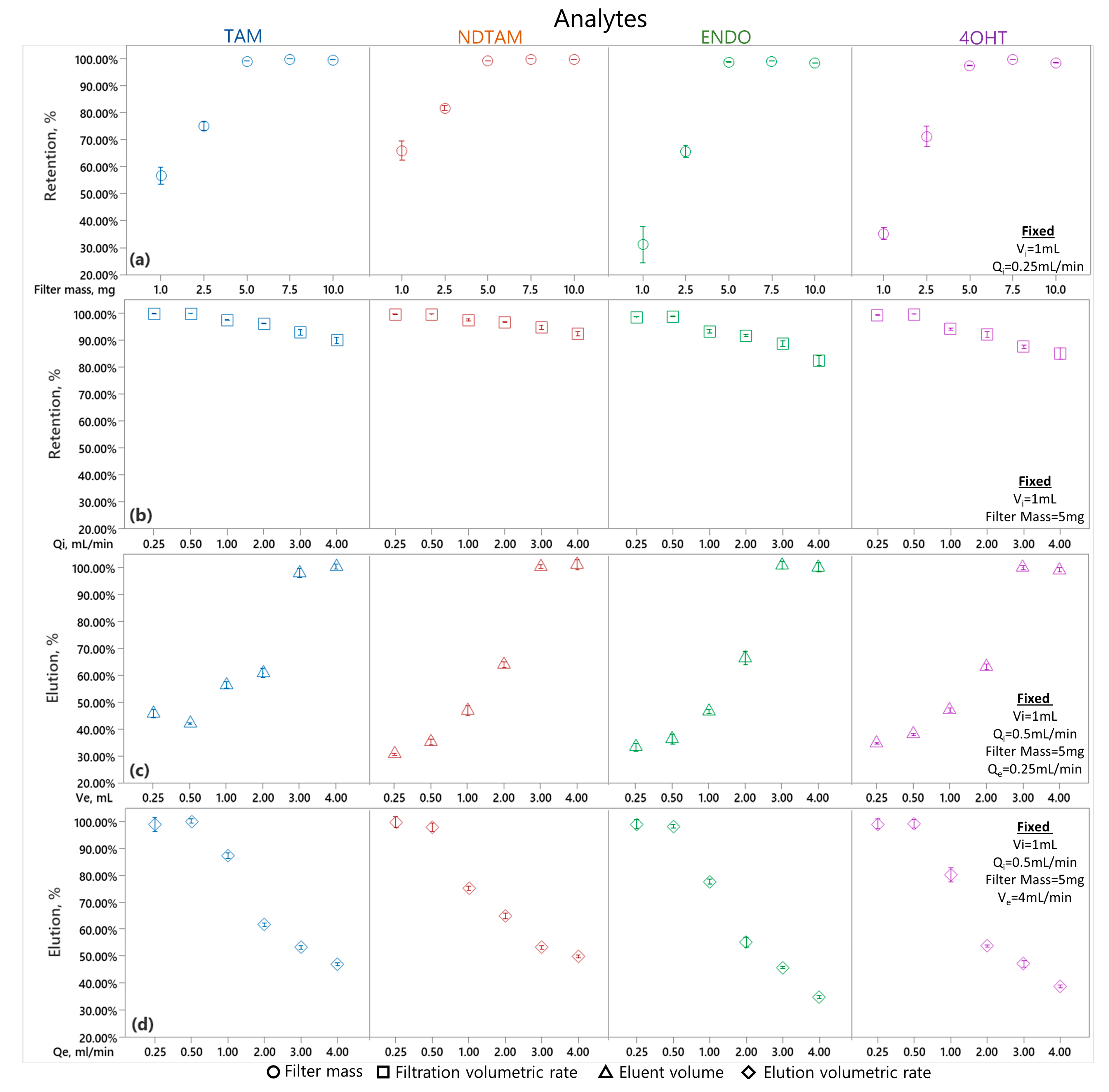
3.5.1. Solid-Phase Extraction Optimization
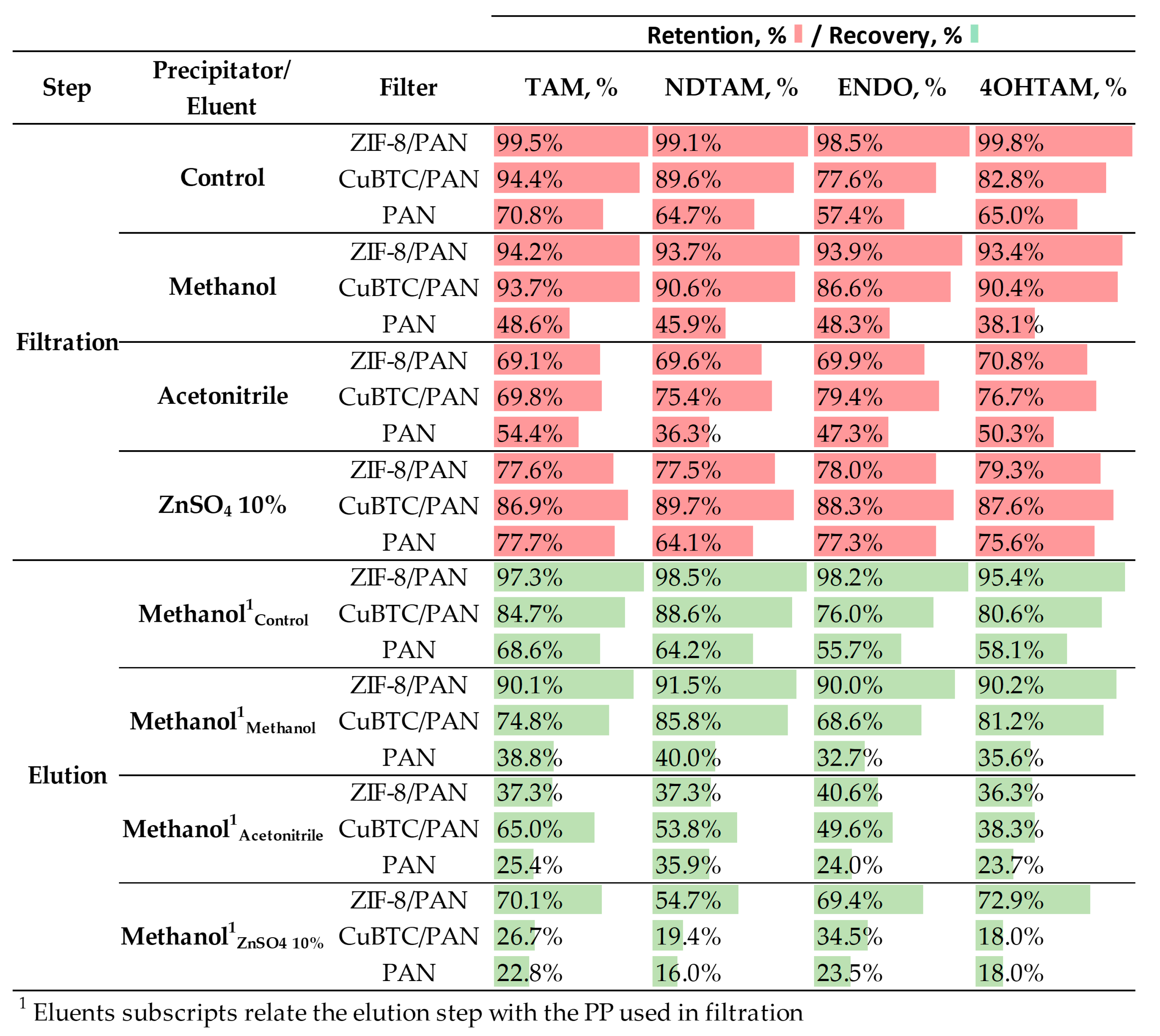
3.5.2. Protein Precipitation for Enhanced Analyte Retention and Elution
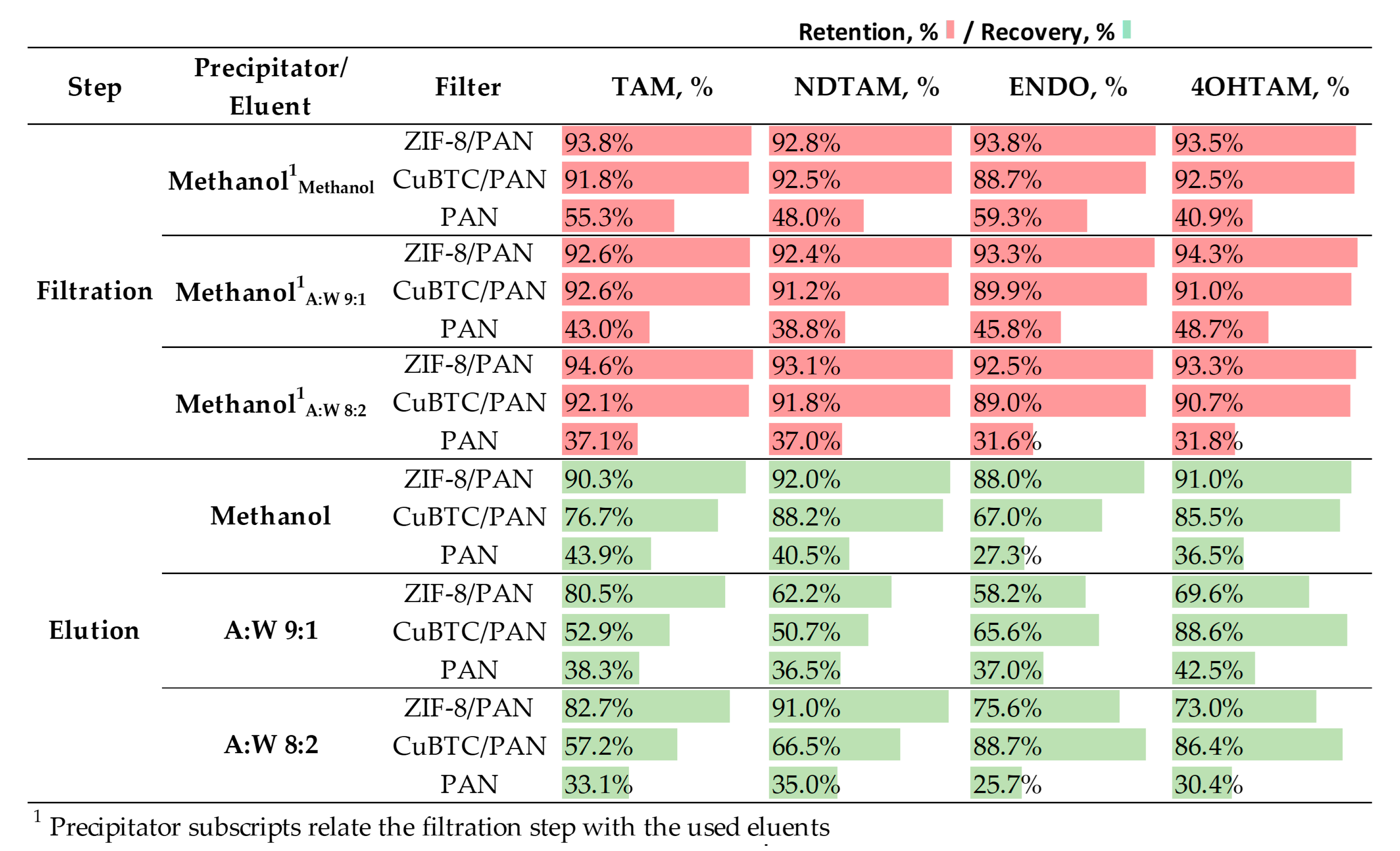
3.5.3. Elution Conditions for Enhanced Analytes Elution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Heath, D.D.; Flatt, S.W.; Wu, A.H.B.; Pruitt, M.A.; Rock, C.L. Evaluation of Tamoxifen and Metabolites by LC-MS/MS and HPLC Methods. Br. J. Biomed. Sci. 2014, 71, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, J.; Gandini, S.; Guerrieri-Gonzaga, A.; Haugan Moi, L.L.; Aristarco, V.; Mellgren, G.; De Censi, A.; Lien, E.A. Tissue Distribution of 4-Hydroxy-N-Desmethyltamoxifen and Tamoxifen-N-Oxide. Breast Cancer Res. Treat. 2012, 134, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, S.F.; Rosing, H.; Schinkel, A.H.; Schellens, J.H.M.; Beijnen, J.H. Bioanalytical Methods for Determination of Tamoxifen and Its Phase I Metabolites: A Review. Anal. Chim. Acta 2010, 683, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.V.; Raymundo, S.; De Oliveira, V.; Staudt, D.E.; Gössling, G.; Peteffi, G.P.; Biazús, J.V.; Cavalheiro, J.A.; Tre-Hardy, M.; Capron, A.; et al. Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometric Method for the Determination of Tamoxifen, N-Desmethyltamoxifen, 4-Hydroxytamoxifen and Endoxifen in Dried Blood Spots—Development, Validation and Clinical Application during Breast. Talanta 2015, 132, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Jager, N.G.L.; Rosing, H.; Schellens, J.H.M.; Linn, S.C.; Beijnen, J.H. Tamoxifen Dose and Serum Concentrations of Tamoxifen and Six of Its Metabolites in Routine Clinical Outpatient Care. Breast Cancer Res. Treat. 2014, 143, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Madlensky, L.; Natarajan, L.; Tchu, S.; Pu, M.; Mortimer, J.; Flatt, S.W.; Nikoloff, D.M.; Hillman, G.; Fontecha, M.R.; Lawrence, H.J.; et al. Tamoxifen Metabolite Concentrations, CYP2D6 Genotype, and Breast Cancer Outcomes. Clin. Pharmacol. Ther. 2011, 89, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Fyles, A.; Sellers, E.M.; Mccready, D.R.; Murphy, J.; Pal, T.; Narod, S.A. Association between CYP3A4 Genotype and Risk of Endometrial Cancer Following Tamoxifen Use. Carcinogenesis 2007, 28, 2139–2142. [Google Scholar] [CrossRef]
- Wu, X.; Hawse, J.R.; Subramaniam, M.; Goetz, M.P.; Ingle, J.N.; Spelsberg, T.C. The Tamoxifen Metabolite, Endoxifen, Is a Potent Antiestrogen That Targets Estrogen Receptor a for Degradation in Breast Cancer Cells. Cancer Res. 2009, 69, 1722–1727. [Google Scholar] [CrossRef]
- Schuurman, T.N.; Witteveen, P.O.; van der Wall, E.; Passier, J.L.M.; Huitema, A.D.R.; Amant, F.; Lok, C.A.R. Tamoxifen and Pregnancy: An Absolute Contraindication? Breast Cancer Res. Treat. 2019, 175, 17–25. [Google Scholar] [CrossRef]
- Bourassa, P.; Dubeau, S.; Maharvi, G.M.; Fauq, A.H.; Thomas, T.J.; Tajmir-Riahi, H.A. Locating the Binding Sites of Anticancer Tamoxifen and Its Metabolites 4-Hydroxytamoxifen and Endoxifen on Bovine Serum Albumin. Eur. J. Med. Chem. 2011, 46, 4344–4353. [Google Scholar] [CrossRef] [PubMed]
- Gerace, E.; Salomone, A.; Abbadessa, G.; Racca, S.; Vincenti, M. Rapid Determination of Anti-Estrogens by Gas Chromatography/Mass Spectrometry in Urine: Method Validation and Application to Real Samples. J. Pharm. Anal. 2012, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Health Promotion. Available online: https://www.who.int/health-topics/health-promotion#tab=tab_2 (accessed on 5 December 2023).
- Smith, S.; Korvink, J.G.; Mager, D.; Land, K. The Potential of Paper-Based Diagnostics to Meet the ASSURED Criteria. RSC Adv. 2018, 8, 34012–34034. [Google Scholar] [CrossRef] [PubMed]
- Mahato, K.; Srivastava, A.; Chandra, P. Paper Based Diagnostics for Personalized Health Care: Emerging Technologies and Commercial Aspects. Biosens. Bioelectron. 2017, 96, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Coatsworth, P.; Shi, X.; Zhi, J.; Hu, L.; Yan, R.; Güder, F.; Yu, H.D. Paper-Based Sensors for Diagnostics, Human Activity Monitoring, Food Safety and Environmental Detection. Sens. Diagn. 2022, 1, 312–342. [Google Scholar] [CrossRef]
- Ostermann, R.; Cravillon, J.; Weidmann, C.; Wiebcke, M.; Smarsly, B.M. Metal-Organic Framework Nanofibers via Electrospinning. Chem. Commun. 2011, 47, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-Phase Extraction of Organic Compounds: A Critical Review (Part I). TrAC Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Buszewski, B.; Szultka, M. Past, Present, and Future of Solid Phase Extraction: A Review. Crit. Rev. Anal. Chem. 2012, 42, 198–213. [Google Scholar] [CrossRef]
- Kaur, H.; Mohanta, G.C.; Gupta, V.; Kukkar, D.; Tyagi, S. Synthesis and Characterization of ZIF-8 Nanoparticles for Controlled Release of 6-Mercaptopurine Drug. J. Drug Deliv. Sci. Technol. 2017, 41, 106–112. [Google Scholar] [CrossRef]
- Arabsorkhi, B.; Sereshti, H.; Abbasi, A. Electrospun Metal-Organic Framework/Polyacrylonitrile Composite Nanofibrous Mat as a Microsorbent for the Extraction of Tetracycline Residue in Human Blood Plasma. J. Sep. Sci. 2019, 42, 1500–1508. [Google Scholar] [CrossRef]
- Yang, Y.; Noviana, E.; Nguyen, M.P.; Geiss, B.J.; Dandy, D.S.; Henry, C.S. Paper-Based Microfluidic Devices: Emerging Themes and Applications. Anal. Chem. 2017, 89, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Lyon, E.; Gastier Foster, J.; Palomaki, G.E.; Pratt, V.M.; Reynolds, K.; Fernanda Sábato, M.; Scott, S.A.; Vitazka, P. Laboratory Testing of CYP2D6 Alleles in Relation to Tamoxifen Therapy. Genet. Med. 2012, 14, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Martín-Calvo, A.; García-Pérez, E.; García-Sánchez, A.; Bueno-Pérez, R.; Hamad, S.; Calero, S. Effect of Air Humidity on the Removal of Carbon Tetrachloride from Air Using Cu-BTC Metal-Organic Framework. Phys. Chem. Chem. Phys. 2011, 13, 11165–11174. [Google Scholar] [CrossRef] [PubMed]
- Najafi Nobar, S. Cu-BTC Synthesis, Characterization and Preparation for Adsorption Studies. Mater. Chem. Phys. 2018, 213, 343–351. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Düren, T. Opening the Gate: Framework Flexibility in ZIF-8 Explored by Experiments and Simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, C.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Electrospun Metal-Organic Framework Derived Hierarchical Carbon Nanofibers with High Performance for Supercapacitors. Chem. Commun. 2017, 53, 1751–1754. [Google Scholar] [CrossRef] [PubMed]
- Shaheed, N.; Javanshir, S.; Esmkhani, M.; Dekamin, M.G.; Naimi-Jamal, M.R. Synthesis of Nanocellulose Aerogels and Cu-BTC/Nanocellulose Aerogel Composites for Adsorption of Organic Dyes and Heavy Metal Ions. Sci. Rep. 2021, 11, 18553. [Google Scholar] [CrossRef] [PubMed]
- Karbownik, I.; Rac-Rumijowska, O.; Fiedot-Toboła, M.; Rybicki, T.; Teterycz, H. The Preparation and Characterization of Polyacrylonitrile-Polyaniline (PAN/PANI) Fibers. Materials 2019, 12, 664. [Google Scholar] [CrossRef]
- Tas, S.; Kaynan, O.; Ozden-Yenigun, E.; Nijmeijer, K. Polyacrylonitrile (PAN)/Crown Ether Composite Nanofibers for the Selective Adsorption of Cations. RSC Adv. 2016, 6, 3608–3616. [Google Scholar] [CrossRef]
- Jenab, A.; Roghanian, R.; Ghorbani, N.; Ghaedi, K.; Emtiazi, G. The Efficacy of Electrospun PAN/Kefiran Nanofiber and Kefir in Mammalian Cell Culture: Promotion of PC12 Cell Growth, Anti-MCF7 Breast Cancer Cells Activities, and Cytokine Production of PBMC. Int. J. Nanomed. 2020, 15, 717–728. [Google Scholar] [CrossRef]
- Homayoonnia, S.; Zeinali, S. Design and Fabrication of Capacitive Nanosensor Based on MOF Nanoparticles as Sensing Layer for VOCs Detection. Sens. Actuators B Chem. 2016, 237, 776–786. [Google Scholar] [CrossRef]
- Bruice, P.Y. Beauchamp Spectroscopy Tables 1. Org. Chem. 2011, 2620, A16–A17. [Google Scholar]
- Pillai, P.; Dharaskar, S.; Sasikumar, S.; Khalid, M. Zeolitic Imidazolate Framework-8 Nanoparticle: A Promising Adsorbent for Effective Fluoride Removal from Aqueous Solution. Appl. Water Sci. 2019, 9, 150. [Google Scholar] [CrossRef]
- Da Silva, J.D.S.F.; Malo, D.L.; Bataglion, G.A.; Eberlin, M.N.; Ronconi, C.M.; Alves, S.; De Sá, G.F. Adsorption in a Fixed-Bed Column and Stability of the Antibiotic Oxytetracycline Supported on Zn(II)-[2-Methylimidazolate] Frameworks in Aqueous Media. PLoS ONE 2015, 10, e0128436. [Google Scholar] [CrossRef]
- Binaeian, E.; Maleki, S.; Motaghedi, N.; Arjmandi, M. Study on the Performance of Cd2+ Sorption Using Dimethylethylenediamine-Modified Zinc-Based MOF (ZIF-8-Mmen): Optimization of the Process by RSM Technique. Sep. Sci. Technol. 2020, 55, 2713–2728. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Q.; Chen, R.; Liu, J.; Zhang, H.; Li, R.; Takahashi, K.; Liu, P.; Wang, J. Fabrication of ZIF-8@SiO2 Micro/Nano Hierarchical Superhydrophobic Surface on AZ31 Magnesium Alloy with Impressive Corrosion Resistance and Abrasion Resistance. ACS Appl. Mater. Interfaces 2017, 9, 11106–11115. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Xuan, X.; Yang, X.; Zhou, J.; Cen, K. Preparation of a Cu(BTC)-RGO Catalyst Loaded on a Pt Deposited Cu Foam Cathode to Reduce CO2 in a Photoelectrochemical Cell. RSC Adv. 2018, 8, 32296–32303. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Gonçalves, A.A.S.; Jaroniec, M. Effect of Metal-Ligand Ratio on the CO2 Adsorption Properties of Cu-BTC Metal-Organic Frameworks. RSC Adv. 2018, 8, 35551–35556. [Google Scholar] [CrossRef]
- Domán, A.; Madarász, J.; László, K. In Situ Evolved Gas Analysis Assisted Thermogravimetric (TG-FTIR and TG/DTA–MS) Studies on Non-Activated Copper Benzene-1,3,5-Tricarboxylate. Thermochim. Acta 2017, 647, 62–69. [Google Scholar] [CrossRef]
- Gimeno-Fabra, M.; Munn, A.S.; Stevens, L.A.; Drage, T.C.; Grant, D.M.; Kashtiban, R.J.; Sloan, J.; Lester, E.; Walton, R.I. Instant MOFs: Continuous Synthesis of Metal–Organic Frameworks by Rapid Solvent Mixing. Chem. Commun. 2012, 48, 10642–10644. [Google Scholar] [CrossRef]
- Chappanda, K.N.; Tchalala, M.R.; Shekhah, O.; Surya, S.G.; Eddaoudi, M.; Salama, K.N. A Comparative Study of Interdigitated Electrode and Quartz Crystal Microbalance Transduction Techniques for Metal–Organic Framework-Based Acetone Sensors. Sensors 2018, 18, 3898. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.A.H.M.; Ismail, A.F.; Yahya, N. Zeolitic Imidazole Framework 8 Decorated Graphene Oxide (ZIF-8/GO) Mixed Matrix Membrane (MMM) for CO2/CH4 Separation. J. Teknol. 2017, 79, 59–63. [Google Scholar] [CrossRef]
- Bobin-Dubigeon, C.; Campone, M.; Rossignol, E.; Salaun, E.; Amiand, M.B.; Bard, J.M. New UPLC-MS/MS Assay for the Determination of Tamoxifen and Its Metabolites in Human Plasma, Application to Patients. Future Sci. OA 2019, 5, FSO374. [Google Scholar] [CrossRef] [PubMed]
- Polson, C.; Sarkar, P.; Incledon, B.; Raguvaran, V.; Grant, R. Optimization of Protein Precipitation Based upon Effectiveness of Protein Removal and Ionization Effect in Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 785, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.A.; Solheim, E.; Lea, O.A.; Lundgren, S.; Kvinnsland, S.; Ueland, P.M. Distribution of 4-Hydroxy-n-Desmethyltamoxifen and Other Tamoxifen Metabolites in Human Biological Fluids during Tamoxifen Treatment. Cancer Res. 1989, 49, 2175–2183. [Google Scholar] [PubMed]
- Sung, W.H.; Tsao, Y.T.; Shen, C.J.; Tsai, C.Y.; Cheng, C.M. Small-Volume Detection: Platform Developments for Clinically-Relevant Applications. J. Nanobiotechnol. 2021, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Li, Y.; Xue, J.; Qiao, R.; Zhang, Z.; Li, Q. Comparison of Heterogeneous Azeotropic Distillation and Energy-Saving Extractive Distillation for Separating the Acetonitrile-Water Mixtures. Sep. Purif. Technol. 2020, 238, 116487. [Google Scholar] [CrossRef]
- Rekharsky, M.; Inoue, Y. Solvation Effects in Supramolecular Recognition. Supramol. Chem. 2012, 117–132. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Hernandez, D.R.; Leija Gutiérrez, H.M.; Hernandez-Hernandez, J.A.; Sánchez-Fernández, J.A.; Bonilla-Rios, J. Enhancing Solid-Phase Extraction of Tamoxifen and Its Metabolites from Human Plasma Using MOF-Integrated Polyacrylonitrile Composites: A Study on CuBTC and ZIF-8 Efficacy. Nanomaterials 2024, 14, 73. https://doi.org/10.3390/nano14010073
Flores-Hernandez DR, Leija Gutiérrez HM, Hernandez-Hernandez JA, Sánchez-Fernández JA, Bonilla-Rios J. Enhancing Solid-Phase Extraction of Tamoxifen and Its Metabolites from Human Plasma Using MOF-Integrated Polyacrylonitrile Composites: A Study on CuBTC and ZIF-8 Efficacy. Nanomaterials. 2024; 14(1):73. https://doi.org/10.3390/nano14010073
Chicago/Turabian StyleFlores-Hernandez, Domingo R., Héctor Manuel Leija Gutiérrez, Jose A. Hernandez-Hernandez, José Antonio Sánchez-Fernández, and Jaime Bonilla-Rios. 2024. "Enhancing Solid-Phase Extraction of Tamoxifen and Its Metabolites from Human Plasma Using MOF-Integrated Polyacrylonitrile Composites: A Study on CuBTC and ZIF-8 Efficacy" Nanomaterials 14, no. 1: 73. https://doi.org/10.3390/nano14010073





