In Vivo Assessment of Hepatic and Kidney Toxicity Induced by Silicon Quantum Dots in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of SiQDs and Their Characteristics
2.2. Administration of SiQDs to Mice
2.3. Histology and Immunohistochemistry
2.4. Obtaining the Tissue Homogenates
2.5. Measurement of Antioxidant Enzyme Activities
2.6. Measurement of Malondialdehyde Level (MDA)
2.7. Measurement of GSH Level
2.8. Western Blot
2.9. Detection of 8-Hyroxy-2′-deoxyguanosine (8-OHdG)
2.10. Quantification of 5-methyl cytosine (5-mC)
2.11. Assessment of Histone Methylation
2.12. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics of SiQDs
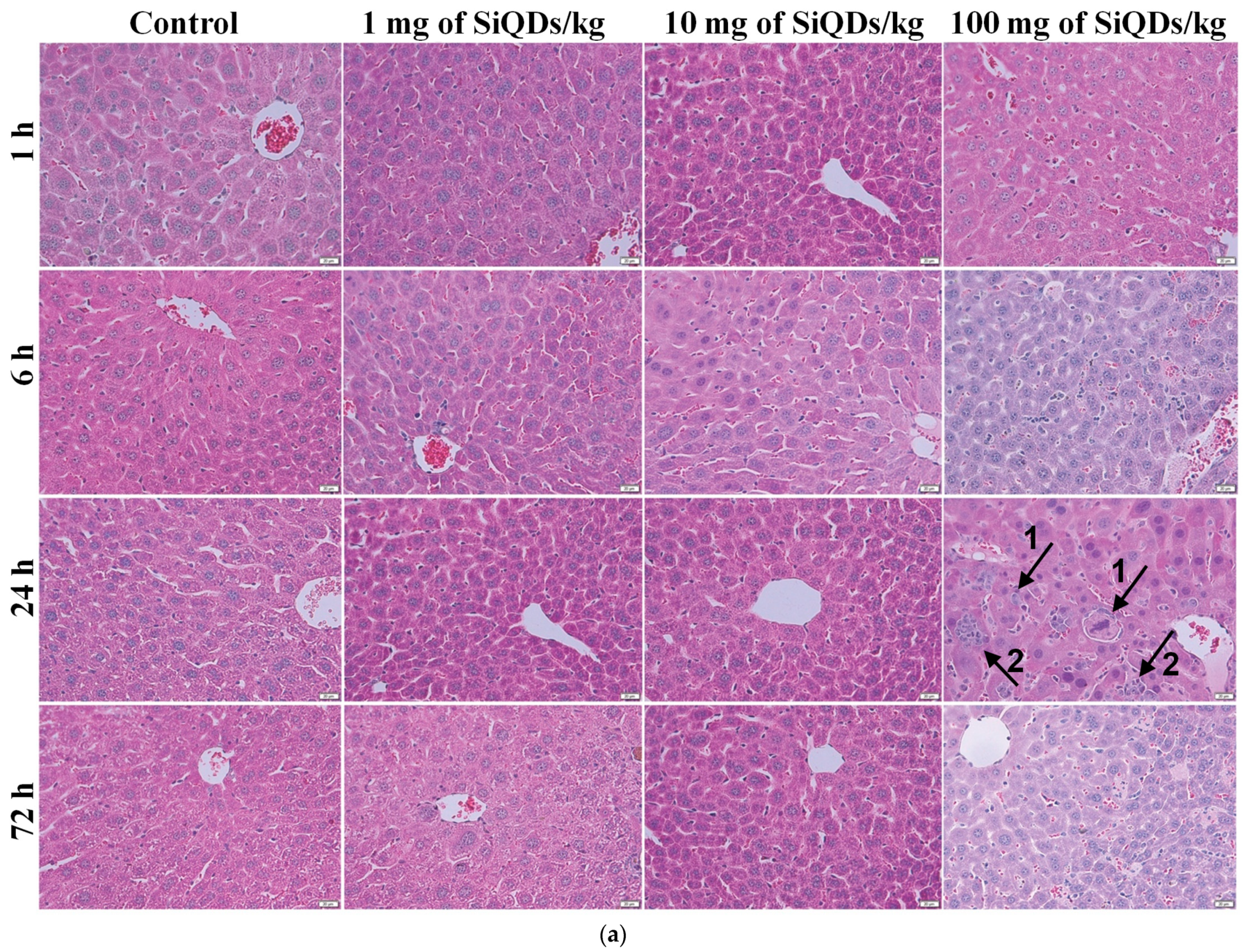
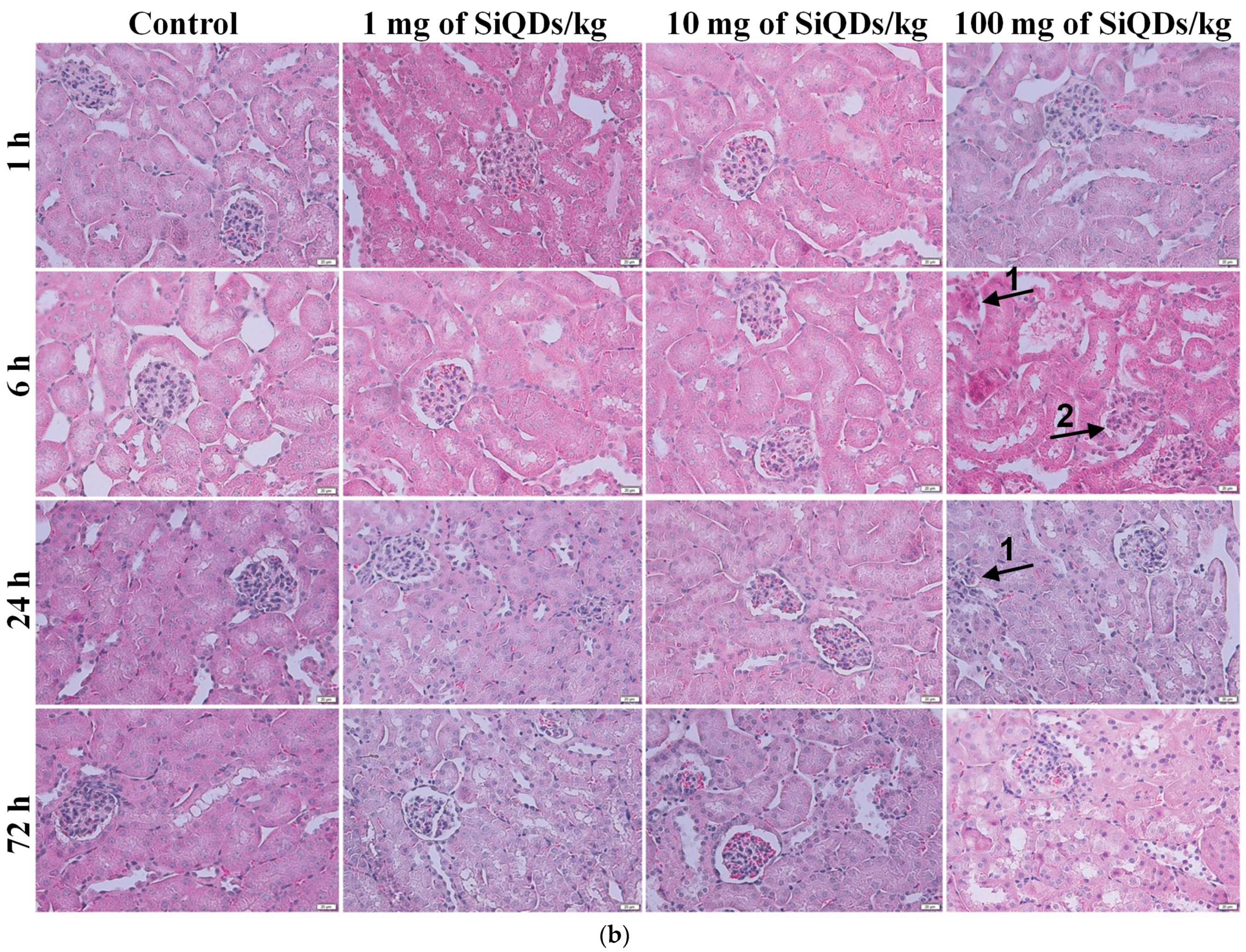
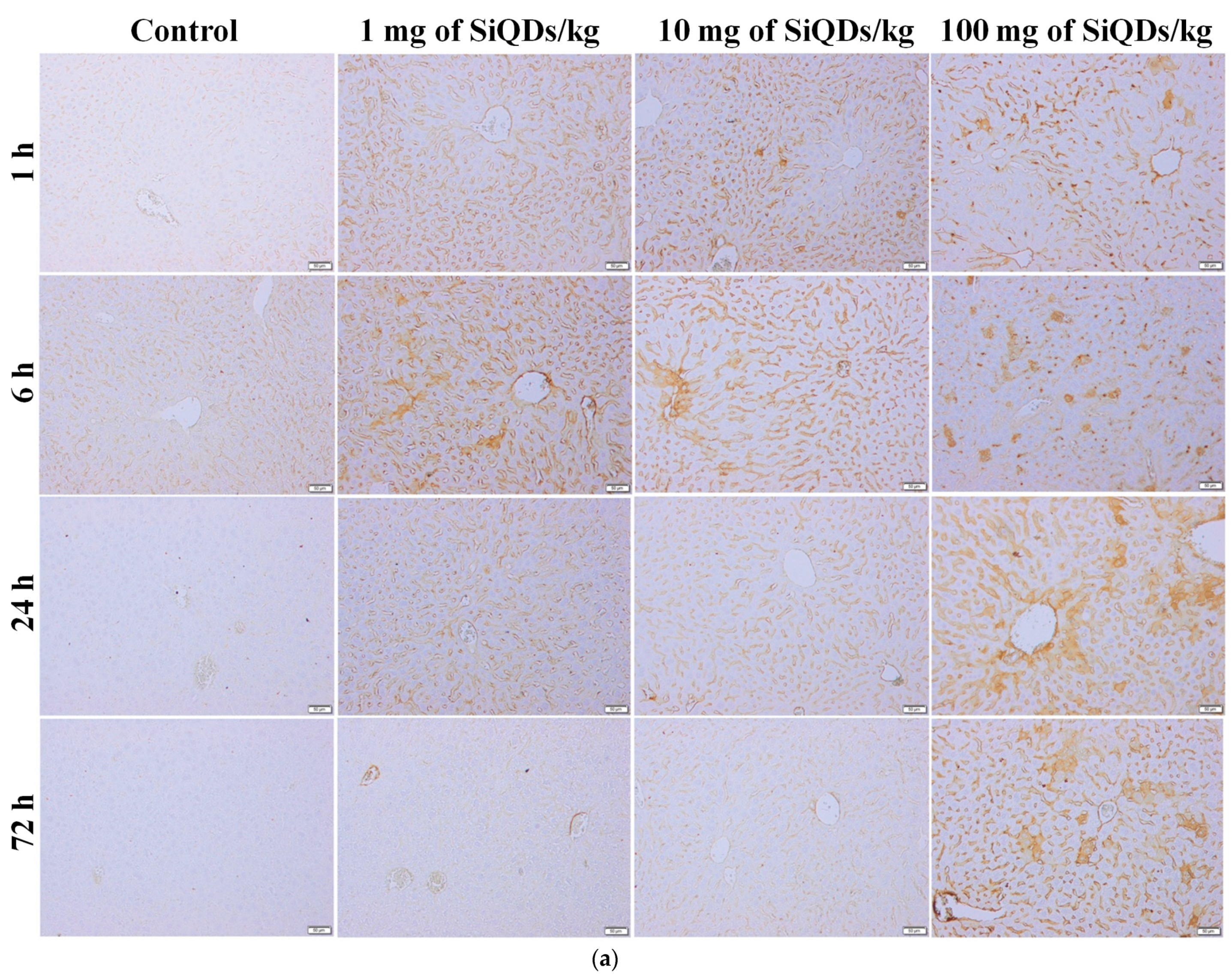
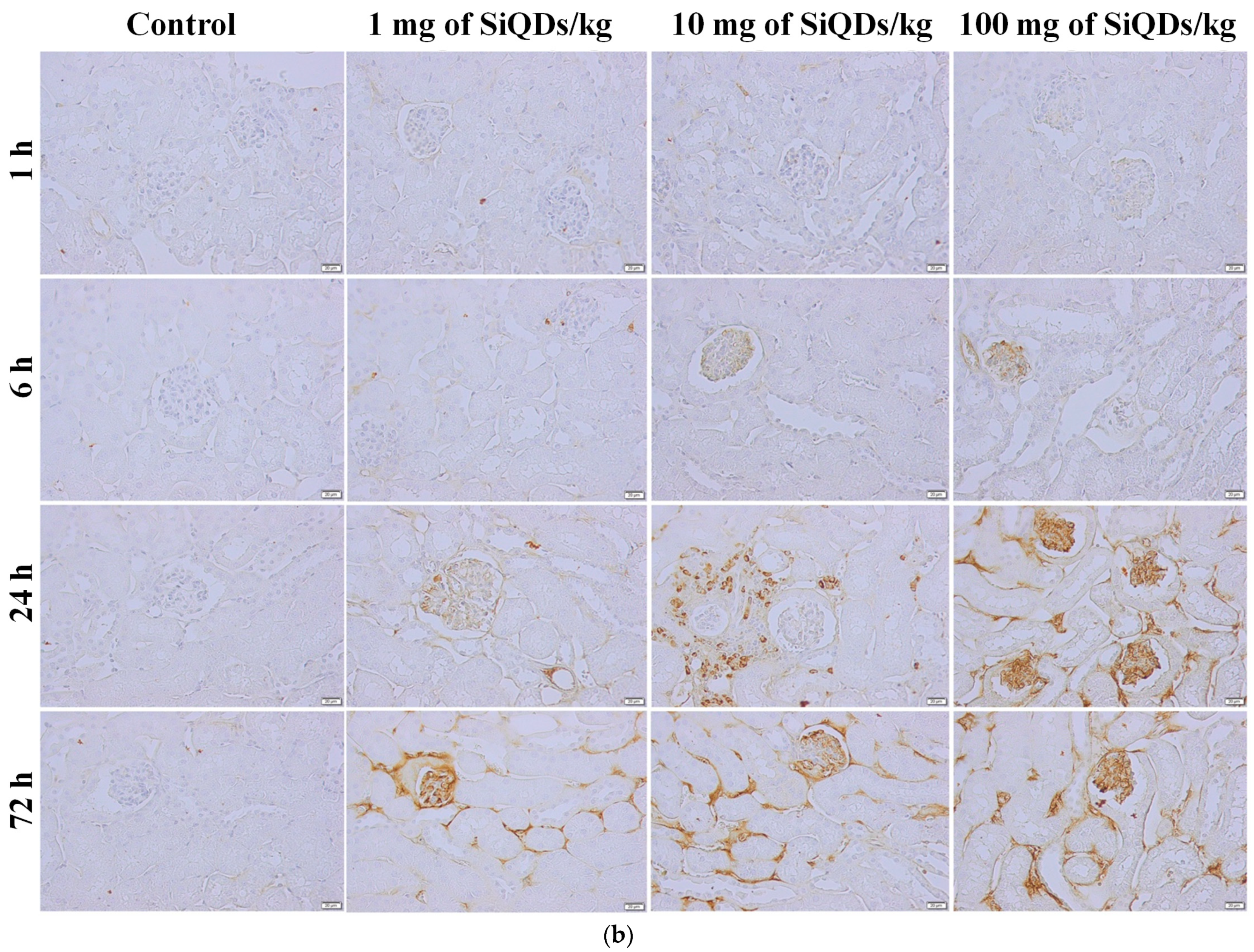
3.2. Histopathology and Immunohistochemical Analysis of the TNF-α in the Mice Liver and Kidneys Exposed to SiQDs
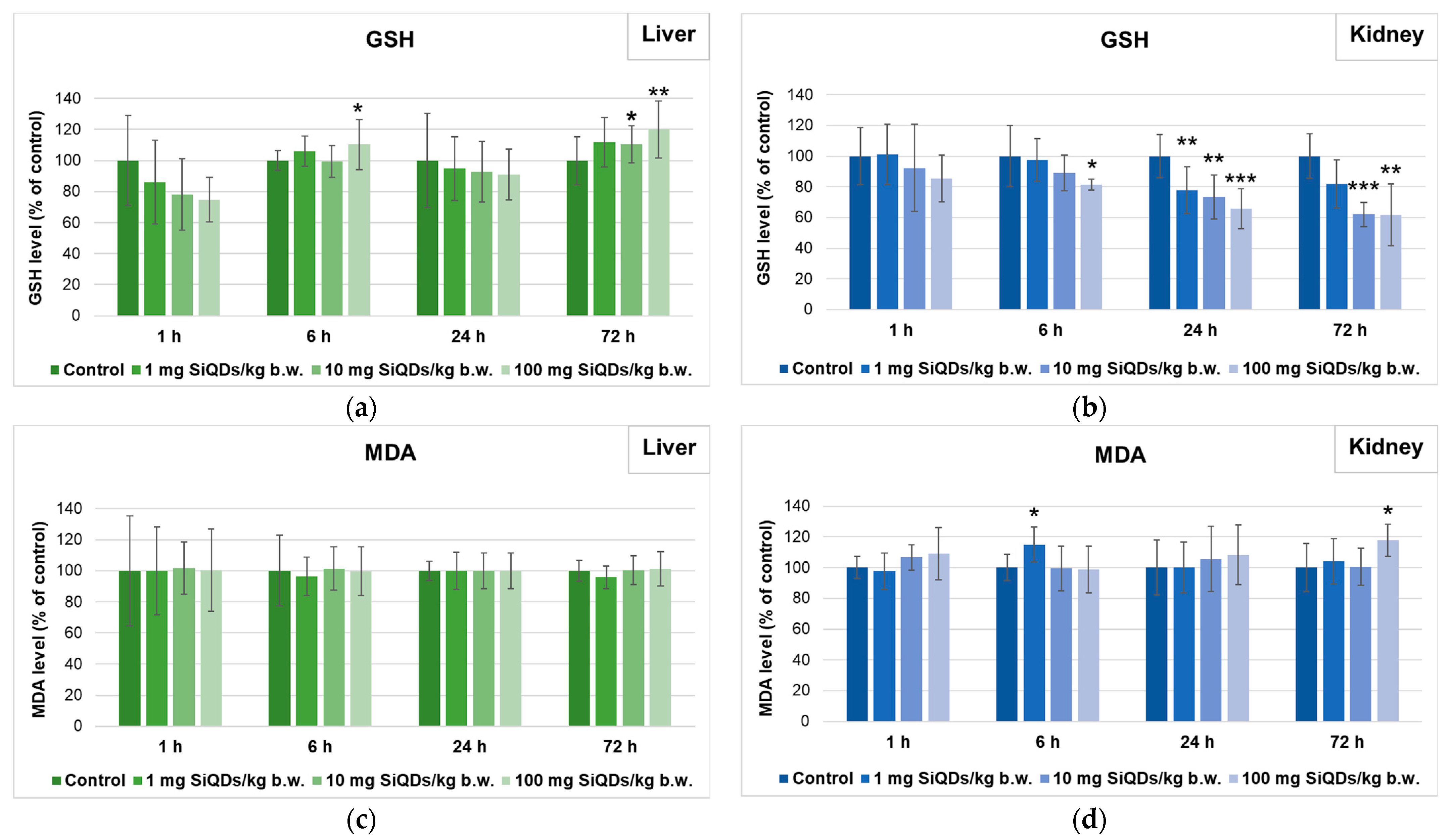
3.3. Analysis of Oxidative Stress Induced by SiQDs Administration
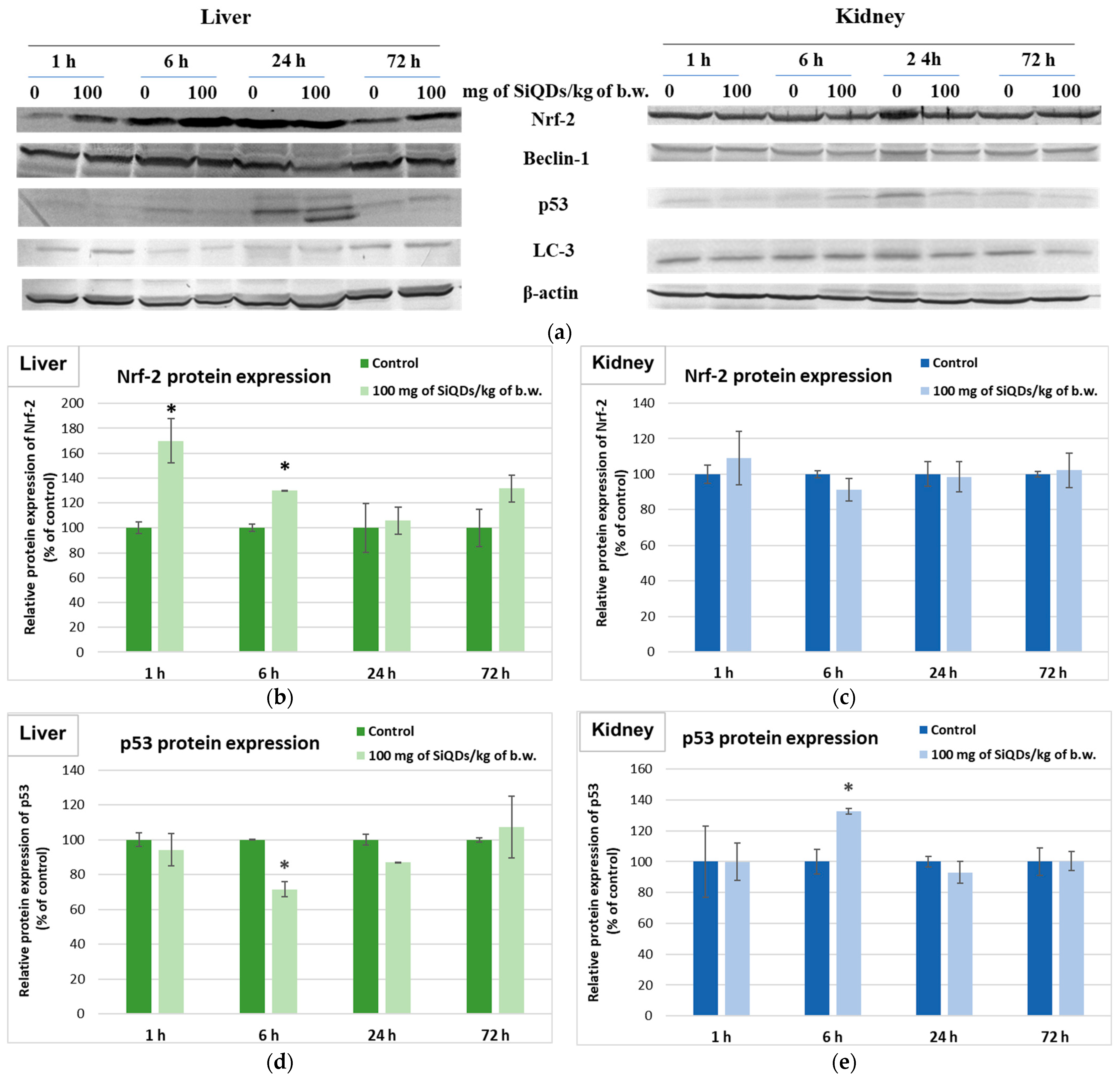
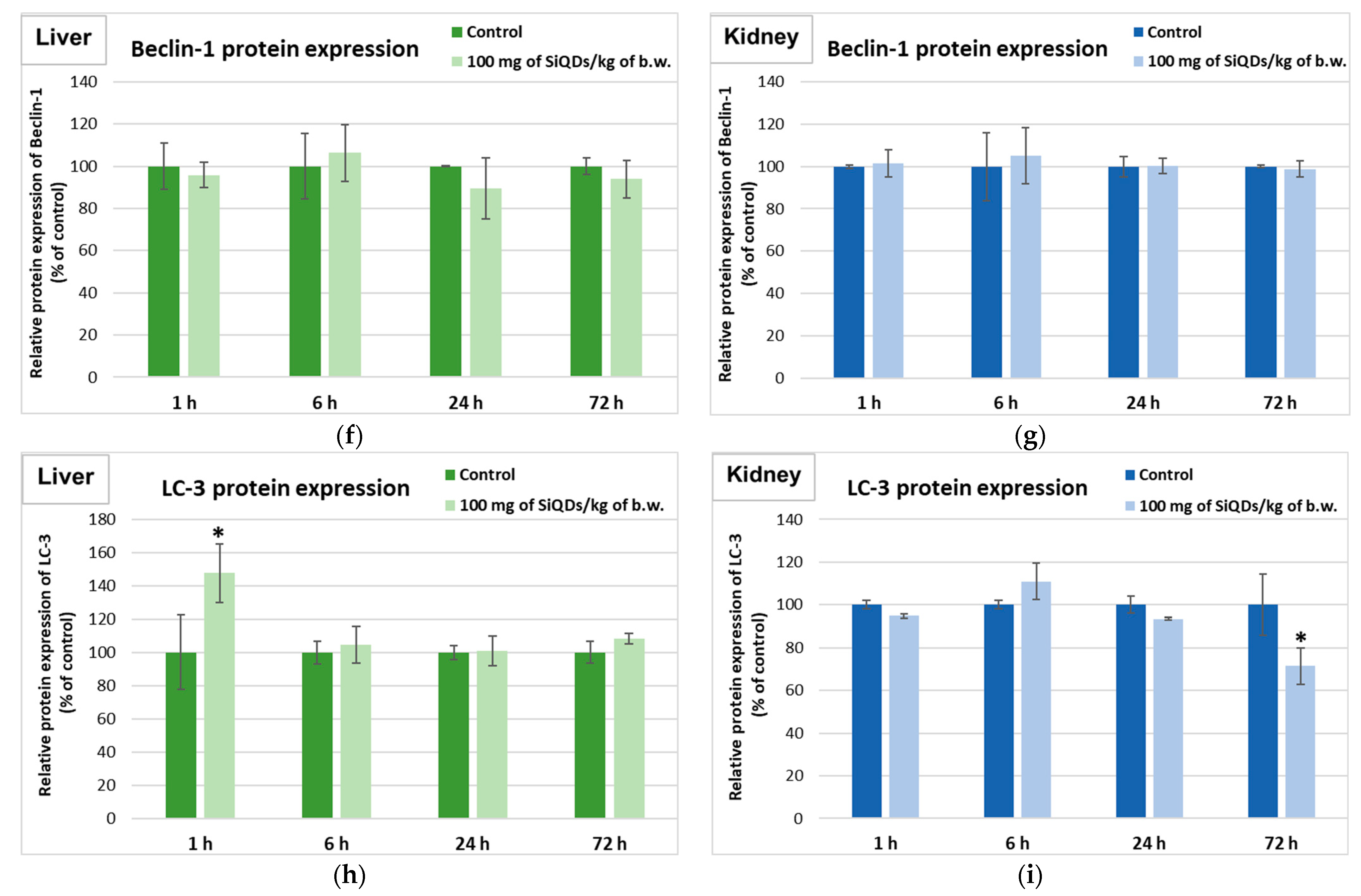
3.4. Analysis of Proteins’ Expression Involved in the Antioxidant Defense System, Apoptosis, and Autophagy
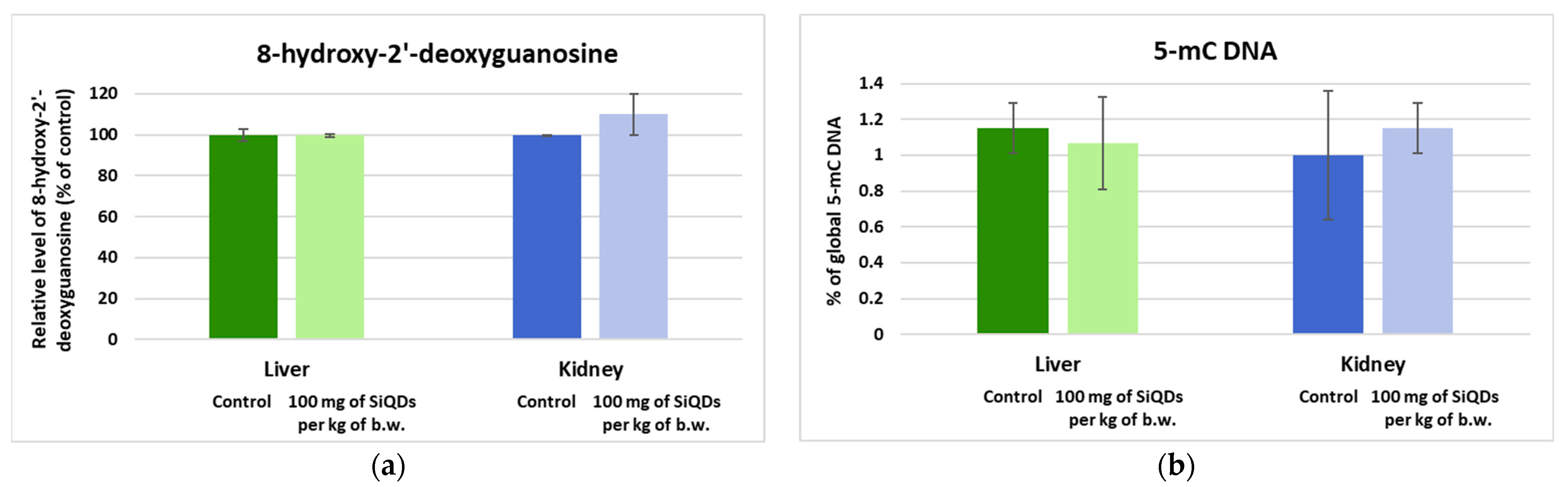
3.5. Genotoxicity Evaluation
3.5.1. Analysis of the 8-OHdG Level
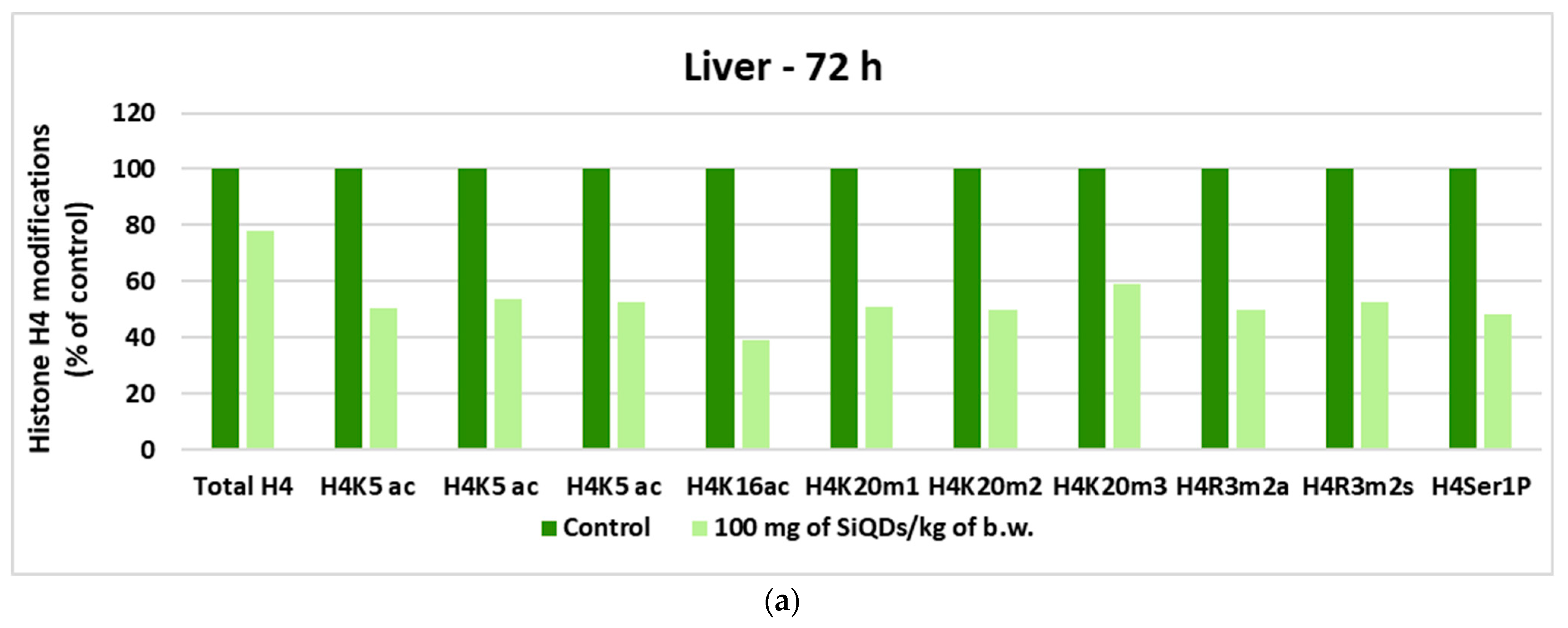
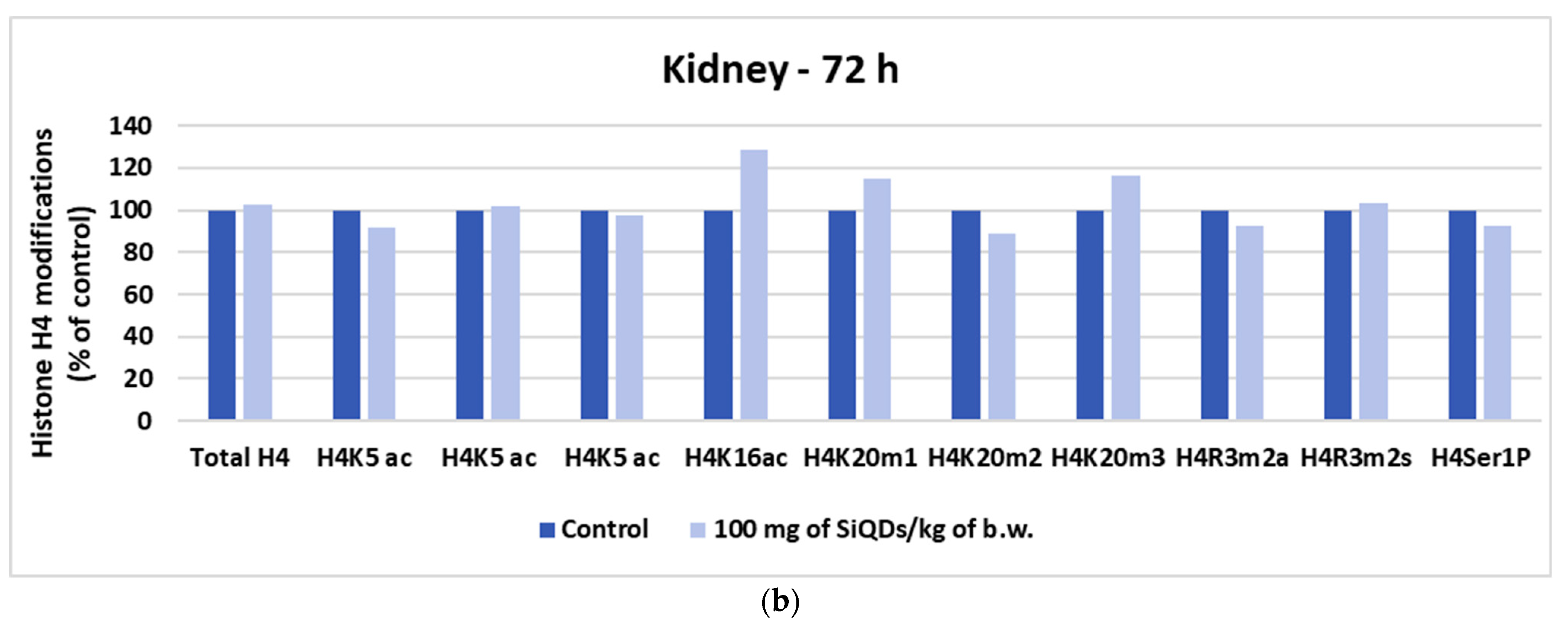
3.5.2. Analysis of Histone H4 Modification
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Montalti, M.; Cantelli, A.; Battistelli, G. Nanodiamonds and silicon quantum dots: Ultrastable and biocompatible luminescent nanoprobes for long-term bioimaging. Chem. Soc. Rev. 2015, 44, 4853–4921. [Google Scholar] [CrossRef]
- Stan, M.S.; Sima, C.; Dinischiotu, A. Silicon Quantum Dots: From synthesis to bioapplications. In Bioactivity of Engineered Nanoparticles; Yan, B., Zhou, H., Gardea-Torresdey, J., Eds.; Springer: Singapore, 2017; pp. 339–359. [Google Scholar]
- Soldado, A.; Barrio, L.C.; Diaz-Gonzalez, M.; de la Escosura-Muniz, A.; Costa-Fernandez, J.M. Advances in quantum dots as diagnostic tools. Adv. Clin. Chem. 2022, 107, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Lowe, S.B.; Reece, P.J.; Gooding, J.J. Colloidal silicon quantum dots: From preparation to the modification of self-assembled monolayers (SAMs) for bio-applications. Chem. Soc. Rev. 2014, 43, 2680–2700. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Su, Y.; Zhong, Y.; Fan, C.; Lee, S.-T.; He, Y. Silicon nanomaterials platform for bioimaging, biosensing, and cancer therapy. Acc. Chem. Res. 2014, 47, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tang, Z.; Dong, Y. Silicon quantum dot involved luminol chemiluminescence and its sensitive detection of dopamine. Anal. Methods 2018, 10, 4129–4135. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Jose, J.; Shanavas, M.S.; Marathakam, A.; Uddin, M.S.; Mathew, B. Silicon Quantum dots: Promising theranostic probes for the future. Curr. Drug Targets 2019, 20, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Ding, Z.; Zhang, J.; Cai, Y.; Bao, X. Recent advances of antioxidant low-dimensional carbon materials for biomedical applications. Front. Bioeng. Biotechnol. 2023, 11, 1121477. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wang, W.; Mattoussi, H. Controlling the spectroscopic properties of quantum dots interactions: Concepts and applications. Nano Today 2016, 11, 98–121. [Google Scholar] [CrossRef]
- Volkov, Y. Quantum dots in nanomedicine: Recent trends, advances and unresolved issues. Biochem. Biophys. Res. Commun. 2015, 468, 419–427. [Google Scholar] [CrossRef]
- González De la Cruz, G.; Rodríguez-Fragoso, L.; Rodríguez-Fragoso, P.; Rodríguez-López, A. Toxicity of Quantum Dots. In Toxicity of Nanoparticles—Recent Advances and New Perspectives; Muzibur Rahman, M., Uddin, J., Mohamed Asiri, A., Rezaur Rahman, M., Eds.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Stan, M.S.; Sima, C.; Cinteză, L.O.; Dinischiotu, A. Silicon-based quantum dots induce inflammation in human lung cells and disrupt extracellular matrix homeostasis. FEBS J. 2015, 282, 2914–2929. [Google Scholar] [CrossRef]
- Stan, M.S.; Memet, I.; Sima, C.; Popescu, T.; Teodorescu, V.S.; Hermenean, A.; Dinischiotu, A. Si/SiO2 quantum dots cause cytotoxicity in lung cells through redox homeostasis imbalance. Chem. Biol. Interact. 2014, 220, 102–115. [Google Scholar] [CrossRef]
- Stanca, L.; Geicu, O.I.; Şerban, A.I.; Dinischiotu, A. Interplay of oxidative stress, inflammation, and autophagy in RAW 264.7 murine macrophage cell line challenged with Si/SiO2 quantum dots. Materials 2023, 16, 5083. [Google Scholar] [CrossRef]
- Șerban, A.I.; Stanca, L.; Sima, C.; Staicu, A.C.; Zărnescu, O.; Dinischiotu, A. Complex responses to Si quantum dots accumulation in carp liver tissue: Beyond oxidative stress. Chem. Biol. Interact. 2015, 239, 56–66. [Google Scholar] [CrossRef]
- Fan, J.-W.; Vankayala, R.; Chang, C.-L.; Chang, C.-H.; Chiang, C.-S.; Hwang, K.C. Preparation, cytotoxicity and in vivo bioimaging of highly luminescent water-soluble silicon quantum dots. Nanotechnology 2015, 26, 215703. [Google Scholar] [CrossRef]
- Wang, Y.W.; Yang, K.; Tang, H.; Chen, D.; Bai, Y.L. Toxicity assessment of repeated intravenous injections of arginine-glycine-aspartic acid peptide conjugated CdSeTe/ZnS quantum dots in mice. Int. J. Nanomed. 2014, 17, 4809–4817. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, S.Y.; Yu, B.; Xu, S.; Shen, J.; Zhao, T.; Zhang, H. Hepatotoxicity assessment of Mn-doped ZnS quantum dots after repeated administration in mice. Int. J. Nanomed. 2015, 15, 5787–5796. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Meng, P.; Wang, M.; Tian, M.; Xiong, Y.; Zhang, X.; Huang, P. Dose and time effect of CdTe quantum dots on antioxidant capacities of the liver and kidneys in mice. Int. J. Nanomed. 2017, 1, 6425–6435. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Tang, M.; Kong, L.; Ying, J.; Wu, T.; Xue, Y.; Pu, Y. Liver toxicity of cadmium telluride quantum dots (CdTe QDs) due to oxidative stress in vitro and in vivo. Int. J. Mol. Sci. 2015, 16, 23279–23299. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Bjornmalm, M.; Thurecht, K.J.; Kent, S.J.; Parton, R.G.; Kavallaris, M.; Jahnstin, A.P.R.; Gooding, J.J.; Corrie, S.R.; Boyd, B.J.; et al. Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol. 2018, 13, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Grigoriu, C.; Kuroki, Y.; Nicolae, I.; Zhu, X.; Hirai, M.; Suematsu, H.; Takata, M.; Yatsui, K. Photo and cathodoluminescence of Si/SiO2 nanoparticles produced by laser ablation. J. Optoelectron. Adv. Mater. 2005, 7, 2979–2984. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1974, 105, 121–126. [Google Scholar] [CrossRef]
- Paoletti, F.; Aldinucci, D.; Mocali, A.; Caparrini, A. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal. Biochem. 1986, 154, 536–541. [Google Scholar] [CrossRef]
- Beutler, E. Glutathione peroxidase. In Red Cell Metabolism; A Manual of Biochemical Methods; Beutler, E., Ed.; Grune and Stratton: Orlando, FL, USA, 1984; pp. 74–76. [Google Scholar]
- Goldberg, D.M.; Spooner, R.J. Assay of Glutathione Reductase. In Methods of Enzymatic Analysis, 3rd ed.; Bergmeyen, H.V., Ed.; Verlog Chemie: Deerfiled Beach, FL, USA, 1983; Volume 3, pp. 258–265. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Omari, S.S.; Nasirzadeh, N. 8-Hydroxy-2′-deoxyguanosine (8-OHdG) as a biomarker of oxidative DNA damage induced by occupational exposure to nanomaterials: A systematic review. Nanotoxicology 2021, 15, 850–864. [Google Scholar] [CrossRef]
- Diz, A.P.; Truebano, M.; Skibinski, D.O.F. The consequences of sample pooling in proteomics: An empirical study. Electrophoresis 2009, 30, 2967–2975. [Google Scholar] [CrossRef] [PubMed]
- Laurin, E.; Thakur, K.; Mohr, P.G.; Hick, P.; Crane, M.S.J.; Gardner, I.A.; Moody, N.J.G.; Colling, A.; Ernst, I. To pool or not to pool? Guidelines for pooling samples for use in surveillance testing of infectious diseases in aquatic animals. J. Fish. Dis. 2019, 42, 1471–1491. [Google Scholar] [CrossRef]
- Tost, J. DNA Methylation: An Introduction to the Biology and the Disease-Associated Changes of a Promising Biomarker. Mol. Biotechnol. 2009, 44, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.S.; Badea, S.; Hermenean, A.; Herman, H.; Trica, B.; Sbarcea, B.G.; Dinischiotu, A. New insights into the cell death signaling pathways triggered by long-Term exposure to silicon-based quantum dots in human lung fibroblasts. Nanomaterials 2021, 11, 323. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Pilger, A.; Rudiger, H.W. 8-Hydroxy-2′-deoxyguanosine as a marker of oxidative DNA damage related to occupational and environmental exposures. Int. Arch. Occup. Environ. Health 2006, 80, 1–15. [Google Scholar] [CrossRef]
- Rim, K.T.; Song, S.W.; Kim, H.Y. Oxidative DNA damage from nanoparticle exposure and its application to workers’ health: A literature review. Saf. Health Work 2013, 4, 177–186. [Google Scholar] [CrossRef]
- Bouwmeester, M.C.; Ruiter, S.; Lommelaars, T.; Sippel, J.; Hodemaekers, H.M.; van den Brandhof, E.J.; Pennings, J.L.; Kamstra, J.H.; Jelinek, J.; Issa, J.P.; et al. Zebrafish embryos as a screen for DNA methylation modifications after compound exposure. Toxicol. Appl. Pharmacol. 2016, 291, 84–96. [Google Scholar] [CrossRef]
- Jiang, S.; Guo, Y. Epigenetic clock: DNA methylation in aging. Stem Cells Int. 2020, 8, 1047896. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Z.; Jia, J.; Du, T.; Zhang, N.; Tang, Y.; Fang, Y.; Fang, D. Overview of histone modification. Adv. Exp. Med. Biol. 2021, 1283, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Andres, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of hydrogen peroxide formation and elimination in mammalian cells, and its role in various pathologies. Stresses 2022, 2, 256–274. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Morris, R.; Cheng, F. Signaling pathway regulated by silica nanoparticles. Molecules 2021, 26, 1398. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Meena, R.; Gaharwar, U.S.; Priyadarshini, E.; Rawat, K.; Paulraj, R.; Mohanta, Y.K.; Saravanan, M.; Bohidar, H.B. Bioaccumulation of CdSe quantum dots show biochemical and oxidative damage in wistar rats. Oxidative Med. Cell. Longev. 2023, 6, 7707452. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, L.; Qu, C.; Zhang, C.; Hong, L.; Jiang, G. CdSe Quantum Dot (QDS)-Induced Morphological and Functional Impairments to Liver in Mice. PLoS ONE 2011, 6, e24406. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.S.; Cinteza, L.O.; Petrescu, L.; Mernea, M.A.; Calborean, O.; Mihailescu, D.F.; Sima, C.; Dinischiotu, A. Dynamic analysis of the interactions between Si/SiO2 quantum dots and biomolecules for improving applications based on nano-bio interfaces. Sci. Rep. 2018, 8, 5289. [Google Scholar] [CrossRef] [PubMed]
- Mills-Goodlet, R.; Johnson, L.; Hoppe, I.J.; Regl, C.; Geppert, M.; Schenck, M.; Huber, S.; Hauser, M.; Ferreira, F.; Hűsing, N.; et al. The nanotopography of SiO2 particles impacts the selectivity and 3D fold of bound allergens. Nanoscale 2021, 13, 20508–20520. [Google Scholar] [CrossRef]
- Tu, C.; Ma, X.; House, A.; Kauzlarich, S.M.; Louie, A.Y. PET imaging and biodistribution of silicon quantum dots in mice. ACS Med. Chem. Lett. 2011, 2, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yang, S.T.; Yang, Y.F.; Ke, D.M.; Liu, J.H.; Chen, X.; Wang, H.; Liu, Y. Blood clearance, distribution, transformation, excretion, and toxicity of near-infrared quantum dots Ag2Se in mice. ACS Appl. Mater. Interfaces 2016, 8, 17859–17869. [Google Scholar] [CrossRef]
- Zhang, M.; Yue, J.; Cui, R.; Ma, Z.; Wan, H.; Wang, F.; Zhu, S.; Zhou, Y.; Kuang, Y.; Zhong, Y.; et al. Bright quantum dots emitting at ~1600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc. Natl. Acad. Sci. USA 2018, 115, 6590–6595. [Google Scholar] [CrossRef] [PubMed]
- Gerasimovich, E.; Bozrova, S.; Baryshnikova, M.; Sokolova, Z.; Samokhvalov, P.; Karaulov, A.; Nabiev, I.; Sukhanova, A. In vivo assessment of acute toxicity and immunotoxicity of quantum dots with different physicochemical properties. Preprints 2023, 2023080032. [Google Scholar] [CrossRef]
- Nguyen, K.C.; Zhang, Y.; Todd, J.; Kittle, K.; Patry, D.; Caldwell, D.; Lalande, M.; Smith, S.; Parks, D.; Navarro, M.; et al. Biodistribution and systemic effects in mice following intravenous administration of cadmium telluride quantum dot nanoparticles. Chem. Res. Toxicol. 2019, 32, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Sun, Y.; Wang, S.; Li, Y.; Zeng, X.; Cao, Z.; Yang, P.; Song, P.; Wang, Z.; Xian, Z.; et al. Inhibition of autophagy overcomes the nanotoxicity elicited by cadmium-based quantum dots. Biomaterials 2016, 78, 102–114. [Google Scholar] [CrossRef]
- Liu, J.; Erogbogbo, F.; Yong, K.T.; Ye, L.; Liu, J.; Hu, R.; Chen, H.; Hu, Y.; Yang, Y.; Yang, J.; et al. Assessing clinical prospects of silicon quantum dots: Studies in mice and monkeys. ACS Nano 2013, 7, 7303–7310. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Wu, Y.; Fu, K.; Chen, Y.; Li, W.; Chu, M. Systematic evaluation of graphene quantum dot toxicity to male mouse sexual behaviors, reproductive and offspring health. Biomaterials 2019, 194, 215–232. [Google Scholar] [CrossRef]
- Geys, J.; Nemmar, A.; Verbeken, E.; Smolders, E.; Ratoi, M.; Hoylaerts, M.F.; Nemery, B.; Hoet, P.H. Acute toxicity and prothrombotic effects of quantum dots: Impact of surface charge. Environ. Health Perspect. 2008, 116, 1607–1613. [Google Scholar] [CrossRef]
- Lin, G.; Ouyang, Q.; Hu, R.; Ding, Z.; Tian, J.; Yin, F.; Xu, G.; Chen, Q.; Wang, X.; Yong, K.-T. In vivo toxicity assessment of non-cadmium quantum dots in BALB/c mice. Nanomedicine 2015, 11, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, Y.; Zhang, C.; Lai, X.; Zhang, Y.; Wu, J.; Hu, C.; Shao, L. Nanomaterial-mediated autophagy: Coexisting hazard and health benefits in biomedicine. Part. Fibre Toxicol. 2020, 17, 53. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, T.; Tang, M. The DNA damage potential of quantum dots: Toxicity, mechanism and challenge. Environ. Pollut. 2023, 317, 120676. [Google Scholar] [CrossRef]
- Singh, N.; Manshian, B.; Jenkins, G.J.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef] [PubMed]
- Magdolenova, Z.; Collins, A.; Kumar, A.; Dhawan, A.; Stone, V.; Dusinska, M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 2013, 8, 233–278. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Tao, G.; Yang, L.; Liu, J.; Liu, Q.; Zhuang, Z. SiO2 nanoparticles induce global genomic hypomethylation in HaCaT cells. Biochem. Biophys. Res. Commun. 2010, 397, 397–400. [Google Scholar] [CrossRef]
- Brown, T.A.; Lee, J.W.; Holian, A.; Porter, V.; Fredriksen, H.; Kim, M.; Cho, Y.H. Alterations in DNA methylation corresponding with lung inflammation and as a biomarker for disease development after MWCNT exposure. Nanotoxicology 2016, 10, 453–461. [Google Scholar] [CrossRef]
- Seidel, C.; Kirsch, A.; Fontana, C.; Visvikis, A.; Remy, A.; Gaté, L.; Darne, C.; Guichard, Y. Epigenetic changes in the early stage of silica-induced cell transformation. Nanotoxicology 2017, 11, 923–935. [Google Scholar] [CrossRef]
- Lu, X.; Miousse, I.R.; Pirela, S.V.; Moore, J.K.; Melnyk, S.; Koturbash, I.; Demokritou, P. In vivo epigenetic effects induced by engineered nanomaterials: A case study of copper oxide and laser printer-emitted engineered nanoparticles. Nanotoxicology 2016, 10, 629–639. [Google Scholar] [CrossRef]
- Hu, J.; Lin, W.; Lin, B.; Wu, K.; Fan, H.; Yu, Y. Persistent DNA methylation changes in zebrafish following graphene quantum dots exposure in surface chemistry-dependent manner. Ecotoxicol. Environ. Saf. 2019, 169, 370–375. [Google Scholar] [CrossRef]
- Gao, F.; Ma, N.; Zhou, H.; Wang, Q.; Zhang, H.; Wang, P.; Hou, H.; Wen, H.; Li, L. Zinc oxide nanoparticles-induced epigenetic change and G2/M arrest are associated with apoptosis in human epidermal keratinocytes. Int. J. Nanomed. 2016, 11, 3859–3874. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristian, R.-E.; Balta, C.; Herman, H.; Trica, B.; Sbarcea, B.G.; Hermenean, A.; Dinischiotu, A.; Stan, M.S. In Vivo Assessment of Hepatic and Kidney Toxicity Induced by Silicon Quantum Dots in Mice. Nanomaterials 2024, 14, 457. https://doi.org/10.3390/nano14050457
Cristian R-E, Balta C, Herman H, Trica B, Sbarcea BG, Hermenean A, Dinischiotu A, Stan MS. In Vivo Assessment of Hepatic and Kidney Toxicity Induced by Silicon Quantum Dots in Mice. Nanomaterials. 2024; 14(5):457. https://doi.org/10.3390/nano14050457
Chicago/Turabian StyleCristian, Roxana-Elena, Cornel Balta, Hildegard Herman, Bogdan Trica, Beatrice G. Sbarcea, Anca Hermenean, Anca Dinischiotu, and Miruna S. Stan. 2024. "In Vivo Assessment of Hepatic and Kidney Toxicity Induced by Silicon Quantum Dots in Mice" Nanomaterials 14, no. 5: 457. https://doi.org/10.3390/nano14050457









