Titanium Dioxide Nanoparticles Doped with Iron for Water Treatment via Photocatalysis: A Review
Abstract
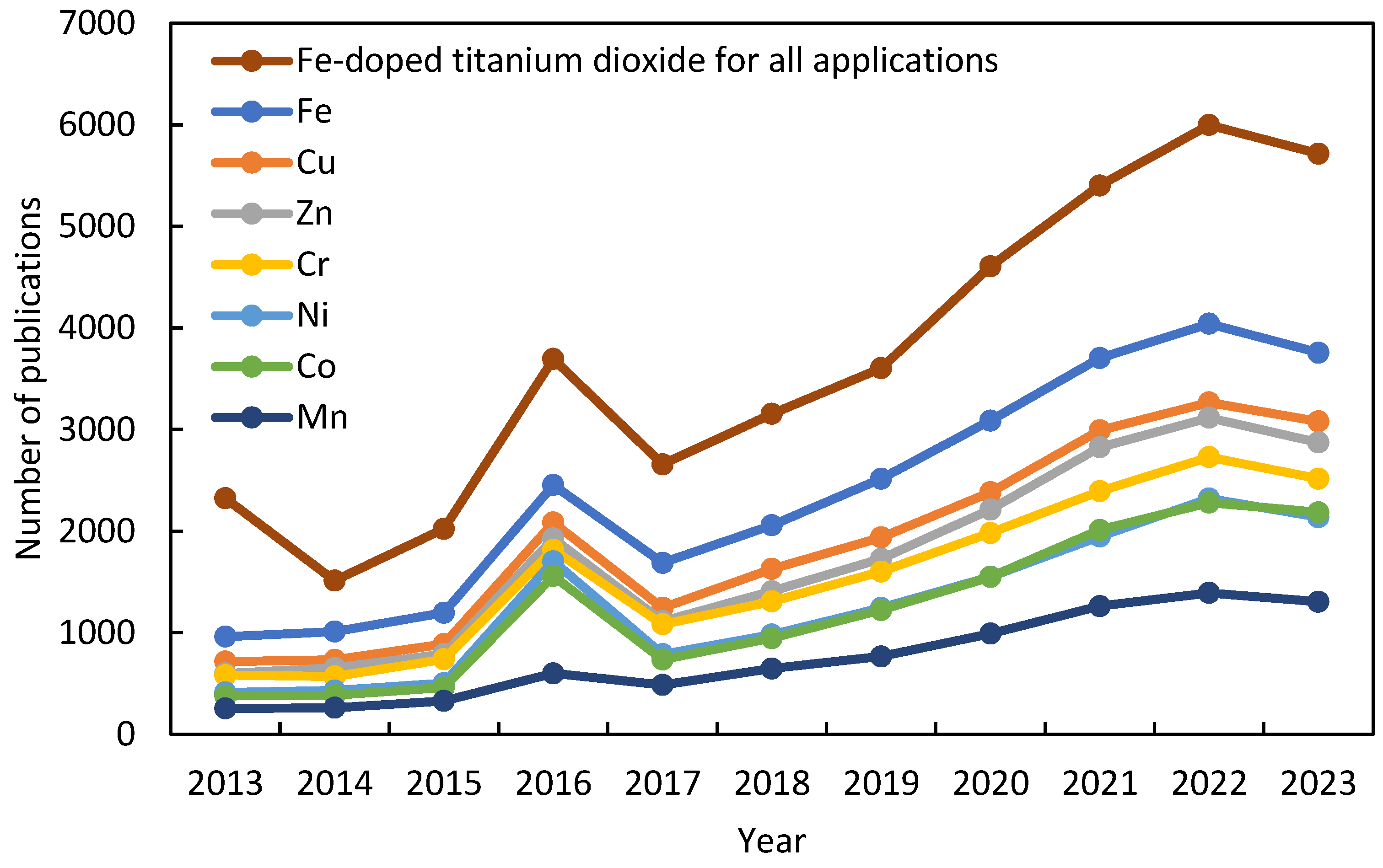
:1. Introduction
2. Advanced Photocatalyst
3. Modification of Titanium Dioxide by Iron
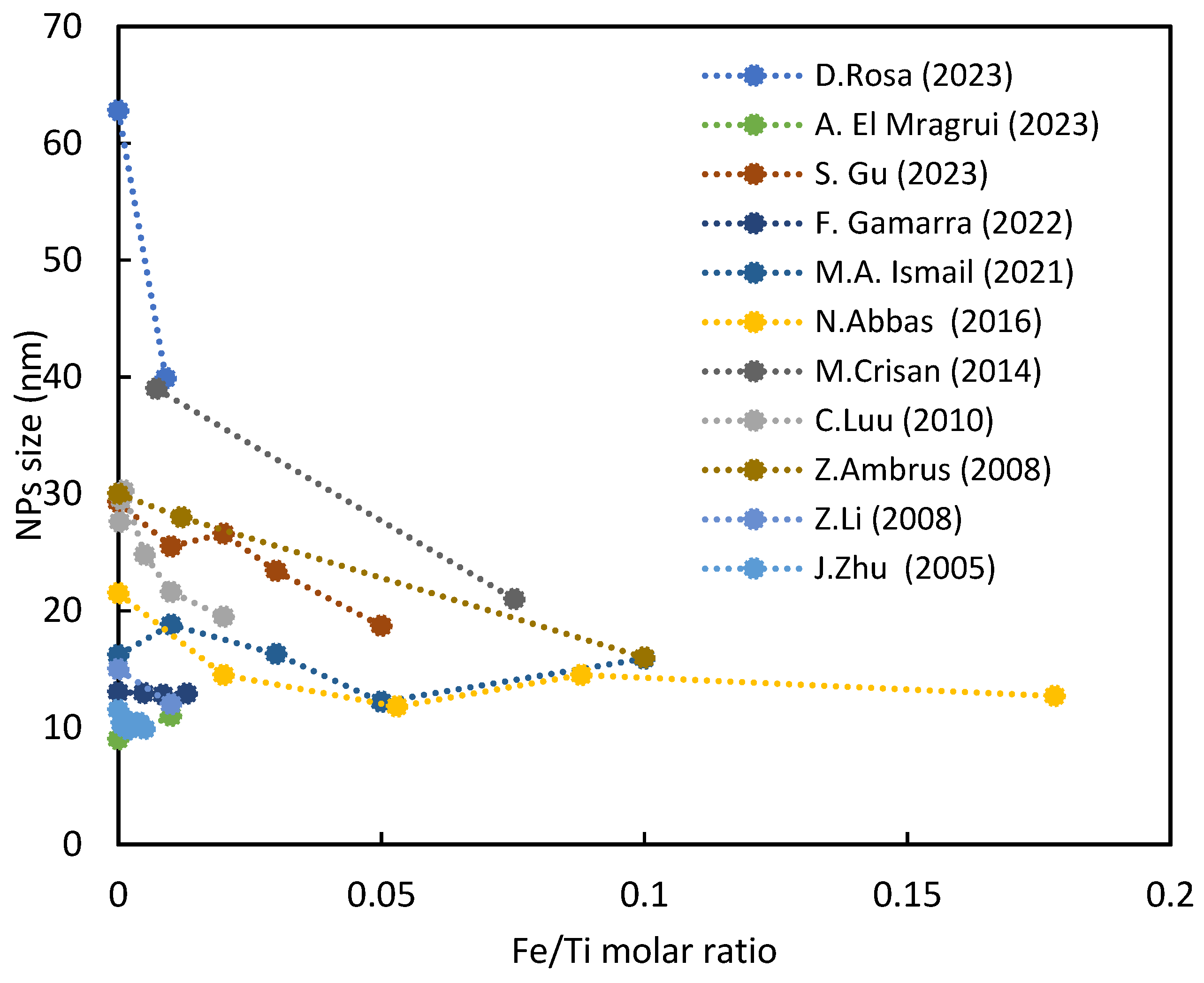
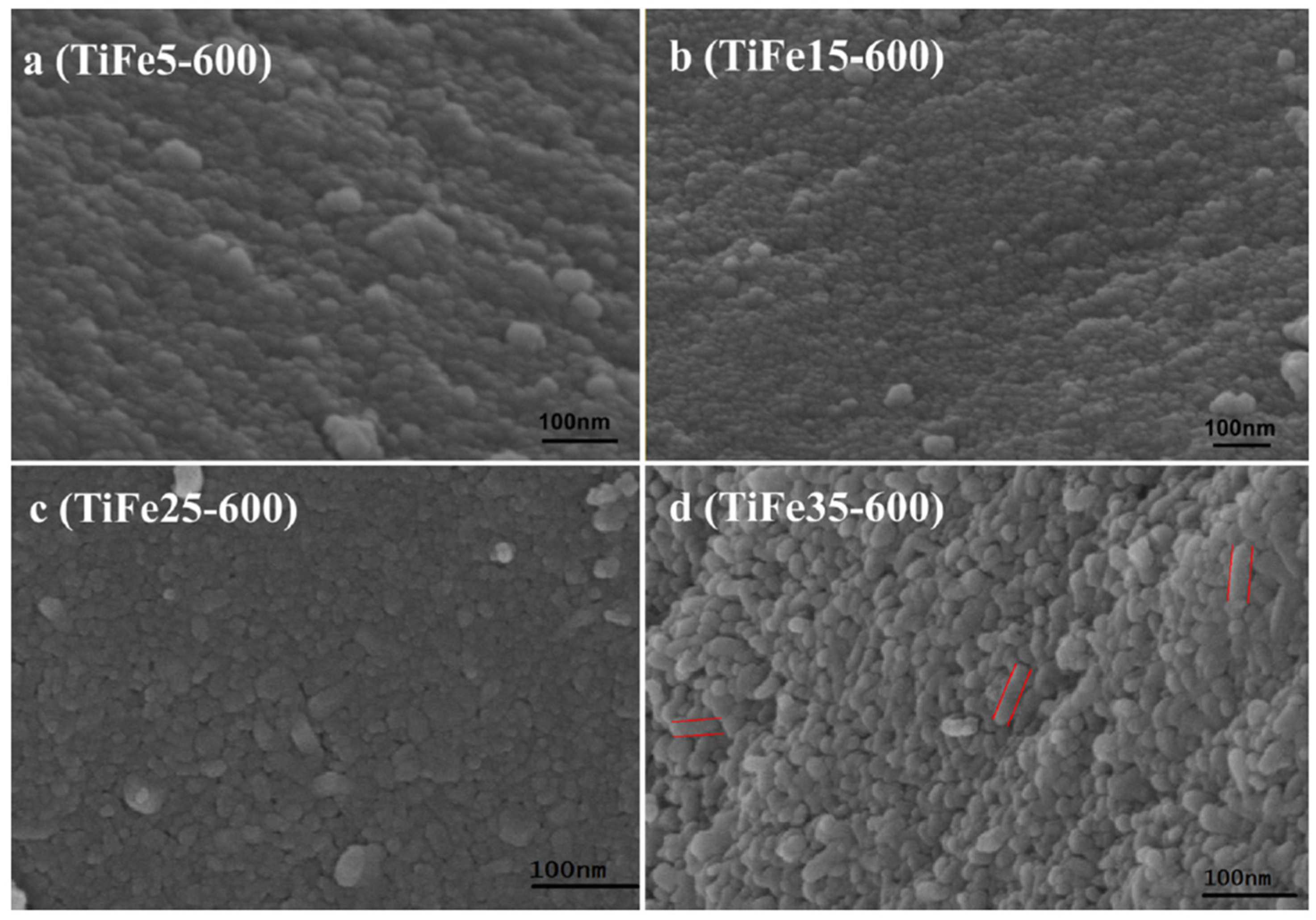
3.1. The Size of Nanoparticles
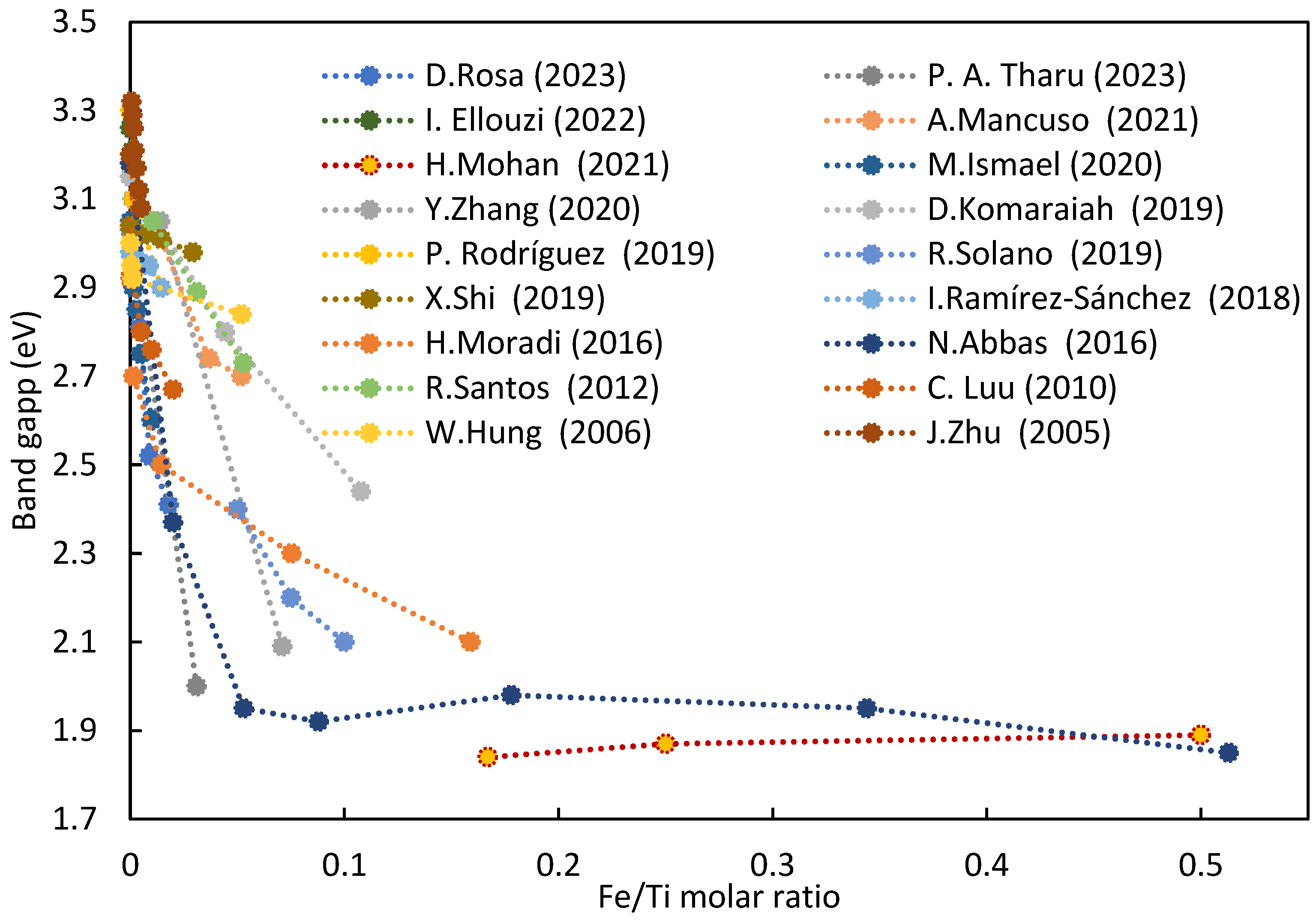
3.2. Band Gap of Semiconductor
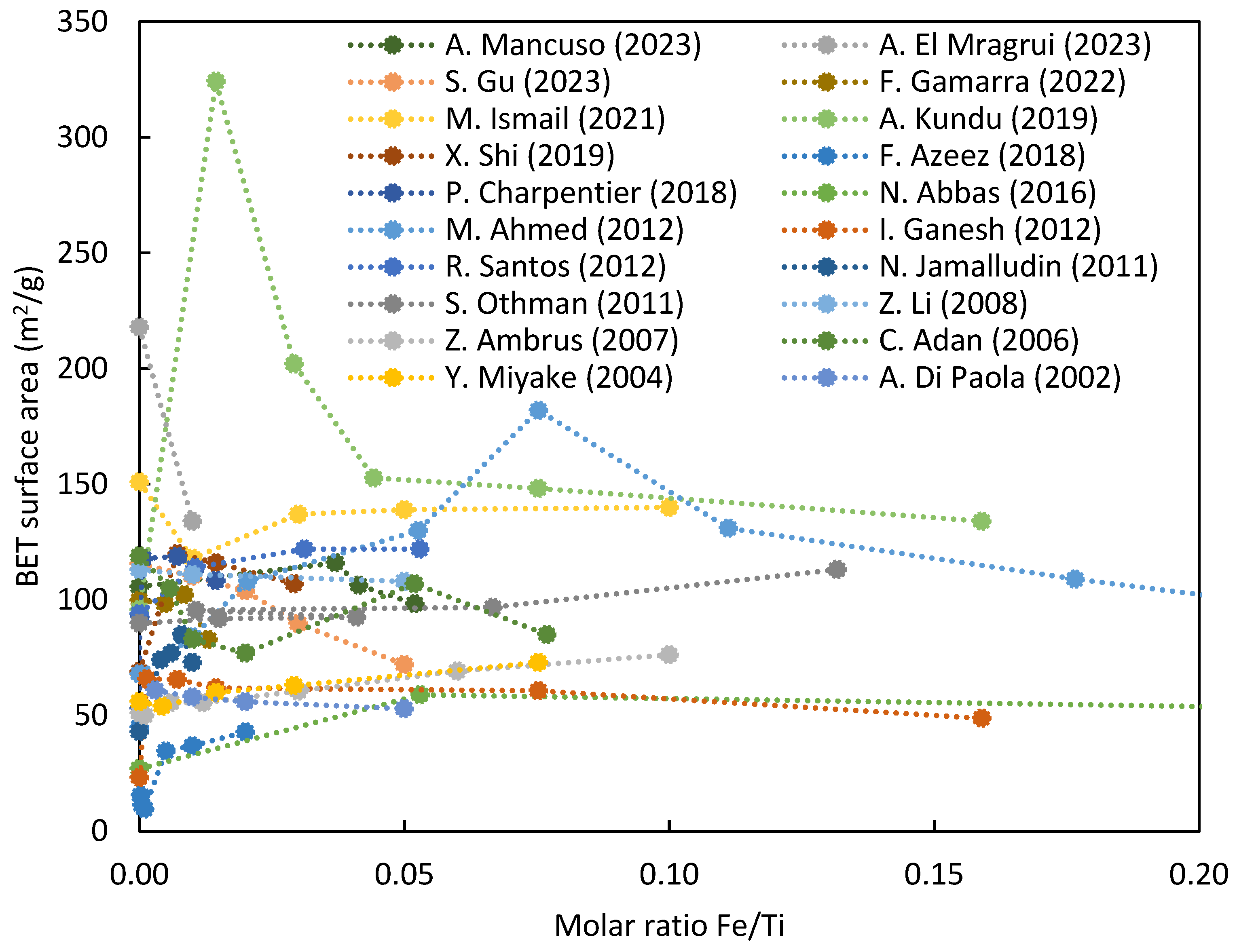
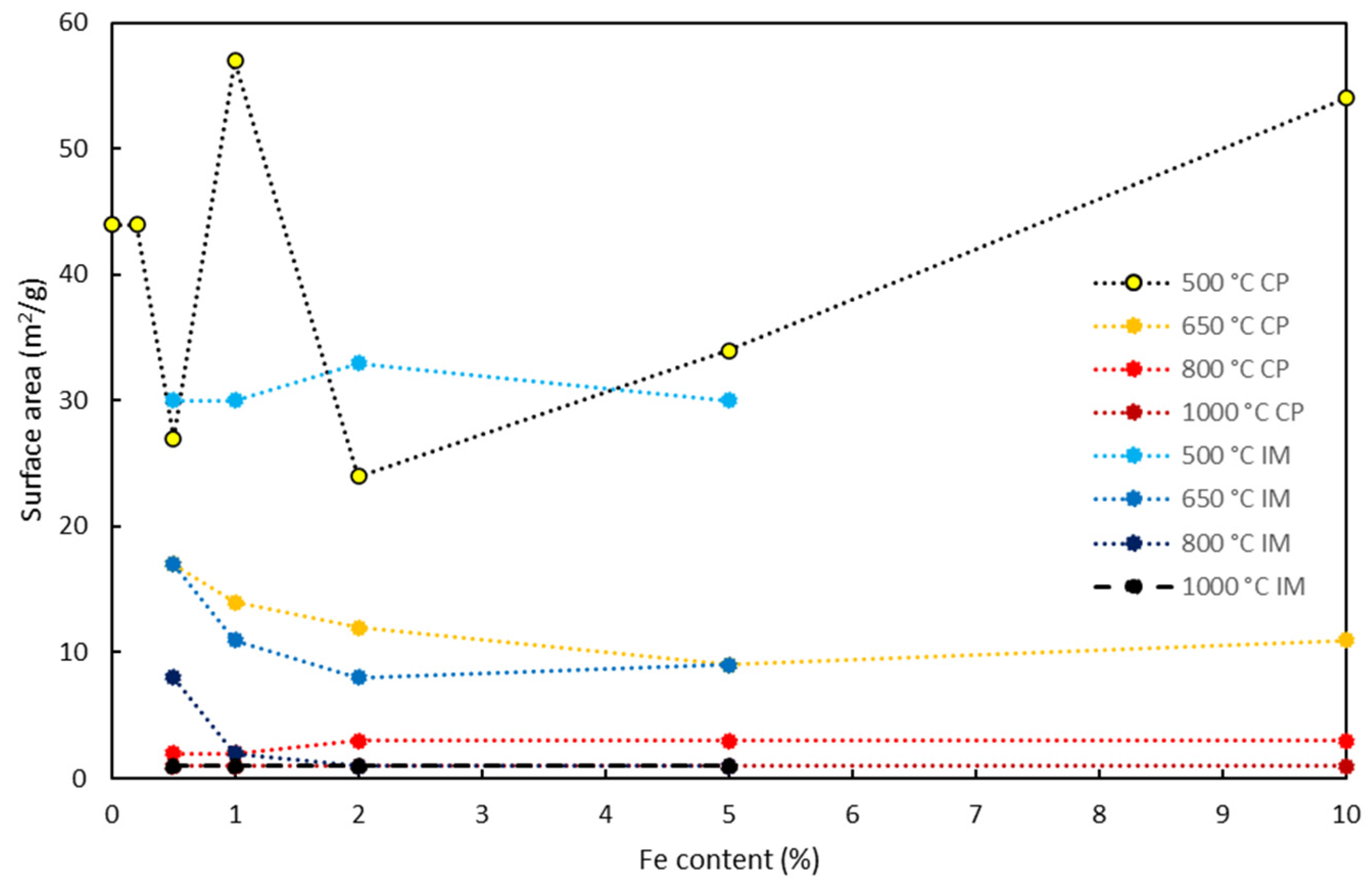
3.3. Surface Area
3.4. Anatase to Rutile Transition
3.5. Charge Recombination
3.6. pHZC
3.7. Photocatalytic Performance
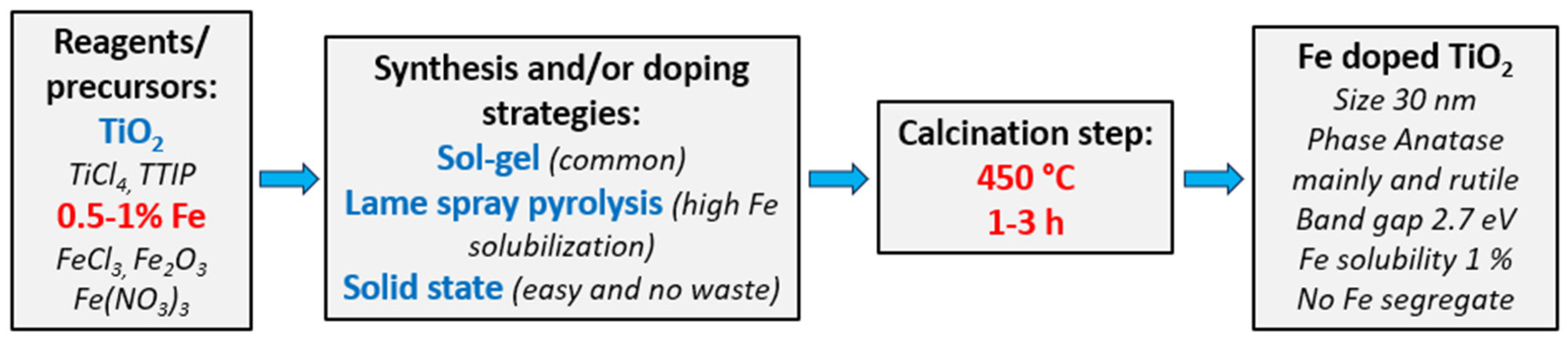
4. A Possible Optimized Synthesis of Nanoparticles Based on Iron-Doped TiO2
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mazzeo, L.; Rosa, D.; Bavasso, I.; Di Palma, L. Entrapped zinc oxide and titania nanoparticles in calcium alginate beads for the removal of Methylene Blue (MB): Adsorption properties and photocatalytic stability. Chem. Eng. Trans. 2021, 84, 181–186. [Google Scholar]
- Ajmal, S.; Kumar, A.; Tabish, M.; Selvaraj, M.; Alam, M.M.; Mushtaq, M.A.; Zhao, J.; Owusu, K.A.; Saad, A.; Nazir, M.T.; et al. Modulating the microenvironment of single atom catalysts with tailored activity to benchmark the CO2 reduction. Mater. Today 2023, 67, 203–228. [Google Scholar] [CrossRef]
- Yasin, G.; Ali, S.; Ibraheem, S.; Kumar, A.; Tabish, M.; Mushtaq, M.A.; Ajmal, S.; Arif, M.; Khan, M.A.; Saad, A.; et al. Simultaneously Engineering the Synergistic-Effects and Coordination-Environment of Dual-Single-Atomic Iron/Cobalt-sites as a Bifunctional Oxygen Electrocatalyst for Rechargeable Zinc-Air Batteries. ACS Catal. 2023, 13, 2313–2325. [Google Scholar] [CrossRef]
- Akerdi, A.G.; Bahrami, S.H. Application of heterogeneous nano-semiconductors for photocatalytic advanced oxidation of organic compounds: A review. J. Environ. Chem. Eng. 2019, 7, 103283. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, I.W.; Huang, F. Toward large-scale water treatment using nanomaterials. Nano Today 2019, 27, 11–27. [Google Scholar] [CrossRef]
- Moradi, H.; Eshaghi, A.; Hosseini, S.R.; Ghani, K. Fabrication of Fe-doped TiO2 nanoparticles and investigation of photocatalytic decolorization of reactive red 198 under visible light irradiation. Ultrason Sonochem. 2016, 32, 314–319. [Google Scholar] [CrossRef]
- Mohseni-Salehi, M.S.; Taheri-Nassaj, E.; Hosseini-Zori, M. Effect of dopant (Co, Ni) concentration and hydroxyapatite compositing on photocatalytic activity of titania towards dye degradation. J. Photochem. Photobiol. A Chem. 2018, 356, 57–70. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Li, K.; Zeng, Y.; Zhao, W.; Zhang, T.; Zhan, Y.; Xie, R.; Leung, D.Y.; Huang, H. Titanium oxide based photocatalytic materials development and their role of in the air pollutants degradation: Overview and forecast. Environ. Int. 2019, 125, 200–228. [Google Scholar] [CrossRef] [PubMed]
- Padovese, E. Effetti di Strutturazione di Materiali a Base di Ossidi Inorganici di Tipo “aerogel-like” per Applicazioni nel Campo di Catalisi e di Isolanti ad alta Efficienza. Ph.D Thesis, Università degli studi di Trieste, Trieste, Italy, 2009. [Google Scholar]
- Kuspanov, Z.; Bakbolat, B.; Baimenov, A.; Issadykov, A.; Yeleuov, M.; Daulbayev, C. Photocatalysts for a sustainable future: Innovations in large-scale environmental and energy applications. Sci. Total Environ. 2023, 885, 163914. [Google Scholar] [CrossRef] [PubMed]
- Likodimos, V. Advanced photocatalytic materials. Materials 2020, 13, 821. [Google Scholar] [CrossRef] [PubMed]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent developments in the use of metal oxides for photocatalytic degradation of pharmaceutical pollutants in water—A review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Alsheheri, S.Z. Nanocomposites containing titanium dioxide for environmental remediation. Des. Monomers Polym. 2021, 24, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, I.S.; Li, Z.; Xia, X.; Lee, W.I.; Dai, S.; Bahnemann, D.W.; Pan, J.H. Nanoporous TiO2 spheres with tailored textural properties: Controllable synthesis, formation mechanism, and photochemical applications. Prog. Mater. Sci. 2020, 109, 100620. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Islam, M.J.; Mokammel, M.A. Visible light induced self-cleaning performance of iron-doped TiO2 nanoparticles for surface applications. Int. J. Appl. Ceram. Technol. 2023. [Google Scholar] [CrossRef]
- Lavand, A.B.; Malghe, Y.S.; Singh, S.H. Synthesis, Characterization, and Investigation of Visible Light Photocatalytic Activity of C Doped TiO2/CdS Core-Shell Nanocomposite. Indian J. Mater. Sci. 2015, 2015, 690568. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Barba-Nieto, I.; Caudillo-Flores, U.; Fernández-García, M.; Kubacka, A. Sunlight-Operated TiO2-Based Photocatalysts. Molecules 2020, 25, 4008. [Google Scholar] [CrossRef]
- Rosa, D.; D’Agostino, F.; Bavasso, I.; Di Palma, L. An Innovative and Easy Method for Iron-doped Titania Synthesis. Chem. Eng. Trans. 2023, 101, 13–18. [Google Scholar]
- Zu, M.; Zhou, X.; Zhang, S.; Qian, S.; Li, D.-S.; Liu, X.; Zhang, S. Sustainable engineering of TiO2-based advanced oxidation technologies: From photocatalyst to application devices. J. Mater. Sci. Technol. 2021, 78, 202–222. [Google Scholar] [CrossRef]
- Adán, C.; Bahamonde, A.; Fernández-García, M.; Martínez-Arias, A. Structure and activity of nanosized iron-doped anatase TiO2 catalysts for phenol photocatalytic degradation. Appl. Catal. B Environ. 2006, 72, 11–17. [Google Scholar] [CrossRef]
- Piera, E.; Tejedor-Tejedor, M.I.; Zorn, M.E.; Anderson, M.A. Relationship concerning the nature and concentration of Fe(III) species on the surface of TiO2particles and photocatalytic activity of the catalyst. Appl. Catal. B Environ. 2003, 46, 671–685. [Google Scholar] [CrossRef]
- Tumbelaka, R.M.; Istiqomah, N.I.; Kato, T.; Oshima, D.; Suharyadi, E. High reusability of green-synthesized Fe3O4/TiO2photocatalyst nanoparticles for efficient degradation of methylene blue dye. Mater. Today Commun. 2022, 33, 104450. [Google Scholar] [CrossRef]
- Abza, T.; Saka, A.; Tesfaye, J.L.; Gudata, L.; Nagaprasad, N.; Krishnaraj, R. Synthesis and Characterization of Iron Doped Titanium Dioxide (Fe: TiO2) Nanoprecipitate at Different pH Values for Applications of Self-Cleaning Materials. Adv. Mater. Sci. Eng. 2022, 2022, 2748908. [Google Scholar] [CrossRef]
- Jack, R.S.; Ayoko, G.A.; Adebajo, M.O.; Frost, R.L. A review of iron species for visible-light photocatalytic water purification. Environ. Sci. Pollut. Res. 2015, 22, 7439–7449. [Google Scholar] [CrossRef] [PubMed]
- Teoh, W.Y.; Amal, R.; Mädler, L.; Pratsinis, S.E. Flame sprayed visible light-active Fe-TiO2 for photomineralisation of oxalic acid. Catal. Today 2007, 120, 203–213. [Google Scholar] [CrossRef]
- Vásquez, G.C.; Peche-Herrero, M.A.; Maestre, D.; Alemán, B.; Ramírez-Castellanos, J.; Cremades, A.; González-Calbet, J.M.; Piqueras, J. Influence of Fe and Al doping on the stabilization of the anatase phase in TiO2nanoparticles. J. Mater. Chem. C Mater. 2014, 2, 10377–10385. [Google Scholar] [CrossRef]
- Litter, M.I.; Navio, J.A. Photocatalytic properties of iron-doped titania semiconductors. J. Photochem. Photobiol. A Chem. 1996, 98, 171–181. [Google Scholar] [CrossRef]
- Afonso, C.; Lima, O.; Segundo, I.R.; Landi, S.; Margalho, É.; Homem, N.; Pereira, M.; Costa, M.F.M.; Freitas, E.; Carneiro, J. Effect of Iron-Doping on the Structure and Photocatalytic Activity of TiO2Nanoparticles. Catalysts 2023, 13, 58. [Google Scholar] [CrossRef]
- Santos, R.d.S.; Faria, G.A.; Giles, C.; Leite, C.A.P.; Barbosa, H.d.S.; Arruda, M.A.Z.; Longo, C. Iron insertion and hematite segregation on Fe-doped TiO2 nanoparticles obtained from sol-gel and hydrothermal methods. ACS Appl. Mater. Interfaces 2012, 4, 5555–5561. [Google Scholar] [CrossRef]
- Fawzi Suleiman Khasawneh, O.; Palaniandy, P. Removal of organic pollutants from water by Fe2O3/TiO2 based photocatalytic degradation: A review. Environ. Technol. Innov. 2021, 21, 101230. [Google Scholar] [CrossRef]
- Kanjana, N.; Maiaugree, W.; Laokul, P. Photocatalytic activity of nanocrystalline Fe3+-doped anatase TiO2hollow spheres in a methylene blue solution under visible-light irradiation. J. Mater. Sci. Mater. Electron. 2022, 33, 4659–4680. [Google Scholar] [CrossRef]
- Cordischi, D.; Burriesci, N.; D’Alba, F.; Petrera, S.M.; Polizzottis, G.; Schiavellos, M. Structural Characterization of Fe/Ti Oxide Photocatalysts by X-Ray, ESR, and Miissbauer Methods. J. Solid State Chem. 1985, 56, 182–190. [Google Scholar] [CrossRef]
- Gracia, F.; Holgado, J.P.; Caballero, A.; Gonzalez-Elipe, A.R. Structural, optical, and photoelectrochemical properties of M n+-TiO2 model thin film photocatalysts. J. Phys. Chem. B 2004, 108, 17466–17476. [Google Scholar] [CrossRef]
- Soria, J.; Conesa, J.C.; Augugliaro, V.; Palmisano, L.; Schiavello, M.; Sclafani, A. Dinitrogen Photoreduction to Ammonia over Titanium Dioxide Powders Doped with Ferric Ions. J. Phys. Chem. 1991, 95, 274–282. [Google Scholar] [CrossRef]
- Navı, J.A.; Colón, G.; Macı, M.; Real, C.; Litter, M.I. Iron-doped titania semiconductor powders prepared by a sol±gel method. Part I: Synthesis and characterization. Appl. Catal. A Gen. 1999, 177, 111–120. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Zhang, J.; Chen, H.; Anpo, M. Fe3+-TiO2photocatalysts prepared by combining sol-gel method with hydrothermal treatment and their characterization. J. Photochem. Photobiol. A Chem. 2006, 180, 196–204. [Google Scholar] [CrossRef]
- Navío, J.A.; Colon, G.; Trillas, M.; Peral, J.; Domenech, X.; Testa, J.J.; Padrón, J.; Rodrı, D.; Litter, M.I. Heterogeneous photocatalytic reactions of nitrite oxidation and Cr(VI) reduction on iron-doped titania prepared by the wet impregnation method. Appl. Catal. B Environ. 1998, 16, 187–196. [Google Scholar] [CrossRef]
- Zolfagharia, A.; Riazian, M.; Ashjari, M. Photodegradation of methylene blue and evans blue by iron and sulphur doped TiO2 nanophotocatalyst under ultraviolet and visible light irradiation. J. Mex. Chem. Soc. 2021, 65, 357–375. [Google Scholar] [CrossRef]
- Nasralla, N.H.S.; Yeganeh, M.; Šiller, L. Photoluminescence study of anatase and rutile structures of Fe-doped TiO2 nanoparticles at different dopant concentrations. Appl. Phys. A Mater. Sci. Process. 2020, 126, 192. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.C.; Zakaria, R.; Ying, J.Y. Role of Particle Size in Nanocrystalline TiO2-Based Photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Li, X.; Yue, P.L.; Kutal, C. Synthesis and photocatalytic oxidation properties of iron doped titanium dioxide nanosemiconductor particles. New J. Chem. 2003, 27, 1264–1269. [Google Scholar] [CrossRef]
- Ikeda, S.; Sugiyama, N.; Murakami, S.Y.; Kominami, H.; Kera, Y.; Noguchi, H.; Uosaki, K.; Torimoto, T.; Ohtani, B. Quantitative analysis of defective sites in titanium(IV) oxide photocatalyst powders. Phys. Chem. Chem. Phys. 2003, 5, 778–783. [Google Scholar] [CrossRef]
- Di Paola, A.; García-López, E.; Marcì, G.; Martín, C.; Palmisano, L.; Rives, V.; Venezia, A.M. Surface characterisation of metal ions loaded TiO2 photocatalysts: Structure-activity relationship. Appl. Catal. B Environ. 2004, 48, 223–233. [Google Scholar] [CrossRef]
- Loan, T.T.; Huong, V.H.; Huyen, N.T.; Van Quyet, L.; Bang, N.A.; Long, N.N. Anatase to rutile phase transformation of iron-doped titanium dioxide nanoparticles: The role of iron content. Opt. Mater. 2021, 111, 110651. [Google Scholar] [CrossRef]
- Mwangi, I.W.; Kinyua, E.M.; Nthumbi, R.; Wanjau, R.N.; Swaleh, S.; Ngila, J.C. Iron (III) doped titanium dioxide coated dimensionally stable graphite anode electrode for electro-chemical treatment of domestic wastewater. Heliyon 2021, 7, e06671. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.; D’Agostino, F.; Bavasso, I.; Bracciale, M.P.; Di Palma, L. Easy way to produce iron-doped titania nanoparticles via the solid-state method and investigation their photocatalytic activity. J. Mater. Res. 2023, 38, 1282–1292. [Google Scholar] [CrossRef]
- Almquist, C.B.; Biswas, P. Role of synthesis method and particle size of nanostructured TiO2 on its photoactivity. J. Catal. 2002, 212, 145–156. [Google Scholar] [CrossRef]
- Luu, C.L.; Nguyen, Q.T.; Ho, S.T. Synthesis and characterization of Fe-doped TiO2 photocatalyst by the sol-gel method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 015008. [Google Scholar] [CrossRef]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shen, W.; He, W.; Zu, X. Effect of Fe-doped TiO2 nanoparticle derived from modified hydrothermal process on the photocatalytic degradation performance on methylene blue. J. Hazard. Mater. 2008, 155, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Anwar Ismail, M.; Hedhili, M.N.; Anjum, D.H.; Singaravelu, V.; Ho Chung, S.; Zielínska-Jurek, A. Synthesis and Characterization of Iron-Doped TiO2 Nanoparticles Using Ferrocene from Flame Spray Pyrolysis. Catalysts 2021, 11, 438. [Google Scholar] [CrossRef]
- Ambrus, Z.; Balázs, N.; Alapi, T.; Wittmann, G.; Sipos, P.; Dombi, A.; Mogyorósi, K. Synthesis, structure and photocatalytic properties of Fe(III)-doped TiO2 prepared from TiCl3. Appl. Catal. B Environ. 2008, 81, 27–37. [Google Scholar] [CrossRef]
- Crişan, M.; Răileanu, M.; Drăgan, N.; Crişan, D.; Ianculescu, A.; Niţoi, I.; Oancea, P.; Şomăcescu, S.; Stănică, N.; Vasile, B.; et al. Sol-gel iron-doped TiO2 nanopowders with photocatalytic activity. Appl. Catal. A Gen. 2015, 504, 130–142. [Google Scholar] [CrossRef]
- Abbas, N.; Shao, G.N.; Haider, M.S.; Imran, S.M.; Park, S.S.; Kim, H.T. Sol–gel synthesis of TiO2-Fe2O3 systems: Effects of Fe2O3 content and their photocatalytic properties. J. Ind. Eng. Chem. 2016, 39, 112–120. [Google Scholar] [CrossRef]
- El Mragui, A.; Aadnan, I.; Zegaoui, O.; Esteves da Silva, J.C.G. Physico-chemical characterization and photocatalytic activity assessment under UV-A and visible-light irradiation of iron-doped TiO2 nanoparticles. Arab. J. Chem. 2023, 16, 105331. [Google Scholar] [CrossRef]
- Gamarra, F.; Medina, J.; Lanchipa, W.; Tamayo, R.; Sacari, E. Structural, Optical, and Arsenic Removal Properties of Sol–Gel Synthesized Fe-Doped TiO2 Nanoparticles. Nanomaterials 2022, 12, 3402. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Zhang, W.; Wu, J.; Liu, X.; Liu, Z.; Xing, H.; Yu, L. Fabrication of iron doped titanium dioxide photocatalytic nanocomposite supported by water-quenched blast furnace slag and photocatalytic degradation of wastewater. Ceram. Int. 2023. [Google Scholar] [CrossRef]
- Egerton, T.A.; Harris, E.; Lawson, E.J.; Mile, B.; Rowlands, C.C. An EPR study of diffusion of iron into rutile. Phys. Chem. Chem. Phys. 2001, 3, 497–504. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Wang, Y.; Zhang, Y.; Wang, H.; Wang, X.; Chen, X.D.; Wu, Z. Highly dispersed titania-supported iron oxide catalysts for efficient heterogeneous photo-Fenton oxidation: Influencing factors, synergistic effects and mechanism insight. J. Colloid Interface Sci. 2021, 587, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; El-Katori, E.E.; Gharni, Z.H. Photocatalytic degradation of methylene blue dye using Fe2O3/TiO2 nanoparticles prepared by sol-gel method. J. Alloys Compd. 2013, 553, 19–29. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics [Internet]. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Husain, S.; Alkhtaby, L.A.; Giorgetti, E.; Zoppi, A.; Muniz Miranda, M. Investigation of the role of iron doping on the structural, optical and photoluminescence properties of sol-gel derived TiO2 nanoparticles. J. Lumin. 2016, 172, 258–263. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S.K. Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visible-light driven photocatalytic degradation of toxic organic compounds. J. Colloid Interface Sci. 2015, 450, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.H.; Kar, A.K.; Chen, K.C.; Chen, C.J. Highly effective Fe-doped TiO2 nanoparticles for removal of toxic organic dyes under visible light illumination. Nanotechnology 2023, 34, 245707. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, G.C.; Peche-Herrero, M.A.; Maestre, D.; Cremades, A.; Ramirez-Castellanos, J.; González-Calbet, J.M.; Piqueras, J. Effects of transition metal doping on the growth and properties of rutile TiO2 nanoparticles. J. Phys. Chem. C 2013, 117, 1941–1947. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, I.M.; Bandala, E.R. Photocatalytic degradation of estriol using iron-doped TiO2 under high and low UV irradiation. Catalysts 2018, 8, 625. [Google Scholar] [CrossRef]
- Hung, W.C.; Fu, S.H.; Tseng, J.J.; Chu, H.; Ko, T.H. Study on photocatalytic degradation of gaseous dichloromethane using pure and iron ion-doped TiO2 prepared by the sol-gel method. Chemosphere 2007, 66, 2142–2151. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Kalarikkal, N.; Sivakumar, J.; Ramana Reddy, M.V.; Sayanna, R. Structural, optical and photoluminescence studies of sol-gel synthesized pure and iron doped TiO2 photocatalysts. Ceram. Int. 2019, 45, 25060–25068. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Vaiano, V.; Bonelli, B.; Esposito, S.; Freyria, F.S.; Blangetti, N.; Sannino, D. Visible light-driven photocatalytic activity and kinetics of fe-doped TiO2 prepared by a three-block copolymer templating approach. Materials 2021, 14, 3105. [Google Scholar] [CrossRef] [PubMed]
- Solano, R.A.; Herrera, A.P.; Maestre, D.; Cremades, A. Fe-TiO2 Nanoparticles Synthesized by Green Chemistry for Potential Application in Waste Water Photocatalytic Treatment. J. Nanotechnol. 2019, 2019, 4571848. [Google Scholar] [CrossRef]
- Ismael, M. Enhanced photocatalytic hydrogen production and degradation of organic pollutants from Fe (III) doped TiO2 nanoparticles. J. Environ. Chem. Eng. 2020, 8, 103676. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Liu, X.; Jin, H.; Lv, H.; He, S.; Hao, H.; Li, C. A mild in-situ method to construct fe-doped cauliflower-like rutile TiO2 photocatalysts for degradation of organic dye in wastewater. Catalysts 2019, 9, 426. [Google Scholar] [CrossRef]
- Mohan, H.; Ramasamy, M.; Ramalingam, V.; Natesan, K.; Duraisamy, M.; Venkatachalam, J.; Shin, T.; Seralathan, K.-K. Enhanced visible light-driven photocatalysis of iron-oxide/titania composite: Norfloxacin degradation mechanism and toxicity study. J. Hazard. Mater. 2021, 412, 125330. [Google Scholar] [CrossRef] [PubMed]
- Ochoa Rodríguez, P.A.; Pecchi, G.A.; Casuscelli, S.G.; Elías, V.R.; Eimer, G.A. A simple synthesis way to obtain iron-doped TiO2 nanoparticles as photocatalytic surfaces. Chem. Phys. Lett. 2019, 732, 136643. [Google Scholar] [CrossRef]
- Anu Tharu, P.; Benoy, M.D.; Dhannia, T. Effect of annealing temperature on the bandgap of TiO2 and Fe- doped titanium dioxide nanoparticles for enhancement of UV shielding performance. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Ellouzi, I.; Regraguy, B.; El Hajjaji, S.; Harir, M.; Schmitt-Kopplin, P.; Lachheb, H.; Laânab, L. Synthesis of Fe-doped TiO2 with improved photocatalytic properties under Vis-L irradiation. Iran. J. Catal. 2022, 12, 283–293. [Google Scholar]
- Bokhimi, X.; Morales, A.; Aguilar, M.; Toledo-Antonio, J.A.; Pedraza, F. Local order in titania polymorphs [Internet]. Int. J. Hydrog. Energy 2001, 26, 1279–1287. [Google Scholar] [CrossRef]
- Rosa, D.; Lattanzio, S.; Bavasso, I.; Di Palma, L. Investigation of the synergistic effect of hydrogen peroxide and ultrasound on the photocatalytic treatment under visible light of dyes wastewater. Chem. Eng. Sci. 2023, 282, 119290. [Google Scholar] [CrossRef]
- Wang, Z.M.; Yang, G.; Biswas, P.; Bresser, W.; Boolchand, P. Processing of iron-doped titania powders in flame aerosol reactors. Powder Technol. 2001, 114, 197–204. [Google Scholar] [CrossRef]
- Babić, B.; Gulicovski, J.; Dohčević-Mitrović, Z.; Bučevac, D.; Prekajski, M.; Zagorac, J.; Matović, B. Synthesis and characterization of Fe3+ doped titanium dioxide nanopowders. Ceram. Int. 2012, 38, 635–640. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, J.; Cheng, B.; Yu, H. Preparation and photocatalytic activity of Fe-doped mesoporous titanium dioxide nanocrystalline photocatalysts. Mater. Chem. Phys. 2005, 93, 159–163. [Google Scholar] [CrossRef]
- Jamalluddin, N.A.; Abdullah, A.Z. Reactive dye degradation by combined Fe(III)/TiO2 catalyst and ultrasonic irradiation: Effect of Fe(III) loading and calcination temperature. Ultrason. Sonochem. 2011, 18, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Murashkina, A.A.; Murzin, P.D.; Rudakova, A.V.; Ryabchuk, V.K.; Emeline, A.V.; Bahnemann, D.W. Influence of the Dopant Concentration on the Photocatalytic Activity: Al-Doped TiO2. J. Phys. Chem. C 2015, 119, 24695–24703. [Google Scholar] [CrossRef]
- Ganesh, I.; Kumar, P.P.; Gupta, A.K.; Sekhar, P.S.; Radha, K.; Padmanabham, G.; Sundararajan, G. Processing and Application of Ceramics 6. Sci. World J. 2012, 2012, 127326. [Google Scholar] [CrossRef]
- Kundu, A.; Mondal, A. Structural, optical, physio-chemical properties and photodegradation study of methylene blue using pure and iron-doped anatase titania nanoparticles under solar-light irradiation. J. Mater. Sci. Mater. Electron. 2019, 30, 3244–3256. [Google Scholar] [CrossRef]
- Miyake, Y.; Tada, H. Photocatalytic Degradation of Methylene Blue with Metal-Doped Mesoporous Titania under Irradiation of White Light. J. Chem. Eng. Jpn. 2004, 37, 630–635. [Google Scholar] [CrossRef]
- Charpentier, P.A.; Chen, C.; Azhie, K.; Grohe, B.; Mumin, M.A.; Lotus, A.F.; Therrien, P.; Mittler, S. Photocatalytic and antibacterial activities of silver and iron doped titania nanoparticles in solution and polyaspartic coatings. Nanotechnology 2019, 30, 085706. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rashid, S.; Othman, S.H.; Mohd Ghazi, T.I.; Abdullah, N. Fe-doped TiO2 nanoparticles produced via MOCVD: Synthesis, characterization, and photocatalytic activity. J. Nanomater. 2011, 2011, 571601. [Google Scholar]
- Di Paola, A.; Marcì, G.; Palmisano, L.; Schiavello, M.; Uosaki, K.; Ikeda, S.; Ohtani, B. Preparation of polycrystalline TiO2 photocatalysts impregnated with various transition metal ions: Characterization and photocatalytic activity for the degradation of 4-nitrophenol. J. Phys. Chem. B 2002, 106, 637–645. [Google Scholar] [CrossRef]
- Mancuso, A.; Blangetti, N.; Sacco, O.; Freyria, F.S.; Bonelli, B.; Esposito, S.; Sannino, D.; Vaiano, V. Photocatalytic Degradation of Crystal Violet Dye under Visible Light by Fe-Doped TiO2 Prepared by Reverse-Micelle Sol–Gel Method. Nanomaterials 2023, 13, 270. [Google Scholar] [CrossRef]
- Wang, J.A.; Limas-Ballesteros, R.; López, T.; Moreno, A.; Gómez, R.; Novaro, O.; Bokhimi, X. Quantitative determination of titanium lattice defects and solid-state reaction mechanism in iron-doped TiO2 photocatalysts. J. Phys. Chem. B 2001, 105, 9692–9698. [Google Scholar] [CrossRef]
- Qamar, M.; Merzougui, B.; Anjum, D.; Hakeem, A.S.; Yamani, Z.H.; Bahnemann, D. Synthesis and photocatalytic activity of mesoporous nanocrystalline Fe-doped titanium dioxide. Catal. Today 2014, 230, 158–165. [Google Scholar] [CrossRef]
- Rosa, D.; Cimini, G.; Bracciale, M.P.; Felici, A.C.; Di Palma, L. Iron-doped titania nanoparticles supported on polystyrene for photocatalytic treatment of contaminated water in a continuous system. J. Photochem. Photobiol. A Chem. 2024, 447, 115241. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Long, Y.; Luo, X.; Zhang, L.; Xue, X.; Yin, Y.; Zhu, Z.; Xu, B. High-temperature transformation behavior of iron-doped titanium dioxide crystal structures. Phase Transit. 2021, 94, 219–235. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Thomas, A.G.; Flavell, W.R.; Mallick, A.K.; Kumarasinghe, A.R.; Tsoutsou, D.; Khan, N.; Chatwin, C.; Rayner, S.; Smith, G.C.; Stockbauer, R.L.; et al. Comparison of the electronic structure of anatase and rutile TiO2 single-crystal surfaces using resonant photoemission and x-ray absorption spectroscopy. Phys. Rev. B 2007, 75, 035105. [Google Scholar] [CrossRef]
- Tanaka, K.; Capule, M.F.; Hisanaga, T. Effect of crystallinity of TiO2 on its photocatalytic action. Chem. Phys. Lett. 1991, 187, 73–76. [Google Scholar] [CrossRef]
- Kissoum, Y.; Mekki, D.E.; Bououdina, M.; Sakher, E.; Bellucci, S. Dependence of Fe Doping and Milling on TiO2 Phase Transformation: Optical and Magnetic Studies. J. Supercond. Nov. Magn. 2020, 33, 427–440. [Google Scholar] [CrossRef]
- Nasralla, N.; Yeganeh, M.; Astuti, Y.; Piticharoenphun, S.; Shahtahmasebi, N.; Kompany, A.; Karimipour, M.; Mendis, B.G.; Poolton, N.R.J.; Šiller, L. Structural and spectroscopic study of Fe-doped TiO2 nanoparticles prepared by sol-gel method. Sci. Iran 2013, 20, 1018–1022. [Google Scholar]
- Nasralla, N.H.S.; Yeganeh, M.; Astuti, Y.; Piticharoenphun, S.; Šiller, L. Systematic study of electronic properties of Fe-doped TiO2 nanoparticles by X-ray photoemission spectroscopy. J. Mater. Sci. Mater. Electron. 2018, 29, 17956–17966. [Google Scholar] [CrossRef]
- Faycal Atitar, M.; Ismail, A.A.; Al-Sayari, S.A.; Bahnemann, D.; Afanasev, D.; Emeline, A.V. Mesoporous TiO2 nanocrystals as efficient photocatalysts: Impact of calcination temperature and phase transformation on photocatalytic performance. Chem. Eng. J. 2015, 264, 417–424. [Google Scholar] [CrossRef]
- Kawahara, T.; Konishi, Y.; Tada, H.; Tohge, N.; Nishii, J.; Ito, S. A Patterned TiO2 (Anatase)/TiO2 (Rutile) Bilayer-Type Photocatalyst: Effect of the Anatase/Rutile Junction on the Photocatalytic Activity. Angew. Chem. Int. Ed. 2002, 41, 2935–2937. [Google Scholar] [CrossRef]
- Tian, B.; Li, C.; Zhang, J. One-step preparation, characterization and visible-light photocatalytic activity of Cr-doped TiO2 with anatase and rutile bicrystalline phases. Chem. Eng. J. 2012, 191, 402–409. [Google Scholar] [CrossRef]
- Romero-Gomez, P.; Borras, A.; Barranco, A.; Espinos, J.P.; Gonzalez-Elipe, A.R. Enhanced photoactivity in bilayer films with buried rutile-anatase heterojunctions. ChemPhysChem 2011, 12, 191–196. [Google Scholar] [CrossRef]
- Li, G.; Gray, K.A. Preparation of mixed-phase titanium dioxide nanocomposites via solvothermal processing. Chem. Mater. 2007, 19, 1143–1146. [Google Scholar] [CrossRef]
- Bacsa, R.R.; Kiwi, J. Effect of rutile phase on the photocatalytic properties of nanocrystalline titania during the degradation of p-coumaric acid. Appl. Catal. B Environ. 1998, 16, 19–29. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemannt, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Niu, Y.; Xing, M.; Zhang, J.; Tian, B. Visible light activated sulfur and iron co-doped TiO2 photocatalyst for the photocatalytic degradation of phenol. Catal. Today 2013, 201, 159–166. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P.; Palaniandy, P.; Ahmadipour, M.; Mohammadi, H.; Bin Hamdan, M.R. Removal of acetaminophen using Fe2O3-TiO2 nanocomposites by photocatalysis under simulated solar irradiation: Optimization study. J. Environ. Chem. Eng. 2021, 9, 104921. [Google Scholar] [CrossRef]
- Wodka, D.; Socha, R.P.; Bielańska, E.; Elżbieciak-Wodka, M.; Nowak, P.; Warszyński, P. Photocatalytic activity of titanium dioxide modified by Fe2O3 nanoparticles. Appl. Surf. Sci. 2014, 319, 173–180. [Google Scholar] [CrossRef]
- Chen, J.; Eberlein, L.; Langford, C.H. Pathways of phenol and benzene photooxidation using TiO2 supported on a zeolite. J. Photochem. Photobiol. A Chem. 2002, 148, 183–189. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photolysis of Chloroform and Other Organic Molecules in Aqueous TiO2 Suspensions [Internet]. Environ. Sci. Technol. 1991, 25, 494–500. [Google Scholar] [CrossRef]
- Isari, A.A.; Payan, A.; Fattahi, M.; Jorfi, S.; Kakavandi, B. Photocatalytic degradation of rhodamine B and real textile wastewater using Fe-doped TiO2 anchored on reduced graphene oxide (Fe-TiO2/rGO): Characterization and feasibility, mechanism and pathway studies. Appl. Surf. Sci. 2018, 462, 549–564. [Google Scholar] [CrossRef]
- Hashemzadeh, F.; Rahimi, R.; Ghaffarinejad, A.; Hashemzadeh, F.; Rahimi, R.; Gaffarinejad, A. Photocatalytic Degradation of Methylene blue and Rhodamine B dyes by Niobium Oxide Nanoparticles synthesized Via Hydrothermal method [Internet]. Int. J. Appl. Chem. Sci. Res. 2013, 1, 95–102. [Google Scholar]
- Su, B.; Wang, K.; Bai, J.; Mu, H.; Tong, Y.; Min, S.; She, S.; Lei, Z. Photocatalytic degradation of methylene blue on Fe3+-doped TiO2 nanoparticles under visible light irradiation. Front. Chem. China 2007, 2, 364–368. [Google Scholar] [CrossRef]
- Nazari, M. Iron-doped Titanium Dioxide Nanomaterials: Synthesis, Characterization and Photodegradation Catalytic Behavior. Ph.D. Thesis, York University, Toronto, ON, Canada, 2019. [Google Scholar]
- Ghorbanpour, M.; Feizi, A. Iron-doped TiO2 Catalysts with Photocatalytic Activity. J. Water Environ. Nanotechnol. 2019, 4, 60–66. [Google Scholar]
- Moradi, V.; Ahmed, F.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Acid-treated Fe-doped TiO2 as a high performance photocatalyst used for degradation of phenol under visible light irradiation. J. Environ. Sci. 2019, 83, 183–194. [Google Scholar] [CrossRef]
- Lin, W.C.; Liu, R.; Yang, W.D. Effect of iron (III)-doping on the photocatalytic activity of titanium dioxide catalysts for methylene blue degradation. Appl. Mech. Mater. 2012, 117–119, 1088–1091. [Google Scholar] [CrossRef]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic performance of Fe-doped TiO2 nanoparticles under visible-light irradiation. Mater. Res. Express. Inst. Phys. Publ. 2017, 4, 015022. [Google Scholar] [CrossRef]
- Xia, Z.; Xing, S.; Wang, H.; Zhao, D.; Wu, S.; Jiang, W.; Wang, N.; Liu, S.; Liu, C.; Ding, W.; et al. Weak-visible-light-driven Fe doped TiO2 photocatalyst prepared by coprecipitation method and degradation of methyl orange. Opt. Mater. 2022, 129, 112522. [Google Scholar] [CrossRef]
- Jamil, T.S.; Gad-Allah, T.A.; Ghaly, M.Y. Parametric study on phenol photocatalytic degradation under pure visible and solar irradiations by Fe-doped TiO2. Desalination Water Treat. 2012, 50, 264–271. [Google Scholar] [CrossRef]
- Saroj, S.; Singh, L.; Singh, S.V. Photodegradation of Direct Blue-199 in carpet industry wastewater using iron-doped TiO2 nanoparticles and regenerated photocatalyst. Int. J. Chem. Kinet. 2019, 51, 189–205. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Li, R.; Jia, Y.; Bu, N.; Wu, J.; Zhen, Q. Photocatalytic degradation of methyl blue using Fe2O3/TiO2 composite ceramics. J Alloys Compd. 2015, 643, 88–93. [Google Scholar] [CrossRef]
- Liu, T. Preparation of modified TiO2 and its photocatalytic properties. Acad. J. Mater. Chem. 2023, 4, 1–14. [Google Scholar]
- Gelover, S.; Mondragón, P.; Jiménez, A. Titanium dioxide sol-gel deposited over glass and its application as a photocatalyst for water decontamination. J. Photochem. Photobiol. A Chem. 2004, 165, 241–246. [Google Scholar] [CrossRef]
- Mansilla, H.; Bravo, C.; Ferreyra, R.; Litter, M.; Jardim, W.; Lizama, C.; Freer, J.; Fernández, J. Photocatalytic EDTA degradation on suspended and immobilized TiO2. J. Photochem. Photobiol. A Chem. 2006, 181, 188–194. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Bideau, M.; Claudel, B.; Dubien, C.; Faure, L.; Kazouan, H. On the “immobilization” of titanium dioxide in the photocatalytic oxidation of spent waters. J. Photochem. Photobiol. A Chem. 1995, 91, 137–144. [Google Scholar] [CrossRef]
- Prakruthi, K.; Ujwal, M.P.; Yashas, S.R.; Mahesh, B.; Kumara Swamy, N.; Shivaraju, H.P. Recent advances in photocatalytic remediation of emerging organic pollutants using semiconducting metal oxides: An overview. In Environmental Science and Pollution Research; Springer Science and Business Media Deutschland GmbH: Berlin, Germany, 2022; Volume 29, pp. 4930–4957. [Google Scholar]


| Contaminant | Atom% Fe | Reference |
|---|---|---|
| Methylene Blue | 0.1 | [65] |
| 0.1 | [122] | |
| 0.3 | [118] | |
| 0.7 | [119] | |
| 0.9 | [48] | |
| 2.7 | [70] | |
| 3.0 | [123] | |
| 4.7 | [56] | |
| Acid Orange 7 | 0.1 | [76] |
| 3.7 | [71] | |
| Methyl Orange | 0.1 | [73] |
| 0.3 | [124] | |
| 0.5 | [120] | |
| Phenol | 0.1 | [125] |
| 0.5 | [121] | |
| 0.6 | [22] | |
| Cyclohexane | 1.0 | [43] |
| Direct Blue 119 | 4.2 | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, D.; Abbasova, N.; Di Palma, L. Titanium Dioxide Nanoparticles Doped with Iron for Water Treatment via Photocatalysis: A Review. Nanomaterials 2024, 14, 293. https://doi.org/10.3390/nano14030293
Rosa D, Abbasova N, Di Palma L. Titanium Dioxide Nanoparticles Doped with Iron for Water Treatment via Photocatalysis: A Review. Nanomaterials. 2024; 14(3):293. https://doi.org/10.3390/nano14030293
Chicago/Turabian StyleRosa, Domenico, Nigar Abbasova, and Luca Di Palma. 2024. "Titanium Dioxide Nanoparticles Doped with Iron for Water Treatment via Photocatalysis: A Review" Nanomaterials 14, no. 3: 293. https://doi.org/10.3390/nano14030293





