Fully Printed Cellulose Nanofiber–Ag Nanoparticle Composite for High-Performance Humidity Sensor
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
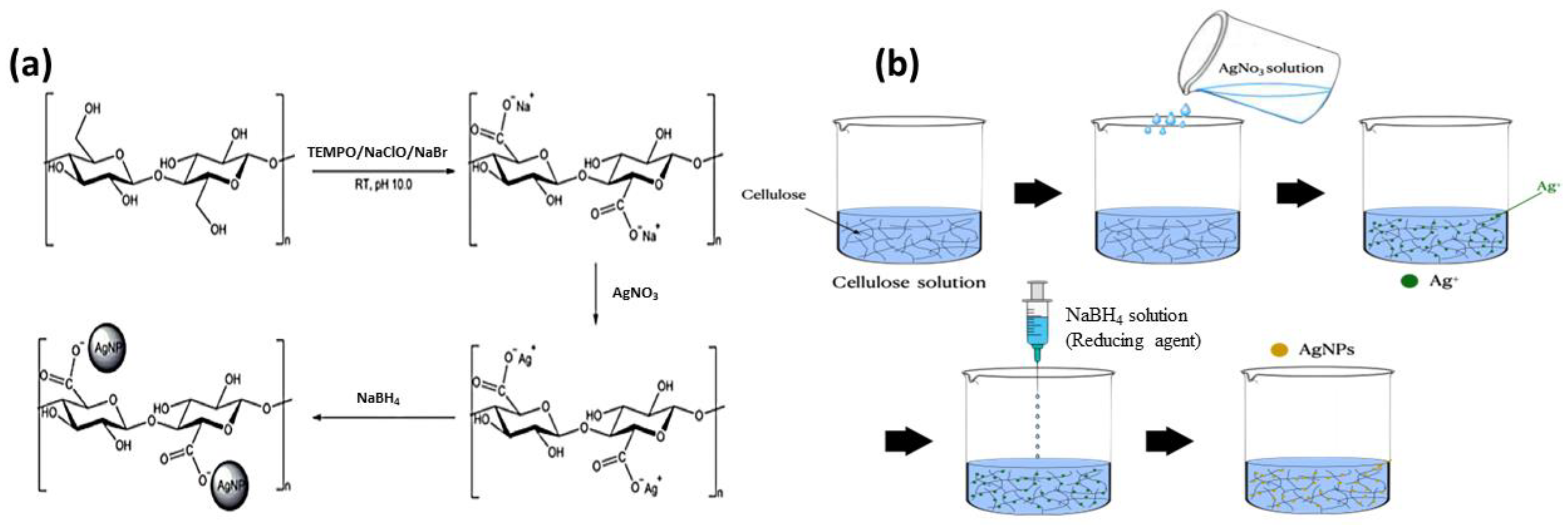
2.2. Synthesis of CNF-AgNP Composite
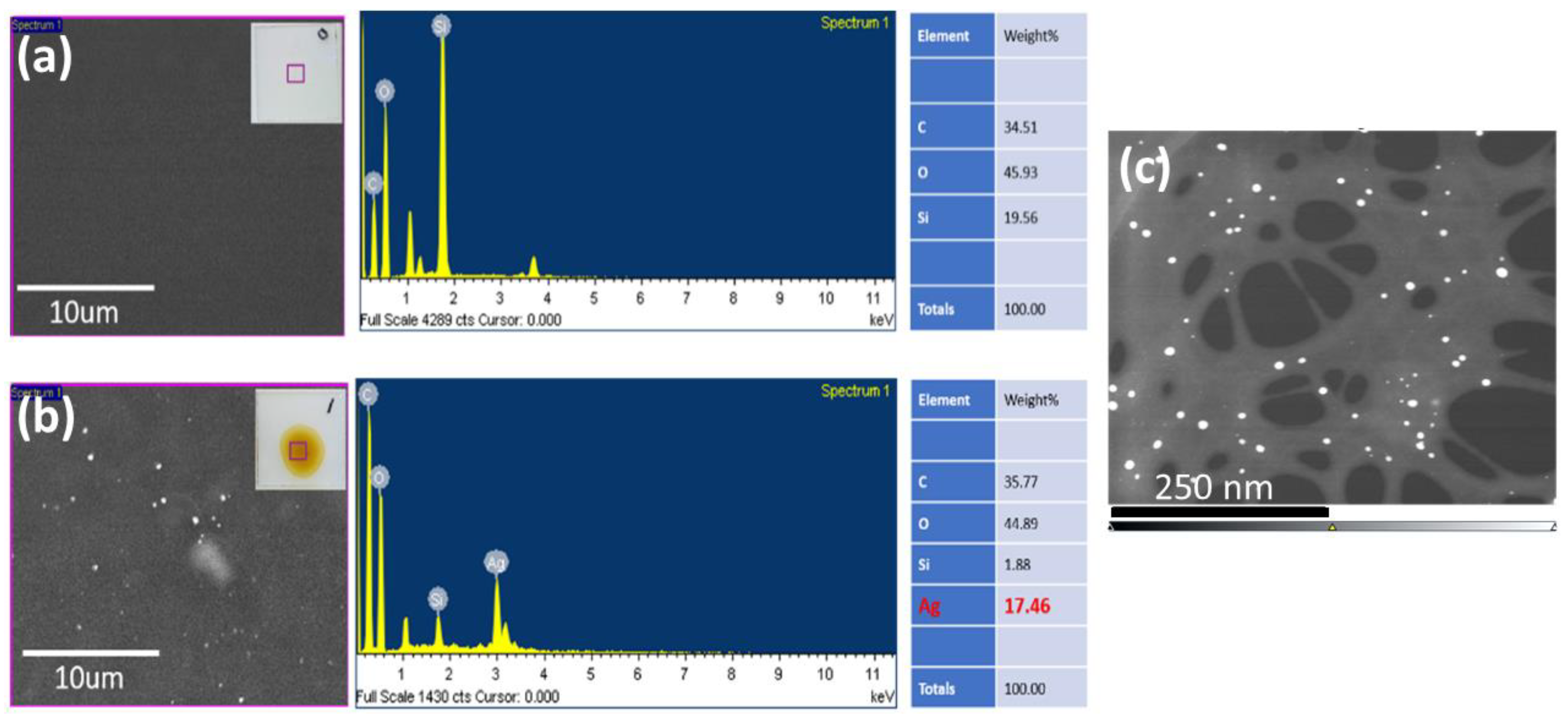
2.3. Characterization of CNF-AgNPs
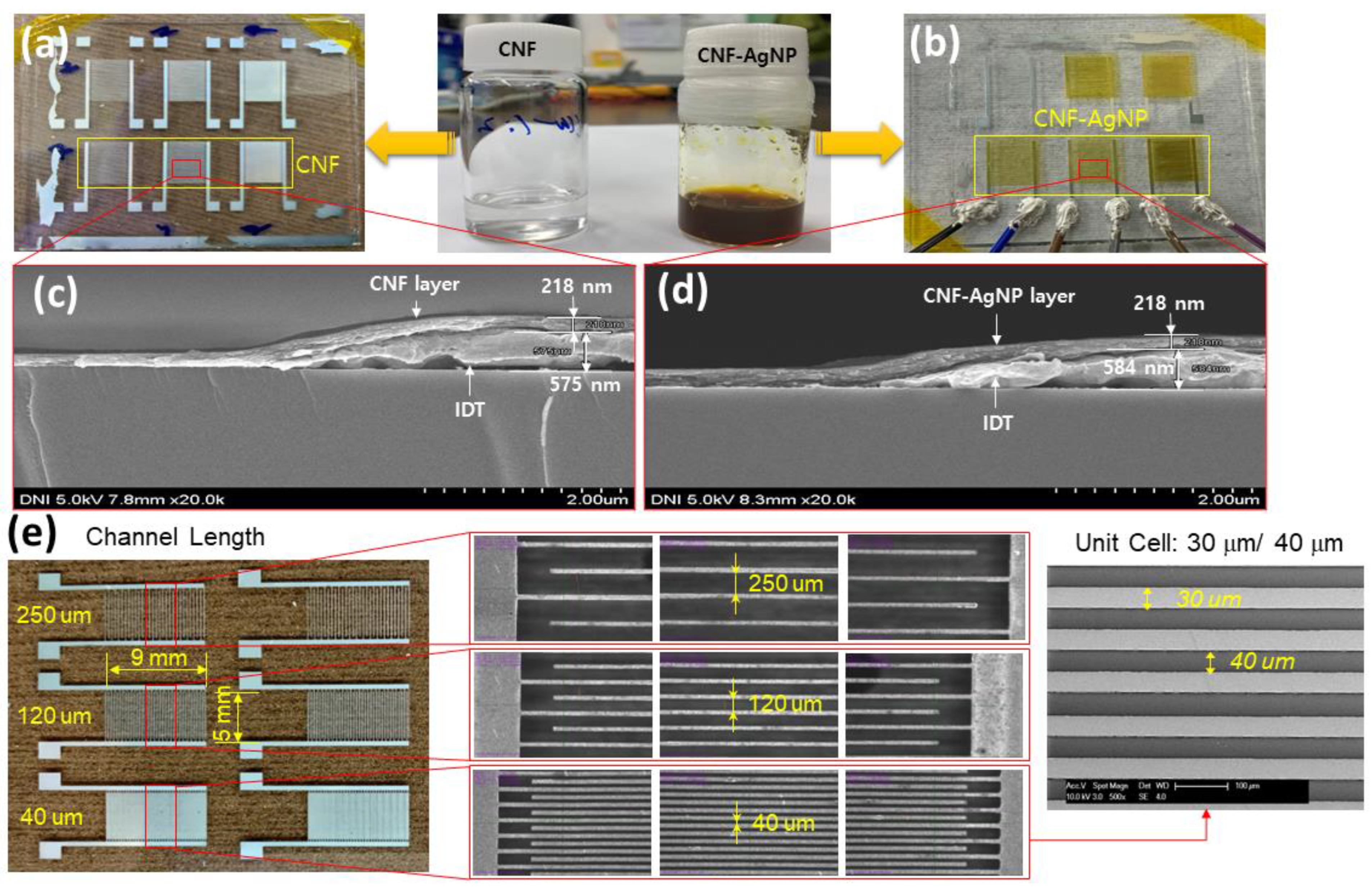
2.4. Humidity Sensor Fabrication
2.5. Performance Evaluation
3. Results and Discussion
3.1. Characterization of the CNF-AgNP Composite
3.2. Fabrication of CNF-AgNPs Sensor
3.3. Humidity Response
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fang, H.; Lin, J.; Hu, Z.; Liu, H.; Tang, Z.; Shi, T.; Liao, G. Cu(OH)2 nanowires/graphene oxide composites based QCM humidity sensor with fast-response for real-time respiration monitoring. Sens. Actuat. B Chem. 2020, 304, 127313. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, K.; Yang, M.; Tang, S.; Yang, T.; Fujita, Y.; Honda, S.; Arie, T.; Akita, S.; Chueh, Y.; et al. Highly stable Pd/HNb3O8 based flexible humidity sensor for perdurable wireless wearable applications. Nanoscale Horiz. 2021, 6, 260–270. [Google Scholar] [CrossRef]
- Dai, J.; Zhao, H.; Lin, X.; Liu, S.; Fei, T.; Zhang, T. Humidity sensors based on 3D porous polyelectrolytes via breath figure method. Adv. Electron. Mater. 2019, 6, 1900846. [Google Scholar] [CrossRef]
- Su, Y.; Li, C.; Li, M.; Li, H.; Xu, S.; Qian, L.; Yang, B. Surface acoustic wave humidity sensor based on three-dimensional architecture graphene/PVA/SiO2 and its application for respiration monitoring. Sens. Actuat. B Chem. 2020, 308, 127693. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, Z.; Zhang, B.; Yuan, Z.; Zhao, Q.; Jiang, Y.; Tai, H. Electrochemical humidity sensor enabled self-powered wireless humidity detection system. Nano Energy 2023, 115, 108745. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Sekine, T.; Takeda, Y.; Yokosawa, K.; Matsui, H.; Kumaki, D.; Shiba, T.; Nishikawa, T.; Tokito, S. Fully printed PEDOT: PSS-based temperature sensor with high humidity stability for wireless healthcare monitoring. Sci. Rep. 2020, 10, 2467. [Google Scholar] [CrossRef]
- Yang, J.; Shi, R.; Lou, Z.; Chai, R.; Jiang, K.; Shen, G. Flexible smart noncontact control systems with ultrasensitive humidity sensors. Small 2019, 15, 1902801. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Sun, H.; Xu, Y.; Wang, X.; Luo, H.; Liu, Z.; Zhang, T. Porous ionic membrane based flexible humidity sensor and its multifunctional applications. Adv. Sci. 2017, 4, 1600404. [Google Scholar] [CrossRef]
- Blank, T.A.; Eksperiandova, L.P.; Belikov, K. Recent trends of ceramic humidity sensors development: A review. Sens. Actuat. B Chem. 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.; Chaudhary, V.; Dahiya, M.; Sharma, A.; Nehra, S.; Duhan, S.; Kailasam, K. An excellent humidity sensor based on In-SnO2 loaded mesoporous graphitic carbon nitride. J. Mater. Chem. A 2017, 5, 14134–14143. [Google Scholar] [CrossRef]
- Cheng, B.; Tian, B.; Xie, C.; Xiao, Y.; Lei, S. Highly sensitive humidity sensor based on amorphous Al2O3 nanotubes. J. Mater. Chem. 2011, 21, 1907–1912. [Google Scholar] [CrossRef]
- Ho, D.H.; Sun, Q.; Kim, S.Y.; Han, J.T.; Kim, D.H.; Cho, J.H. Stretchable and multimodal all graphene electronic skin. Adv. Mater. 2016, 28, 2601–2608. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-H.; Kang, D.; Ruoff, R.S.; Shin, H.S.; Park, Y.-B. Poly(vinyl alcohol) reinforced and toughened with poly(dopamine)-treated graphene oxide and its use for humidity sensing. ACS Nano 2014, 8, 6739–6747. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Shim, B.S.; Kim, H.S.; Lee, Y.-J.; Min, S.-K.; Jang, D.; Abas, Z.; Kim, J. Review of Nanocellulose for Sustainable Future Materials. Int. J. Precis. Eng. Manufactur.-Green Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef]
- Kim, J.; Yun, S.; Ounaies, Z. Discovery of cellulose as a smart material. Macromolecules 2006, 39, 4202–4206. [Google Scholar] [CrossRef]
- Zhao, B.; Sivasankar, V.S.; Subudhi, S.K.; Dasgupta, A.; Das, S. Printed Carbon Nanotube-Based Humidity Sensors Deployable on Surfaces of Widely Varying Curvatures. ACS Appl. Nano Mater. 2023, 6, 1459–1474. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Yun, S.; Kim, J. Flexible humidity and temperature sensor based on cellulose–polypyrrole nanocomposite. Sens. Actuat. A Phys. 2011, 165, 194–199. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.; Kasuga, T.; Uetani, K.; Nogi, M.; Koga, H. All-cellulose-derived humidity sensor prepared via direct laser writing of conductive and moisture-stable electrodes on TEMPO-oxidized cellulose paper. J. Mater. Chem. C 2022, 10, 3712–3719. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Bui, T.T.; Hua, S.H.; Chun, H.; Kim, Y.S. New stand-alone hygrometers based on the moisture-driven bending of a nanoclay and polyimide bilayer. Sens. Actuat. B Chem. 2022, 359, 131559. [Google Scholar] [CrossRef]
- Hai, L.V.; Zhai, L.; Kim, H.C.; Kim, J.S.; Choi, E.S.; Kim, J. Cellulose nanofibers isolated by TEMPO-oxidation and aqueous counter collision methods. Carbohydr. Polym. 2018, 191, 65–70. [Google Scholar]
- Zhao, X.; Xia, Y.; Li, Q.; Ma, X.; Quan, F.; Geng, C.; Han, Z. Microwave-assisted synthesis of silver nanoparticles using sodium alginate and their antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 180–188. [Google Scholar] [CrossRef]
- Bazmandeh, A.Z.; Rezaei, A.; Jafarbigloo, H.R.G.; Javar, A.M.A.; Hassanzadeh, A.; Amirian, A.; Niakan, M.H.; Nave, H.H.; Mehrabi, M. Green synthesis and characterization of biocompatible silver nanoparticles using Stachys lavandulifolia Vahl. extract and their antimicrobial performance study. J. Environ. Treat. Tech. 2020, 8, 284–290. [Google Scholar]
- Gusrizal, G.; Santosa, S.J.; Kunarti, E.S.; Rusdiarso, B. Silver nanoparticles capped with p-hydroxy benzoic acid as a colorimetric sensor for the determination of paraquat. Indones. J. Chem. 2020, 20, 688–696. [Google Scholar] [CrossRef]
- González, A.L.; Noguez, C.; Beránek, J.; Barnard, A.S. Size, shape, stability, and color of plasmonic silver nanoparticles. J. Phys. Chem. C 2014, 118, 9128–9136. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Ding, A.; Ren, N.; Li, G.; Liang, H. Fabrication and characterization of thin-film composite (TFC) nanofiltration membranes incorporated with cellulose nanocrystals (CNCs) for enhanced desalination performance and dye removal. Chem. Eng. J. 2019, 358, 1519–1528. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kim, N.-J.; Ross, L., Jr.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef]
- Cho, N.-B.; Lim, T.-H.; Jeon, Y.-M.; Gong, M.-S. Inkjet printing of polymeric resistance humidity sensor using UV-curable electrolyte inks. Macromol. Res. 2008, 16, 149–154. [Google Scholar] [CrossRef]
- Lee, C.-W.; Rhee, H.-W.; Gong, M.-S. Humidity sensitive properties of copolymers containing phosphonium salts. Synth. Met. 1999, 106, 177–182. [Google Scholar] [CrossRef]
- Yuan, J.-K.; Yao, S.-H.; Dong, Z.-M.; Sylvestre, A.; Genestoux, M.; Bai, J. Giant dielectric permittivity nanocomposites: Realizing true potential of pristine carbon nanotubes in polyvinylidene fluoride matrix through an enhanced interfacial interaction. J. Phys. Chem. C 2011, 115, 5515–5521. [Google Scholar] [CrossRef]



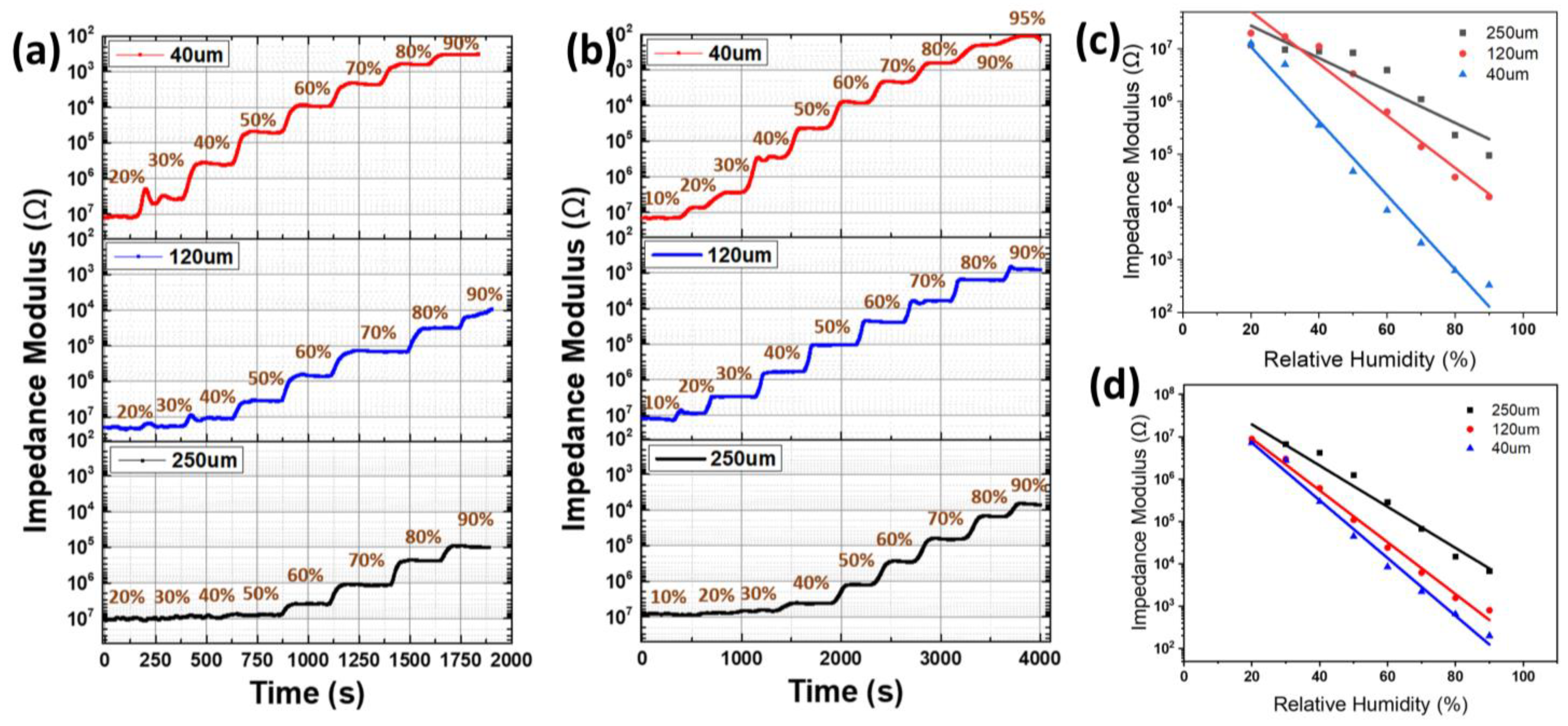

| Name | Humidity Range (%RH) | Response (Ω) | Response/Recovery Time (s) | Reference | |
|---|---|---|---|---|---|
| CNF | 10~90 | 1 × 107~3 × 102 | 0.0565 | 7/75 | This work |
| CNF-AgNP | 10~90 | 1.7 × 107~1 × 102 | 0.0654 | 4/34 | |
| TEMPO-cellulose | 11~98 | 9 × 108~8 × 105 | 0.0351 | 60/495 | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, M.; Jung, M.; Kim, J.; Kim, D.-S. Fully Printed Cellulose Nanofiber–Ag Nanoparticle Composite for High-Performance Humidity Sensor. Nanomaterials 2024, 14, 343. https://doi.org/10.3390/nano14040343
Won M, Jung M, Kim J, Kim D-S. Fully Printed Cellulose Nanofiber–Ag Nanoparticle Composite for High-Performance Humidity Sensor. Nanomaterials. 2024; 14(4):343. https://doi.org/10.3390/nano14040343
Chicago/Turabian StyleWon, Mijin, Minhun Jung, Jaehwan Kim, and Dong-Soo Kim. 2024. "Fully Printed Cellulose Nanofiber–Ag Nanoparticle Composite for High-Performance Humidity Sensor" Nanomaterials 14, no. 4: 343. https://doi.org/10.3390/nano14040343





