Nanocrystalline Cubic Phase Scandium-Stabilized Zirconia Thin Films
Abstract
:1. Introduction
2. Methods and Materials
2.1. Reactive Magnetron Co-Sputtering of ScSZ
2.2. Sample Characterization
3. Results and Discussion
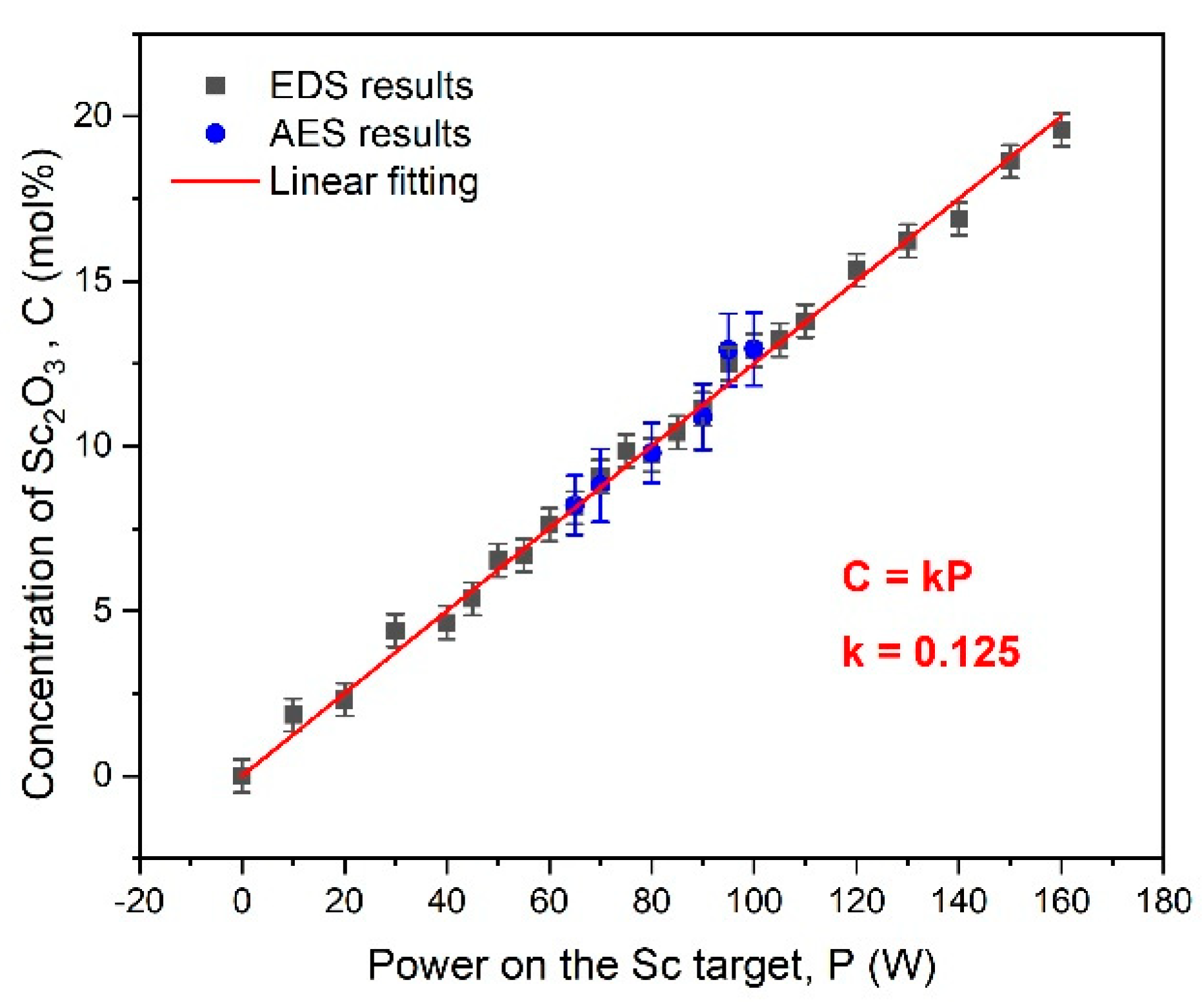
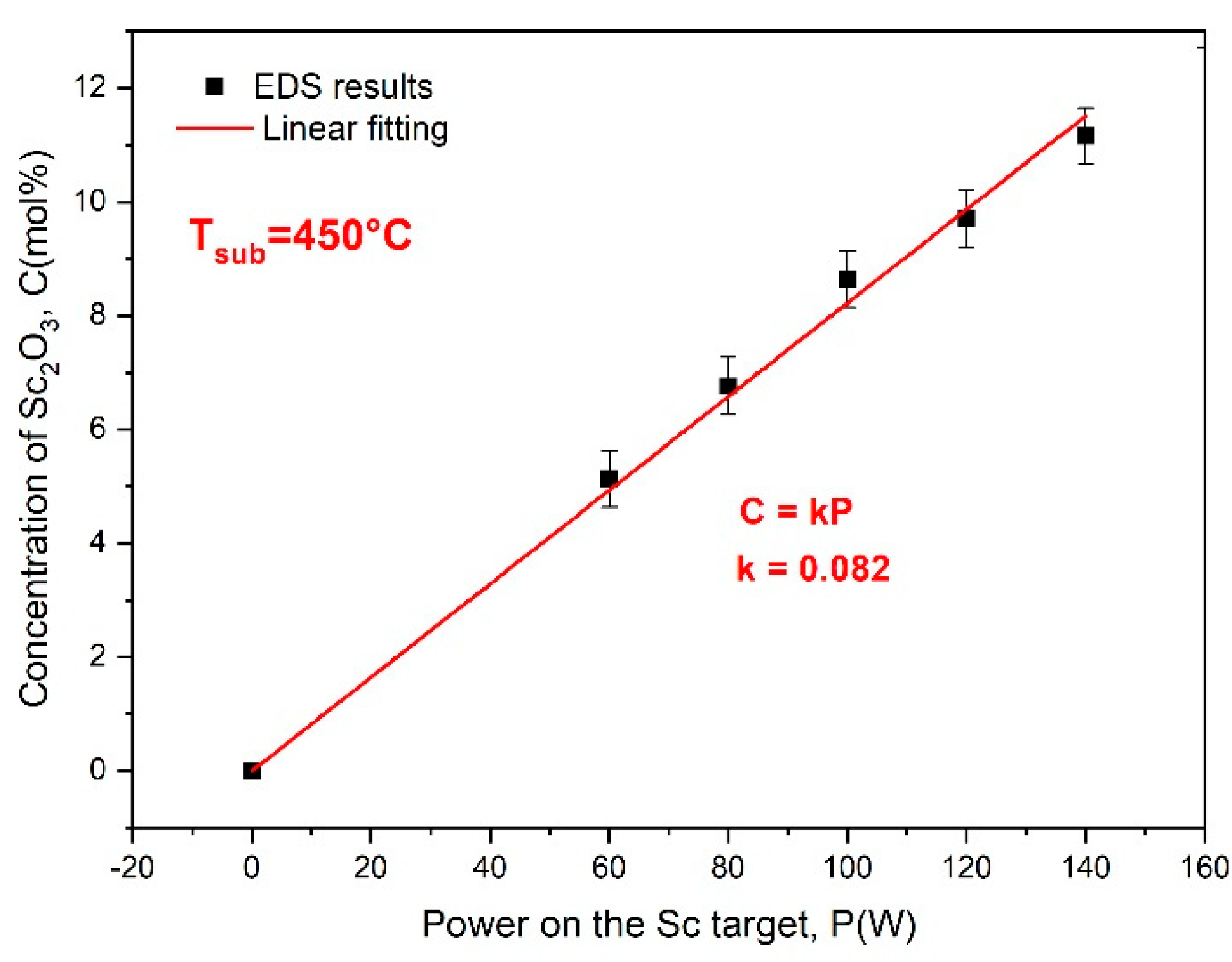
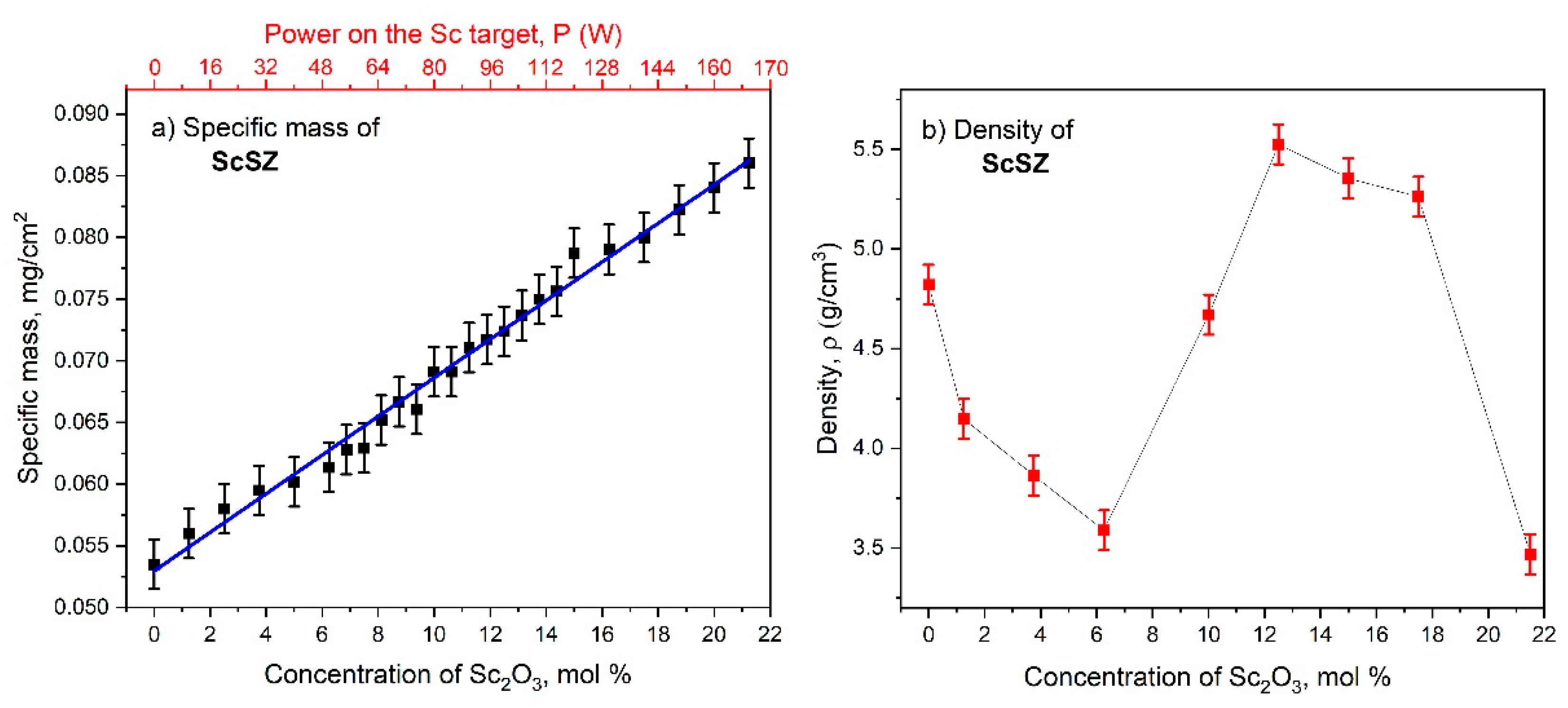
3.1. Concentration Analysis
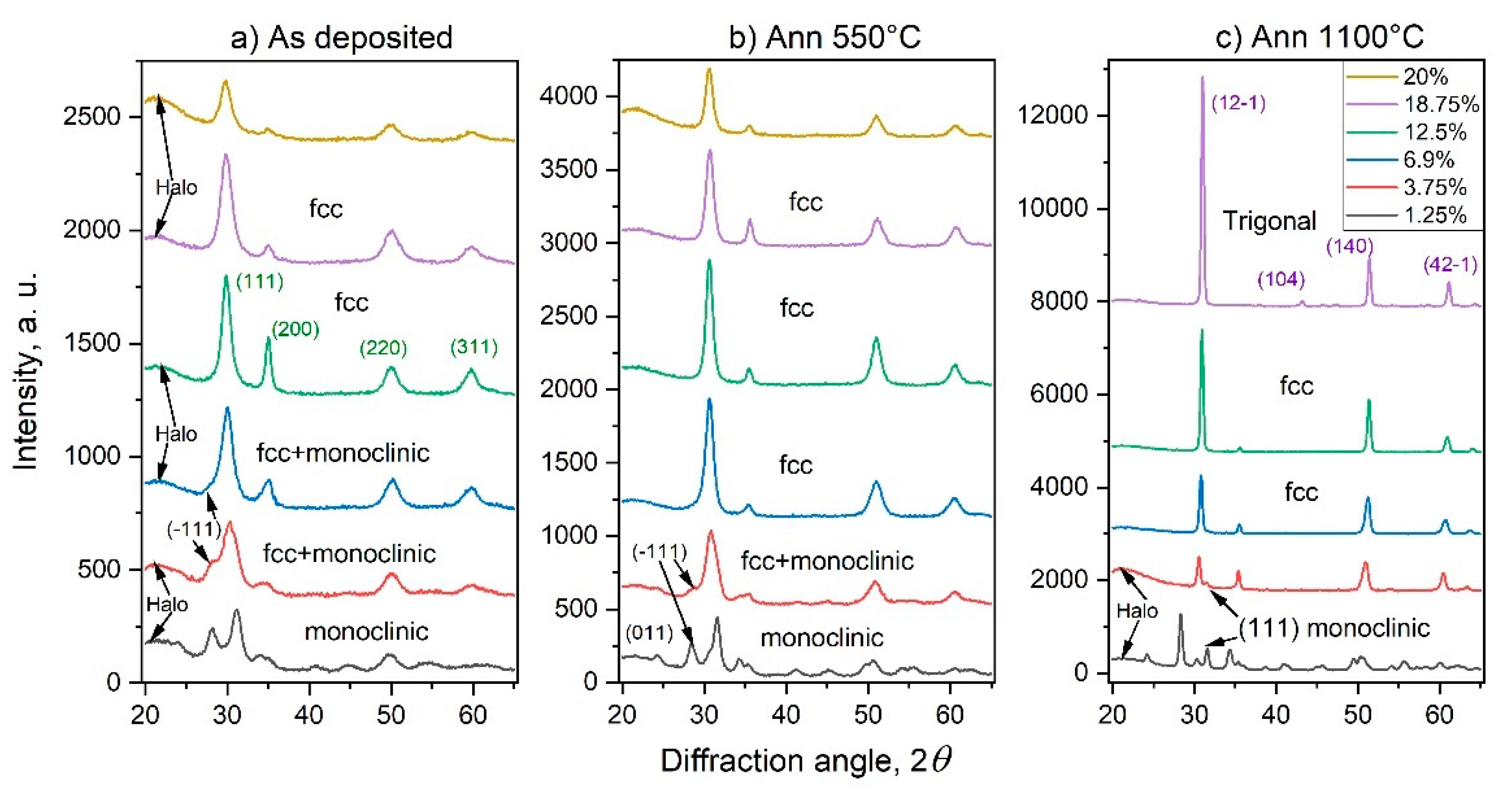
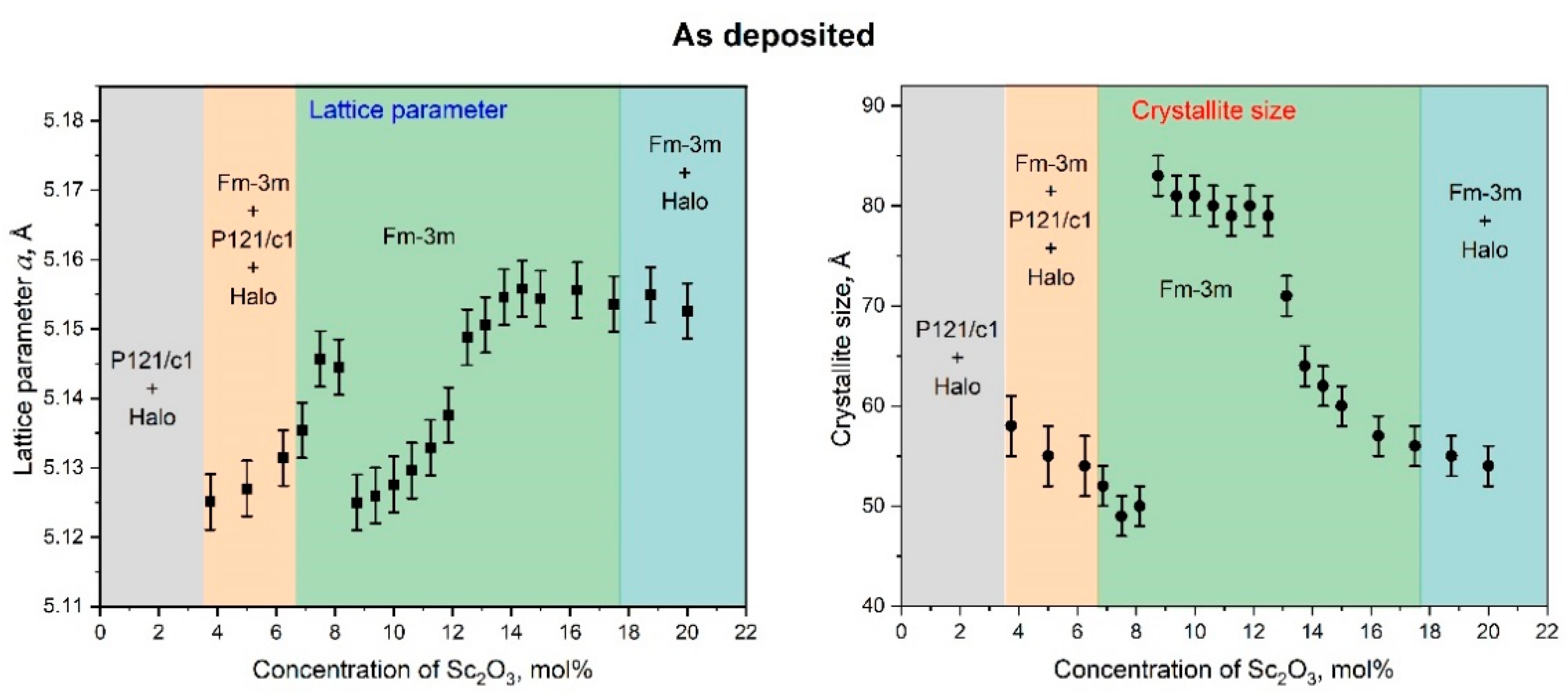
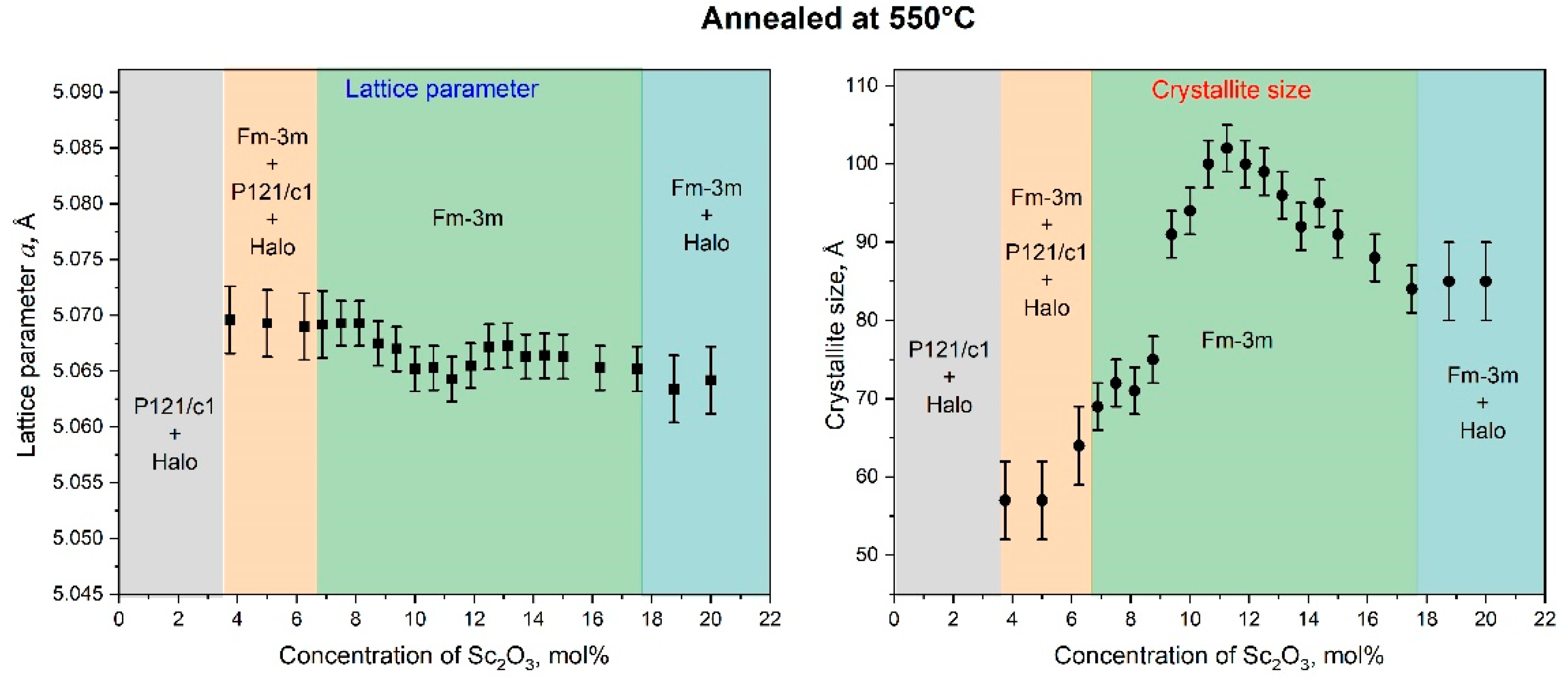
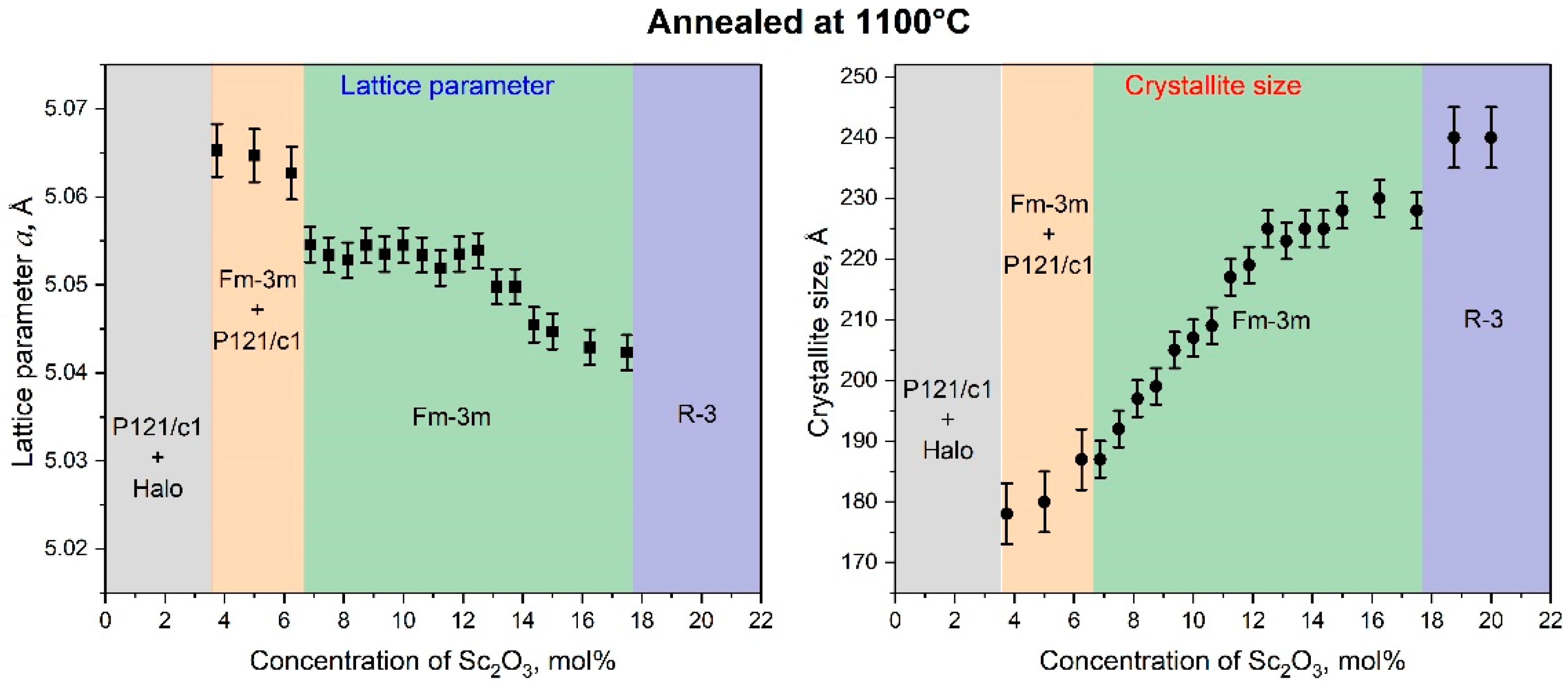
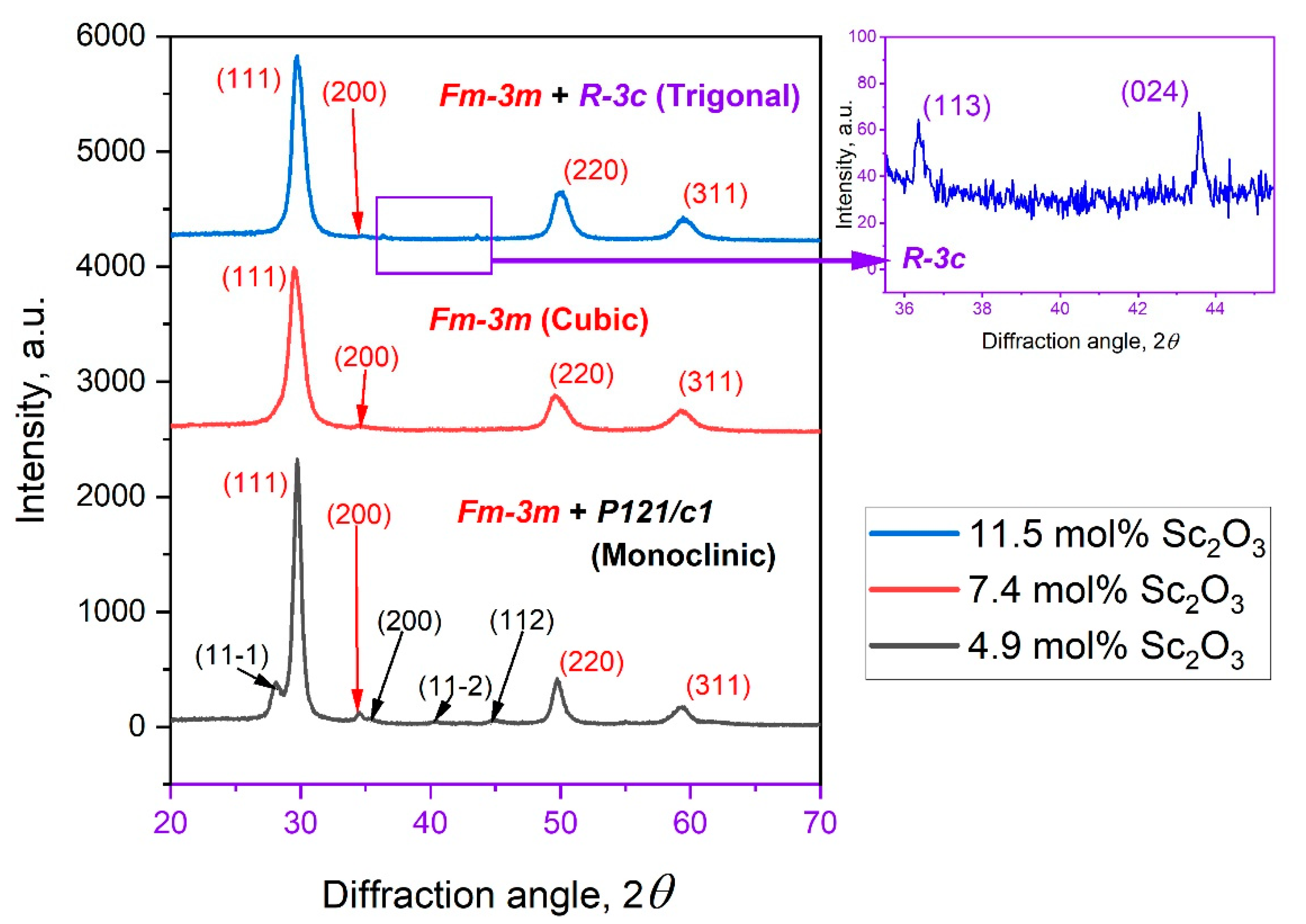
3.2. Crystal Structure, Crystallite Sizes, and Lattice Parameters
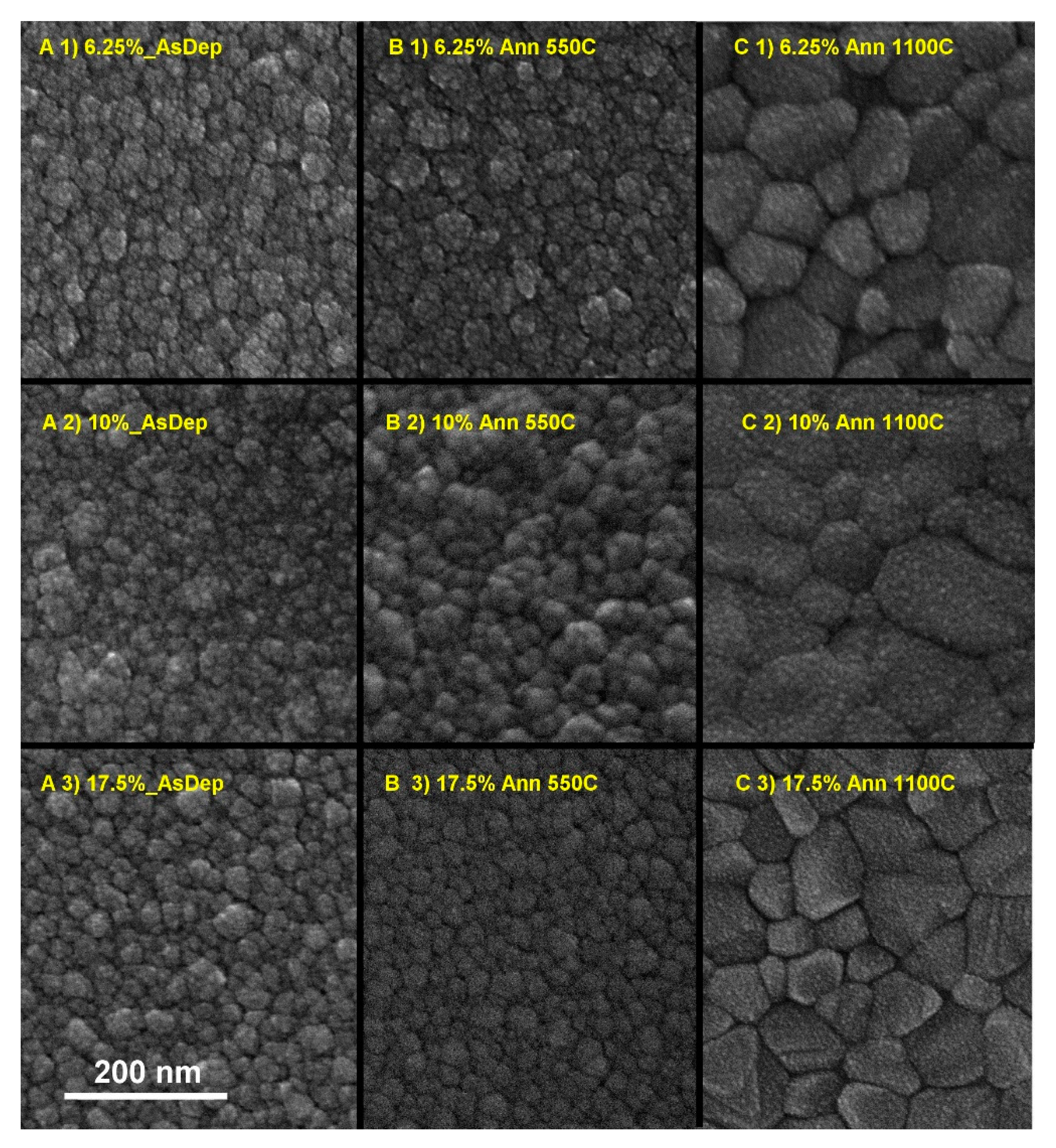
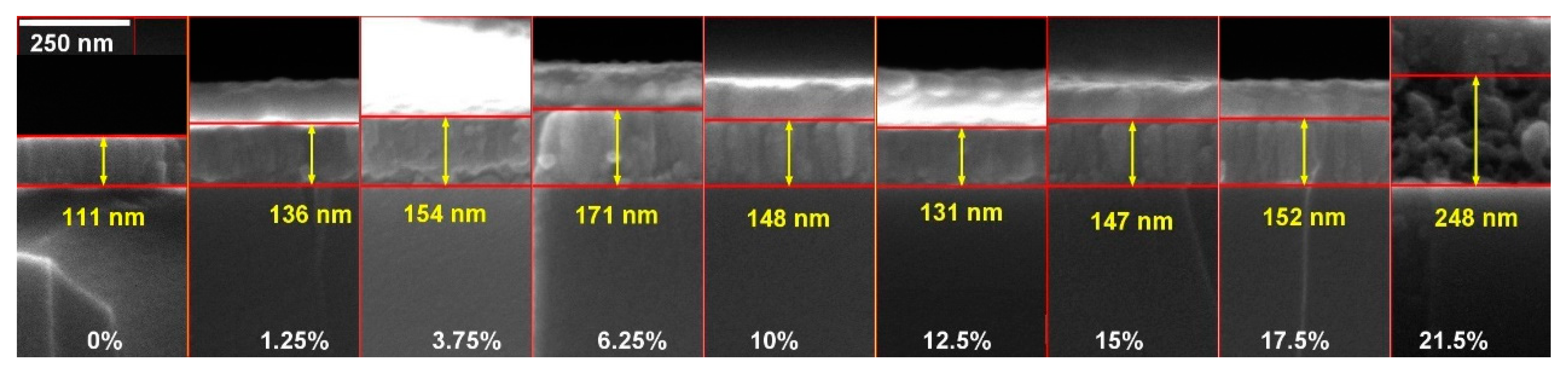
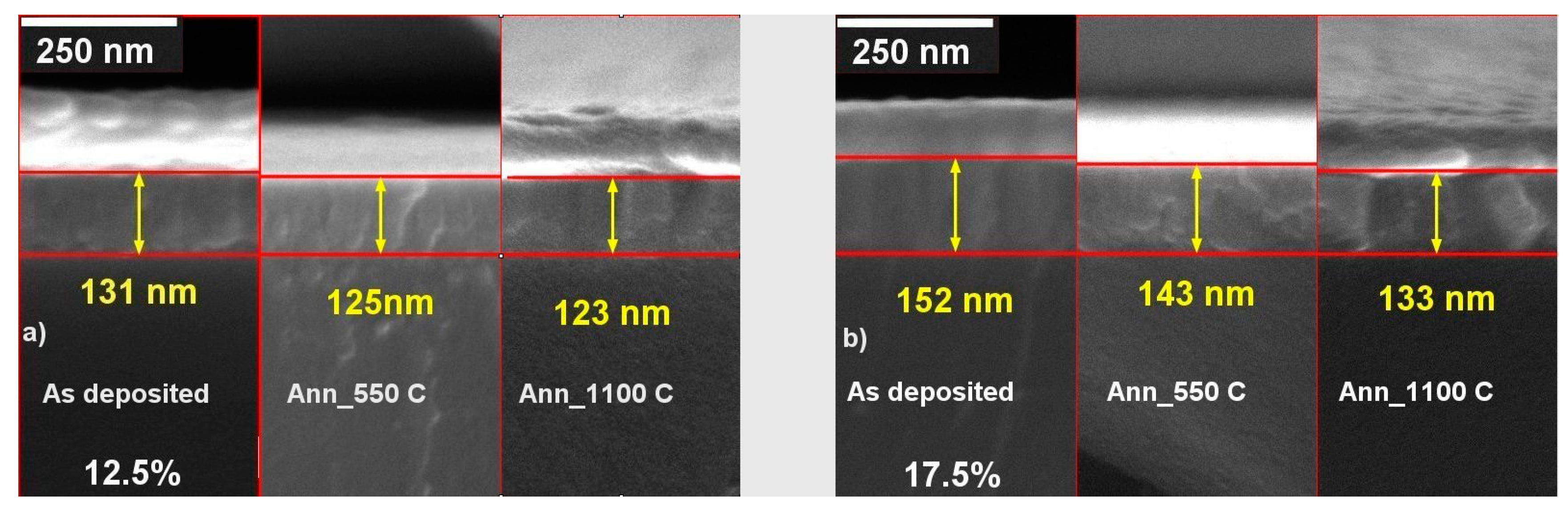
3.3. Morphology of the As-Deposited and Annealed RT Co-Sputtered ScSZ Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van de Walle, A. A complete representation of structure-property relationships in crystals. Nat. Mater. 2008, 7, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Yashima, M.; Kakihana, M.; Yoshimura, M. Metastable-stable phase diagrams in the zirconia-containing systems utilized in solid-oxide fuel cell application. Solid State Ion. 1996, 86–88, 1131–1149. [Google Scholar] [CrossRef]
- Guan, Y.; Zhou, J.; Zhong, H.; Wang, W.; Zhang, Z.; Luo, F.; Ning, S. Thickness dependence of the crystallization and phase transition in ZrO2 thin films. J. Adv. Ceram. 2023, 12, 822–829. [Google Scholar] [CrossRef]
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic steels? Nature 1975, 258, 703. [Google Scholar] [CrossRef]
- Wilk, G.D.; Wallace, R.M.; Anthony, J.M. High-k gate dielectrics: Current status and materials properties considerations. J. Appl. Phys. 2001, 89, 5243. [Google Scholar] [CrossRef]
- Tanabe, K. Surface and catalytic properties of ZrO2. Mater. Chem. Phys. 1985, 13, 347–364. [Google Scholar] [CrossRef]
- Peuchert, U.; Okano, Y.; Menke, Y.; Reichel, S.; Ikesue, A. Transparent cubic-ZrO2 ceramics for application as optical lenses. J. Eur. Ceram. Soc. 2009, 29, 283–291. [Google Scholar] [CrossRef]
- Manicone, P.F.; Iommetti, P.R.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef]
- Burkhard, D.J.M.; Hanson, B.; Ulmer, G.C. ZrO2 oxygen sensors: An evaluation of behavior at temperatures as low as 300 °C. Solid State Ion. 1991, 47, 169–175. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Wright, P.K.; Evans, A.G. Mechanisms governing the performance of thermal barrier coatings. Curr. Opin. Solid State Mater. Sci. 1999, 4, 255. [Google Scholar] [CrossRef]
- Clarke, D.R.; Phillpot, S.R. Thermal barrier coating materials. Mater. Today 2005, 8, 22–29. [Google Scholar] [CrossRef]
- Vasylyev, O.; Brychevskyi, M.; Brodnikovskyi, Y. The structural optimisation of ceramic fuel cell. Univers. J. Chem. 2016, 4, 31–54. [Google Scholar] [CrossRef]
- Haile, S.M. Fuel cell materials and components. Acta Mater. 2003, 51, 5981–6000. [Google Scholar] [CrossRef]
- Cho, V.; Lee, Y.H.; Hong, S.W.; Bae, J.; An, J.; Kim, Y.B.; Cha, S.W. High-performance thin film solid oxide fuel cells with scandia-stabilized zirconia (ScSZ) thin film electrolyte. Int. J. Hydrogen Energy 2015, 40, 15704–15708. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, J.; Wang, J.; Wu, G.; Chen, L. Sol-gel derived ZrO2-SiO2 highly reflective coatings. Int. J. Inorg. Mater. 2000, 2, 319. [Google Scholar] [CrossRef]
- Ruff, O.; Ebert, F.; Stephan, E. Beitrage Zur Keramik Hochfeuerfester Stoffe II. Das System ZrO-CaO. ZAAC 1929, 180, 215–224. [Google Scholar] [CrossRef]
- Namavar, F.; Wang, G.; Cheung, C.L.; Sabirianov, R.F.; Zeng, X.C.; Mei, W.N.; Bai, J.; Brewer, J.R.; Haider, H.; Garvin, K.L. Thermal stability of nanostructurally stabilized zirconium oxide. Nanotechnology 2007, 18, 415702. [Google Scholar] [CrossRef]
- Goedike, K.; Liebig, J.; Zywitzki, O.; Sahm, H. Influence of process parameters on the structure and the properties of ZrO2 coatings deposited by reactive pulsed magnetron sputtering. Thin Solid Films 2000, 337–338, 37–42. [Google Scholar] [CrossRef]
- Ngaruiya, J.M.; Kappertz, O.; Mohamed, S.H.; Wuttig, M. Structure formation upon reactive direct current magnetron sputtering of transition metal oxide films. Appl. Phys. Lett. 2004, 85, 748. [Google Scholar] [CrossRef]
- Fabris, S.; Paxton, A.T.; Finnis, M.W. A stabilization mechanism of zirconia based on oxygen vacancies only. Acta Mater. 2002, 50, 5171–5178. [Google Scholar] [CrossRef]
- Magunov, R.L.; Shklyar, G.L.; Katridi, V.F. Phase diagrams of ZrO2(HfO2)-Sc2O3 systems. Inorg. Mater. 1989, 25, 1035. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Yamamoto, O.; Arachi, Y.; Sakai, H.; Takeda, Y.; Imanishi, N.; Mizutani, Y.; Kawai, M.; Nakamura, Y. Zirconia based ion conductors for solid oxide fuel cell. Ionics 1998, 4, 403–408. [Google Scholar] [CrossRef]
- Kharton, V.V.; Marques, F.M.B.; Atkinson, A. Transport properties of solid oxide electrolyte ceramics: A brief review. Solid State Ion. 2004, 174, 135–149. [Google Scholar] [CrossRef]
- Arifin, N.A.; Afifi, A.A.; Samreen, A.; Hafriz, R.S.R.M.; Muchtar, A. Characteristic and challenges of scandia stabilized zirconia as solid oxide fuel cell material–In depth review. Solid State Ion. 2023, 399, 116302. [Google Scholar] [CrossRef]
- Singhal, S.C. Advances in solid oxide fuel cell technology. Solid State Ion. 2000, 135, 305–315. [Google Scholar] [CrossRef]
- Will, J.; Mitterdorfer, A.; Kleinlogel, C.; Perendis, D.; Gauckler, L.J. Fabrication of thin electrolytes for second-generation solid oxide fuel cell. Solid State Ion. 2000, 131, 76–79. [Google Scholar] [CrossRef]
- Zhigachev, A.O.; Rodaev, V.V.; Zhigacheva, D.V.; Lyskov, N.V.; Shchukina, M.A. Doping of scandia-stabilized zirconia electrolytes for intermediate-temperature solid oxide fuel cell: A review. Ceram. Int. 2021, 47, 32490–32504. [Google Scholar] [CrossRef]
- Mathur, L.; Shin, D.; Hanantyo, M.P.G.; Namgung, Y.; Singh, B.; Sengodan, S.; Song, S.J. Role of Divalent Co-Dopant as Structure Stabilizer in Scandia-Stabilized Zirconia Electrolyte for SOFC. J. Electrochem. Soc. 2023, 170, 124519. [Google Scholar] [CrossRef]
- Mathur, L.; Jeon, S.-Y.; Namgung, Y.; Hanantyo, M.P.G.; Park, J.; Islam, M.S.; Sengodan, S.; Song, S.-J. Ternary co-doped ytterbium-scandium stabilized zirconia electrolyte for solid oxide fuel cells. Solid State Ion. 2024, 408, 116507. [Google Scholar] [CrossRef]
- Cha, S.W.; Cho, G.Y.; Lee, Y.; Park, T.; Kim, Y.; Lee, J. Effects of carbon contaminations on Y2O3-stabilized ZrO2 thin films electrolyte prepared by atomic layer deposition for thin film solid oxide fuel cell. CIRP Ann. Manuf. Technol. 2016, 65, 515–518. [Google Scholar] [CrossRef]
- Cho, G.; Yu, W.; Lee, Y.; Tanveer, W.; Kim, Y.; Lee, S.; Cha, S. Effects of nanoscale PEALD YSZ interlayer for AAO based thin film solid oxide fuel cells. Int. J. Presic. Eng. Manuf. Green Technol. 2020, 7, 423–430. [Google Scholar] [CrossRef]
- Hagiwara, A.; Hobara, N.; Takizava, K.; Sato, K.; Abe, H.; Naito, M. Preparation of LSM/ScSZ composite powder materials by spray pyrolysis for the pre-fabrication SOFC cathodes. Solid State Ion. 2007, 178, 1552–1562. [Google Scholar] [CrossRef]
- Suciu, C.; Erichsen, E.; Hoffman, A.; Dorolti, E.; Tetean, R. Modified sol-gel method used for obtaining SOFC electrolyte materials. ECS Trans. 2009, 25, 1679–1697. [Google Scholar] [CrossRef]
- Sobolev, A.; Musin, A.; Whyman, G.; Borodianskiy, K.; Krichevski, O.; Kalashnikov, A.; Zinigrad, M. Stabilization of cubic phase in scandium-doped zirconia nanocrystals synthesized with sol-gel method. J. Am. Ceram. Soc. 2019, 102, 3236–3243. [Google Scholar] [CrossRef]
- Kwon, C.; Son, J.; Lee, J.; Kim, H.; Lee, H.; Kim, K. High-performance micro-solid oxide fuel cells fabricated on nanoporous anodic aluminium oxide templates. Adv. Funct. Mater. 2011, 21, 1154–1159. [Google Scholar] [CrossRef]
- Kainbayev, N.; Sriubas, M.; Bockute, K.; Virbukas, D.; Laukaitis, G. E-beam deposition of scandia-stabilized zirconia thin films co-doped with Al. Coatings 2020, 10, 870. [Google Scholar] [CrossRef]
- Duwez, P.; Brown, F.H., Jr.; Odell, F. The zirconia-yttria system. J. Electrochem. Soc. 1951, 98, 356–362. [Google Scholar] [CrossRef]
- Ruh, R.; Garret, H.J.; Domagala, R.F.; Patel, V.A. The system zirconia-scandia. J. Am. Ceram. Soc. 1977, 60, 399–403. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Devanathan, R.; Jiang, W.; Nachimuthu, P.; Shutthanandan, V.; Saraf, L.; Wang, C.M.; Kuchibhatla, S.V.N.T.; Thevuthasan, S. Integrated experimental and modeling study of ionic conductivity of scandia-stabilized zirconia thin films. Solid State Ion. 2010, 181, 367–371. [Google Scholar] [CrossRef]
- Solovyev, A.A.; Sochugov, N.S.; Rabotkin, S.V.; Shipilova, A.V.; Ionov, I.V.; Kovalchuk, A.N.; Borduleva, A.O. Application of PVD methods to solid oxide fuel cells. Appl. Surf. Sci. 2014, 310, 272–277. [Google Scholar] [CrossRef]
- Grosso, R.L.; Muccillo, E.N.S.; Castro, R.H.R. Phase stability in scandia-zirconia nanocrystals. J. Am. Ceram. Soc. 2017, 100, 2199–2208. [Google Scholar] [CrossRef]
- Lee, Y.H.; Ren, H.; Wu, E.A.; Fullerton, E.E.; Meng, Y.S.; Minh, N.Q. All-sputtered, superior power density thin-film solid oxide fuel cells with a novel nanofibrous ceramic cathode. Nano Lett. 2020, 20, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.K.; Kossenko, A.; Danchuk, V.; Shatalov, M.; Rahumi, O.; Borodianskiy, K.; Zinigrad, M.; Sahoo, T.; Mishra, S. Development of highly efficient and durable large-area solid oxide fuel cell by direct-ink-writing three-dimensional printer. J. Power Source 2022, 552, 232225. [Google Scholar] [CrossRef]
- Danchuk, V.; Shatalov, N.; Pogreb, R.; Musin, A. Characterization of sputtered ZnO blocking layers with surface plasmon resonance method. In Proceedings of the Eighteenth Russian-Israeli Bi-National Workshop, Ein Bokek, Israel, 17–22 February 2019; pp. 186–193. [Google Scholar]
- Shekunov, B. Kinetics of crystallization and glass transition in amorphous materials. Cryst. Growth Des. 2020, 20, 95–106. [Google Scholar] [CrossRef]
- Lee, B.; Burr, G.W.; Shelby, R.M.; Raoux, S.; Rettner, C.T.; Bogle, S.N.; Darmawikarta, K.; Bishop, S.G.; Abelson, J.R. Observation of the role of subcritical nuclei in crystallization of a glassy solid. Science 2009, 326, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Solodovnik, A.A.; Danchuk, V.V. Orientational order in solid N2O-Kr solutions. J. Low Temp. Phys. 2001, 122, 493–499. [Google Scholar] [CrossRef]
- Krivanek, O.L.; Gaskel, P.H.; Howie, A. Seeing order in amorphous materials. Nature 1976, 262, 454–457. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Gibson, J.M.; Fan, L.; Paterson, D.J.; McNulty, I. Fluctuation microscopy: A probe of medium range order. Rep. Prog. Phys. 2005, 68, 2899–2944. [Google Scholar] [CrossRef]
- Vainshtein, B.K. Structural Analysis by Electron Diffraction, 1st ed.; Pergamon Press: Oxford, UK, 1964. [Google Scholar]
- Urusov, V.S. The phenomenological theory of solid solutions. In Solid Solutions in Silicate and Oxide Systems; Geiger, C.A., Ed.; GeoScienceWorld: McLean, VA, USA, 2001. [Google Scholar] [CrossRef]
- Yamamura, Y.; Tawara, H. Energy dependence of ion-induced sputtering yields from monoatomic solids at normal incidence. At. Data Nucl. Data Tables 1996, 62, 149–253. [Google Scholar] [CrossRef]
- Schaffer, J.P. The Science and Design of Engineering Materials, Featured ed.; McGraw-Hill Education: New York, NY, USA, 2000. [Google Scholar]
- Palenzona, A.; Cirafici, S. The Sc-Zr (scandium-zirconium) system. J. Phase Equilibria 1991, 12, 53–56. [Google Scholar] [CrossRef]
- Jarvis, J.F.; Meyer, H.; Ramm, D. Measurement of (∂P/∂T)V and related properties in solidified gases. II. Solid H2. Phys. Rev. 1969, 178, 1461. [Google Scholar] [CrossRef]
- Danchuk, V.V.; Solodovnik, A.A.; Strzhemechny, M.A. Lattice parameter of CO2-Kr cryoalloys. Low Temp. Phys. 2010, 36, 254. [Google Scholar] [CrossRef]
- Solodovnik, A.A.; Danchuk, V.V.; Mysko, N.S. Cluster approach to formation of nitrogen-rare gas cryoalloys. Low Temp. Phys. 2013, 39, 456. [Google Scholar] [CrossRef]
- Vegard, L. Die Konstitution der mishkristalle und die raumfullung der atom. Z. fur Phusik 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Whyman, G.; Kalashnikov, A.; Zinigrad, M. On the dependence of the ionic conductivity on dopant concentration in the cubic zirconium oxide doped with oxides of trivalent metals. Solid State Ion. 2018, 316, 34–37. [Google Scholar] [CrossRef]
- Yamamoto, O.; Arati, Y.; Takeda, Y.; Imanishi, N.; Mizutani, Y.; Kawai, M.; Nakamura, Y. Electrical conductivity of stabilized zirconia with ytterbia and scandia. Solid State Ion. 1995, 79, 137–142. [Google Scholar] [CrossRef]
- Arachi, Y.; Sakai, H.; Yamamoto, O.; Takeda, Y.; Imanishai, N. Electrical conductivity of the ZrO2-Ln2O3 (Ln = lanthanides) system. Solid State Ion. 1999, 121, 133–139. [Google Scholar] [CrossRef]
- Lajavardi, M.; Kenney, D.J.; Lin, S.H. Time-resolved phase transition of the nanocrystalline cubic to submicron monoclinic phase in zirconia. J. Chin. Chem. Soc. 2000, 47, 1043. [Google Scholar] [CrossRef]
- Gateshki, M.; Petkov, V.; Williams, G.; Pradhan, S.K.; Ren, Y. Atomic-scale structure of nanocrystalline ZrO2 prepared by high-energy ball milling. Phys. Rev. B 2005, 71, 224107. [Google Scholar] [CrossRef]
- Kusano, E. Structure-Zone Modeling of Sputter-Deposited Thin Films: A Brief Review. Appl. Sci. Converg. Technol. 2019, 28, 179–185. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danchuk, V.; Shatalov, M.; Zinigrad, M.; Kossenko, A.; Brider, T.; Le, L.; Johnson, D.; Strzhemechny, Y.M.; Musin, A. Nanocrystalline Cubic Phase Scandium-Stabilized Zirconia Thin Films. Nanomaterials 2024, 14, 708. https://doi.org/10.3390/nano14080708
Danchuk V, Shatalov M, Zinigrad M, Kossenko A, Brider T, Le L, Johnson D, Strzhemechny YM, Musin A. Nanocrystalline Cubic Phase Scandium-Stabilized Zirconia Thin Films. Nanomaterials. 2024; 14(8):708. https://doi.org/10.3390/nano14080708
Chicago/Turabian StyleDanchuk, Victor, Mykola Shatalov, Michael Zinigrad, Alexey Kossenko, Tamara Brider, Luc Le, Dustin Johnson, Yuri M. Strzhemechny, and Albina Musin. 2024. "Nanocrystalline Cubic Phase Scandium-Stabilized Zirconia Thin Films" Nanomaterials 14, no. 8: 708. https://doi.org/10.3390/nano14080708







