Plasma-Engineered CeOx Nanosheet Array with Nitrogen-Doping and Porous Architecture for Efficient Electrocatalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Direct Growth of CeO2 Nanosheets on Ni Foam
2.2. Plasma-Induced Fabrication of N-Doped CeOx
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
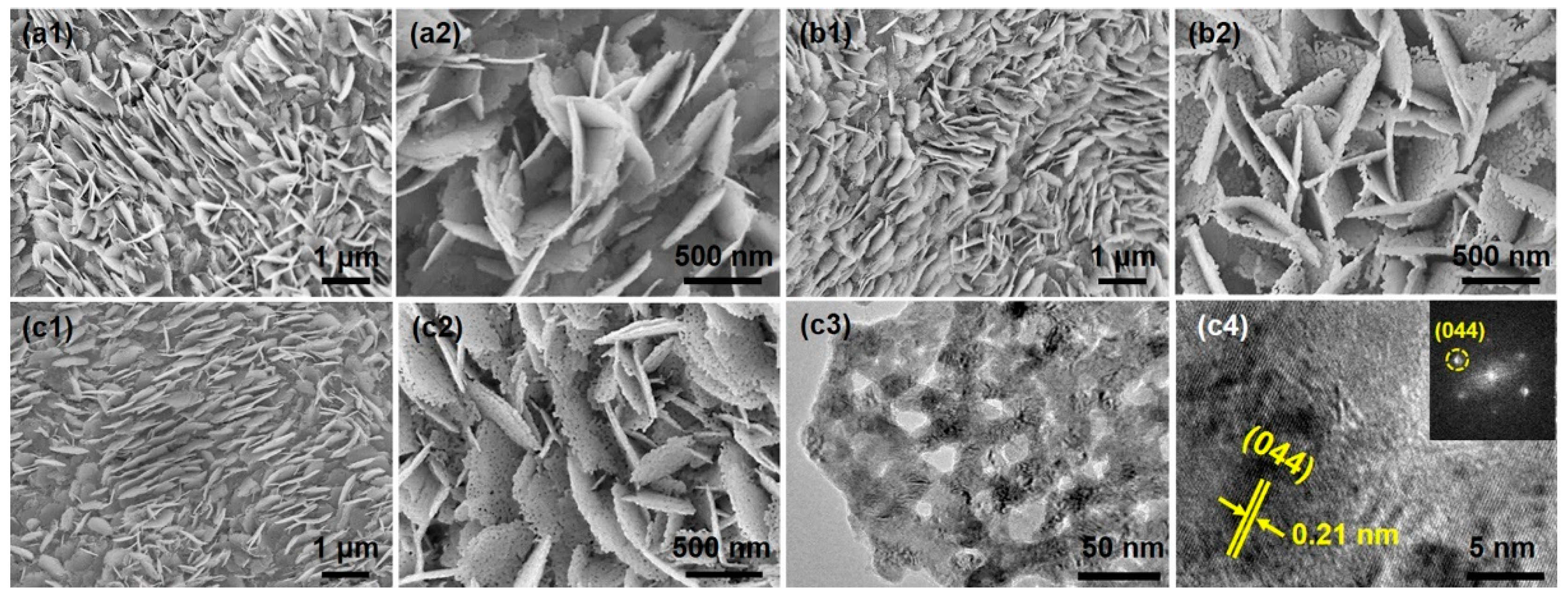
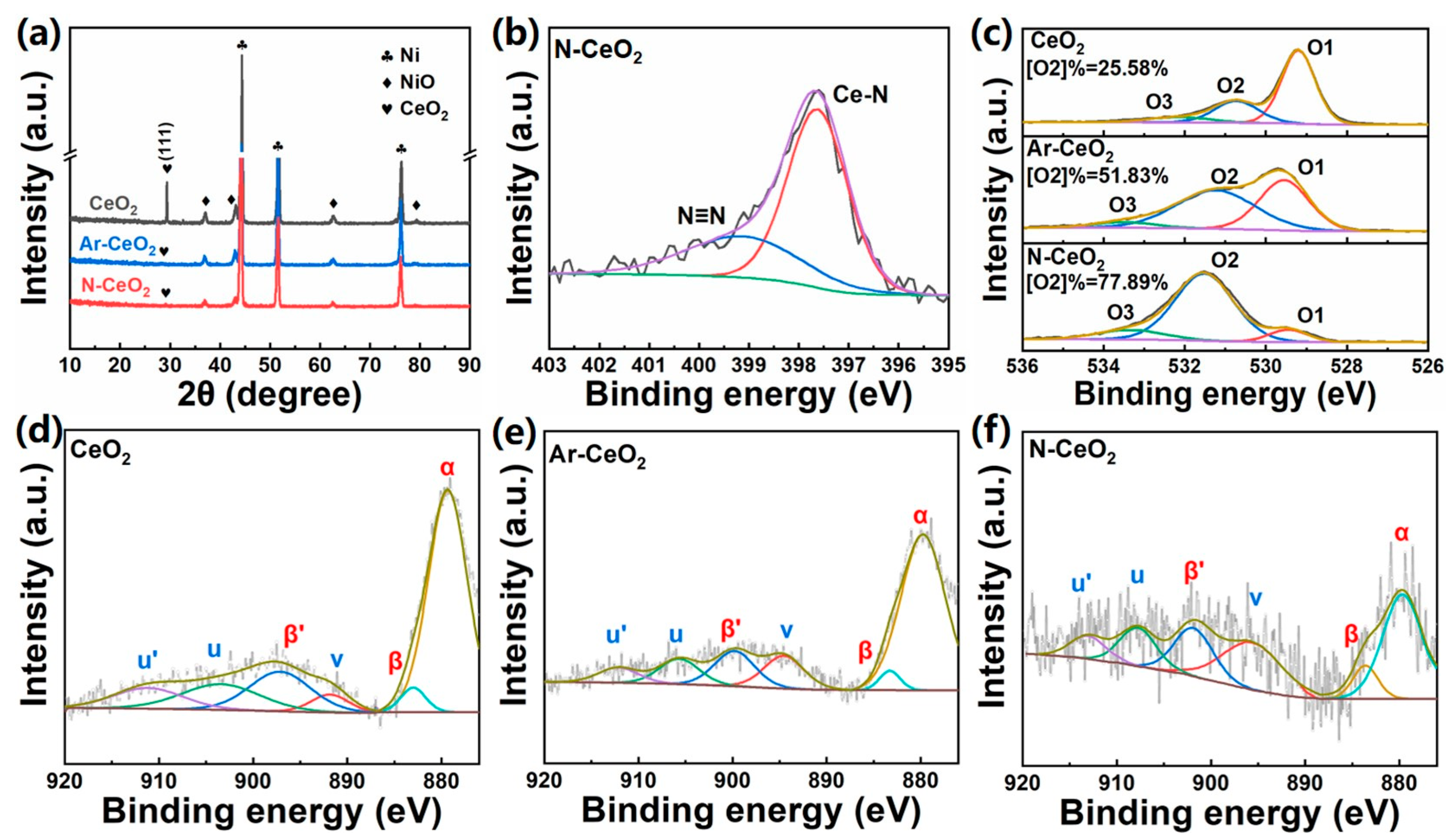
3.1. Physicochemical Properties
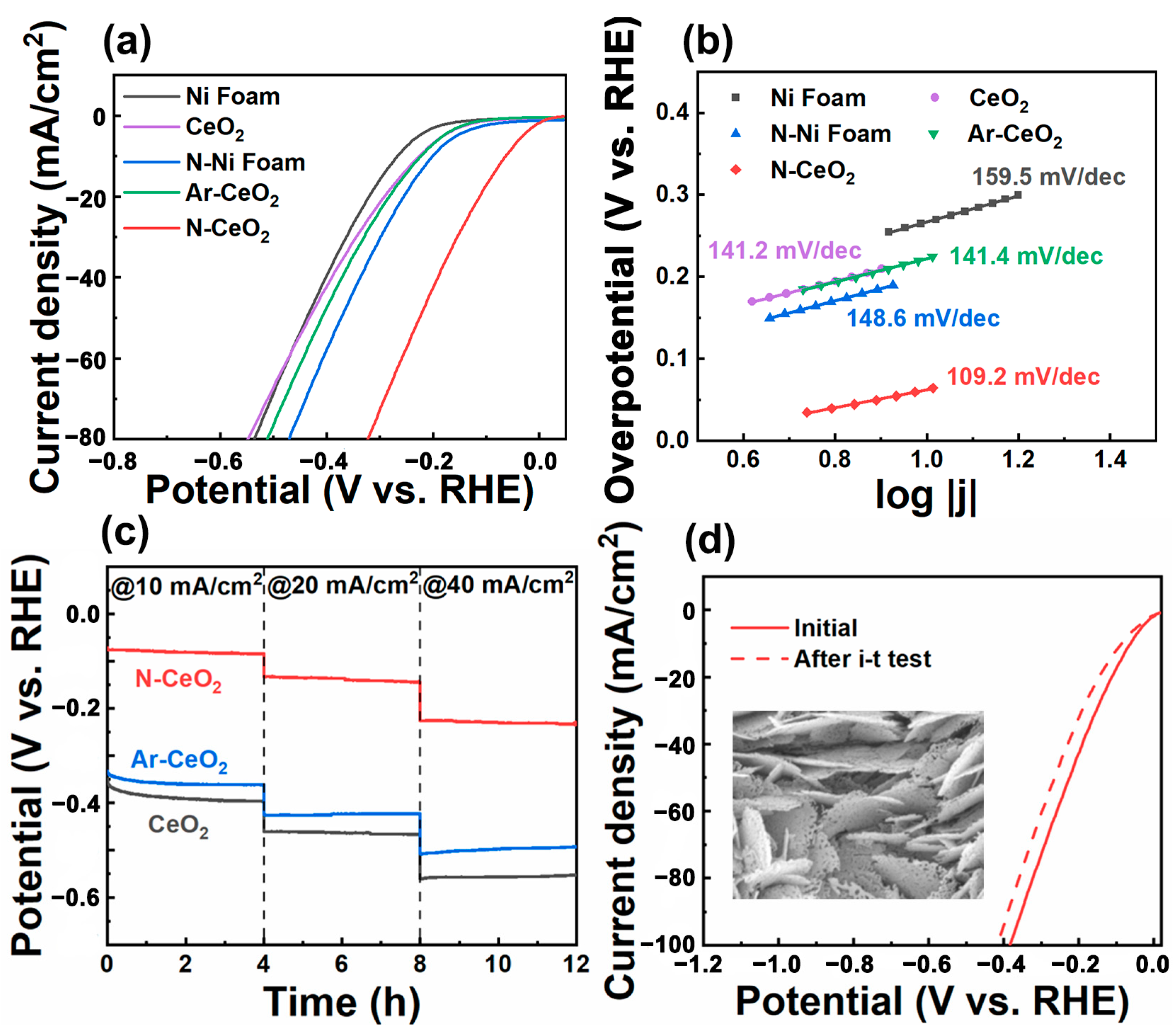
3.2. HER Performance
3.3. Mechanism Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Suryadi, B.; Yan, J.; Feng, J.; Bhaskoro, A.G.A. strategic roadmap for ASEAN to develop hydrogen energy: Economic prospects and carbon emission reduction. Inter. J. Hydrogen Energ. 2023, 48, 11113–11130. [Google Scholar] [CrossRef]
- Liu, G. Forward perspective on the development and strategic pathway of green hydrogen in China. Clean Energy 2023, 7, 1–7. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, R.; Lv, Z.; Liu, J.; Zhou, H.; Xu, C. Green hydrogen: A promising way to the carbon-free society. Chin. J. Chem. Eng. 2022, 43, 2–13. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Wang, Y.; Xiong, Y.; Yang, P.; Qin, J.; Xiong, X.; Lei, Y. Designing a built-in electric gield for efficient energy electrocatalysis. ACS Nano 2022, 16, 19959–19979. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, P.; Song, Y.; Hou, Y.; Xu, B.; Liao, T.; Zhang, H.; Guo, J.; Sun, Z. heterostructure engineering of 2D superlattice materials for electrocatalysis. Adv. Sci. 2022, 9, e2204297. [Google Scholar] [CrossRef]
- Zhao, X.; Levell, Z.H.; Yu, S.; Liu, Y. Atomistic understanding of two-dimensional electrocatalysts from first principles. Chem. Rev. 2022, 122, 10675–10709. [Google Scholar] [CrossRef]
- Liu, F.; Cai, X.; Tang, Y.; Liu, W.; Chen, Q.; Dong, P.; Xu, M.; Tan, Y.; Bao, S. Nano-Ni-induced electronic modulation of MoS2 nanosheets enables energy-saving H2 production and sulfide degradation. Energy Environ. Mater. 2023, 0, e12644. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, J.; Yang, J. Fiber materials for electrocatalysis applications. Adv. Fiber Mater. 2022, 4, 720–735. [Google Scholar] [CrossRef]
- You, B.; Tang, M.T.; Tsai, C.; Abild-Pedersen, F.; Zheng, X.; Li, H. Enhancing electrocatalytic water splitting by strain engineering. Adv. Mater. 2019, 31, e1807001. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Zhang, L.; Shen, L.; Xiao, Y.; Zhang, Y.; Au, C.; Jiang, L. Insight into the effect of morphology on catalytic performance of porous CeO2 nanocrystals for H2S selective oxidation. Appl. Catal. B Environ. 2019, 252, 98–110. [Google Scholar] [CrossRef]
- Li, T.; Wang, Q.; Wang, Z. Oxygen Vacancy Injection on (111) CeO2 Nanocrystal Facets for Efficient H2O2 Detection. Biosensors 2022, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Spezzati, G.; Benavidez, A.D.; DeLaRiva, A.T.; Su, Y.; Hofmann, J.P.; Asahina, S.; Olivier, E.J.; Neethling, J.H.; Miller, J.T.; Datye, A.K.; et al. CO oxidation by Pd supported on CeO2(100) and CeO2(111) facets. Appl. Catal. B Environ. 2019, 243, 36–46. [Google Scholar] [CrossRef]
- Wang, M.; Shen, M.; Jin, X.; Tian, J.; Li, M.; Zhou, Y.; Zhang, L.; Li, Y.; Shi, J. Oxygen vacancy generation and stabilization in CeO2–x by Cu introduction with improved CO2 photocatalytic reduction activity. ACS Catal. 2019, 9, 4573–4581. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Z.; Brosnahan, J.T.; Zhang, S.; Guo, Y.; Guo, Y.; Wang, L.; Wang, Y.; Zhan, W. Ru/CeO2 catalyst with optimized CeO2 support morphology and surface facets for propane combustion. Environ. Sci. Technol. 2019, 53, 5349–5358. [Google Scholar] [CrossRef]
- Su, Z.; Yang, W.; Wang, C.; Xiong, S.; Cao, X.; Peng, Y.; Si, W.; Weng, Y.; Xue, M.; Li, J. Roles of oxygen vacancies in the bulk and surface of CeO2 for toluene catalytic combustion. Environ. Sci. Technol. 2020, 54, 12684–12692. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xu, K.; Fang, Z.; Tong, Y.; Wu, J.; Lu, X.; Peng, X.; Ding, H.; Wu, C.; Xie, Y. Metallic Co4N porous nanowire arrays activated by surface oxidation as electrocatalysts for the oxygen evolution reaction. Angew. Chem. Int. Ed. Engl. 2015, 54, 14710–14714. [Google Scholar] [CrossRef] [PubMed]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Souza Filho, E.A.d.; Pieretti, E.F.; Bento, R.T.; Pillis, M.F. Effect of nitrogen-doping on the surface chemistry and corrosion stability of TiO2 films. J. Mater. Res. Technol. 2020, 9, 922–934. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, T.; Wang, Z. Plasma-Engineered N-CoOx Nanowire Array as a Bifunctional Electrode for Supercapacitor and Electrocatalysis. Nanomaterials 2022, 12, 2984. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Niu, P.; Wang, S.; Shi, J.; Li, L. Porous two-dimensional materials for photocatalytic and electrocatalytic applications. Matter 2020, 2, 1377–1413. [Google Scholar] [CrossRef]
- Yin, C.; Liu, Y.; Xia, Q.; Kang, S.; Li, X.; Wang, Y.; Cui, L. Oxygen vacancy-rich nitrogen-doped Co3O4 nanosheets as an efficient water-resistant catalyst for low temperature CO oxidation. J. Colloid Interface Sci. 2019, 553, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, W.; Chen, X.; Peng, Y.; Song, Y.; Lv, C.; Liu, H.; Sun, J.; Yuan, D.; Li, X.; et al. Defect-rich nitrogen doped Co3O4/C porous nanocubes enable high-efficiency bifunctional oxygen electrocatalysis. Adv. Funct. Mater. 2019, 29, 1902875. [Google Scholar] [CrossRef]
- Tang, J.; Su, C.; Shao, Z. Nonthermal plasma treatment for electrocatalysts structural and surface engineering. Energy Technol. 2022, 10, 2200235. [Google Scholar] [CrossRef]
- Li, D.; Xu, K.; Zhang, Y. A review on research progress in plasma-controlled superwetting surface structure and properties. Polymers 2022, 14, 3759. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Song, B.; Li, X.; Wang, R.; Wang, S.; Xu, S.; Reniers, F.; Lam, C.H. Enhancing the properties of photocatalysts via nonthermal plasma modification: Recent advances, treatment variables, mechanisms, and perspectives. Ind. Eng. Chem. 2021, 60, 16813–16826. [Google Scholar] [CrossRef]
- Liu, C.; Sun, H.; Qian, J.; Chen, Z.; Lv, Y.; Chen, F.; Lu, X.; Wu, Z. Biotemplating synthesis and photocatalytic activities of N-doped CeO2 microcapsule tailored by hemerocallis pollen. Adv. Powder Technol. 2017, 28, 2741–2746. [Google Scholar] [CrossRef]
- Kesarla, M.K.; Fuentez-Torres, M.O.; Alcudia-Ramos, M.A.; Ortiz-Chi, F.; Espinosa-González, C.G.; Aleman, M.; Torres-Torres, J.G.; Godavarthi, S. Synthesis of g-C3N4/N-doped CeO2 composite for photocatalytic degradation of an herbicide. J. Mater. Res. Technol. 2019, 8, 1628–1635. [Google Scholar] [CrossRef]
- Lei, X.; Wang, J.; Wang, T.; Wang, X.; Xie, X.; Huang, H.; Li, D.; Ao, Z. Toluene decomposition by non-thermal plasma assisted CoOx-gamma-Al2O3: The relative contributions of specific energy input of plasma, Co3+ and oxygen vacancy. J. Hazard. Mater. 2023, 456, 131613. [Google Scholar] [CrossRef]
- Yun, J.; Wu, L.; Hao, Q.; Teng, Z.; Gao, X.; Dou, B.; Bin, F. Non-equilibrium plasma enhanced oxygen vacancies of CuO/CeO2 nanorod catalysts for toluene oxidation. J. Environ. Chem. Eng. 2022, 10, 107847. [Google Scholar] [CrossRef]
- Jang, D.; Maeng, J.; Kim, J.; Han, H.; Park, G.H.; Ha, J.; Shin, D.; Hwang, Y.J.; Kim, W.B. Boosting electrocatalytic nitrate reduction reaction for ammonia synthesis by plasma-induced oxygen vacancies over MnCuOx. Appl. Surf. Sci. 2023, 610, 155521. [Google Scholar] [CrossRef]
- Lin, J.H.; Yan, Y.T.; Xu, T.X.; Qu, C.Q.; Li, J.; Cao, J.; Feng, J.C.; Qi, J.L. S doped NiCo2O4 nanosheet arrays by Ar plasma: An efficient and bifunctional electrode for overall water splitting. J. Colloid Interface Sci. 2020, 560, 34–39. [Google Scholar] [CrossRef]
- Wang, Q.; Li, M.; Wang, Z. Supercapacitive performance of TiO2 boosted by a unique porous TiO2/Ti network and activated Ti3+. RSC. Adv. 2019, 9, 7811–7817. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Diao, L.; Tang, Y.; He, F.; Liu, E.; He, C.; Shi, C.; Li, J.; Sha, J.; et al. CeOx-decorated NiFe-layered double hydroxide for efficient alkaline hydrogen evolution by oxygen vacancy engineering. ACS Appl. Mater. Interf. 2018, 10, 35145–35153. [Google Scholar] [CrossRef]
- Sung, M.-C.; Lee, G.-H.; Kim, D.-W. CeO2/Co(OH)2 hybrid electrocatalysts for efficient hydrogen and oxygen evolution reaction. J. Alloys Compd. 2019, 800, 450–455. [Google Scholar] [CrossRef]
- Liu, M.; Ji, Z.; Shen, X.; Zhou, H.; Zhu, J.; Xie, X.; Song, C.; Miao, X.; Kong, L.; Zhu, G. An electrocatalyst for a hydrogen evolution reaction in an alkaline medium: Three-dimensional graphene supported CeO2 hollow microspheres. Eur. J. Inorg. Chem. 2018, 2018, 3952–3959. [Google Scholar] [CrossRef]
- Zhiani, M.; Kamali, S. Synergistic effect of ceria on the structure and hydrogen evolution activity of nickel nanoparticles grown on reduced graphene oxide. J. Mater. Chem. A 2017, 5, 8108–8116. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, J.; Xie, J.; Zheng, X.; Wang, M.; Li, X.; Tang, B. High-performance alkaline hydrogen evolution electrocatalyzed by a Ni3N–CeO2 nanohybrid. Inorg. Chem. Front. 2018, 5, 3042–3045. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.; Dong, H.; Wang, L.; Chen, Z.; Wang, Y.; Li, Z.; Xia, M.; Shao, G. The effects of CeO2 nanorods and CeO2 nanoflakes on Ni–S alloys in hydrogen evolution reactions in alkaline solutions. Catalysts 2017, 7, 197. [Google Scholar] [CrossRef]
- Sun, H.; Tian, C.; Fan, G.; Qi, J.; Liu, Z.; Yan, Z.; Cheng, F.; Chen, J.; Li, C.P.; Du, M. Boosting activity on Co4N porous nanosheet by coupling CeO2 for efficient electrochemical overall water splitting at high current densities. Adv. Funct. Mater. 2020, 30, 1910596. [Google Scholar] [CrossRef]
- Zhang, R.; Ren, X.; Hao, S.; Ge, R.; Liu, Z.; Asiri, A.M.; Chen, L.; Zhang, Q.; Sun, X. Selective phosphidation: An effective strategy toward CoP/CeO2 interface engineering for superior alkaline hydrogen evolution electrocatalysis. J. Mater. Chem. A 2018, 6, 1985–1990. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, F.-T.; Chen, J.-H.; Fu, X.-Z.; Sun, R.; Wong, C.-P. Amorphous NiFe nanotube arrays bifunctional electrocatalysts for efficient electrochemical overall water splitting. ACS Appl. Energ. Mater. 2018, 1, 1210–1217. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Qian, K.; Wei, W.; Sun, C.; Xie, J. In situ growth of N-doped carbon coated CoNi alloy with graphene decoration for enhanced HER performance. J. Energ. Chem. 2019, 29, 129–135. [Google Scholar] [CrossRef]


| Active Materials | Overpotential (mV vs. RHE) | Tafel Slope (mV/dec) | Ref. |
|---|---|---|---|
| N-CeO2 | 65 | 109.2 | Our work |
| CeO2/Co(OH)2 | 317 | 144 | [34] |
| 3D CeO2/rGO | 341 | 112. 8 | [35] |
| Ni-CeO2/rGO | 111 | 107.3 | [36] |
| Ni3N-CeO2 | 80 | 122 | [37] |
| Ni-S/CeO2 | 180 | 158 | [38] |
| Co4N/CeO2 | 24 | 61 | [39] |
| CoP/CeO2 | 43 | 45 | [40] |
| NiFe nanotube | 181 | 147 | [41] |
| CoNi@N-C/rGO | 510 | 133.7 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, T.; Wang, Q. Plasma-Engineered CeOx Nanosheet Array with Nitrogen-Doping and Porous Architecture for Efficient Electrocatalysis. Nanomaterials 2024, 14, 185. https://doi.org/10.3390/nano14020185
Wang Z, Li T, Wang Q. Plasma-Engineered CeOx Nanosheet Array with Nitrogen-Doping and Porous Architecture for Efficient Electrocatalysis. Nanomaterials. 2024; 14(2):185. https://doi.org/10.3390/nano14020185
Chicago/Turabian StyleWang, Zhou, Tong Li, and Qi Wang. 2024. "Plasma-Engineered CeOx Nanosheet Array with Nitrogen-Doping and Porous Architecture for Efficient Electrocatalysis" Nanomaterials 14, no. 2: 185. https://doi.org/10.3390/nano14020185





