Near-Plasma Chemical Surface Engineering
Abstract
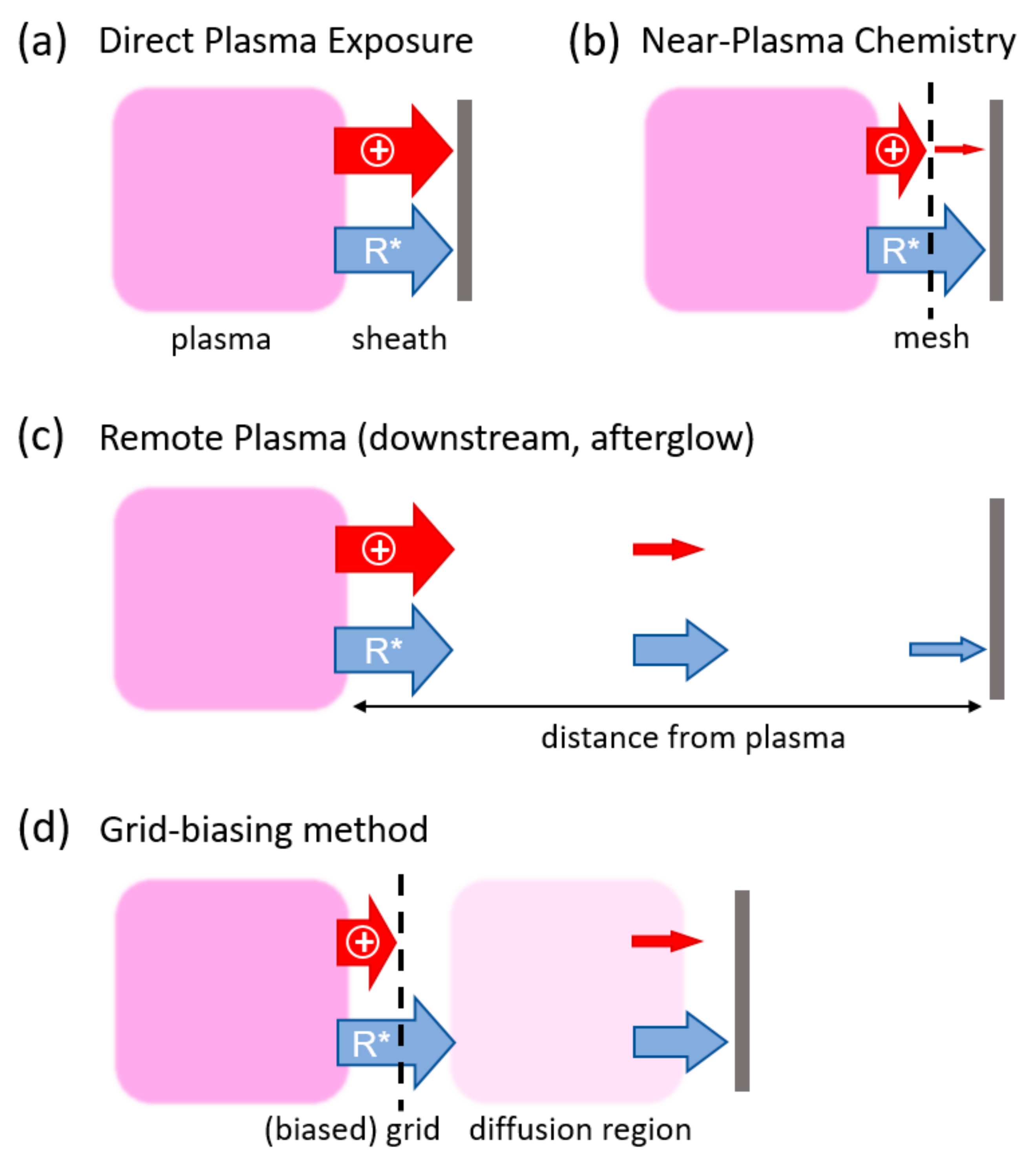
:1. Introduction
2. Materials and Methods
3. Results
3.1. Activation of PTFE for Wettability Control
3.2. Cyclic Polymerization and Etching of HMDSO Coatings
4. Discussion
4.1. Characteristics of Near-Plasma Chemistry
4.2. Impact of Near-Plasma Chemistry on Plasma Etching
4.3. Impact of Near-Plasma Chemistry on Deposition/Etching Processes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tabarés, F.L. (Ed.) Plasma Applications for Material Modification: From Microelectronics to Biological Materials; Jenny Stanford Publishing: New York, NY, USA, 2021; ISBN 978-1-00-311920-3. [Google Scholar]
- Palumbo, F.; Fanelli, F.; Milella, A.; Favia, P.; Fracassi, F. Plasma Processes for the Surface Engineering of Polymers. In Plasma Applications in Gases, Liquids and Solids; World Scientific: Singapore, 2023; pp. 57–120. ISBN 9789811275920. [Google Scholar]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2008; ISBN 978-0-521-84735-3. [Google Scholar]
- Adamovich, I.; Agarwal, S.; Ahedo, E.; Alves, L.L.; Baalrud, S.; Babaeva, N.; Bogaerts, A.; Bourdon, A.; Bruggeman, P.J.; Canal, C.; et al. The 2022 Plasma Roadmap: Low Temperature Plasma Science and Technology. J. Phys. D Appl. Phys. 2022, 55, 373001. [Google Scholar] [CrossRef]
- Lee, W.J.; Cho, T.-Y.; Choa, S.-H.; Cho, S.-K. Environmental Reliability and Moisture Barrier Properties of Silicon Nitride and Silicon Oxide Films Using Roll-to-Roll Plasma Enhanced Chemical Vapor Deposition. Thin Solid Films 2021, 720, 138524. [Google Scholar] [CrossRef]
- Booth, J.-P.; Mozetič, M.; Nikiforov, A.; Oehr, C. Foundations of Plasma Surface Functionalization of Polymers for Industrial and Biological Applications. Plasma Sources Sci. Technol. 2022, 31, 103001. [Google Scholar] [CrossRef]
- Friedrich, J.L.; Meichsner, J. (Eds.) Nonthermal Plasmas for Materials Processing; Wiley-Scrivener Publishing: Hoboken, NJ, USA, 2022; ISBN 978-1-119-36476-4. [Google Scholar]
- Montes, L.; Román, J.M.; García-Casas, X.; Castillo-Seoane, J.; Sánchez-Valencia, J.R.; Barranco, Á.; López-Santos, C.; Borrás, A. Plasma-Assisted Deposition of TiO2 3D Nanomembranes: Selective Wetting, Superomniphobicity, and Self-Cleaning. Adv. Mater. Interfaces 2021, 8, 2100767. [Google Scholar] [CrossRef]
- Ma, C.; Nikiforov, A.; Hegemann, D.; De Geyter, N.; Morent, R.; Ostrikov, K. (Ken) Plasma-Controlled Surface Wettability: Recent Advances and Future Applications. Int. Mater. Rev. 2023, 68, 82–119. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Guo, Z.; Huo, L.; Lei, M. Wettability and Water-Droplet Contact Electrification of Nanostructural Polypropylene (PP) by Plasma Modification. Plasma Process. Polym. 2023, 20, 2200117. [Google Scholar] [CrossRef]
- Ghosh, R.; Baut, A.; Belleri, G.; Kappl, M.; Butt, H.-J.; Schutzius, T.M. Photocatalytically Reactive Surfaces for Simultaneous Water Harvesting and Treatment. Nat. Sustain. 2023, 6, 1663–1672. [Google Scholar] [CrossRef]
- Slobodian, O.M.; Okholin, P.N.; Lytvyn, P.M.; Malyuta, S.V.; Khyzhun, O.Y.; Vasin, A.V.; Rusavsky, A.V.; Gomeniuk, Y.V.; Glotov, V.I.; Nazarova, T.M.; et al. Plasma Treatment as a Versatile Tool for Tuning of Sorption Properties of Thin Nanoporous Carbon Films. Appl. Surf. Sci. 2021, 544, 148876. [Google Scholar] [CrossRef]
- Alvarez, R.; Lopez-Santos, C.; Ferrer, F.J.; Rico, V.; Cotrino, J.; Gonzalez-Elipe, A.R.; Palmero, A. Modulating Low Energy Ion Plasma Fluxes for the Growth of Nanoporous Thin Films. Plasma Process. Polym. 2015, 12, 719–724. [Google Scholar] [CrossRef]
- Navascués, P.; Buchtelová, M.; Zajícková, L.; Rupper, P.; Hegemann, D. Polymerization Mechanisms of Hexamethyldisiloxane in Low-Pressure Plasmas Involving Complex Geometries. Appl. Surf. Sci. 2024, 645, 158824. [Google Scholar] [CrossRef]
- Michlíček, M.; Blahová, L.; Dvořáková, E.; Nečas, D.; Zajíčková, L. Deposition Penetration Depth and Sticking Probability in Plasma Polymerization of Cyclopropylamine. Appl. Surf. Sci. 2021, 540, 147979. [Google Scholar] [CrossRef]
- Blanchard, N.E.; Hanselmann, B.; Drosten, J.; Heuberger, M.; Hegemann, D. Densification and Hydration of HMDSO Plasma Polymers: Densification and Hydration of HMDSO Films. Plasma Process. Polym. 2015, 12, 32–41. [Google Scholar] [CrossRef]
- Barranco, A.; Groening, P. Fluorescent Plasma Nanocomposite Thin Films Containing Nonaggregated Rhodamine 6G Laser Dye Molecules. Langmuir 2006, 22, 6719–6722. [Google Scholar] [CrossRef] [PubMed]
- Vandencasteele, N.; Nisol, B.; Viville, P.; Lazzaroni, R.; Castner, D.G.; Reniers, F. Plasma-Modified PTFE for Biological Applications: Correlation between Protein-Resistant Properties and Surface Characteristics. Plasma Process. Polym. 2008, 5, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Shenton, M.J.; Stevens, G.C. Surface Modification of Polymer Surfaces: Atmospheric Plasma versus Vacuum Plasma Treatments. J. Phys. D Appl. Phys. 2001, 34, 2761. [Google Scholar] [CrossRef]
- Lopaev, D.V.; Zyryanov, S.M.; Zotovich, A.I.; Rakhimova, T.V.; Mankelevich, Y.A.; Voronina, E.N. Damage to Porous SiCOH Low-k Dielectrics by O, N and F Atoms at Lowered Temperatures. J. Phys. D Appl. Phys. 2020, 53, 175203. [Google Scholar] [CrossRef]
- Chen, X.; Hogan, C.J. Nanoparticle Dynamics in the Spatial Afterglows of Nonthermal Plasma Synthesis Reactors. Chem. Eng. J. 2021, 411, 128383. [Google Scholar] [CrossRef]
- Obrero, J.M.; Filippin, A.N.; Alcaire, M.; Sanchez-Valencia, J.R.; Jacob, M.; Matei, C.; Aparicio, F.J.; Macias-Montero, M.; Rojas, T.C.; Espinos, J.P.; et al. Supported Porous Nanostructures Developed by Plasma Processing of Metal Phthalocyanines and Porphyrins. Front. Chem. 2020, 8, 520. [Google Scholar] [CrossRef]
- Alcaire, M.; Aparicio, F.J.; Obrero, J.; López-Santos, C.; Garcia-Garcia, F.J.; Sánchez-Valencia, J.R.; Frutos, F.; Ostrikov, K.; Borrás, A.; Barranco, A. Plasma Enabled Conformal and Damage Free Encapsulation of Fragile Molecular Matter: From Surface-Supported to On-Device Nanostructures. Adv. Funct. Mater. 2019, 29, 1903535. [Google Scholar] [CrossRef]
- Kim, H.-J.; Bae, J.H.; Lee, S.; Kim, J.; Jung, S.; Jo, C.; Lee, J.Y.; Seo, J.H.; Park, S. Structural and Functional Changes in Soybean Protein via Remote Plasma Treatments. Molecules 2023, 28, 3882. [Google Scholar] [CrossRef]
- Korzec, D.; Theirich, D.; Werner, F.; Traub, K.; Engemann, J. Remote and Direct Microwave Plasma Deposition of HMDSO Films: Comparative Study. Surf. Coat. Technol. 1995, 74–75, 67–74. [Google Scholar] [CrossRef]
- Lin, K.-Y.; Preischl, C.; Hermanns, C.F.; Rhinow, D.; Solowan, H.-M.; Budach, M.; Edinger, K.; Oehrlein, G.S. SiO2 Etching and Surface Evolution Using Combined Exposure to CF4/O2 Remote Plasma and Electron Beam. J. Vac. Sci. Technol. A 2022, 40, 063004. [Google Scholar] [CrossRef]
- Bai, K.H.; Choi, C.K.; Chang, H.Y. Electron Temperature Control with a Small Mesh Number Grid in Inductively Coupled Plasmas. Plasma Sources Sci. Technol. 2004, 13, 662. [Google Scholar] [CrossRef]
- Ikada, R.; Nishimura, G.; Kato, K.; Iizuka, S. Production of High Density and Low Electron-Temperature Plasma by a Modified Grid-Biasing Method Using Inductively Coupled RF Discharge. Thin Solid Films 2004, 457, 55–58. [Google Scholar] [CrossRef]
- Gergs, T.; Monti, C.; Gaiser, S.; Amberg, M.; Schütz, U.; Mussenbrock, T.; Trieschmann, J.; Heuberger, M.; Hegemann, D. Nanoporous SiOx Plasma Polymer Films as Carrier for Liquid-Infused Surfaces. Plasma Process. Polym. 2022, 19, 2200049. [Google Scholar] [CrossRef]
- Primc, G. Recent Advances in Surface Activation of Polytetrafluoroethylene (PTFE) by Gaseous Plasma Treatments. Polymers 2020, 12, 2295. [Google Scholar] [CrossRef] [PubMed]
- Okubo, M. Enhanced Fluoropolymer Surface Adhesion by a Plasma Hybrid Process—Metal Plating Technology and Its Application to Millimeter-Wave Devices. In Plasma Modification of Polyolefins: Synthesis, Characterization and Applications; Baneesh, N.S., Sari, P.S., Vackova, T., Thomas, S., Eds.; Engineering Materials; Springer International Publishing: Cham, Switzerland, 2022; pp. 215–240. ISBN 978-3-030-52264-3. [Google Scholar]
- Yasuda, H.; Marsh, H.C.; Brandt, E.S.; Reilley, C.N. ESCA Study of Polymer Surfaces Treated by Plasma. J. Polym. Sci. Polym. Chem. Ed. 1977, 15, 991–1019. [Google Scholar] [CrossRef]
- Pachchigar, V.; Gaur, U.K.; Amrutha, T.V.; Sooraj, K.P.; Hans, S.; Srivastava, S.K.; Ranjan, M. Hydrophobic to Superhydrophobic and Hydrophilic Transitions of Ar Plasma-Nanostructured PTFE Surfaces. Plasma Process. Polym. 2022, 19, 2200037. [Google Scholar] [CrossRef]
- Zanini, S.; Barni, R.; Pergola, R.D.; Riccardi, C. Modification of the PTFE Wettability by Oxygen Plasma Treatments: Influence of the Operating Parameters and Investigation of the Ageing Behaviour. J. Phys. D Appl. Phys. 2014, 47, 325202. [Google Scholar] [CrossRef]
- Felix, T.; Soldi, V.; Debacher, N.A. Surface Modification and Hydrophobic Recovery (Aging) of Polyolefin Exposed to Plasma. In Plasma Modification of Polyolefins: Synthesis, Characterization and Applications; Baneesh, N.S., Sari, P.S., Vackova, T., Thomas, S., Eds.; Engineering Materials; Springer International Publishing: Cham, Switzerland, 2022; pp. 197–214. ISBN 978-3-030-52264-3. [Google Scholar]
- Hunke, H.; Soin, N.; Shah, T.H.; Kramer, E.; Pascual, A.; Karuna, M.S.L.; Siores, E. Low-Pressure H2, NH3 Microwave Plasma Treatment of Polytetrafluoroethylene (PTFE) Powders: Chemical, Thermal and Wettability Analysis. Materials 2015, 8, 2258–2275. [Google Scholar] [CrossRef]
- König, U.; Nitschke, M.; Pilz, M.; Simon, F.; Arnhold, C.; Werner, C. Stability and Ageing of Plasma Treated Poly(Tetrafluoroethylene) Surfaces. Colloids Surf. B Biointerfaces 2002, 25, 313–324. [Google Scholar] [CrossRef]
- Hai, W.; Hi, T.; Shimizu, K.; Yajima, T. Preparation of a Super Hydrophilic Polytetrafluoroethylene Surface Using a Gaseous Ammonia-Water Low-Temperature Plasma. J. Photopol. Sci. Technol. 2015, 28, 479–483. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Gu, Y.; Chen, B.; Wang, B.; Yan, J.; Liu, J.; Chen, F.; Zhao, D.; Liu, X. Improving Surface Wettability and Adhesion Property of Polytetrafluoroethylene by Atmospheric-Pressure Ammonia Water-Mixed Plasma Treatment. Vacuum 2022, 196, 110763. [Google Scholar] [CrossRef]
- Xie, X.; de los Arcos, T.; Grundmeier, G. Comparative Analysis of Hexamethyldisiloxane and Hexamethyldisilazane Plasma Polymer Thin Films before and after Plasma Oxidation. Plasma Process. Polym. 2022, 19, 2200052. [Google Scholar] [CrossRef]
- Hegemann, D.; Bülbül, E.; Hanselmann, B.; Schütz, U.; Amberg, M.; Gaiser, S. Plasma Polymerization of Hexamethyldisiloxane: Revisited. Plasma Process. Polym. 2021, 18, 2000176. [Google Scholar] [CrossRef]
- Hegemann, D.; Michlíček, M.; Blanchard, N.E.; Schütz, U.; Lohmann, D.; Vandenbossche, M.; Zajíčková, L.; Drábik, M. Deposition of Functional Plasma Polymers Influenced by Reactor Geometry in Capacitively Coupled Discharges. Plasma Process. Polym. 2016, 13, 279–286. [Google Scholar] [CrossRef]
- Piwowarczyk, J.; Jędrzejewski, R.; Moszyński, D.; Kwiatkowski, K.; Niemczyk, A.; Baranowska, J. XPS and FTIR Studies of Polytetrafluoroethylene Thin Films Obtained by Physical Methods. Polymers 2019, 11, 1629. [Google Scholar] [CrossRef]
- De Los Arcos, T.; Müller, H.; Wang, F.; Damerla, V.R.; Hoppe, C.; Weinberger, C.; Tiemann, M.; Grundmeier, G. Review of Infrared Spectroscopy Techniques for the Determination of Internal Structure in Thin SiO2 Films. Vib. Spectrosc. 2021, 114, 103256. [Google Scholar] [CrossRef]
- Hoppe, C.; Mitschker, F.; Awakowicz, P.; Kirchheim, D.; Dahlmann, R.; De Los Arcos, T.; Grundmeier, G. Adhesion of Plasma-Deposited Silicon Oxide Barrier Layers on PDMS Containing Polypropylene. Surf. Coat. Technol. 2018, 335, 25–31. [Google Scholar] [CrossRef]
- He, Y.; Jin, W.; Wang, Y.; Lv, S.; Wang, R.; Liu, J.; Liu, H. Electron Density and Electron Temperature Control with a Magnetic Field and a Grid in Inductively Coupled Argon Plasma. Plasma Chem. Plasma Process. 2023, 43, 381–400. [Google Scholar] [CrossRef]
- Trieschmann, J.; Hegemann, D. Plasma Polymerization at Different Positions in an Asymmetric Ethylene Discharge. J. Phys. D Appl. Phys. 2011, 44, 475201. [Google Scholar] [CrossRef]
- Bormashenko, E.; Whyman, G.; Multanen, V.; Shulzinger, E.; Chaniel, G. Physical Mechanisms of Interaction of Cold Plasma with Polymer Surfaces. J. Colloid Interface Sci. 2015, 448, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Lojen, D.; Zaplotnik, R.; Primc, G.; Mozetič, M.; Vesel, A. Effect of VUV Radiation and Reactive Hydrogen Atoms on Depletion of Fluorine from Polytetrafluoroethylene Surface. Appl. Surf. Sci. 2020, 533, 147356. [Google Scholar] [CrossRef]
- Rubner, J.; Stellmann, L.; Mertens, A.-K.; Tepper, M.; Roth, H.; Kleines, L.; Dahlmann, R.; Wessling, M. Organosilica Coating Layer Prevents Aging of a Polymer with Intrinsic Microporosity. Plasma Process. Polym. 2022, 19, 2200016. [Google Scholar] [CrossRef]
- Zhou, W.; Bailey, S.; Sooryakumar, R.; King, S.; Xu, G.; Mays, E.; Ege, C.; Bielefeld, J. Elastic Properties of Porous Low-k Dielectric Nano-Films. J. Appl. Phys. 2011, 110, 043520. [Google Scholar] [CrossRef]
- Lopaev, D.V.; Rakhlinsky, V.V.; Zyryanov, S.M.; Mankelevich, Y.A.; Rakhimova, T.V.; Kurchikov, K.A.; Baklanov, M.R. Photoabsorption and Damage of OSG Low-k Films by VUV Emission at 140–160 Nm. Plasma Process. Polym. 2018, 15, 1700166. [Google Scholar] [CrossRef]



| HMDSO/O2 | Power Deposition [W] | Power Etching [W] | Refractive Index/Porosity * |
|---|---|---|---|
| 4/40 | 100 | 100 | 1.416/20% |
| 100 | 300 | 1.418/20% | |
| 300 | 100 | 1.456/11% | |
| 300 | 300 | 1.443/14% | |
| 5/40 | 80 | 100 | 1.434/16% |
| 100 | 100 | 1.405/23% | |
| 125 | 100 | 1.412/21% | |
| 6/40 | 100 | 100 | 1.424/18% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navascués, P.; Schütz, U.; Hanselmann, B.; Hegemann, D. Near-Plasma Chemical Surface Engineering. Nanomaterials 2024, 14, 195. https://doi.org/10.3390/nano14020195
Navascués P, Schütz U, Hanselmann B, Hegemann D. Near-Plasma Chemical Surface Engineering. Nanomaterials. 2024; 14(2):195. https://doi.org/10.3390/nano14020195
Chicago/Turabian StyleNavascués, Paula, Urs Schütz, Barbara Hanselmann, and Dirk Hegemann. 2024. "Near-Plasma Chemical Surface Engineering" Nanomaterials 14, no. 2: 195. https://doi.org/10.3390/nano14020195





