Tissue Nanotransfection Silicon Chip and Related Electroporation-Based Technologies for In Vivo Tissue Reprogramming
Abstract
:1. Introduction
2. Microneedle-Based Electroporation for In Vivo Gene Transfer
2.1. Microneedle-Type Electrodes-Based Bulk Electroporation for In Vivo Gene Transfer
2.2. TNT for In Vivo Gene Transfer
3. Delivery Mechanisms of Microchip-Based In Vivo Gene Transfer
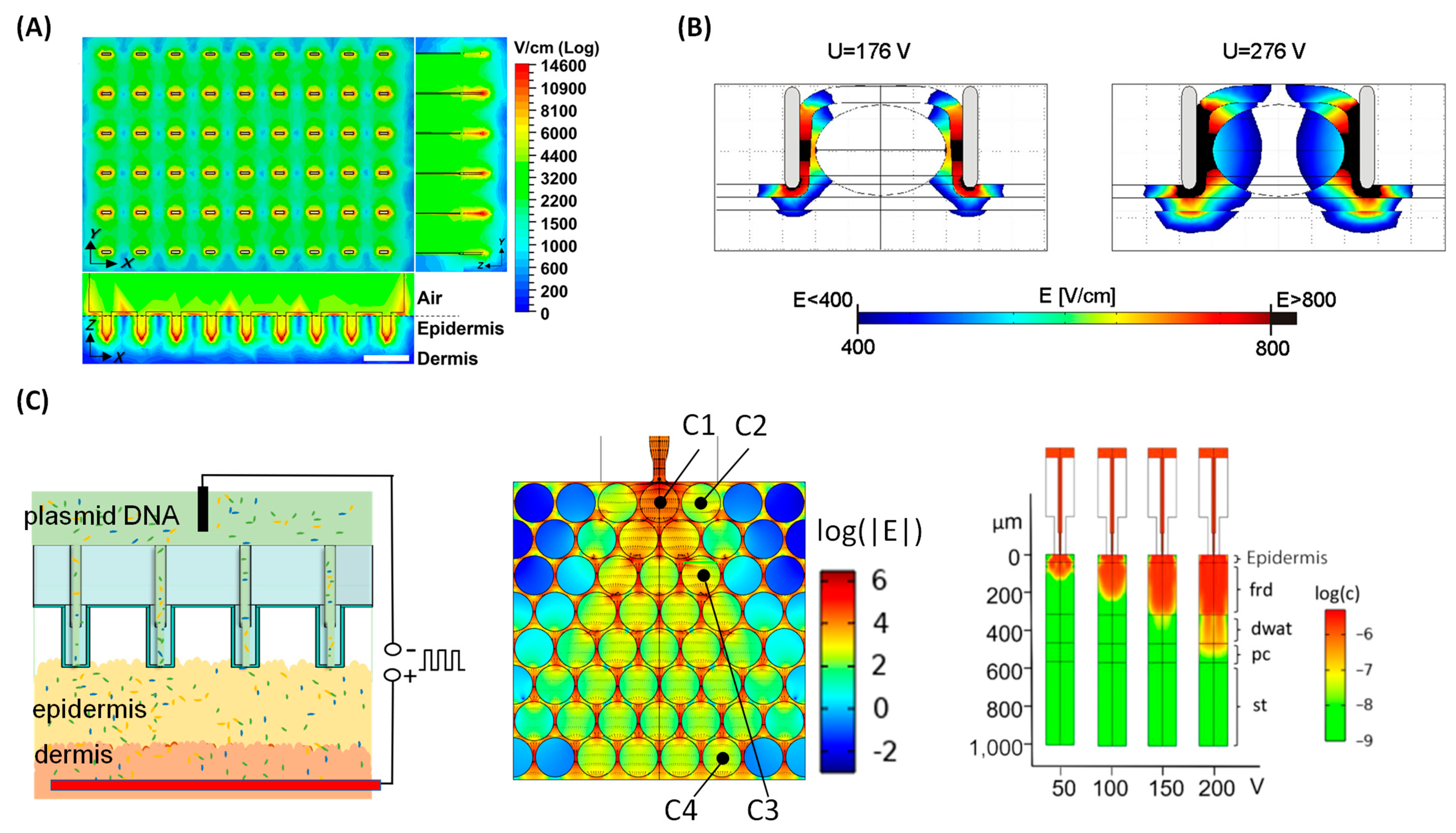
3.1. Bulk Electroporation for In Vivo Gene Transfer
3.2. Tissue Nanotransfection for In Vivo Gene Transfer
4. Fabrication of Microneedle Chips for Electroporation-Based In Vivo Gene Transfer
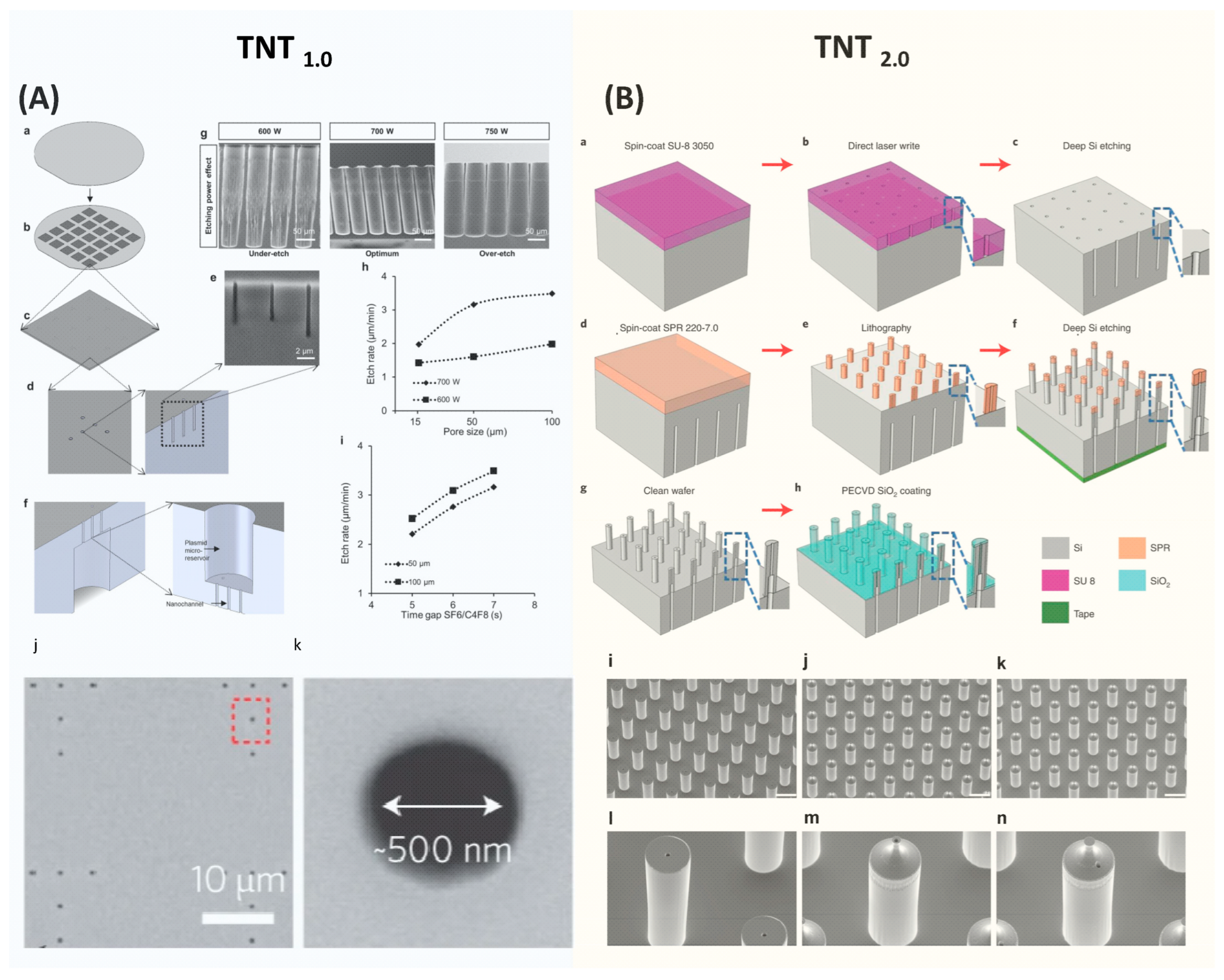
4.1. Fabrication of Microneedle-Type Electrodes-Based BEP Chips

4.2. Fabrication of TNT Si Chips
5. Applications of Microneedle-Based Electroporation Gene Transfer
6. Challenge and Future Prospects
6.1. Industrialization
6.2. Electroporation Protocol Optimization
6.3. Bulk Electroporation System
6.4. Clinical Translational
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Neumann, E.; Schaeferridder, M.; Wang, Y.; Hofschneider, P.H. Gene-Transfer into Mouse Lyoma Cells by Electroporation in High Electric-Fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.L.; Byrne, B.J.; Tung, L. Electroporation-mediated gene transfer in cardiac tissue. FEBS Lett. 1998, 435, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Tang, J.G.; Xie, X.L.; Yang, J.C.; Li, S.; Ji, J.G.; Gu, J. A comprehensive study of optimal conditions for naked plasmid DNA transfer into skeletal muscle by electroporation. J. Gene Med. 2005, 7, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.O.; Kim, Y.C.; Park, J.H.; Hutcheson, J.; Gill, H.S.; Yoon, Y.K.; Prausnitz, M.R.; Allen, M.G. An electrically active microneedle array for electroporation. Biomed. Microdevices 2010, 12, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.O.; Kim, Y.C.; Lee, J.W.; Park, J.H.; Prausnitz, M.R.; Allen, M.G. Intracellular Protein Delivery and Gene Transfection by Electroporation Using a Microneedle Electrode Array. Small 2012, 8, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.W.; Zheng, S.Q.; Wang, R.X.; Bu, X.L.; Ma, H.L.; Wu, Y.D.; Zhu, L.; Hu, Z.Y.; Liang, Z.C.; Li, Z.H. A flexible microneedle array as low-voltage electroporation electrodes for DNA and siRNA delivery. Lab Chip 2014, 14, 4093–4102. [Google Scholar] [CrossRef]
- Roy, S.; Sen, C.K.; Ghatak, S.; Higuita-Castro, N.; Palakurti, R.; Nalluri, N.; Clark, A.; Stewart, R.; Gallego-Perez, D.; Prater, D.N.; et al. Neurogenic tissue nanotransfection in the management of cutaneous diabetic polyneuropathy. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102220. [Google Scholar] [CrossRef]
- Xia, D.N.; Jin, R.; Byagathvalli, G.; Yu, H.; Ye, L.; Lu, C.Y.; Bhamla, M.S.; Yang, C.L.; Prausnitz, M.R. An ultra-low-cost electroporator with microneedle electrodes (ePatch) for SARS-CoV-2 vaccination. Proc. Natl. Acad. Sci. USA 2021, 118, e2110817118. [Google Scholar] [CrossRef]
- Yang, T.R.; Huang, D.; Li, C.H.; Zhao, D.Y.; Li, J.S.; Zhang, M.J.; Chen, Y.F.; Wang, Q.N.; Liang, Z.C.; Liang, X.J.; et al. Rolling microneedle electrode array (RoMEA) empowered nucleic acid delivery and cancer immunotherapy. Nano Today 2021, 36, 101017. [Google Scholar] [CrossRef]
- Gallego-Perez, D.; Pal, D.; Ghatak, S.; Malkoc, V.; Higuita-Castro, N.; Gnyawali, S.; Chang, L.Q.; Liao, W.C.; Shi, J.F.; Sinha, M.; et al. Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nat. Nanotechnol. 2017, 12, 974–979. [Google Scholar] [CrossRef]
- Wei, D.; Pu, N.; Li, S.Y.; Wang, Y.G.; Tao, Y. Application of iontophoresis in ophthalmic practice: An innovative strategy to deliver drugs into the eye. Drug Deliv. 2023, 30, 2165736. [Google Scholar] [CrossRef] [PubMed]
- Do, H.D.; Menager, C.; Michel, A.; Seguin, J.; Korichi, T.; Dhotel, H.; Marie, C.; Doan, B.T.; Mignet, N. Development of Theranostic Cationic Liposomes Designed for Image-Guided Delivery of Nucleic Acid. Pharmaceutics 2020, 12, 854. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.G.; Alam, U.; Tang, Q.; Shi, Y.D.; Zhang, Y.; Wang, R.; Lu, Z.L. Functional lipids based on [12]aneN3 and naphthalimide as efficient non-viral gene vectors. Org. Biomol. Chem. 2016, 14, 6346–6354. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, X.; Mo, J.; Wang, H.; Huang, Q.; Yang, C.; Zhang, T.; Chen, H.J.; Hang, T.; Liu, F.; et al. A Fully Integrated Closed-Loop System Based on Mesoporous Microneedles-Iontophoresis for Diabetes Treatment. Adv. Sci. 2021, 8, e2100827. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Brown, B.A.; Siegel, A.P.; El Masry, M.S.; Zeng, X.; Song, W.; Das, A.; Khandelwal, P.; Clark, A.; Singh, K.; et al. Exosome-Mediated Crosstalk between Keratinocytes and Macrophages in Cutaneous Wound Healing. ACS Nano 2020, 14, 12732–12748. [Google Scholar] [CrossRef]
- Clark, A.; Ghatak, S.; Guda, P.R.; El Masry, M.S.; Xuan, Y.; Sato, A.Y.; Bellido, T.; Sen, C.K. Myogenic tissue nanotransfection improves muscle torque recovery following volumetric muscle loss. npj Regen. Med. 2022, 7, 63. [Google Scholar] [CrossRef]
- Briz, P.; López-Alonso, B.; Sarnago, H.; Burdío, J.M.; Lucía, O. Tumor location on electroporation therapies by means of multi-electrode structures and machine learning. Bioelectrochemistry 2023, 154, 108510. [Google Scholar] [CrossRef]
- Ghatak, S.; Khanna, S.; Roy, S.; Thirunavukkarasu, M.; Pradeep, S.R.; Wulff, B.C.; El Masry, M.S.; Sharma, A.; Palakurti, R.; Ghosh, N.; et al. Driving adult tissue repair via re-engagement of a pathway required for fetal healing. Mol. Ther. 2023, 31, 454–470. [Google Scholar] [CrossRef]
- Gordillo, G.M.; Guda, P.R.; Singh, K.; Biswas, A.; Abouhashem, A.S.; Rustagi, Y.; Sen, A.; Kumar, M.; Das, A.; Ghatak, S.; et al. Tissue nanotransfection causes tumor regression by its effect on nanovesicle cargo that alters microenvironmental macrophage state. Mol. Ther. 2023, 31, 1402–1417. [Google Scholar] [CrossRef]
- Singh, K.; Rustagi, Y.; Abouhashem, A.S.; Tabasum, S.; Verma, P.; Hernandez, E.; Pal, D.; Khona, D.K.; Mohanty, S.K.; Kumar, M.; et al. Genome-wide DNA hypermethylation opposes healing in patients with chronic wounds by impairing epithelial-mesenchymal transition. J. Clin. Investig. 2022, 132, 157279. [Google Scholar] [CrossRef]
- Pal, D.; Ghatak, S.; Singh, K.; Abouhashem, A.S.; Kumar, M.; El Masry, M.S.; Mohanty, S.K.; Palakurti, R.; Rustagi, Y.; Tabasum, S.; et al. Identification of a physiologic vasculogenic fibroblast state to achieve tissue repair. Nat. Commun. 2023, 14, 1129. [Google Scholar] [CrossRef] [PubMed]
- Rustagi, Y.; Abouhashem, A.S.; Verma, P.; Verma, S.S.; Hernandez, E.; Liu, S.; Kumar, M.; Guda, P.R.; Srivastava, R.; Mohanty, S.K.; et al. Endothelial Phospholipase Cgamma2 Improves Outcomes of Diabetic Ischemic Limb Rescue Following VEGF Therapy. Diabetes 2022, 71, 1149–1165. [Google Scholar] [CrossRef] [PubMed]
- Abrbekoh, F.N.; Salimi, L.; Saghati, S.; Amini, H.; Karkan, S.F.; Moharamzadeh, K.; Sokullu, E.; Rahbarghazi, R. Application of microneedle patches for drug delivery; doorstep to novel therapies. J. Tissue Eng. 2022, 13, 20417314221085390. [Google Scholar]
- Liu, G.S.; Kong, Y.F.; Wang, Y.S.; Luo, Y.H.; Fan, X.D.; Xie, X.; Yang, B.R.; Wu, M.X. Microneedles for transdermal diagnostics: Recent advances and new horizons. Biomaterials 2020, 232, 119740. [Google Scholar] [CrossRef] [PubMed]
- Erdem, Ö.; Es, I.; Akceoglu, G.A.; Saylan, Y.; Inci, F. Recent Advances in Microneedle-Based Sensors for Sampling, Diagnosis and Monitoring of Chronic Diseases. Biosensors 2021, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Potocnik, T.; Rems, L.; Miklavcic, D. Revisiting the role of pulsed electric fields in overcoming the barriers to gene electrotransfer. Bioelectrochemistry 2022, 144, 107994. [Google Scholar] [CrossRef] [PubMed]
- Lemmerman, L.R.; Balch, M.H.H.; Moore, J.T.; Alzate-Correa, D.; Rincon-Benavides, M.A.; Salazar-Puerta, A.; Gnyawali, S.; Harris, H.N.; Lawrence, W.; Ortega-Pineda, L.; et al. Nanotransfection-based vasculogenic cell reprogramming drives functional recovery in a mouse model of ischemic stroke. Sci. Adv. 2021, 7, abd4735. [Google Scholar] [CrossRef]
- Toner, M.; Cravalho, E.G. Modeling of Electroporation in Membranes by the Nucleation Theory in Condensed Systems. Biophys. J. 1990, 57, A76. [Google Scholar]
- Tsong, T.Y. On Electroporation of Cell-Membranes and Some Related Phenomena. Bioelectrochem. Bioenerg. 1990, 24, 271–295. [Google Scholar] [CrossRef]
- Barnett, A.; Weaver, J.C. Electroporation—A Unified, Quantitative Theory of Reversible Electrical Breakdown and Mechanical Rupture in Artificial Planar Bilayer-Membranes. Bioelectrochem. Bioenerg. 1991, 25, 163–182. [Google Scholar] [CrossRef]
- Weaver, J.C. Theory of Electroporation. Biomembr. Electrochem. 1994, 235, 447–470. [Google Scholar]
- Weaver, J.C.; Chizmadzhev, Y.A. Theory of electroporation: A review. Bioelectrochem. Bioenerg. 1996, 41, 135–160. [Google Scholar] [CrossRef]
- Neu, J.C.; Krassowska, W. Asymptotic model of electroporation. Phys. Rev. E 1999, 59, 3471–3482. [Google Scholar] [CrossRef]
- Pucihar, G.; Miklavcic, D.; Kotnik, T. A Time-Dependent Numerical Model of Transmembrane Voltage Inducement and Electroporation of Irregularly Shaped Cells. IEEE Trans. Biomed. Eng. 2009, 56, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, T.; Kramar, P.; Pucihar, G.; Miklavcic, D.; Tarek, M. Cell Membrane Electroporation-Part 1: The Phenomenon. IEEE Electr. Insul. Mag. 2012, 28, 14–23. [Google Scholar] [CrossRef]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavcic, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.C.; Neu, J.C.; Krassowska, W. Model of creation and evolution of stable electropores for DNA delivery. Biophys. J. 2004, 86, 2813–2826. [Google Scholar] [CrossRef]
- Upadhyay, P. Electroporation of the skin to deliver antigen by using a piezo ceramic gas igniter. Int. J. Pharm. 2001, 217, 249–253. [Google Scholar] [CrossRef]
- Schwan, H.P. Electrical properties of tissue and cell suspensions. Adv. Biol. Med. Phys. 1957, 5, 147–209. [Google Scholar]
- Kotnik, T.; Miklavcic, D. Analytical description of transmembrane voltage induced by electric fields on spheroidal cells. Biophys. J. 2000, 79, 670–679. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, D.Y.; Wang, X.X.; Li, C.H.; Yang, T.R.; Du, L.L.; Wei, Z.W.; Cheng, Q.; Cao, H.Q.; Liang, Z.C.; et al. Efficient delivery of nucleic acid molecules into skin by combined use of microneedle roller and flexible interdigitated electroporation array. Theranostics 2018, 8, 2361–2376. [Google Scholar] [CrossRef]
- Miklavcic, D.; Semrov, D.; Mekid, H.; Mir, L.M. A validated model of in vivo electric field distribution in tissues for electrochemotherapy and for DNA electrotransfer for gene therapy. Biochim. Biophys. Acta Gen. Subj. 2000, 1523, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Ma, O.; Zhang, M.J. Dynamics Modeling and Control of Electroporation-Mediated Gene Delivery. IEEE Trans. Autom. Sci. Eng. 2009, 6, 228–238. [Google Scholar] [CrossRef]
- Li, Z.; Xuan, Y.; Ghatak, S.; Guda, P.R.; Roy, S.; Sen, C.K. Modeling the gene delivery process of the needle array-based tissue nanotransfection. Nano Res. 2022, 15, 3409–3421. [Google Scholar] [CrossRef]
- Corovic, S.; Lackovic, I.; Sustaric, P.; Sustar, T.; Rodic, T.; Miklavcic, D. Modeling of electric field distribution in tissues during electroporation. Biomed. Eng. Online 2013, 12, 16. [Google Scholar] [CrossRef]
- Forjanic, T.; Markelc, B.; Marcan, M.; Bellard, E.; Couillaud, F.; Golzio, M.; Miklavcic, D. Electroporation-Induced Stress Response and Its Effect on Gene Electrotransfer Efficacy: Imaging and Numerical Modeling. IEEE Trans. Biomed. Eng. 2019, 66, 2671–2683. [Google Scholar] [CrossRef] [PubMed]
- Zupanic, A.; Kos, B.; Miklavcic, D. Treatment planning of electroporation-based medical interventions: Electrochemotherapy, gene electrotransfer and irreversible electroporation. Phys. Med. Biol. 2012, 57, 5425–5440. [Google Scholar] [CrossRef] [PubMed]
- Granot, Y.; Rubinsky, B. Mass Transfer Model for Drug Delivery in Tissue Cells with Reversible Electroporation. Int. J. Heat Mass Transf. 2008, 51, 5610–5616. [Google Scholar] [CrossRef]
- Mahnic-Kalamiza, S.; Miklavcic, D.; Vorobiev, E. Dual-porosity model of solute diffusion in biological tissue modified by electroporation. Biochim. Biophys. Acta 2014, 1838, 1950–1966. [Google Scholar] [CrossRef]
- Krassowska, W.; Filev, P.D. Modeling electroporation in a single cell. Biophys. J. 2007, 92, 404–417. [Google Scholar] [CrossRef]
- Qiu, B.L.; Gong, L.Y.; Li, Z.R.; Han, J. Electrokinetic flow in the U-shaped micro-nanochannels. Theor. Appl. Mech. Lett. 2019, 9, 36–42. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Dave, K.; Venuganti, V.V.K. Microneedles in the clinic. J. Control. Release 2017, 260, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, D.F.; Xuan, X.; He, H.C. Advances of Microneedles in Biomedical Applications. Molecules 2021, 26, 5912. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mat. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Sharma, S.; Hatware, K.; Bhadane, P.; Sindhikar, S.; Mishra, D.K. Recent advances in microneedle composites for biomedical applications: Advanced drug delivery technologies. Mat. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109717. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Microneedles: A New Frontier in Nanomedicine Delivery. Pharm. Res. 2016, 33, 1055–1073. [Google Scholar] [CrossRef]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. Macromol. Biosci. 2021, 21, 2000307. [Google Scholar] [CrossRef]
- Tucak, A.; Sirbubalo, M.; Hindija, L.; Rahic, O.; Hadziabdic, J.; Muhamedagic, K.; Cekic, A.; Vranic, E. Microneedles: Characteristics, Materials, Production Methods and Commercial Development. Micromachines 2020, 11, 961. [Google Scholar] [CrossRef]
- Liu, B.; Yi, X.; Zheng, Y.; Yuan, Z.S.; Yang, J.B.; Yang, J.; Yu, X.; Jiang, L.L.; Wang, C.Y. A Review of Nano/Micro/Milli Needles Fabrications for Biomedical Engineering. Chin. J. Mech. Eng. 2022, 35, 1–29. [Google Scholar] [CrossRef]
- Mo, J.; Liu, J.; Huang, S.; Liang, B.; Huang, X.; Yang, C.; Chen, M.; Liu, J.; Zhang, T.; Xie, X.; et al. Determination of Transdermal Rate of Metallic Microneedle Array through an Impedance Measurements-Based Numerical Check Screening Algorithm. Micromachines 2022, 13, 718. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Ghatak, S.; Clark, A.; Li, Z.G.; Khanna, S.; Pak, D.; Agarwal, M.; Roy, S.; Duda, P.; Sen, C.K. Fabrication and use of silicon hollow-needle arrays to achieve tissue nanotransfection in mouse tissue in vivo. Nat. Protoc. 2021, 16, 5707–5738. [Google Scholar] [CrossRef] [PubMed]
- Wilke, N.; Mulcahy, A.; Ye, S.R.; Morrissey, A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectron. J. 2005, 36, 650–656. [Google Scholar] [CrossRef]
- Held, J.; Gaspar, J.; Ruther, P.; Hagner, M.; Cismak, A.; Heilmann, A.; Paul, O. Design of experiment characterization of microneedle fabrication processes based on dry silicon etching. J. Micromech. Microeng. 2010, 20, 025024. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef]
- Badnikar, K.; Jayadevi, S.N.; Pahal, S.; Sripada, S.; Nayak, M.M.; Vemula, P.K.; Subrahmanyam, D.N. Generic Molding Platform for Simple, Low-Cost Fabrication of Polymeric Microneedles. Macromol. Mater. Eng. 2020, 305, 2000072. [Google Scholar] [CrossRef]
- Rad, Z.F.; Prewett, P.D.; Davies, G.J. An overview of microneedle applications, materials, and fabrication methods. Beilstein J. Nanotech. 2021, 12, 1034–1046. [Google Scholar]
- Ji, J.; Tay, F.E.; Miao, J.; Iliescu, C. Microfabricated Silicon Microneedle Array for Transdermal Drug Delivery; IOP Publishing: Bristol, UK, 2006. [Google Scholar]
- Huff, M. Recent Advances in Reactive Ion Etching and Applications of High-Aspect-Ratio Microfabrication. Micromachines 2021, 12, 991. [Google Scholar] [CrossRef]
- Gottscho, R.A.; Jurgensen, C.W.; Vitkavage, D.J. Microscopic uniformity in plasma etching. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1992, 10, 2133–2147. [Google Scholar] [CrossRef]
- Blauw, M.A.; Zijlstra, T.; Bakker, R.A.; van der Drift, E. Kinetics and crystal orientation dependence in high aspect ratio silicon dry etching. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2000, 18, 3453–3461. [Google Scholar] [CrossRef]
- Coburn, J.W.; Winters, H.F. Conductance considerations in the reactive ion etching of high aspect ratio features. Appl. Phys. Lett. 1989, 55, 2730–2732. [Google Scholar] [CrossRef]
- Lai, S.L.; Johnson, D.; Westerman, R. Aspect ratio dependent etching lag reduction in deep silicon etch processes. J. Vac. Sci. Technol. A 2006, 24, 1283–1288. [Google Scholar] [CrossRef]
- Tang, Y.; Sandoughsaz, A.; Owen, K.J.; Najafi, K. Ultra deep reactive ion etching of high aspect-ratio and thick silicon using a ramped-parameter process. J. Microelectromech. Syst. 2018, 27, 686–697. [Google Scholar] [CrossRef]
- Wilke, N.; Hibert, C.; O’Brien, J.; Morrissey, A. Silicon microneedle electrode array with temperature monitoring for electroporation. Sens. Actuators A Phys. 2005, 123–124, 319–325. [Google Scholar] [CrossRef]
- Moore, J.T.; Wier, C.G.; Lemmerman, L.R.; Ortega-Pineda, L.; Dodd, D.J.; Lawrence, W.R.; Duarte-Sanmiguel, S.; Dathathreya, K.; Diaz-Starokozheva, L.; Harris, H.N.; et al. Nanochannel-Based Poration Drives Benign and Effective Nonviral Gene Delivery to Peripheral Nerve Tissue. Adv. Biosyst. 2020, 4, e2000157. [Google Scholar] [CrossRef]
- Brown, B.A.; Guda, P.R.; Zeng, X.; Anthony, A.; Couse, A.; Barnes, L.F.; Sharon, E.M.; Trinidad, J.C.; Sen, C.K.; Jarrold, M.F.; et al. Analysis of Keratinocytic Exosomes from Diabetic and Nondiabetic Mice by Charge Detection Mass Spectrometry. Anal. Chem. 2022, 94, 8909–8918. [Google Scholar] [CrossRef]
- Diaz-Starokozheva, L.; Das, D.; Gu, X.; Moore, J.T.; Lemmerman, L.R.; Valerio, I.; Powell, H.M.; Higuita-Castro, N.; Go, M.R.; Palmer, A.F.; et al. Early Intervention in Ischemic Tissue with Oxygen Nanocarriers Enables Successful Implementation of Restorative Cell Therapies. Cell Mol. Bioeng. 2020, 13, 435–446. [Google Scholar] [CrossRef]
- Duarte-Sanmiguel, S.; Salazar-Puerta, A.I.; Panic, A.; Dodd, D.; Francis, C.; Alzate-Correa, D.; Ortega-Pineda, L.; Lemmerman, L.; Rincon-Benavides, M.A.; Dathathreya, K.; et al. ICAM-1-decorated extracellular vesicles loaded with miR-146a and Glut1 drive immunomodulation and hinder tumor progression in a murine model of breast cancer. Biomater. Sci. 2023, 11, 6834–6847. [Google Scholar] [CrossRef]
- Hassanein, A.H.; Sinha, M.; Neumann, C.R.; Mohan, G.; Khan, I.; Sen, C.K. A Murine Tail Lymphedema Model. J. Vis. Exp. 2021, 168, e61848. [Google Scholar]
- Davalos, R.V.; Mir, I.L.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223–231. [Google Scholar] [CrossRef]
- Murugesan, M.; Mori, K.; Bea, J.C.; Koyanagi, M.; Fukushima, T. High aspect ratio through-silicon-via formation by using low-cost electroless-Ni as barrier and seed layers for 3D-LSI integration and packaging applications. Jpn. J. Appl. Phys. 2020, 59, SGGC02. [Google Scholar] [CrossRef]
- Kim, S.K.; Popovici, M. Future of dynamic random-access memory as main memory. Mrs. Bull. 2018, 43, 334–339. [Google Scholar] [CrossRef]
- Zappatore, M.; Cerfeda, G.; Merla, C.; Tarricone, L. Machine Learning for H-FIRE Protocols. IEEE Microw. Magn. 2021, 22, 42–59. [Google Scholar] [CrossRef]
- Rems, L.; Tang, X.R.; Zhao, F.W.; Pérez-Conesa, S.; Testa, I.; Delemotte, L. Identification of electroporation sites in the complex lipid organization of the plasma membrane. eLife 2022, 11, e74773. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Patino, C.A.; Pathak, N.; Lemaitre, V.; Espinosa, H.D. Deep Learning-Assisted Automated Single Cell Electroporation Platform for Effective Genetic Manipulation of Hard-to-Transfect Cells. Small 2022, 18, 202107795. [Google Scholar] [CrossRef] [PubMed]
- Byagathvalli, G.; Sinha, S.; Zhang, Y.; Styczynski, M.P.; Standeven, J.; Bhamla, M.S. ElectroPen: An ultra-low-cost, electricity-free, portable electroporator. PLoS Biol. 2020, 18, 3000589. [Google Scholar] [CrossRef]
- Amante, D.H.; Smith, T.R.F.; Mendoza, J.M.; Schultheis, K.; McCoy, J.R.; Khan, A.S.; Sardesai, N.Y.; Broderick, K.E. Skin Transfection Patterns and Expression Kinetics of Electroporation-Enhanced Plasmid Delivery Using the CELLECTRA-3P, a Portable Next-Generation Dermal Electroporation Device. Hum. Gene Ther. Method 2015, 26, 134–146. [Google Scholar] [CrossRef]
- Ching, C.T.S.; Sun, T.P.; Huang, W.T.; Huang, S.H.; Hsiao, C.S.; Chang, K.M. A circuit design of a low-cost, portable and programmable electroporation device for biomedical applications. Sens. Actuators B Chem. 2012, 166, 292–300. [Google Scholar] [CrossRef]
- Schmitt, M.A.; Friedrich, O.; Gilbert, D.F. Portoporator®: A portable low-cost electroporation device for gene transfer to cultured cells in biotechnology, biomedical research and education. Biosens. Bioelectron. 2019, 131, 95–103. [Google Scholar] [CrossRef]
- Clinical Trials Website. Available online: https://clinicaltrials.gov/ (accessed on 14 January 2024).


| Applications | Target | Function of TNT | Reagent | Ref |
|---|---|---|---|---|
| Wound Healing | Mechanism | Fibroblast state change | Anti-miR-200b | [21] |
| Rescue muscle loss | Fibroblast into myogenic cells | MyoD | [16] | |
| Rescue Necrotizing Tissues | Fibroblast into neuronal Cells and Fibroblast into endothelial cells | Ascl1, Brn2 and Myt1l Etv2, Foxc2 and Fli1 | [10] | |
| Wound Closure | Significant Acceleration in wound recovery | LNA-anti-pan-miR-29 [18], Etv2, Foxc2 and Fli1 [76] | [18,76] | |
| Exosome | Exosome labeling for wound mechanism study | CD9, CD63 and CD81 | [15,77] | |
| Diabetes | Management of cutaneous Diabetic Polyneuropathy | Fibroblast into neuronal cells | Ascl1, Brn2 and Myt1l | [7] |
| Diabetic Ischemic Limb Rescue | effective in limb rescue | 2 | [22] | |
| Ischemic Diseases | Ischemic Tissue | Fibroblast into vasculogenic cells | Etv2, Foxc2 and Fli1 | [27,78] |
| Cancer | Breast Cancer | Decrease in Tumor Growth | EV with ICAM-1+miR-146a and Glut1 [79] | [79] |
| Tumor | Tumor regression | tumor-originating EV-borne angio-miR [19] | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, Y.; Wang, C.; Ghatak, S.; Sen, C.K. Tissue Nanotransfection Silicon Chip and Related Electroporation-Based Technologies for In Vivo Tissue Reprogramming. Nanomaterials 2024, 14, 217. https://doi.org/10.3390/nano14020217
Xuan Y, Wang C, Ghatak S, Sen CK. Tissue Nanotransfection Silicon Chip and Related Electroporation-Based Technologies for In Vivo Tissue Reprogramming. Nanomaterials. 2024; 14(2):217. https://doi.org/10.3390/nano14020217
Chicago/Turabian StyleXuan, Yi, Cong Wang, Subhadip Ghatak, and Chandan K. Sen. 2024. "Tissue Nanotransfection Silicon Chip and Related Electroporation-Based Technologies for In Vivo Tissue Reprogramming" Nanomaterials 14, no. 2: 217. https://doi.org/10.3390/nano14020217





