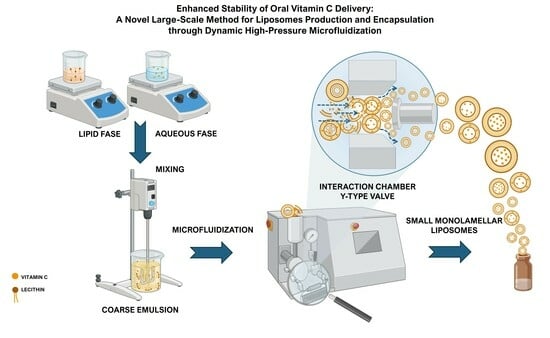
Enhanced Stability of Oral Vitamin C Delivery: A Novel Large-Scale Method for Liposomes Production and Encapsulation through Dynamic High-Pressure Microfluidization
Abstract
:1. Introduction
2. Materials and Methods
2.1. HPH for Vitamin C-Loaded Liposomes Production
2.2. Characterization of Lipo-C: Morphological, Physicochemical Analysis and Vitamin C Encapsulation Efficiency
2.3. Stability Studies of Lipo-C
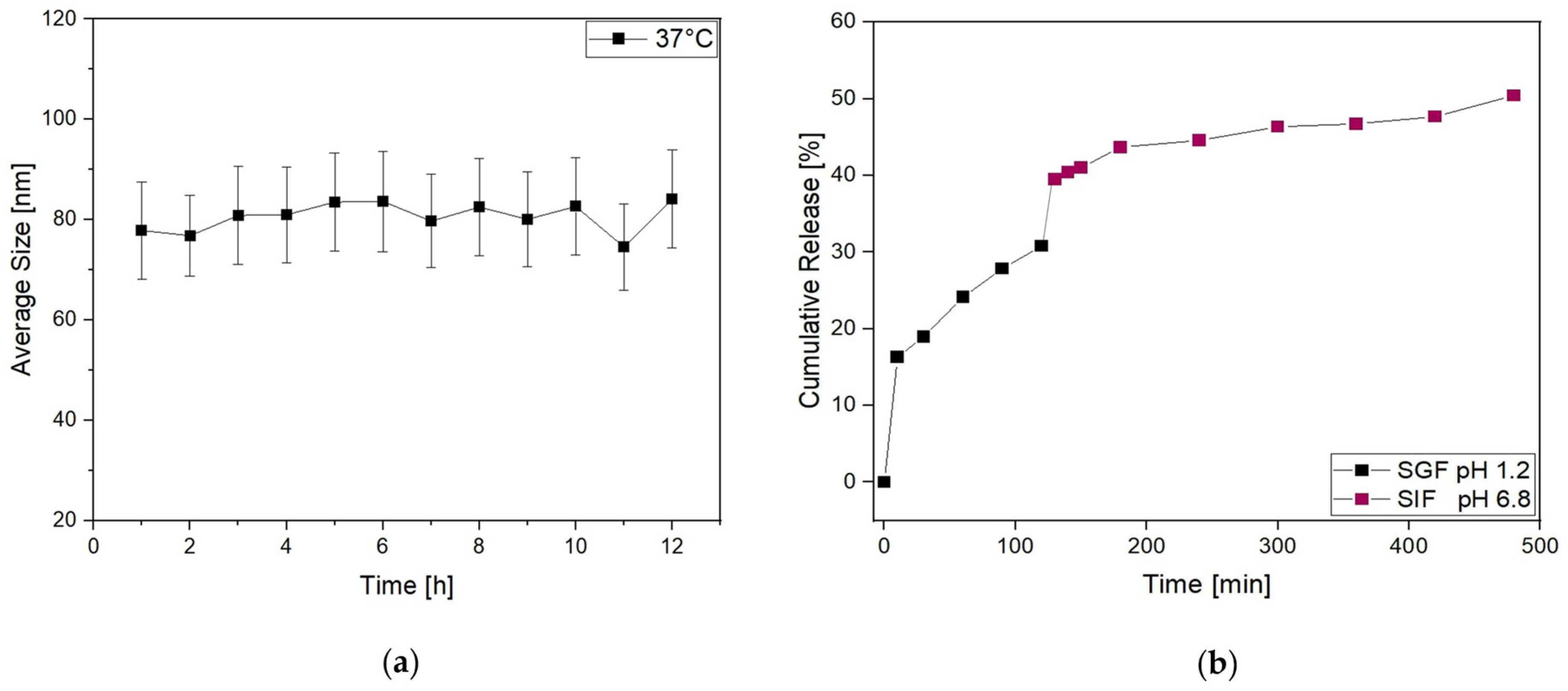
2.3.1. Storage Stability by Size and Antioxidant Activity Monitoring
2.3.2. Release Studies in Simulated GIF
2.3.3. Statistical Analysis
3. Results
3.1. Effect of HPH Operative Parameters on Lipo-C Preparation
3.2. Lipo-C-20 Storage Stability and Behavior in Simulated GIF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katouzian, I.; Esfanjani, A.F.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Lamparelli, E.P.; Ciardulli, M.C.; Scala, P.; Scognamiglio, M.; Charlier, B.; Di Pietro, P.; Izzo, V.; Vecchione, C.; Maffulli, N.; Della Porta, G. Lipid nano-vesicles for thyroid hormone encapsulation: A comparison between different fabrication technologies, drug loading, and an in vitro delivery to human tendon stem/progenitor cells in 2D and 3D culture. Int. J. Pharm. 2022, 624, 122007. [Google Scholar] [CrossRef]
- Keller, B.C. Liposomes in nutrition. Trends Food Sci. Technol. 2001, 12, 25–31. [Google Scholar] [CrossRef]
- Subramanian, P. Lipid-based nanocarrier system for the effective delivery of nutraceuticals. Molecules 2021, 26, 5510. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrini, L.; Maestrelli, F.; Mennini, N.; Mura, P.; Ghelardini, C.; Mannelli, L.D.C. Design, characterization and in vivo evaluation of nanostructured lipid carriers (NLC) as a new drug delivery system for hydrochlorothiazide oral administration in pediatric therapy. Drug Deliv. 2018, 25, 1910–1921. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of nanoliposomal vitamin D3 for potential application in beverage fortification. Adv. Pharm. Bull. 2014, 4 (Suppl. 2), 569. [Google Scholar] [PubMed]
- Sebaaly, C.; Jraij, A.; Fessi, H.; Charcosset, C.; Greige-Gerges, H. Preparation and characterization of clove essential oil-loaded liposomes. Food Chem. 2015, 178, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Lee, K.-E.; Kim, J.-J.; Lim, S.-H. The effect of cholesterol in the liposome bilayer on the stabilization of incorporated retinol. J. Liposome Res. 2005, 15, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.; Babazadeh, A.; Rehman, A.; Shaddel, R.; Akbari-Alavijeh, S.; Boostani, S.; Jafari, S. Protection and controlled release of vitamin C by different micro/nanocarriers. Crit. Rev. Food Sci. Nutr. 2020, 62, 3301–3322. [Google Scholar] [CrossRef] [PubMed]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and anti-Inflammatory activity of ascorbic acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Drouin, G.; Godin, J.-R.; Page, B. The genetics of vitamin C loss in vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef] [PubMed]
- Herbig, A.-L.; Renard, C.M. Factors that impact the stability of vitamin C at intermediate temperatures in a food matrix. Food Chem. 2017, 220, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.A.; Farshi, P.; Ahmadi, P.; Ahmadi, A.; Yousefi, M.; Ghorbani, M.; Hosseini, S.M. Encapsulation of Vitamins Using Nanoliposome: Recent Advances and Perspectives. Adv. Pharm. Bull. 2023, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Kirby, C.J.; Whittle, C.J.; Rigby, N.; Coxon, D.T.; Law, B.A. Stabilization of ascorbic acid by microencapsulation in liposomes. Int. J. Food Sci. Technol. 1991, 26, 437–449. [Google Scholar] [CrossRef]
- Marsanasco, M.; Márquez, A.L.; Wagner, J.R.; Alonso, S.d.V.; Chiaramoni, N.S. Liposomes as vehicles for vitamins E and C: An alternative to fortify orange juice and offer vitamin C protection after heat treatment. Food Res. Int. 2011, 44, 3039–3046. [Google Scholar] [CrossRef]
- Łukawski, M.; Dałek, P.; Borowik, T.; Foryś, A.; Langner, M.; Witkiewicz, W.; Przybyło, M. New oral liposomal vitamin C formulation: Properties and bioavailability. J. Liposome Res. 2020, 30, 227–234. [Google Scholar] [CrossRef]
- Chen, J.; Dehabadi, L.; Ma, Y.-C.; Wilson, L.D. Development of novel lipid-based formulations for water-soluble vitamin C versus fat-soluble vitamin D3. Bioengineering 2022, 9, 819. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Romano, E.; Netti, P.A.; Torino, E. A high throughput approach based on dynamic high pressure for the encapsulation of active compounds in exosomes for precision medicine. Int. J. Mol. Sci. 2021, 22, 9896. [Google Scholar] [CrossRef]
- Melchior, S.; Codrich, M.; Gorassini, A.; Mehn, D.; Ponti, J.; Verardo, G.; Tell, G.; Calzolai, L.; Calligaris, S. Design and advanced characterization of quercetin-loaded nano-liposomes prepared by high-pressure homogenization. Food Chem. 2023, 428, 136680. [Google Scholar] [CrossRef]
- Li, Y.; Deng, L.; Dai, T.; Li, Y.; Chen, J.; Liu, W.; Liu, C. Microfluidization: A promising food processing technology and its challenges in industrial application. Food Control 2022, 137, 108794. [Google Scholar] [CrossRef]
- Yadav, K.S.; Kale, K. High pressure homogenizer in pharmaceuticals: Understanding its critical processing parameters and applications. J. Pharm. Innov. 2019, 15, 690–701. [Google Scholar] [CrossRef]
- Vinchhi, P.; Patel, J.K.; Patel, M.M. High-pressure homogenization techniques for nanoparticles. In Emerging Technologies for Nanoparticle Manufacturing; Springer: Cham, Switzerland, 2021; pp. 263–285. [Google Scholar]
- Hidajat, M.J.; Jo, W.; Kim, H.; Noh, J. Effective Droplet Size Reduction and Excellent Stability of Limonene Nanoemulsion Formed by High-Pressure Homogenizer. Colloids Interfaces 2020, 4, 5. [Google Scholar] [CrossRef]
- Romano, E.; Campagnuolo, C.; Palladino, R.; Schiavo, G.; Maglione, B.; Luceri, C.; Mennini, N. Technical Evaluation of a New Medical Device Based on Rigenase in the Treatment of Chronic Skin Lesions. Bioengineering 2023, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, P.; Zou, Y.X.; Luo, Z.G.; Tamer, T.M. Co-encapsulation of Vitamin C and β-Carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef] [PubMed]
- Németh, Z.; Csóka, I.; Jazani, R.S.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef] [PubMed]
- Pochapski, D.J.; dos Santos, C.C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: Effects of experimental conditions and electrokinetic models on the interpretation of results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef] [PubMed]
- Roffo, F.; Ponsiglione, A.M.; Netti, P.A.; Torino, E. coupled Hydrodynamic Flow Focusing (cHFF) to Engineer Lipid–Polymer Nanoparticles (LiPoNs) for Multimodal Imaging and Theranostic Applications. Biomedicines 2022, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation—Guidance for Industry; US Food and Drug Administration: Silver Spring, MD, USA, 2018.
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef]
- Gopi, S.; Balakrishnan, P. Evaluation and clinical comparison studies on liposomal and non-liposomal ascorbic acid (vitamin C) and their enhanced bioavailability. J. Liposome Res. 2020, 31, 356–364. [Google Scholar] [CrossRef]
- Chaves, M.A.; Ferreira, L.S.; Baldino, L.; Pinho, S.C.; Reverchon, E. Current Applications of Liposomes for the Delivery of Vitamins: A Systematic Review. Nanomaterials 2023, 13, 1557. [Google Scholar] [CrossRef] [PubMed]
- Unlu, A.; Kirca, O.; Ozdogan, M.; Nayır, E. High-dose vitamin C and cancer. J. Oncol. Sci. 2016, 1, 10–12. [Google Scholar] [CrossRef]
- Carr, A.C.; Lykkesfeldt, J. Factors Affecting the Vitamin C Dose-Concentration Relationship: Implications for Global Vitamin C Dietary Recommendations. Nutrients 2023, 15, 1657. [Google Scholar] [CrossRef]
- Bedhiafi, T.; Idoudi, S.; Fernandes, Q.; Al-Zaidan, L.; Uddin, S.; Dermime, S.; Billa, N.; Merhi, M. Nano-vitamin C: A promising candidate for therapeutic applications. Biomed. Pharmacother. 2023, 158, 114093. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Nguyen, D.H. Development and in vitro evaluation of liposomes using soy lecithin to encapsulate paclitaxel. Int. J. Biomater. 2017, 2017, 8234712. [Google Scholar] [CrossRef]
- Shade, C.W. Liposomes as advanced delivery systems for nutraceuticals. Integr. Med. A Clin. J. 2016, 15, 33. [Google Scholar]
- Widayanti, A.; Elfiyani, R.; Lestari, D. Effect of Lecithin’s concentration of entrapment vitamin E acetate liposomes using thin layers hydration method. Adv. Sci. Lett. 2017, 23, 12510–12513. [Google Scholar] [CrossRef]
- Villalobos-Castillejos, F.; Granillo-Guerrero, V.G.; Leyva-Daniel, D.E.; Alamilla-Beltrán, L.; Gutiérrez-López, G.F.; Monroy-Villagrana, A.; Jafari, S.M. Fabrication of nanoemulsions by microfluidization. In Nanoemulsions; Academic Press: Cambridge, MA, USA, 2018; pp. 207–232. [Google Scholar]
- Ocampo-Salinas, I.O.; Tellez-Medina, D.I.; Jimenez-Martinez, C.; Davila-Ortiz, G. Application of high pressure homogenization to improve stability and decrease droplet size in emulsion-flavor systems. Int. J. Environ. Agric. Biotechnol. 2016, 1, 238581. [Google Scholar]
- Microfluidics. Microfluidizer Processor User Guide; Microfluidics Corporation: Newton, MA, USA, 2008. [Google Scholar]
- Chung, S.K.; Shin, G.H.; Jung, M.K.; Hwang, I.C.; Park, H.J. Factors influencing the physicochemical characteristics of cationic polymer-coated liposomes prepared by high-pressure homogenization. Colloids Surf. A Physicochem. Eng. Asp. 2014, 454, 8–15. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef] [PubMed]
- Carrión, F.; De La Maza, A.; Parra, J. The influence of ionic strength and lipid bilayer charge on the stability of liposomes. J. Colloid Interface Sci. 1994, 164, 78–87. [Google Scholar] [CrossRef]
- Daeihamed, M.; Dadashzadeh, S.; Haeri, A.; Akhlaghi, M. Potential of liposomes for enhancement of oral drug absorption. Curr. Drug Deliv. 2017, 14, 289–303. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical stability of ascorbic acid integrated into commercial products: A review on bioactivity and delivery technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Encapsulation of vitamin C in tripolyphosphate cross-linked chitosan microspheres by spray drying. J. Microencapsul. 2005, 22, 179–192. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Y. Encapsulation of vitamin C by a double-layer zein/chitosan structure with improved stability and controlled release. Int. Nanomed. Nanosurg. 2016, 2. [Google Scholar] [CrossRef]
- Baek, J.; Ramasamy, M.; Willis, N.C.; Kim, D.S.; Anderson, W.A.; Tam, K.C. Encapsulation and controlled release of vitamin C in modified cellulose nanocrystal/chitosan nanocapsules. Curr. Res. Food Sci. 2021, 4, 215–223. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, E.; Palladino, R.; Cannavale, M.; Lamparelli, E.P.; Maglione, B. Enhanced Stability of Oral Vitamin C Delivery: A Novel Large-Scale Method for Liposomes Production and Encapsulation through Dynamic High-Pressure Microfluidization. Nanomaterials 2024, 14, 516. https://doi.org/10.3390/nano14060516
Romano E, Palladino R, Cannavale M, Lamparelli EP, Maglione B. Enhanced Stability of Oral Vitamin C Delivery: A Novel Large-Scale Method for Liposomes Production and Encapsulation through Dynamic High-Pressure Microfluidization. Nanomaterials. 2024; 14(6):516. https://doi.org/10.3390/nano14060516
Chicago/Turabian StyleRomano, Eugenia, Roberta Palladino, Mariagabriella Cannavale, Erwin Pavel Lamparelli, and Barbara Maglione. 2024. "Enhanced Stability of Oral Vitamin C Delivery: A Novel Large-Scale Method for Liposomes Production and Encapsulation through Dynamic High-Pressure Microfluidization" Nanomaterials 14, no. 6: 516. https://doi.org/10.3390/nano14060516