Bayesian Optimization of Wet-Impregnated Co-Mo/Al2O3 Catalyst for Maximizing the Yield of Carbon Nanotube Synthesis
Abstract
:1. Introduction
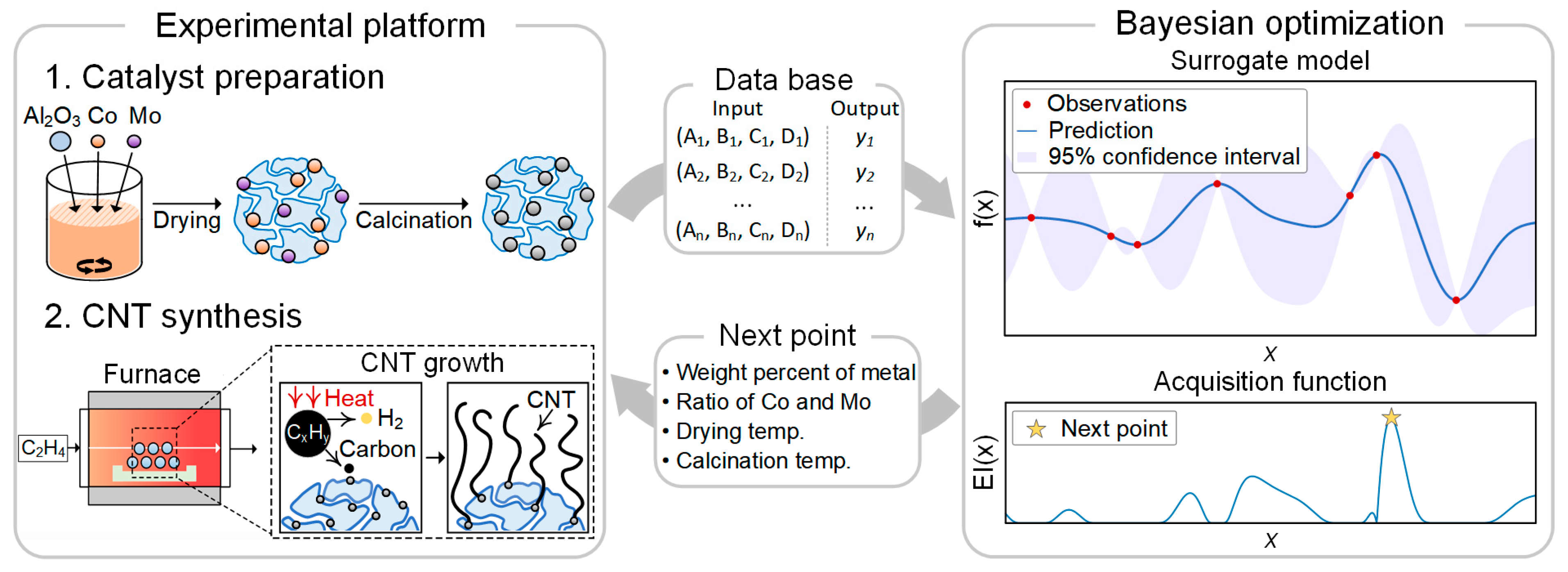
2. Materials and Methods
2.1. Catalyst Preparation
2.2. CNT Synthesis
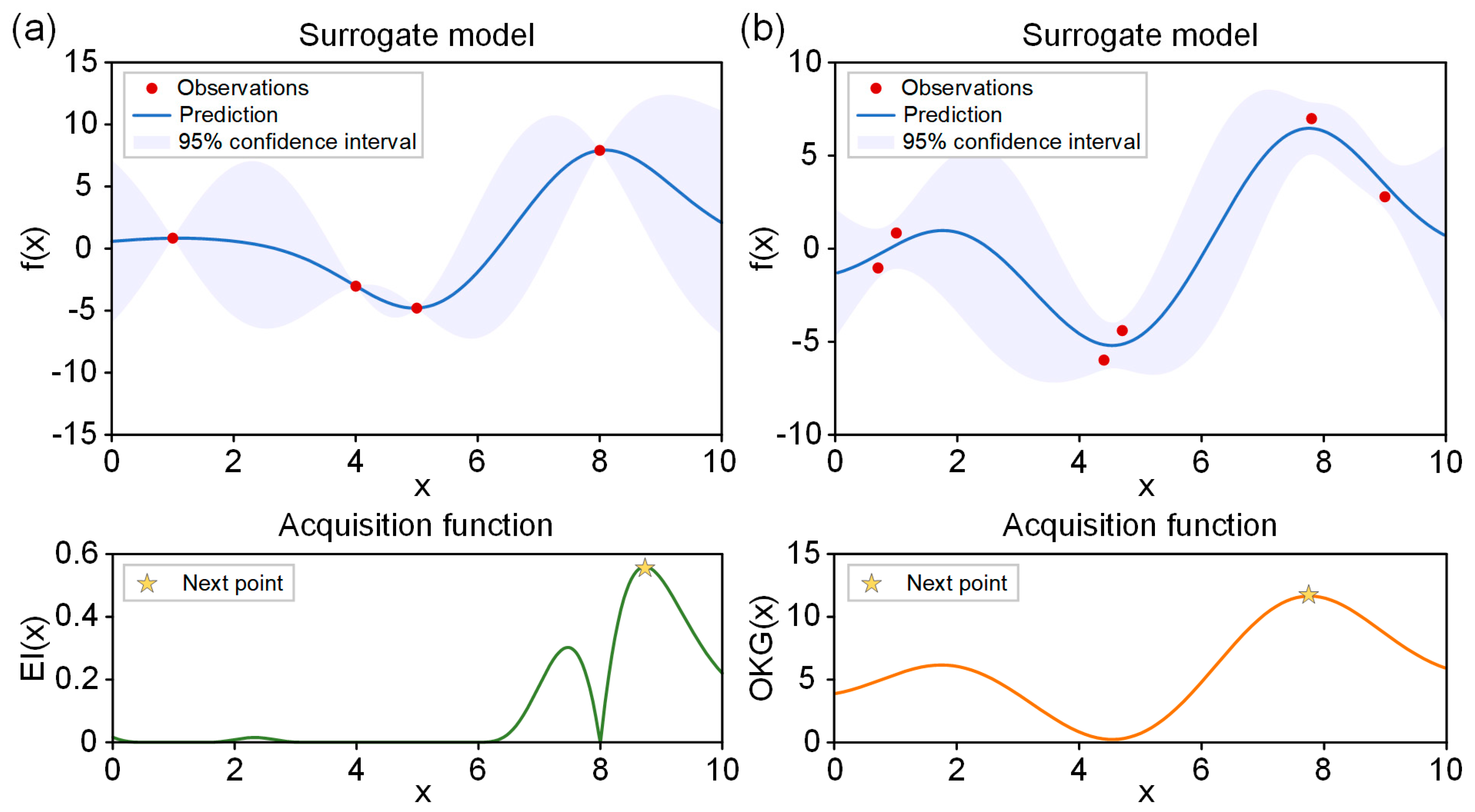
2.3. Bayesian Optimization
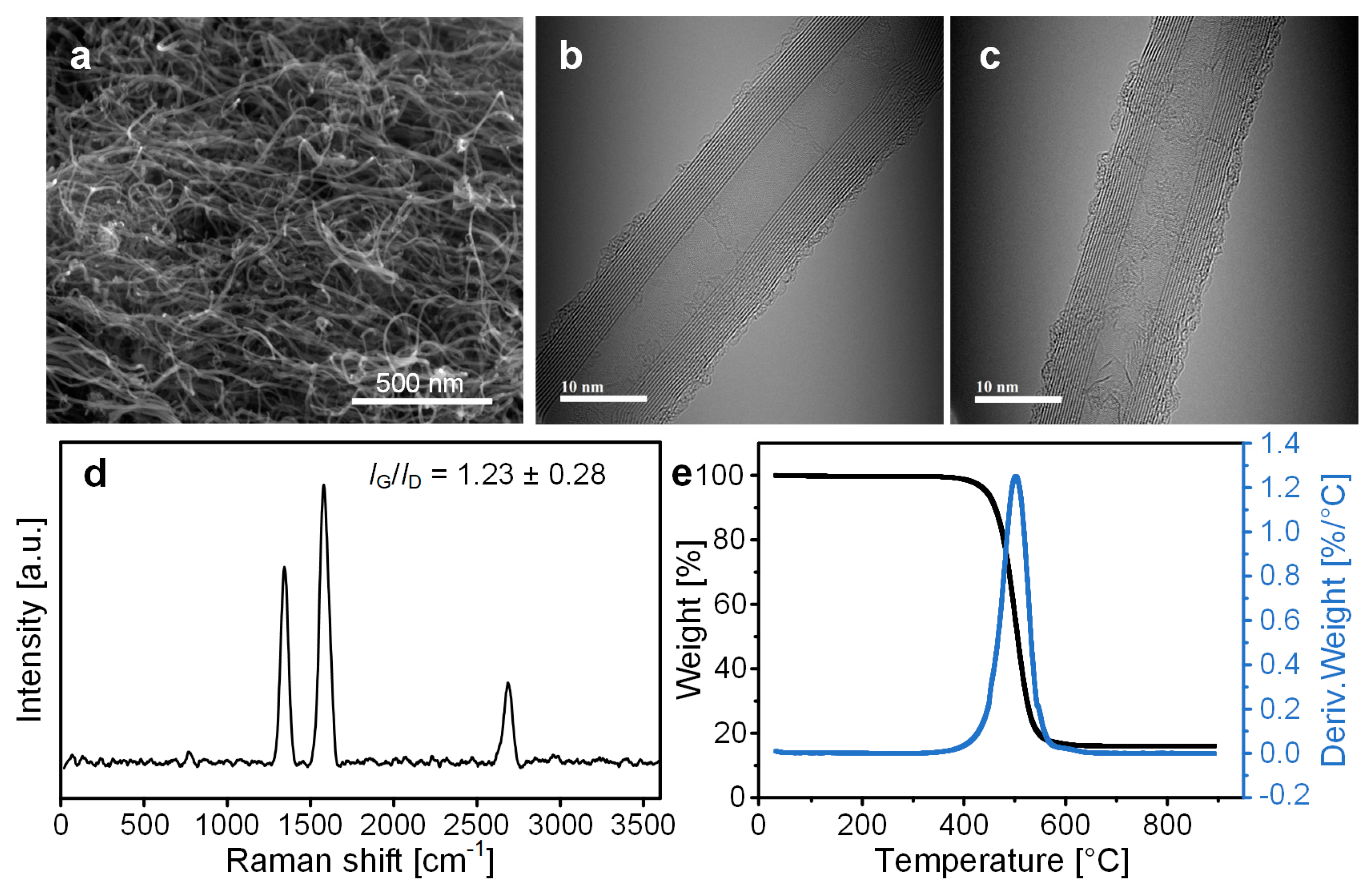
2.4. Characterization
3. Results and Discussion
3.1. Building the Initial Database
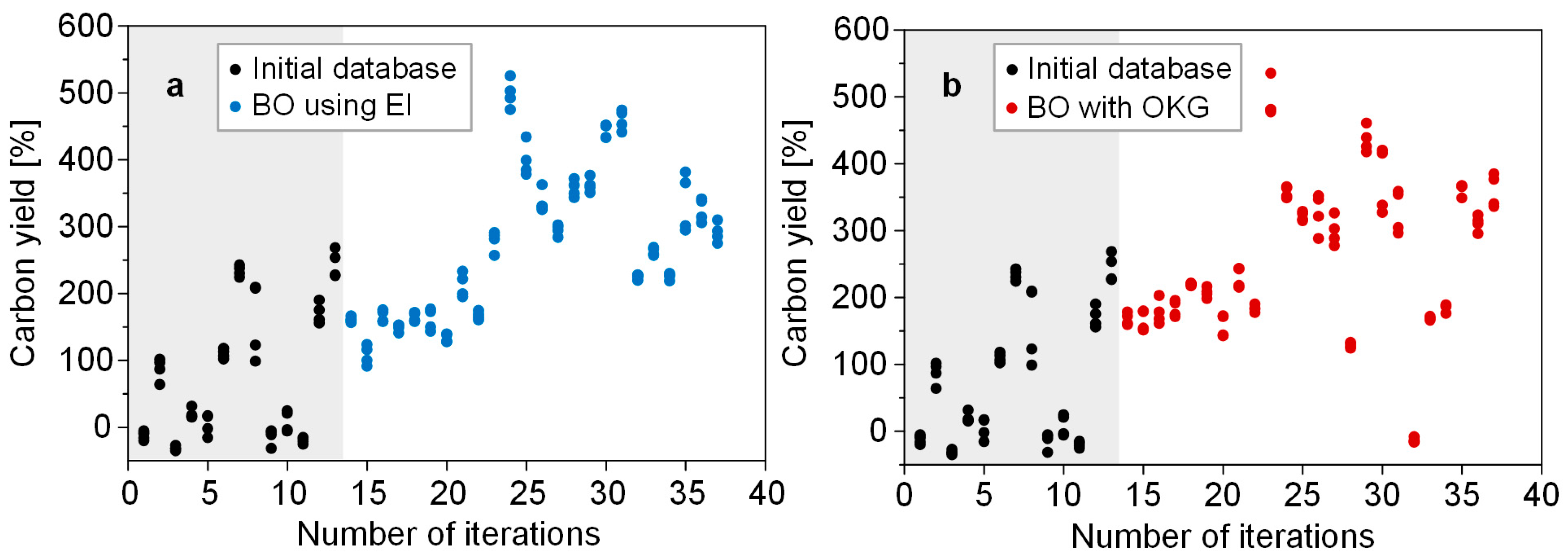
3.2. Parallel Bayesian Optimization Processes
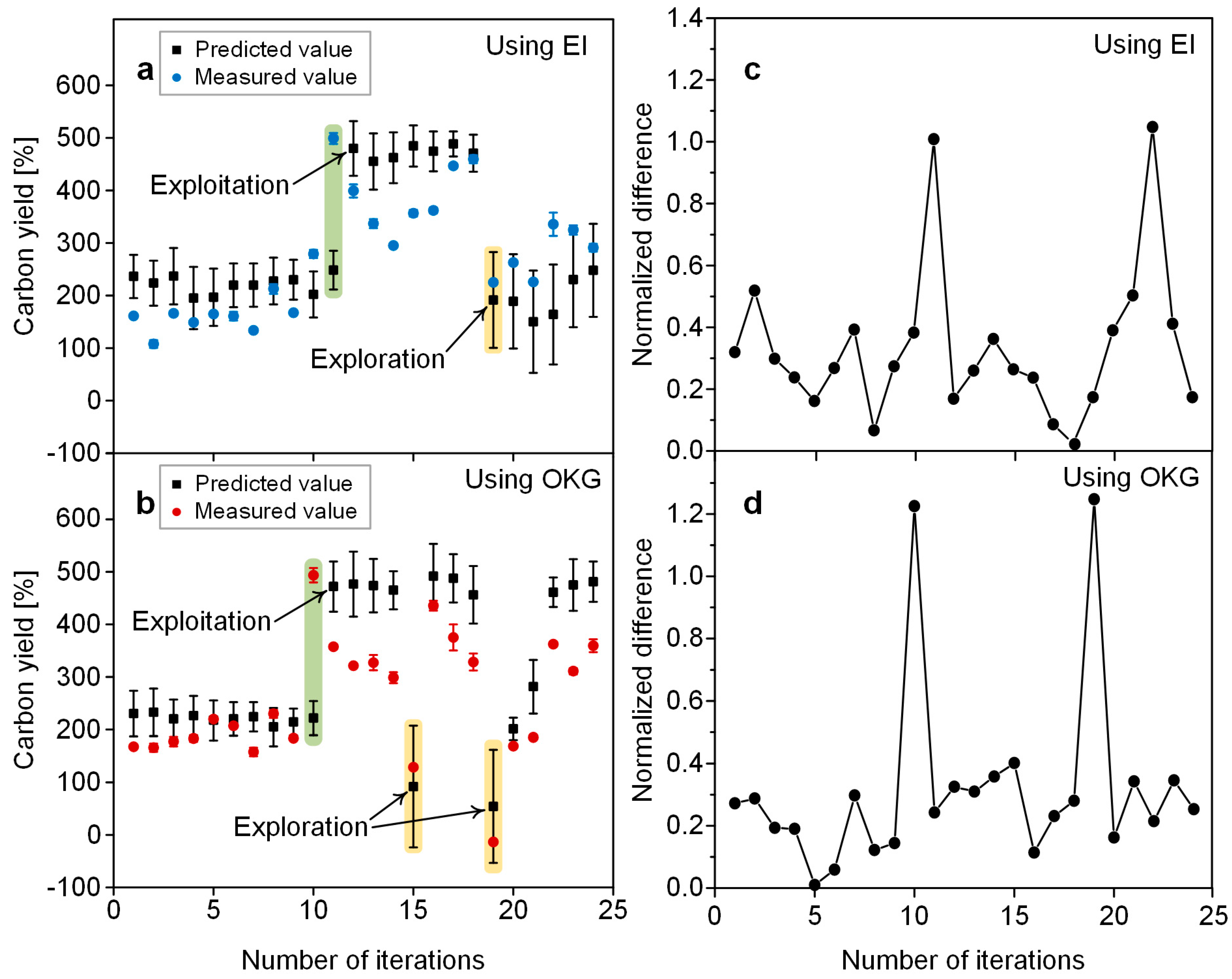
3.3. Predictive Performance
3.4. Analysis of As-Synthesized CNTs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical Conductivity of Individual Carbon Nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Berber, S.; Kwon, Y.K.; Tománek, D. Unusually High Thermal Conductivity of Carbon Nanotubes. Phys. Rev. Lett. 2000, 84, 4613–4616. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes under Tensile Load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, R.; Ye, X.; Zhu, Z.; Xie, H.; Shen, B.; Cai, D.; Liu, B.; Zhang, C.; Jia, Z.; et al. Carbon Nanotube Bundles with Tensile Strength over 80 GPa. Nat. Nanotechnol. 2018, 13, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Kleinerman, O.; Wang, X.; Ma, A.W.K.; Bengio, E.A.; Ter Waarbeek, R.F.; De Jong, J.J.; Hoogerwerf, R.E.; et al. Strong, Light, Multifunctional Fibers of Carbon Nanotubes with Ultrahigh Conductivity. Science 2013, 339, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Cho, Y.S.; Park, J.H.; Cheon, J.Y.; Lee, J.W.; Kim, J.H.; Park, C.R.; Kim, T.; Yang, S.J. Selective Interbundle Cross-Linking for Lightweight and Superstrong Carbon Nanotube Yarns. Nano Lett. 2023, 23, 3128–3136. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, D.M.; Jung, Y.; Park, J.; Lee, H.S.; Kim, Y.K.; Park, C.R.; Jeong, H.S.; Kim, S.M. Direct Spinning and Densification Method for High-Performance Carbon Nanotube Fibers. Nat. Commun. 2019, 10, 2962. [Google Scholar] [CrossRef]
- Im, J.; Jeong, Y.H.; Kim, M.C.; Oh, D.; Son, J.; Hyun, K.; Jeong, B.; Hong, S.; Lee, J. Wet Spinning of Multi-Walled Carbon Nanotube Fibers. Carbon 2024, 216, 118532. [Google Scholar] [CrossRef]
- Jessl, S.; Beesley, D.; Engelke, S.; Valentine, C.J.; Stallard, J.C.; Fleck, N.; Ahmad, S.; Cole, M.T.; De Volder, M. Carbon Nanotube Conductive Additives for Improved Electrical and Mechanical Properties of Flexible Battery Electrodes. Mater. Sci. Eng. A 2018, 735, 269–274. [Google Scholar] [CrossRef]
- Hills, G.; Lau, C.; Wright, A.; Fuller, S.; Bishop, M.D.; Srimani, T.; Kanhaiya, P.; Ho, R.; Amer, A.; Stein, Y.; et al. Modern Microprocessor Built from Complementary Carbon Nanotube Transistors. Nature 2019, 572, 595–602. [Google Scholar] [CrossRef]
- Lee, J.; Oh, E.; Kim, T.; Sa, J.H.; Lee, S.H.; Park, J.; Moon, D.; Kang, I.S.; Kim, M.J.; Kim, S.M.; et al. The Influence of Boundary Layer on the Growth Kinetics of Carbon Nanotube Forests. Carbon 2015, 93, 217–225. [Google Scholar] [CrossRef]
- da Cunha, T.; Maulu, A.; Guillot, J.; Fleming, Y.; Duez, B.; Lenoble, D.; Arl, D. Design of Silica Nanoparticles-Supported Metal Catalyst by Wet Impregnation with Catalytic Performance for Tuning Carbon Nanotubes Growth. Catalysts 2021, 11, 986. [Google Scholar] [CrossRef]
- Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Kinloch, I.A.; Windle, A.H. Direct Spinning of Carbon Nanotube Fibers from Chemical Vapor Deposition Synthesis. Science 2004, 304, 276–278. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.R.; Lee, T.; Lee, H.; Lee, J.; Lee, J.; Park, J.; Lee, K.H. Synthesis of Carbon Nanotube Fibers from Carbon Precursors with Low Decomposition Temperatures Using a Direct Spinning Process. Carbon 2017, 124, 219–227. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, F.; Luo, G.; Yu, H.; Gu, A. The Large-Scale Production of Carbon Nanotubes in a Nano-Agglomerate Fluidized-Bed Reactor. Chem. Phys. Lett. 2002, 364, 568–572. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, Q.; Qian, W.Z.; Yu, H.; Wang, Y.; Luo, G.H.; Xu, G.H.; Wang, D.Z. The Mass Production of Carbon Nanotubes Using a Nano-Agglomerate Fluidized Bed Reactor: A Multiscale Space-Time Analysis. Powder Technol. 2008, 183, 10–20. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, M.Q.; Huang, J.Q.; Nie, J.Q.; Wei, F. Mass Production of Aligned Carbon Nanotube Arrays by Fluidized Bed Catalytic Chemical Vapor Deposition. Carbon 2010, 48, 1196–1209. [Google Scholar] [CrossRef]
- Zhang, K.; Xue, P.; Wu, J.; Wu, L.; Wang, N.; Xu, T.; Zhu, K.; Pu, J.; Li, Q.; Deng, Y.; et al. Reaction Pathway Analysis of B/Li2O in a Li-B-O System for Boron Nitride Nanotube Growth. Chem. Mater. 2023, 35, 4857–4864. [Google Scholar] [CrossRef]
- Wang, N.; Ding, L.; Li, T.; Zhang, K.; Wu, L.; Zhou, Z.; He, Q.; He, X.; Wang, X.; Hu, Y.; et al. Self-Catalytic Ternary Compounds for Efficient Synthesis of High-Quality Boron Nitride Nanotubes. Small 2023, 19, 2206933. [Google Scholar] [CrossRef]
- Bankole, M.T.; Mohammed, I.A.; Abdulkareem, A.S.; Tijani, J.O.; Ochigbo, S.S.; Abubakre, O.K.; Afolabi, A.S. Optimization of Supported Bimetallic (Fe-Co/CaCO3) Catalyst Synthesis Parameters for Carbon Nanotubes Growth Using Factorial Experimental Design. J. Alloys Compd. 2018, 749, 85–102. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Fakeeha, A.H.; Ibrahim, A.A.; Khan, W.U.; Atia, H.; Eckelt, R.; Seshan, K.; Chowdhury, B. Decomposition of Methane over Alumina Supported Fe and Ni–Fe Bimetallic Catalyst: Effect of Preparation Procedure and Calcination Temperature. J. Saudi Chem. Soc. 2018, 22, 239–247. [Google Scholar] [CrossRef]
- Ning, G.; Wei, F.; Wen, Q.; Luo, G.; Wang, Y.; Jin, Y. Improvement of Fe/MgO Catalysts by Calcination for the Growth of Single- and Double-Walled Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Harris, A.T. Synthesis of Coiled Carbon Nanotubes on Co/Al2O3 Catalysts in a Fluidised-Bed. J. Nanoparticle Res. 2010, 12, 645–653. [Google Scholar] [CrossRef]
- Corrias, M.; Caussat, B.; Ayral, A.; Durand, J.; Kihn, Y.; Kalck, P.; Serp, P. Carbon Nanotubes Produced by Fluidized Bed Catalytic CVD: First Approach of the Process. Chem. Eng. Sci. 2003, 58, 4475–4482. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Lin, Y.T.; Chen, W.Y.; Wei, J.L. Parameter Setting on Growth of Carbon Nanotubes over Transition Metal/Alumina Catalysts in a Fluidized Bed Reactor. Powder Technol. 2009, 192, 16–22. [Google Scholar] [CrossRef]
- Kitiyanan, B.; Alvarez, W.E.; Harwell, J.H.; Resasco, D.E. Controlled Production of Single-Wall Carbon Nanotubes by Catalytic Decomposition of CO on Bimetallic Co-Mo Catalysts. Chem. Phys. Lett. 2000, 317, 497–503. [Google Scholar] [CrossRef]
- Chiang, W.H.; Sankaran, R.M. Synergistic Effects in Bimetallic Nanoparticles for Low Temperature Carbon Nanotube Growth. Adv. Mater. 2008, 20, 4857–4861. [Google Scholar] [CrossRef]
- Sato, S.; Kawabata, A.; Kondo, D.; Nihei, M.; Awano, Y. Carbon Nanotube Growth from Titanium-Cobalt Bimetallic Particles as a Catalyst. Chem. Phys. Lett. 2005, 402, 149–154. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Zhang, D.; Yang, J.; Luo, D.; Xu, Z.; Wei, J.; Wang, J.Q.; Xu, Z.; Peng, F.; et al. Chirality-Specific Growth of Single-Walled Carbon Nanotubes on Solid Alloy Catalysts. Nature 2014, 510, 522–524. [Google Scholar] [CrossRef]
- He, M.; Chernov, A.I.; Obraztsova, E.D.; Jiang, H.; Kauppinen, E.I.; Lehtonen, J. Synergistic Effects in FeCu Bimetallic Catalyst for Low Temperature Growth of Single-Walled Carbon Nanotubes. Carbon 2013, 52, 590–594. [Google Scholar] [CrossRef]
- Cui, K.; Kumamoto, A.; Xiang, R.; An, H.; Wang, B.; Inoue, T.; Chiashi, S.; Ikuhara, Y.; Maruyama, S. Synthesis of Subnanometer-Diameter Vertically Aligned Single-Walled Carbon Nanotubes with Copper-Anchored Cobalt Catalysts. Nanoscale 2016, 8, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Frazier, P.I. A Tutorial on Bayesian Optimization. arXiv 2018, arXiv:1807.02811. [Google Scholar]
- Greenhill, S.; Rana, S.; Gupta, S.; Vellanki, P.; Venkatesh, S. Bayesian Optimization for Adaptive Experimental Design: A Review. IEEE Access 2020, 8, 13937–13948. [Google Scholar] [CrossRef]
- Snoek, J.; Larochelle, H.; Adams, R.P. Practical Bayesian Optimization of Machine Learning Algorithms. arXiv 2012, arXiv:1206.2944. [Google Scholar] [CrossRef]
- Frazier, P.I.; Wang, J. Bayesian Optimization for Materials Design. Inf. Sci. Mater. Discov. Des. 2015, 225, 45–75. [Google Scholar] [CrossRef]
- Burhenne, S.; Jacob, D.; Henze, G.P. Sampling Based on Sobol′ Sequences for Monte Carlo Techniques Applied to Building Simulations. In Proceedings of the Building Simulation 2011: 12th Conference of International Building Performance Simulation Association, Sydney, Australia, 14–16 November 2011; pp. 1816–1823. [Google Scholar]
- Esteves, L.M.; Smarzaro, J.L.; Caytuero, A.; Oliveira, H.A.; Passos, F.B. Catalyst Preparation Methods to Reduce Contaminants in a High-Yield Purification Process of Multiwalled Carbon Nanotubes. Braz. J. Chem. Eng. 2019, 36, 1587–1600. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Awadallah, A.E. Impact of Co/Mo Ratio on the Activity of CoMo/MgO Catalyst for Production of High-Quality Multi-Walled Carbon Nanotubes from Polyethylene Waste. Mater. Chem. Phys. 2019, 238, 121879. [Google Scholar] [CrossRef]
- Yardimci, A.I.; Yilmaz, S.; Selamet, Y. The Effects of Catalyst Pretreatment, Growth Atmosphere and Temperature on Carbon Nanotube Synthesis Using Co-Mo/MgO Catalyst. Diam. Relat. Mater. 2015, 60, 81–86. [Google Scholar] [CrossRef]
- Kludpantanapan, T.; Nantapong, P.; Rattanaamonkulchai, R.; Srifa, A.; Koo-Amornpattana, W.; Chaiwat, W.; Sakdaronnarong, C.; Charinpanitkul, T.; Assabumrungrat, S.; Wongsakulphasatch, S.; et al. Simultaneous Production of Hydrogen and Carbon Nanotubes from Biogas: On the Effect of Ce Addition to CoMo/MgO Catalyst. Int. J. Hydrogen Energy 2021, 46, 38175–38190. [Google Scholar] [CrossRef]
- Fazle Kibria, A.K.M.; Shajahan, M.; Mo, Y.H.; Kim, M.J.; Nahm, K.S. Long Activity of Co-Mo/MgO Catalyst for the Synthesis of Carbon Nanotubes in Large-Scale and Application Feasibility of the Grown Tubes. Diam. Relat. Mater. 2004, 13, 1865–1872. [Google Scholar] [CrossRef]
- Song, H.; Kim, D.H.; Park, C.W.; Jae, J.; Hong, S.; Lee, J. Statistical Analysis of the Synthesis of Carbon Nanotubes Using Wet-Impregnated Catalysts for Improved Robustness. Carbon Lett. 2023, 33, 921–929. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman Spectroscopy of Carbon Nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]

| Number | Metal wt.% | Co wt.% | Mo wt.% | Drying Temperature [°C] | Calcination Temperature [°C] | Carbon Yield [%] |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 0 | 228 | 829 | −11.8 ± 6.0 |
| 2 | 70 | 61 | 9 | 205 | 789 | 87.2 ± 16.8 |
| 3 | 50 | 2 | 48 | 224 | 755 | −31.6 ± 3.5 |
| 4 | 9 | 8 | 1 | 209 | 747 | 20.2 ± 7.8 |
| 5 | 10 | 1 | 9 | 155 | 729 | 4.0 ± 15.7 |
| 6 | 63 | 35 | 28 | 92 | 579 | 109.8 ± 7.0 |
| 7 | 45 | 44 | 1 | 150 | 567 | 233.7 ± 8.2 |
| 8 | 41 | 32 | 9 | 270 | 539 | 159.7 ± 57.3 |
| 9 | 22 | 0 | 22 | 145 | 431 | −13.9 ± 12.1 |
| 10 | 60 | 21 | 39 | 233 | 426 | 8.9 ± 15.8 |
| 11 | 43 | 2 | 41 | 291 | 406 | −19.8 ± 4.3 |
| 12 | 45 | 44 | 1 | 132 | 354 | 170.6 ± 15.3 |
| 13 | 65 | 54 | 11 | 114 | 311 | 244.0 ± 20.5 |
| Number | Metal wt.% | Co wt.% | Mo wt.% | Drying Temperature [°C] | Calcination Temperature [°C] | Carbon Yield [%] |
|---|---|---|---|---|---|---|
| 1 | 61 | 57 | 4 | 123 | 433 | 161.0 ± 4.2 |
| 2 | 70 | 52 | 18 | 80 | 300 | 107.7 ± 14.8 |
| 3 | 59 | 48 | 11 | 154 | 300 | 166.1 ± 8.9 |
| 4 | 42 | 42 | 0 | 183 | 502 | 148.8 ± 5.6 |
| 5 | 40 | 33 | 7 | 142 | 531 | 164.8 ± 6.8 |
| 6 | 70 | 51 | 19 | 124 | 300 | 160.8 ± 16.5 |
| 7 | 58 | 47 | 11 | 108 | 300 | 133.6 ± 6.2 |
| 8 | 48 | 42 | 6 | 145 | 568 | 212.5 ± 18.1 |
| 9 | 66 | 59 | 7 | 151 | 300 | 167.1 ± 6.0 |
| 10 | 45 | 45 | 0 | 129 | 634 | 279.1 ± 15.4 |
| 11 | 46 | 46 | 0 | 139 | 766 | 499.0 ± 21.1 |
| 12 | 46 | 46 | 0 | 138 | 832 | 399.1 ± 24.9 |
| 13 | 46 | 46 | 0 | 164 | 759 | 337.0 ± 17.3 |
| 14 | 51 | 51 | 0 | 134 | 756 | 295.1 ± 8.1 |
| 15 | 44 | 44 | 0 | 134 | 767 | 356.7 ± 12.6 |
| 16 | 47 | 43 | 4 | 136 | 755 | 362.0 ± 10.8 |
| 17 | 47 | 47 | 0 | 143 | 737 | 446.7 ± 8.9 |
| 18 | 46 | 46 | 0 | 125 | 766 | 459.6 ± 15.2 |
| 19 | 49 | 49 | 0 | 91 | 663 | 224.9 ± 3.8 |
| 20 | 49 | 49 | 0 | 93 | 865 | 262.8 ± 5.7 |
| 21 | 56 | 56 | 0 | 95 | 813 | 225.7 ± 4.9 |
| 22 | 52 | 40 | 12 | 93 | 800 | 335.7 ± 44.1 |
| 23 | 53 | 42 | 11 | 117 | 916 | 324.8 ± 17.6 |
| 24 | 55 | 41 | 14 | 127 | 779 | 290.9 ± 14.8 |
| Number | Metal wt.% | Co wt.% | Mo wt.% | Drying Temperature [°C] | Calcination Temperature [°C] | Carbon Yield [%] |
|---|---|---|---|---|---|---|
| 1 | 64 | 59 | 5 | 86 | 400 | 167.8 ± 8.8 |
| 2 | 61 | 51 | 10 | 174 | 341 | 165.9 ± 15.4 |
| 3 | 43 | 43 | 0 | 157 | 501 | 177.5 ± 18.2 |
| 4 | 52 | 44 | 8 | 123 | 479 | 183.2 ± 12.0 |
| 5 | 39 | 39 | 0 | 119 | 563 | 219.6 ± 1.9 |
| 6 | 50 | 50 | 0 | 125 | 578 | 207.1 ± 7.4 |
| 7 | 60 | 46 | 14 | 99 | 308 | 157.7 ± 16.5 |
| 8 | 43 | 38 | 5 | 134 | 625 | 229.8 ± 15.3 |
| 9 | 40 | 34 | 6 | 130 | 512 | 183.5 ± 5.1 |
| 10 | 41 | 41 | 0 | 132 | 753 | 493.6 ± 27.7 |
| 11 | 41 | 41 | 0 | 154 | 774 | 357.4 ± 8.5 |
| 12 | 47 | 47 | 0 | 121 | 803 | 321.4 ± 6.6 |
| 13 | 36 | 36 | 0 | 124 | 758 | 327.2 ± 29.2 |
| 14 | 41 | 41 | 0 | 125 | 797 | 298.7 ± 21.1 |
| 15 | 70 | 49 | 21 | 242 | 353 | 128.7 ± 3.9 |
| 16 | 46 | 46 | 0 | 102 | 730 | 435.7 ± 18.8 |
| 17 | 44 | 44 | 0 | 142 | 719 | 375.2 ± 49.4 |
| 18 | 43 | 37 | 6 | 120 | 751 | 328.4 ± 32.5 |
| 19 | 1 | 1 | 0 | 260 | 626 | −13.4 ± 3.7 |
| 20 | 50 | 50 | 0 | 131 | 587 | 168.8 ± 2.5 |
| 21 | 48 | 47 | 1 | 96 | 539 | 185.1 ± 5.9 |
| 22 | 40 | 40 | 0 | 119 | 738 | 362.2 ± 8.9 |
| 23 | 50 | 50 | 0 | 133 | 757 | 310.1 ± 11.7 |
| 24 | 38 | 38 | 0 | 138 | 744 | 359.4 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Song, H.; Shin, Y.S.; Lee, J.; Seo, T.H. Bayesian Optimization of Wet-Impregnated Co-Mo/Al2O3 Catalyst for Maximizing the Yield of Carbon Nanotube Synthesis. Nanomaterials 2024, 14, 75. https://doi.org/10.3390/nano14010075
Shin S, Song H, Shin YS, Lee J, Seo TH. Bayesian Optimization of Wet-Impregnated Co-Mo/Al2O3 Catalyst for Maximizing the Yield of Carbon Nanotube Synthesis. Nanomaterials. 2024; 14(1):75. https://doi.org/10.3390/nano14010075
Chicago/Turabian StyleShin, Sangsoo, Hyeongyun Song, Yeon Su Shin, Jaegeun Lee, and Tae Hoon Seo. 2024. "Bayesian Optimization of Wet-Impregnated Co-Mo/Al2O3 Catalyst for Maximizing the Yield of Carbon Nanotube Synthesis" Nanomaterials 14, no. 1: 75. https://doi.org/10.3390/nano14010075





