Effect of Synchrotron X-ray Irradiation Time on the Particle Size and DFAFC Performance of Pd/CNT Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CNT and Synthesis of Pd-Decorated CNT
2.2. Characterization of R-CNT, A-CNT, and Pd/CNT
2.3. Electrocatalytic Activity of Pd/CNT
2.4. DFAFC Assembly and Evaluation
3. Results and Discussion
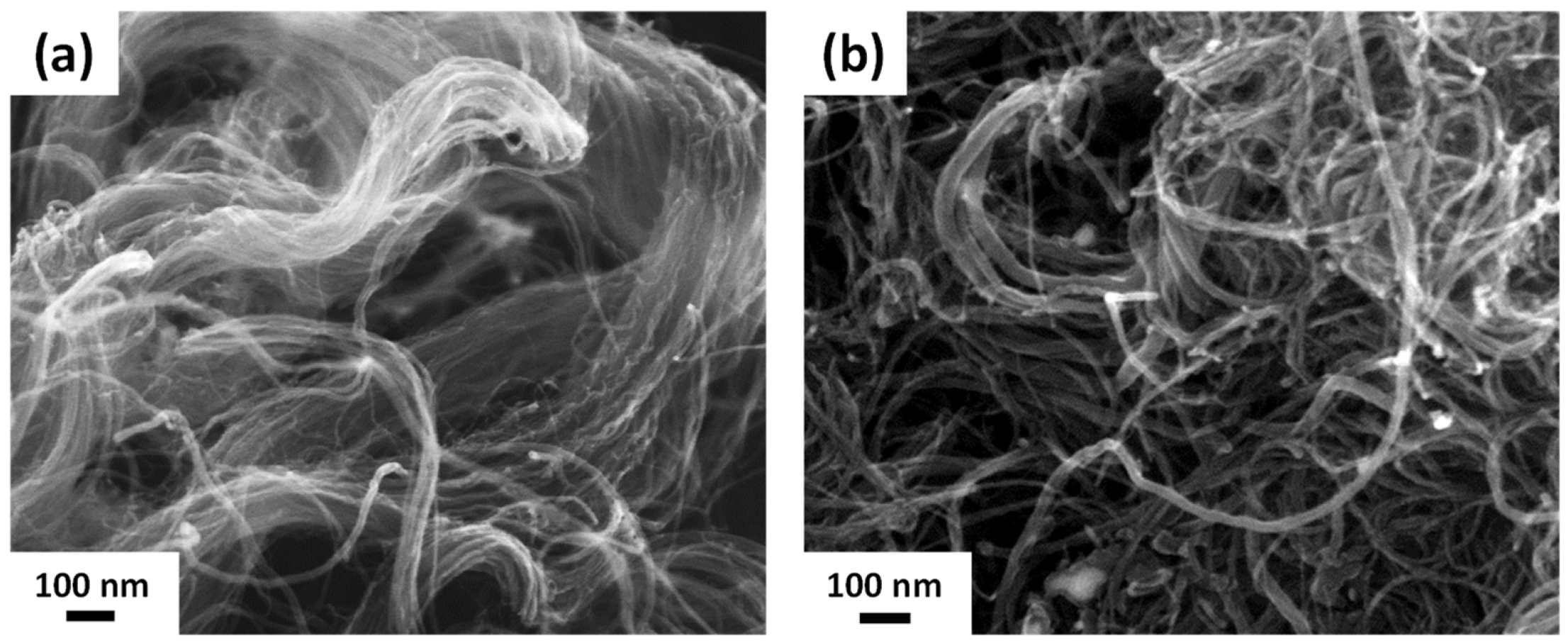
3.1. Characterization of R-CNT and A-CNT
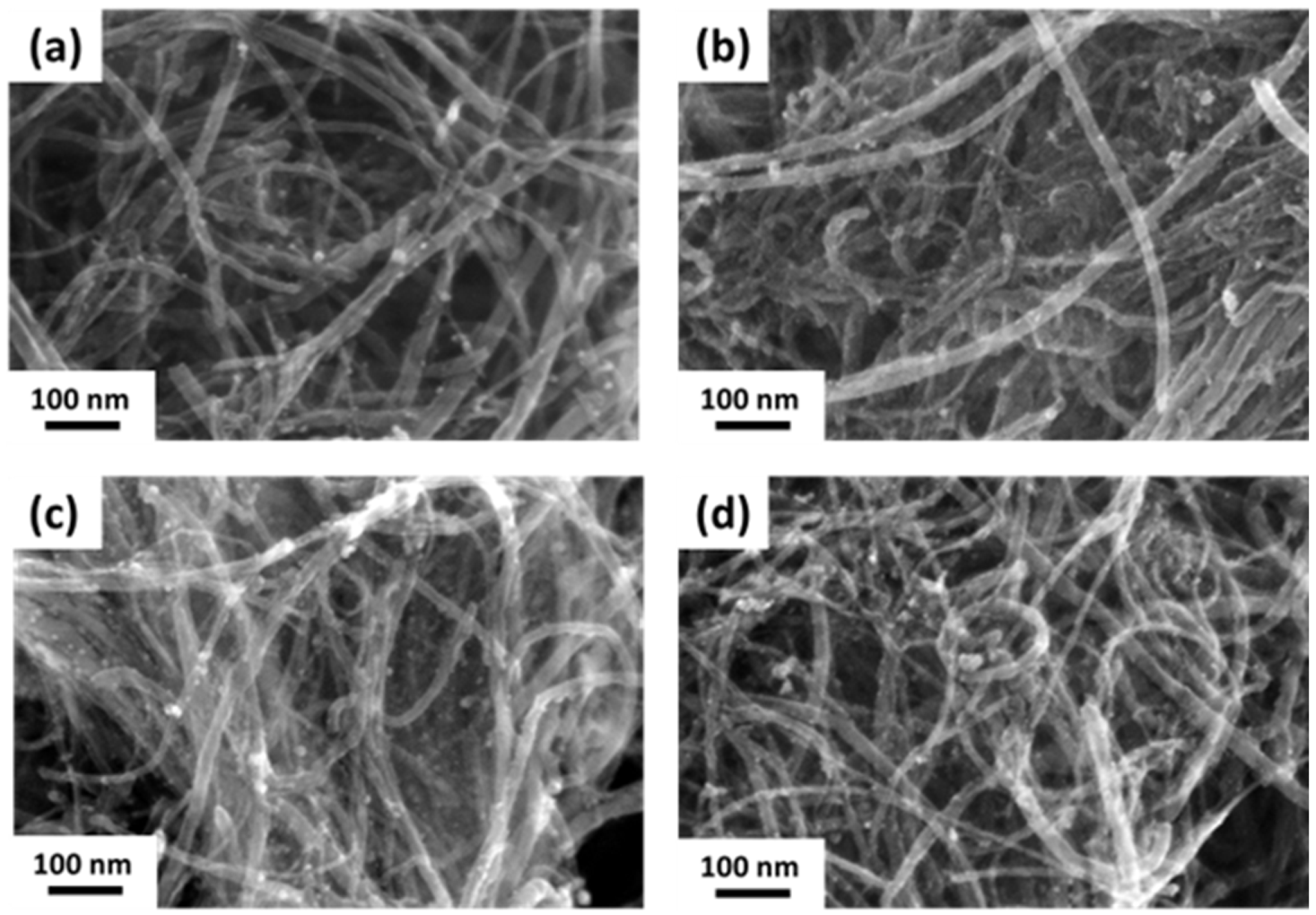
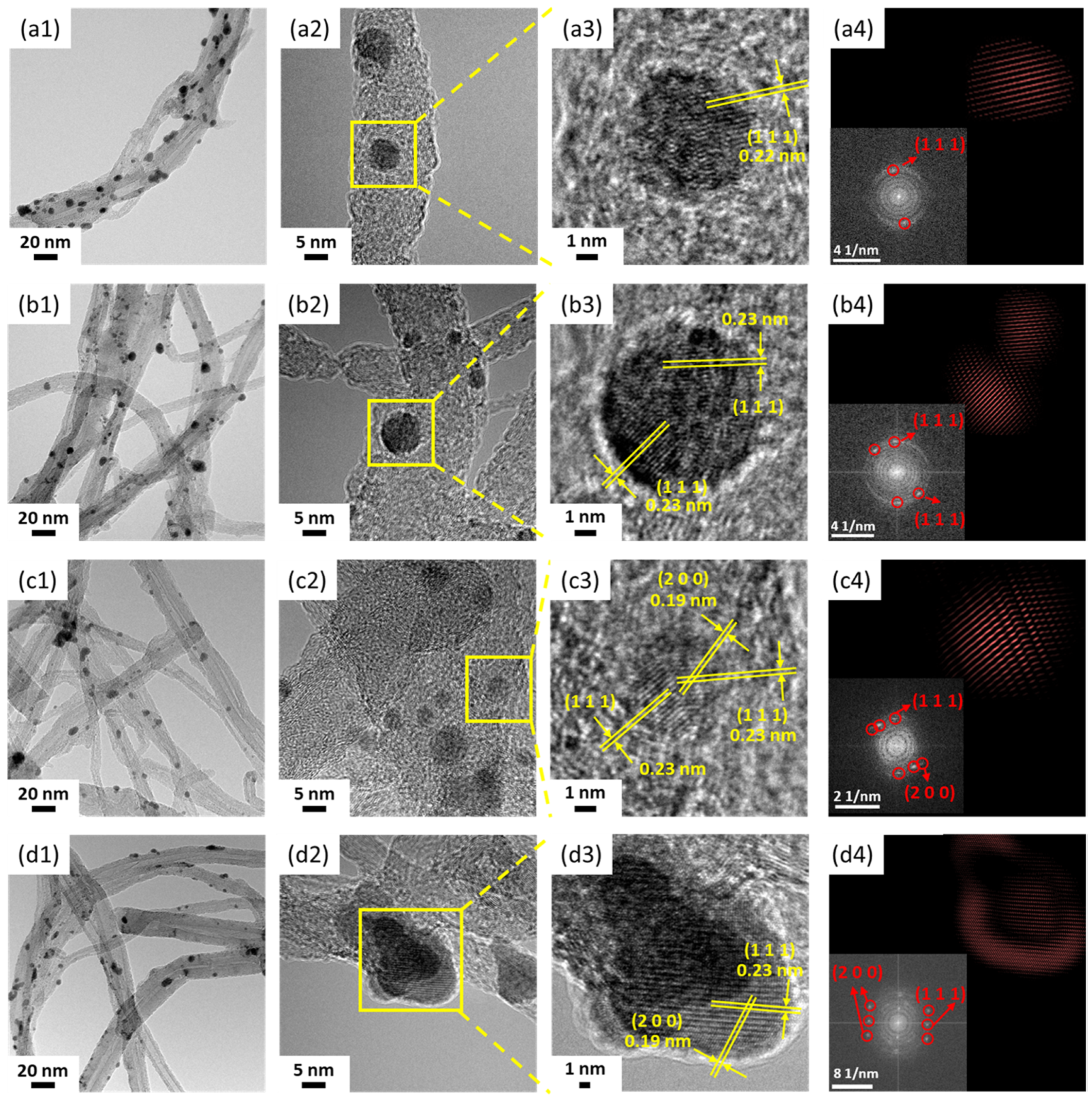
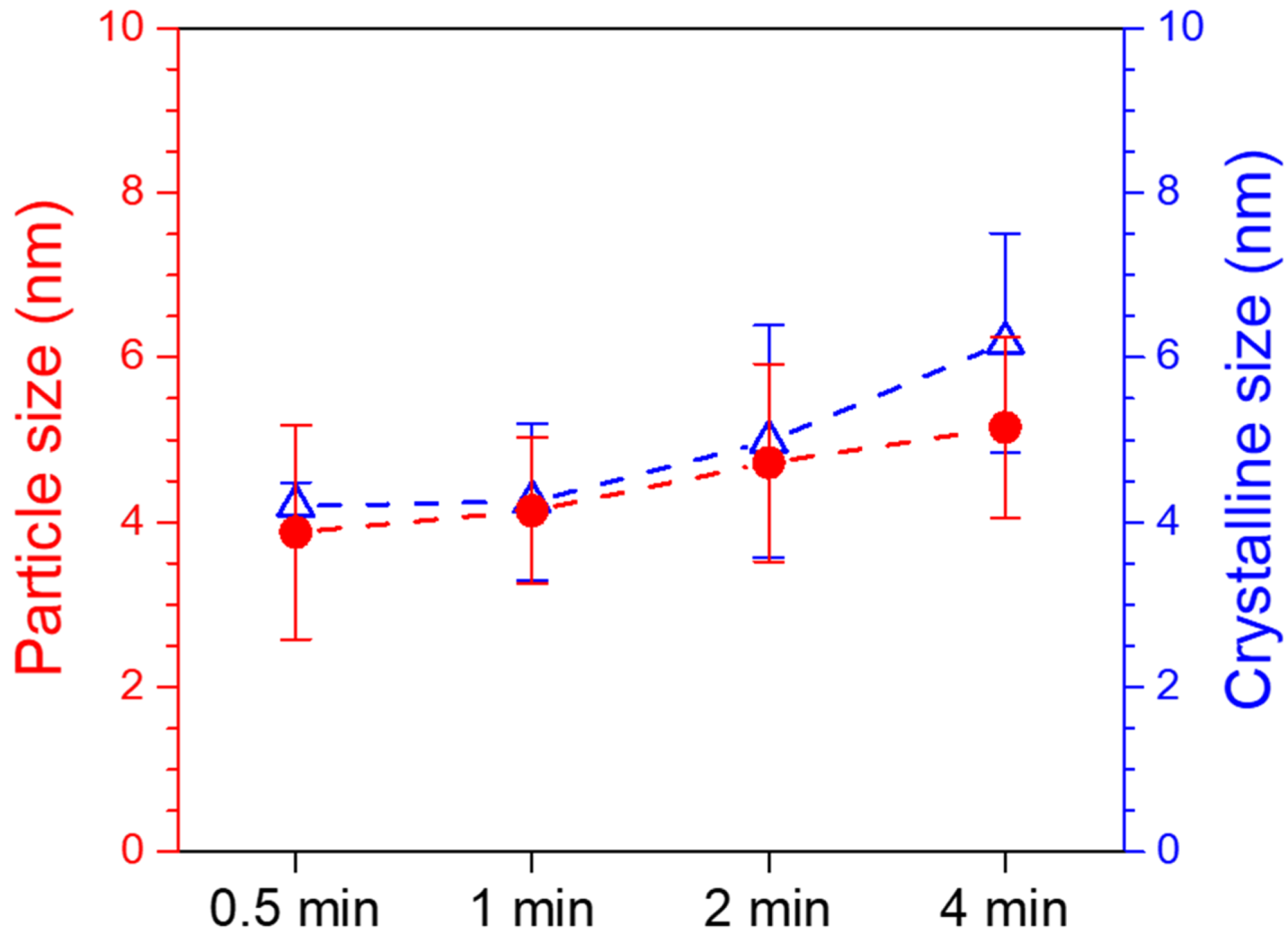
3.2. Characterization of Pd/CNT
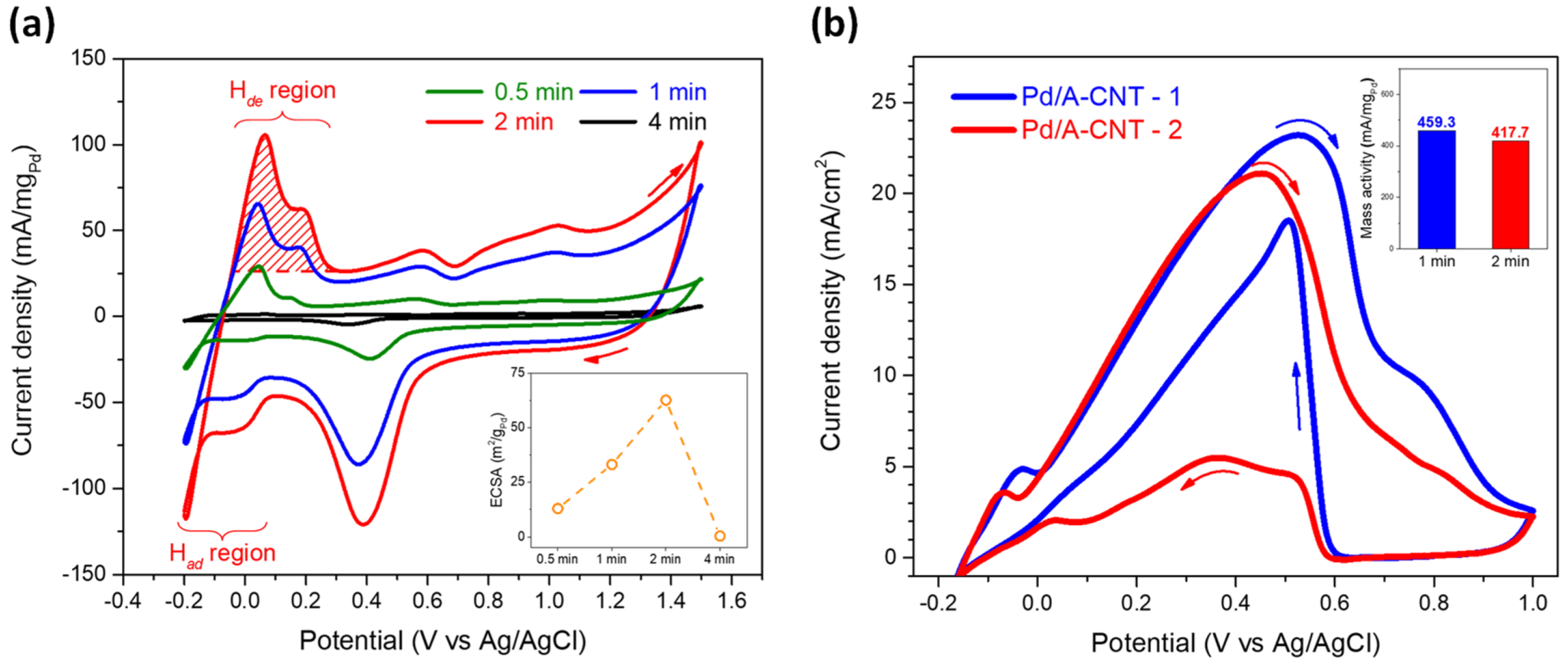
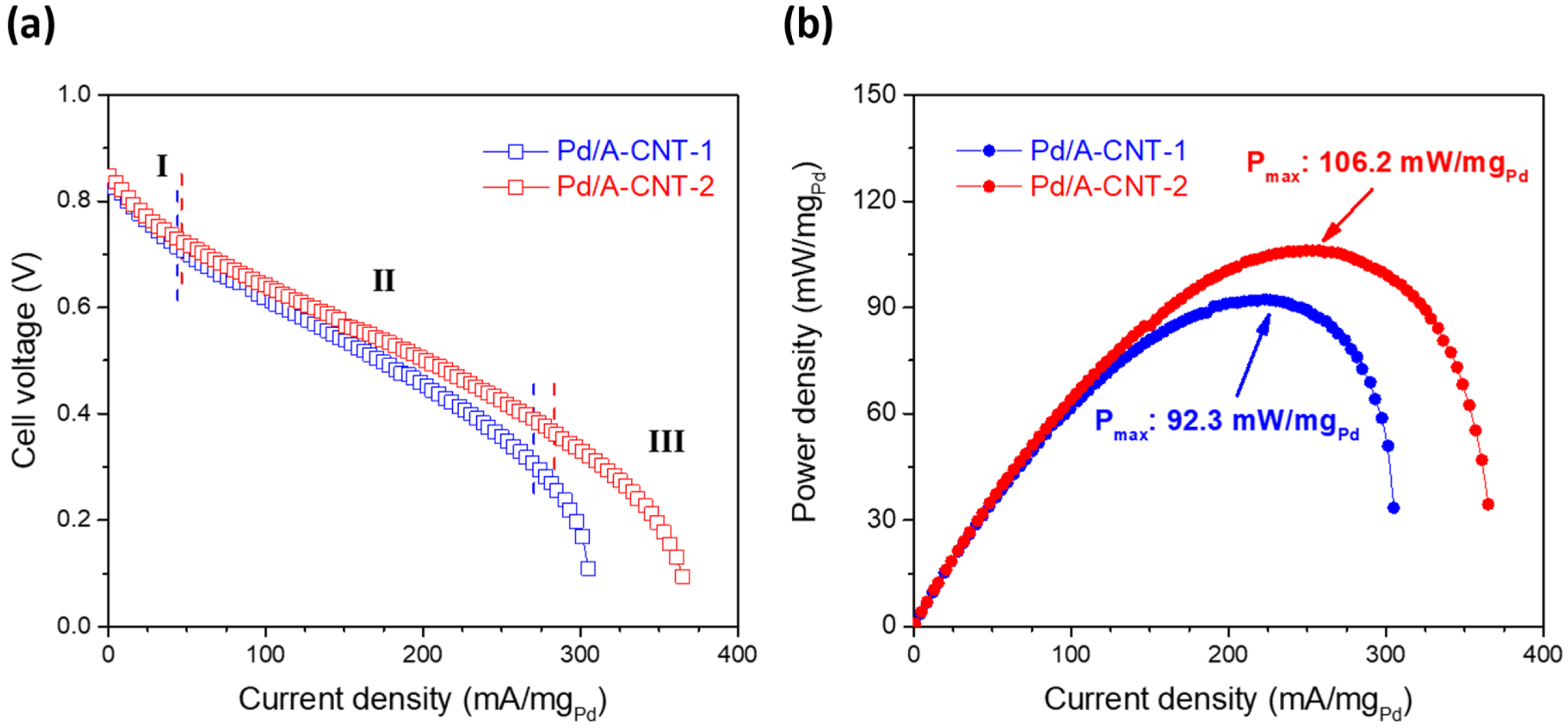
3.3. Electrocatalytic Activity and DFAFC Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dell, R.M.; Rand, D.A.J. Energy storage—A key technology for global energy sustainability. J. Power Sources 2001, 100, 2–17. [Google Scholar] [CrossRef]
- Sovacool, B.K. The intermittency of wind, solar, and renewable electricity generators: Technical barrier or rhetorical excuse? Util. Policy 2009, 17, 288–296. [Google Scholar] [CrossRef]
- Safari, A.; Das, N.; Langhelle, O.; Roy, J.; Assadi, M. Natural gas: A transition fuel for sustainable energy system transformation? Energy Sci. Eng. 2019, 7, 1075–1094. [Google Scholar] [CrossRef]
- Ibrahim, H.; Ilinca, A.; Perron, J. Energy storage systems—Characteristics and comparisons. Renew. Sust. Energ. Rev. 2008, 12, 1221–1250. [Google Scholar] [CrossRef]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Kalair, A.; Abas, N.; Saleem, M.S.; Kalair, A.R.; Khan, N. Role of energy storage systems in energy transition from fossil fuels to renewables. Energy Storage 2021, 3, e135. [Google Scholar] [CrossRef]
- Khan, N.; Dilshad, S.; Khalid, R.; Kalair, A.R.; Abas, N. Review of energy storage and transportation of energy. Energy Storage 2019, 1, e49. [Google Scholar] [CrossRef]
- Hulton, A.T.; Malone, J.J.; Clarke, N.D.; MacLaren, D.P. Energy requirements and nutritional strategies for male soccer players: A review and suggestions for practice. Nutrients 2022, 14, 657. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrog. Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Elsaid, K.; Wilberforce, T.; Kamil, M.; Sayed, E.T.; Olabi, A. Environmental aspects of fuel cells: A review. Sci. Total Environ. 2021, 752, 141803. [Google Scholar] [CrossRef] [PubMed]
- Ferriday, T.B.; Middleton, P.H. Alkaline fuel cell technology—A review. Int. J. Hydrog. Energy 2021, 46, 18489–18510. [Google Scholar] [CrossRef]
- Wang, H.-S.; Chang, C.-P.; Huang, Y.-J.; Su, Y.-C.; Tseng, F.-G. A high-yield and ultra-low-temperature methanol reformer integratable with phosphoric acid fuel cell (PAFC). Energy 2017, 133, 1142–1152. [Google Scholar] [CrossRef]
- Peng, J.; Huang, J.; Wu, X.-l.; Xu, Y.-w.; Chen, H.; Li, X. Solid oxide fuel cell (SOFC) performance evaluation, fault diagnosis and health control: A review. J. Power Sources 2021, 505, 230058. [Google Scholar] [CrossRef]
- Bischoff, M. Molten carbonate fuel cells: A high temperature fuel cell on the edge to commercialization. J. Power Sources 2006, 160, 842–845. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, Z.; Chan, S.H. Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): A review. J. Power Sources 2021, 488, 229434. [Google Scholar] [CrossRef]
- Alias, M.; Kamarudin, S.; Zainoodin, A.; Masdar, M. Active direct methanol fuel cell: An overview. Int. J. Hydrog. Energy 2020, 45, 19620–19641. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, W.; Mansoor, M.; Liu, Y.; Wang, X.; Zhang, K.; Xiao, C.; Liu, Q.; Mao, L.; Wang, M. Recent Progress in Using Mesoporous Carbon Materials as Catalyst Support for Proton Exchange Membrane Fuel Cells. Nanomaterials 2023, 13, 2818. [Google Scholar] [CrossRef]
- Shin, J.; Hwang, W.-S.; Choi, H. Can hydrogen fuel vehicles be a sustainable alternative on vehicle market?: Comparison of electric and hydrogen fuel cell vehicles. Technol. Forecast. Soc. Change 2019, 143, 239–248. [Google Scholar] [CrossRef]
- Morrison, G.; Stevens, J.; Joseck, F. Relative economic competitiveness of light-duty battery electric and fuel cell electric vehicles. Transp. Res. Part C Emerg. Technol. 2018, 87, 183–196. [Google Scholar] [CrossRef]
- Shekhawat, D.; Spivey, J.J.; Berry, D.A. Fuel Cells: Technologies for Fuel Processing; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Ijaodola, O.; Ogungbemi, E.; Khatib, F.N.; Wilberforce, T.; Ramadan, M.; El Hassan, Z.; Thompson, J.; Olabi, A.G. Evaluating the effect of metal bipolar plate coating on the performance of proton exchange membrane fuel cells. Energies 2018, 11, 3203. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, W. Recent advances in formic acid electro-oxidation: From the fundamental mechanism to electrocatalysts. Nanoscale Adv. 2021, 3, 94–105. [Google Scholar] [CrossRef]
- Chen, X.; Granda-Marulanda, L.P.; McCrum, I.T.; Koper, M.T. How palladium inhibits CO poisoning during electrocatalytic formic acid oxidation and carbon dioxide reduction. Nat. Commun. 2022, 13, 38. [Google Scholar] [CrossRef]
- Goodman, E.D.; Dai, S.; Yang, A.-C.; Wrasman, C.J.; Gallo, A.; Bare, S.R.; Hoffman, A.S.; Jaramillo, T.F.; Graham, G.W.; Pan, X. Uniform Pt/Pd bimetallic nanocrystals demonstrate platinum effect on palladium methane combustion activity and stability. ACS Catal. 2017, 7, 4372–4380. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Guo, P.; Li, Q.; Ding, R.; Wang, B.; Li, H.; Liu, J.; Zhao, X. Formic acid-assisted synthesis of palladium nanocrystals and their electrocatalytic properties. Langmuir 2014, 30, 440–446. [Google Scholar] [CrossRef]
- Chiou, Y.J.; Juchniewicz, K.; Kupiec, K.R.; Mikolajczuk-Zychora, A.; Mierzwa, B.; Lin, H.M.; Borodzinski, A. Pd Nanoparticle Size Effect of Anodic Catalysts on Direct Formic Acid Fuel Cell Initial Performance: Development of a Mathematical Model and Comparison with Experimental Results. ChemElectroChem 2021, 8, 3348–3359. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.H. Understanding the effect of Pd size on formic acid dehydrogenation via size-controlled Pd/C catalysts prepared by NaBH4 treatment. Appl. Catal. B Environ. 2019, 244, 684–693. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, J.; Wiley, B.; Xia, Y.; Aloni, S.; Yin, Y. Understanding the role of oxidative etching in the polyol synthesis of Pd nanoparticles with uniform shape and size. J. Am. Chem. Soc. 2005, 127, 7332–7333. [Google Scholar] [CrossRef]
- Basnayake Pussepitiyalage, V.; Hemmati, S. Sustainable, Green, and Continuous Synthesis of Fivefold Palladium Nanorods Using l-Ascorbic Acid in a Segmented Millifluidic Flow Reactor. Langmuir 2022, 38, 4200–4212. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, K.; Liu, Y.; He, Y.; Wu, C.; Jin, M. Hydrothermal synthesis of palladium nitrides as robust multifunctional electrocatalysts for fuel cells. J. Mater. Chem. A 2021, 9, 6196–6204. [Google Scholar] [CrossRef]
- Boxall, D.L.; Lukehart, C. Rapid synthesis of Pt or Pd/carbon nanocomposites using microwave irradiation. Chem. Mater. 2001, 13, 806–810. [Google Scholar] [CrossRef]
- Navaladian, S.; Viswanathan, B.; Varadarajan, T.; Viswanath, R. A rapid synthesis of oriented palladium nanoparticles by UV irradiation. Nanoscale Res. Lett. 2009, 4, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.-J.; Chen, M.-Y.; Chang, Y.-L.; Lin, H.-M.; Borodzinski, A. Zirconia Modified Pd Electrocatalysts for DFAFCs. Adv. Chem. Eng. Sci. 2020, 10, 99–112. [Google Scholar] [CrossRef]
- Harada, M.; Kizaki, S. Formation mechanism of gold nanoparticles synthesized by photoreduction in aqueous ethanol solutions of polymers using in situ quick scanning x-ray absorption fine structure and small-angle X-ray scattering. Cryst. Growth Des. 2016, 16, 1200–1212. [Google Scholar] [CrossRef]
- Harada, M.; Katagiri, E. Mechanism of silver particle formation during photoreduction using in situ time-resolved SAXS analysis. Langmuir 2010, 26, 17896–17905. [Google Scholar] [CrossRef] [PubMed]
- Ozkaraoglu, E.; Tunc, I.; Suzer, S. Preparation of Au and Au–Pt nanoparticles within PMMA matrix using UV and X-ray irradiation. Polymer 2009, 50, 462–466. [Google Scholar] [CrossRef]
- Li, M.-T.; Lai, S.-F.; Yang, S.-M.; Chen, Y.-S.; Chen, Y.-J.; Tok, E.S.; Margaritondo, G.; Hwu, Y. Gold nano-mesh synthesis by continuous-flow X-ray irradiation. J. Synchrotron Rad. 2019, 26, 1929–1935. [Google Scholar] [CrossRef]
- Zaman, S. X-ray characterization of just one atom: A pioneering accomplishment. Matter 2023, 6, 2632–2634. [Google Scholar] [CrossRef]
- Peters, G.; Zakharchenko, O.; Konarev, P.; Karmazikov, Y.; Smirnov, M.; Zabelin, A.; Mukhamedzhanov, E.; Veligzhanin, A.; Blagov, A.; Kovalchuk, M. The small-angle X-ray scattering beamline BioMUR at the Kurchatov synchrotron radiation source. Nucl. Instrum. Methods Phys. Res. A Accel. Spectrom. Detect. Assoc. Equip. 2019, 945, 162616. [Google Scholar] [CrossRef]
- Bhuvanendran, N.; Ravichandran, S.; Zhang, W.; Ma, Q.; Xu, Q.; Khotseng, L.; Su, H. Highly efficient methanol oxidation on durable PtxIr/MWCNT catalysts for direct methanol fuel cell applications. Int. J. Hydrog. Energy 2020, 45, 6447–6460. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Cellat, K.; Arıkan, K.; Savk, A.; Karimi, F.; Şen, F. Palladium–Nickel nanoparticles decorated on Functionalized-MWCNT for high precision non-enzymatic glucose sensing. Mater. Chem. Phys. 2020, 250, 123042. [Google Scholar] [CrossRef]
- Göksu, H.; Zengin, N.; Burhan, H.; Cellat, K.; Şen, F. A novel hydrogenation of nitroarene compounds with multi wall carbon nanotube supported palladium/copper nanoparticles (PdCu@ MWCNT NPs) in aqueous medium. Sci. Rep. 2020, 10, 8043. [Google Scholar] [CrossRef]
- Navlani-García, M.; Salinas-Torres, D.; Vázquez-Álvarez, F.D.; Cazorla-Amorós, D. Formic acid dehydrogenation attained by Pd nanoparticles-based catalysts supported on MWCNT-C3N4 composites. Catal. Today 2022, 397, 428–435. [Google Scholar] [CrossRef]
- Bagherzade, A.; Jamshidi, M. Thermo-mechanical properties of epoxy nanocomposites incorporating amino acid and acid functionalized multi-walled carbon nanotubes. J. Compos. Mater. 2020, 54, 1847–1861. [Google Scholar] [CrossRef]
- Chen, W.; Zou, C.; Li, X. Application of large-scale prepared MWCNTs nanofluids in solar energy system as volumetric solar absorber. Sol. Energy Mater Sol. Cells 2019, 200, 109931. [Google Scholar] [CrossRef]
- Özütok, F.; Er, I.K.; Acar, S.; Demiri, S. Enhancing the Co gas sensing properties of ZnO thin films with the decoration of MWCNTs. J. Mater. Sci. Mater. Electron. 2019, 30, 259–265. [Google Scholar] [CrossRef]
- Zenou, V.Y.; Bakardjieva, S. Microstructural analysis of undoped and moderately Sc-doped TiO2 anatase nanoparticles using Scherrer equation and Debye function analysis. Mater. Charact. 2018, 144, 287–296. [Google Scholar] [CrossRef]
- Chen, M.-S.; Lin, H.-N.; Cheng, Y.-C.; Fang, A.; Chen, C.-Y.; Lee, P.-Y.; Lin, C.-K. Effects of milling time, zirconia addition, and storage environment on the radiopacity performance of mechanically milled Bi2O3/ZrO2 composite powders. Materials 2020, 13, 563. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, K.; Yan, B.; Wang, J.; Wang, C.; Li, S.; Gu, Z.; Du, Y.; Yang, P. Ultra-uniform PdBi nanodots with high activity towards formic acid oxidation. J. Power Sources 2017, 356, 27–35. [Google Scholar] [CrossRef]
- Betts, A.; Briega-Martos, V.; Cuesta, A.; Herrero, E. Adsorbed formate is the last common intermediate in the dual-path mechanism of the electrooxidation of formic acid. ACS Catal. 2020, 10, 8120–8130. [Google Scholar] [CrossRef]
- Mikolajczuk-Zychora, A.; Borodzinski, A.; Kedzierzawski, P.; Mierzwa, B.; Mazurkiewicz-Pawlicka, M.; Stobinski, L.; Ciecierska, E.; Zimoch, A.; Opałło, M. Highly active carbon supported Pd cathode catalysts for direct formic acid fuel cells. Appl. Surf. Sci. 2016, 388, 645–652. [Google Scholar] [CrossRef]
- Li, Y.; Yan, Y.; Yao, M.-S.; Wang, F.; Li, Y.; Collins, S.M.; Chiu, Y.-L.; Du, S. Porous electrodes from self-assembled 3D jointed Pd polyhedra for direct formic acid fuel cells. Chem. Eng. J. 2023, 462, 142244. [Google Scholar] [CrossRef]
- Li, Y.-S.; Liao, J.-L.; Wang, S.-Y.; Chiang, W.-H. Intercalation-assisted longitudinal unzipping of carbon nanotubes for green and scalable synthesis of graphene nanoribbons. Sci. Rep. 2016, 6, 22755. [Google Scholar] [CrossRef] [PubMed]
- Bielawski, C.W.; Dreyer, D.; Park, S.; Ruoff, R. The chemistry of grapheme oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar]
- Kim, S.J.; Park, Y.J.; Ra, E.J.; Kim, K.K.; An, K.H.; Lee, Y.H.; Choi, J.Y.; Park, C.H.; Doo, S.K.; Park, M.H. Defect-induced loading of Pt nanoparticles on carbon nanotubes. Appl. Phys. Lett. 2007, 90, 023114. [Google Scholar] [CrossRef]
- Chiou, Y.-J.; Chung, M.-Y.; Lin, H.-M.; Liu, H.-Y.; Borodzinski, A.; Stobinski, L.; Lin, C.-K.; Kupiec, K.R. Synthesis and characterization of PANI-MWCNTs supported nano hybrid electrocatalysts. Mater. Sci. Eng. A 2017, 7, 1–8. [Google Scholar]
- Yamaguchi, A.; Okada, I.; Fukuoka, T.; Ishihara, M.; Sakurai, I.; Utsumi, Y. One-step synthesis of copper and cupric oxide particles from the liquid phase by X-ray radiolysis using synchrotron radiation. J. Nanomater. 2016, 2016, 8584304. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Okada, I.; Fukuoka, T.; Sakurai, I.; Utsumi, Y. Synthesis of metallic nanoparticles through X-ray radiolysis using synchrotron radiation. Jpn. J. Appl. Phys. 2016, 55, 055502. [Google Scholar] [CrossRef]
- Karthik, T.; Olvera-Amador, M.d.l.L.; Maldonado, A.; Hernandez, A.G.; Gómez-Pozos, H. Propane gas-sensing properties of pure and Pd-doped tin oxide nanostructures. J. Mater. Sci. Mater. Electron. 2023, 34, 228. [Google Scholar] [CrossRef]
- Mittermeier, T.; Weiß, A.; Hasché, F.; Gasteiger, H.A. Activity, stability and degradation of carbon supported palladium (Pd/C) fuel cell electrocatalysts for the oxygen reduction. ECS Trans. 2015, 69, 303. [Google Scholar] [CrossRef]
- Zhou, W.; Li, M.; Ding, O.L.; Chan, S.H.; Zhang, L.; Xue, Y. Pd particle size effects on oxygen electrochemical reduction. Int. J. Hydrog. Energy 2014, 39, 6433–6442. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Tiwary, C.S.; Ma, Z.; Huang, H.; Zhang, J.; Lu, Z.; Huang, W.; Wu, Y. Palladium nanoparticles supported on nitrogen and sulfur dual-doped graphene as highly active electrocatalysts for formic acid and methanol oxidation. ACS Appl. Mater. Iterfaces 2016, 8, 10858–10865. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Zaman, S.; Majeed, I.; Kanodarwala, F.K.; Nadeem, M.A.; Stride, J.A.; Nadeem, M.A. Porous carbon/rGO composite: An ideal support material of highly efficient palladium electrocatalysts for the formic acid oxidation reaction. ChemElectroChem 2017, 4, 3126–3133. [Google Scholar] [CrossRef]
- Mahesh, K.N.; Balaji, R.; Dhathathreyan, K. Palladium nanoparticles as hydrogen evolution reaction (HER) electrocatalyst in electrochemical methanol reformer. Int. J. Hydrog. Energy 2016, 41, 46–51. [Google Scholar] [CrossRef]
- Narreddula, M.; Balaji, R.; Ramya, K.; Rajalakshmi, N.; Ramachandraiah, A. Nitrogen doped graphene supported Pd as hydrogen evolution catalyst for electrochemical methanol reformation. Int. J. Hydrog. Energy 2019, 44, 4582–4591. [Google Scholar] [CrossRef]
- Sharma, R.; Gyergyek, S.; Andersen, S.M. Critical thinking on baseline corrections for electrochemical surface area (ECSA) determination of Pt/C through H-adsorption/H-desorption regions of a cyclic voltammogram. Appl. Catal. B Environ. 2022, 311, 121351. [Google Scholar] [CrossRef]
- Ma, Y.; Li, T.; Chen, H.; Chen, X.; Deng, S.; Xu, L.; Sun, D.; Tang, Y. A general strategy to the synthesis of carbon-supported PdM (M = Co, Fe and Ni) nanodendrites as high-performance electrocatalysts for formic acid oxidation. J. Energy Chem. 2017, 26, 1238–1244. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, X.; Chen, X.; Yu, H.; Chen, D.; Yu, B.; Jin, Y. Enhanced formic acid oxidation with Pd nanoparticles deposited on boron-doped graphene: A comprehensive electrochemical and spectroscopic investigation. Int. J. Electrochem. Sci. 2023, 18, 100156. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, H.; Wang, J.; Chang, L.; Gao, Y.; Liu, W.; Tang, Z. Shape-dependent electrocatalytic activity of monodispersed palladium nanocrystals toward formic acid oxidation. Nanoscale 2013, 5, 8392–8397. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, Y.; Zhu, Y.; Jiang, H.; Shen, J.; Li, C. Efficient electrocatalytic formic acid oxidation over PdAu-manganese oxide/carbon. J. Colloid Interface Sci. 2021, 593, 244–250. [Google Scholar] [CrossRef]
- Hu, S.; Scudiero, L.; Ha, S. Electronic effect on oxidation of formic acid on supported Pd–Cu bimetallic surface. Electrochim. Acta 2012, 83, 354–358. [Google Scholar] [CrossRef]
- Yeom, J.; Jayashree, R.; Rastogi, C.; Shannon, M.; Kenis, P. Passive direct formic acid microfabricated fuel cells. J. Power Sources 2006, 160, 1058–1064. [Google Scholar] [CrossRef]
- Larsen, R.; Ha, S.; Zakzeski, J.; Masel, R.I. Unusually active palladium-based catalysts for the electrooxidation of formic acid. J. Power Sources 2006, 157, 78–84. [Google Scholar] [CrossRef]
- Zhu, Y.; Khan, Z.; Masel, R. The behavior of palladium catalysts in direct formic acid fuel cells. J. Power Sources 2005, 139, 15–20. [Google Scholar] [CrossRef]
- Chang, J.; Li, S.; Feng, L.; Qin, X.; Shao, G. Effect of carbon material on Pd catalyst for formic acid electrooxidation reaction. J. Power Sources 2014, 266, 481–487. [Google Scholar] [CrossRef]
- Jałowiecka, M.; Bojarska, Z.; Małolepszy, A.; Makowski, Ł. Mass transport enhancement in direct formic acid fuel cell with a novel channel design. Chem. Eng. J. 2023, 451, 138474. [Google Scholar] [CrossRef]
- Lesiak, B.; Mazurkiewicz, M.; Malolepszy, A.; Stobinski, L.; Mierzwa, B.; Mikolajczuk-Zychora, A.; Juchniewicz, K.; Borodzinski, A.; Zemek, J.; Jiricek, P. Effect of the Pd/MWCNTs anode catalysts preparation methods on their morphology and activity in a direct formic acid fuel cell. Appl. Surf. Sci. 2016, 387, 929–937. [Google Scholar] [CrossRef]
- Mikołajczuk, A.; Borodzinski, A.; Kedzierzawski, P.; Stobinski, L.; Mierzwa, B.; Dziura, R. Deactivation of carbon supported palladium catalyst in direct formic acid fuel cell. Appl. Surf. Sci. 2011, 257, 8211–8214. [Google Scholar] [CrossRef]
- Ha, S.; Larsen, R.; Masel, R. Performance characterization of Pd/C nanocatalyst for direct formic acid fuel cells. J. Power Sources 2005, 144, 28–34. [Google Scholar] [CrossRef]




| Sample (Anode) | Preparation | Cathode | Max. Power Density (mW/mgPd) | Ref. |
|---|---|---|---|---|
| Pd | Electrodeposit | Pt | 7.5 | [73] |
| Pd Black | NaBH4 reduction | Pt nanoparticle | 108.3 | [74] |
| Pd Black | Commercial | Pt nanoparticle | 31.6 | [75] |
| Pd polyhedron | HCOOH reduction | Pt GDE | 202.0 | [53] |
| Pd/C | Commercial Pd and C mixture | Pt nanoparticle | 81.8 | [76] |
| Pd/C | Commercial | 40 wt.% Pt/C | 22.9 | [77] |
| Pd/MWCNT | X-ray photoreduction | 60 wt.% Pt/C | 241.0 | [27] |
| Pd/f-MWCNT | Polyol method | 60 wt.% Pt/C | 175.8 | [27] |
| Pd/purified MWCNT | NaBH4 | 60 wt.% Pt/C | 199.4 | [27] |
| Pd/f-CNT | High-pressure microwave | 60 wt.% Pt/C | 75.0 | [78] |
| Pd/f-CNT | Microwave-assisted polyol | 60 wt.% Pt/C | 57.0 | [78] |
| Pd/f-CNT | NaBH4 reduction | 60 wt.% Pt/C | 119.0 | [78] |
| Pd/A-CNT-1 | X-ray photoreduction | 40 wt.% Pt/C | 92.3 | This work |
| Pd/A-CNT-2 | X-ray photoreduction | 40 wt.% Pt/C | 106.2 | This work |
| Sample (Anode) | Formic Acid Concentration | Lifetime (Hour) | Percentage of Initial Power Density | Ref. |
|---|---|---|---|---|
| Commercial Pd | 5 M | 0.95 | 62% | [80] |
| Commercial Pd | 5 M | 0.95 | 63% | [74] |
| Pd/C | 3 M | 2.00 | 55% | [76] |
| Pd Black | 10 M | 6.20 | 44% | [75] |
| Pd/CNT-2 | 3 M | 2.90 | 10% | This work * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsou, S.-J.; Mazurkiewicz-Pawlicka, M.; Chiou, Y.-J.; Lin, C.-K. Effect of Synchrotron X-ray Irradiation Time on the Particle Size and DFAFC Performance of Pd/CNT Catalysts. Nanomaterials 2024, 14, 162. https://doi.org/10.3390/nano14020162
Tsou S-J, Mazurkiewicz-Pawlicka M, Chiou Y-J, Lin C-K. Effect of Synchrotron X-ray Irradiation Time on the Particle Size and DFAFC Performance of Pd/CNT Catalysts. Nanomaterials. 2024; 14(2):162. https://doi.org/10.3390/nano14020162
Chicago/Turabian StyleTsou, Sheng-Jung, Marta Mazurkiewicz-Pawlicka, Yuh-Jing Chiou, and Chung-Kwei Lin. 2024. "Effect of Synchrotron X-ray Irradiation Time on the Particle Size and DFAFC Performance of Pd/CNT Catalysts" Nanomaterials 14, no. 2: 162. https://doi.org/10.3390/nano14020162





