Physiochemical Characterization of Lipidic Nanoformulations Encapsulating the Antifungal Drug Natamycin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Natamycin-Loaded Transethosomes
2.2.2. Synthesis of Natamycin-Loaded Lipid Nanoparticles
2.2.3. Size and Zeta Potential Measurements
2.2.4. NMR Analysis
2.2.5. SAXS/WAXS
2.2.6. Determination of the Encapsulation Efficiency
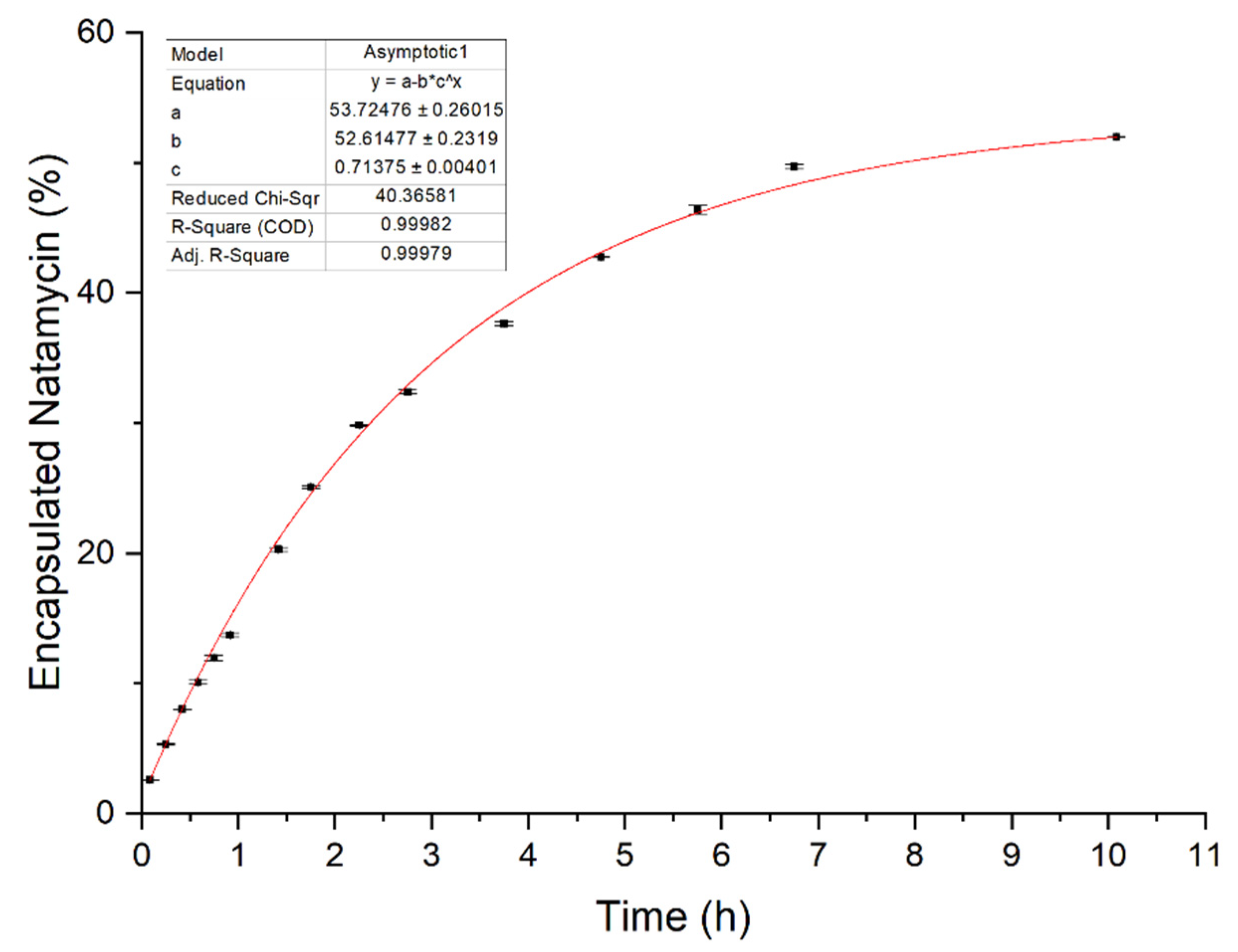
2.2.7. In Vitro Release
3. Results and Discussion
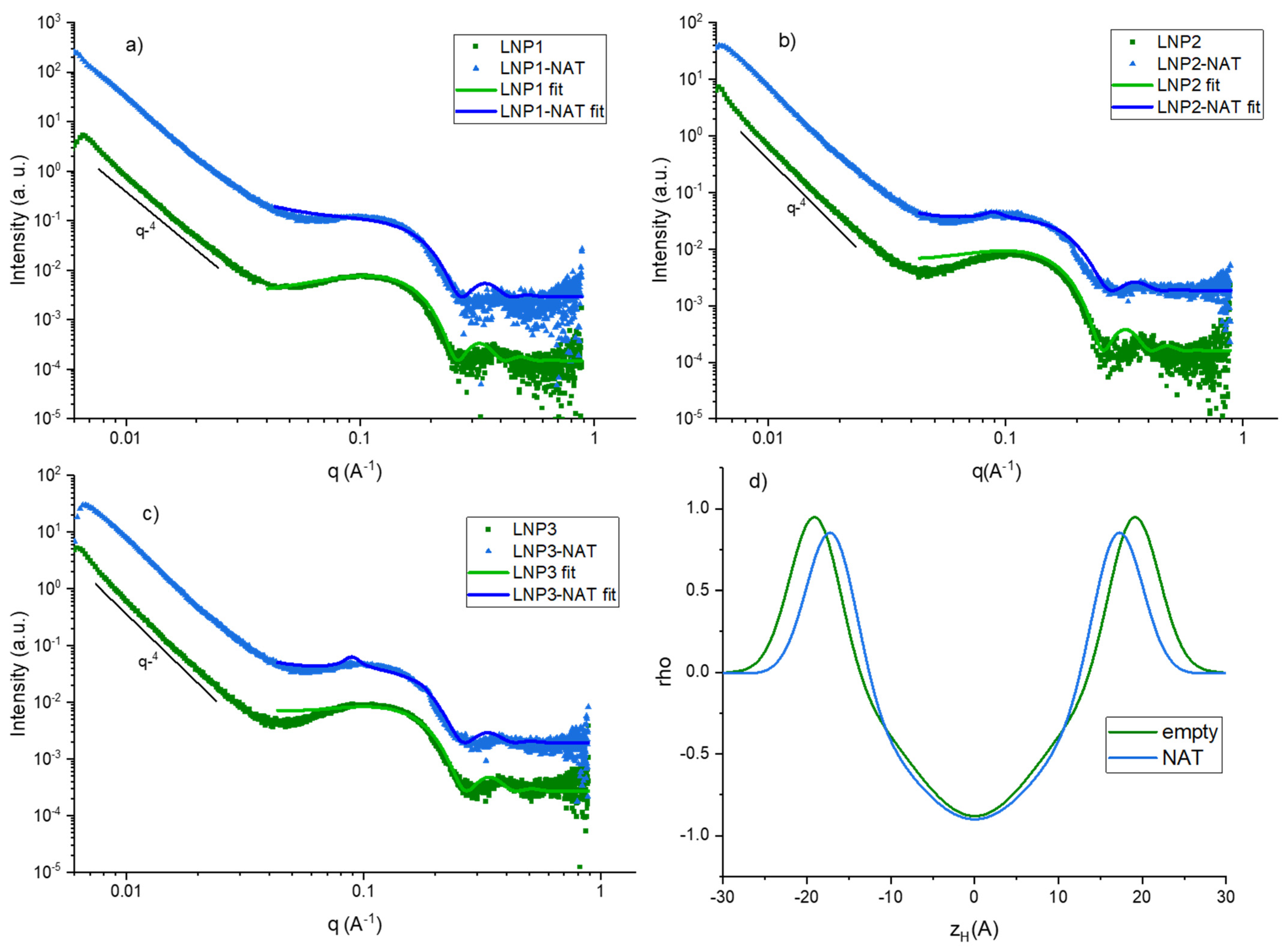
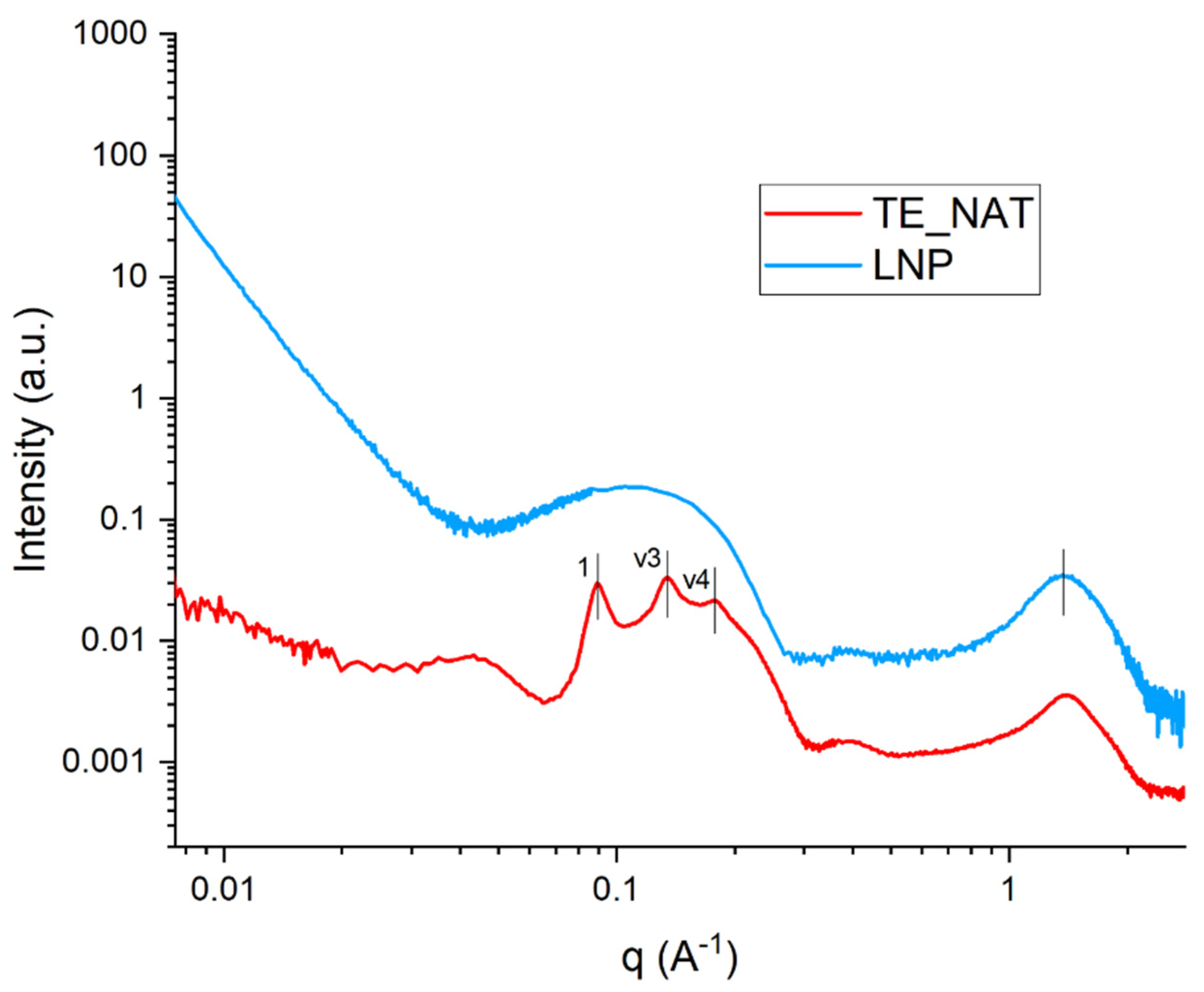
3.1. Size, Zeta Potential and SAXS/WAXS
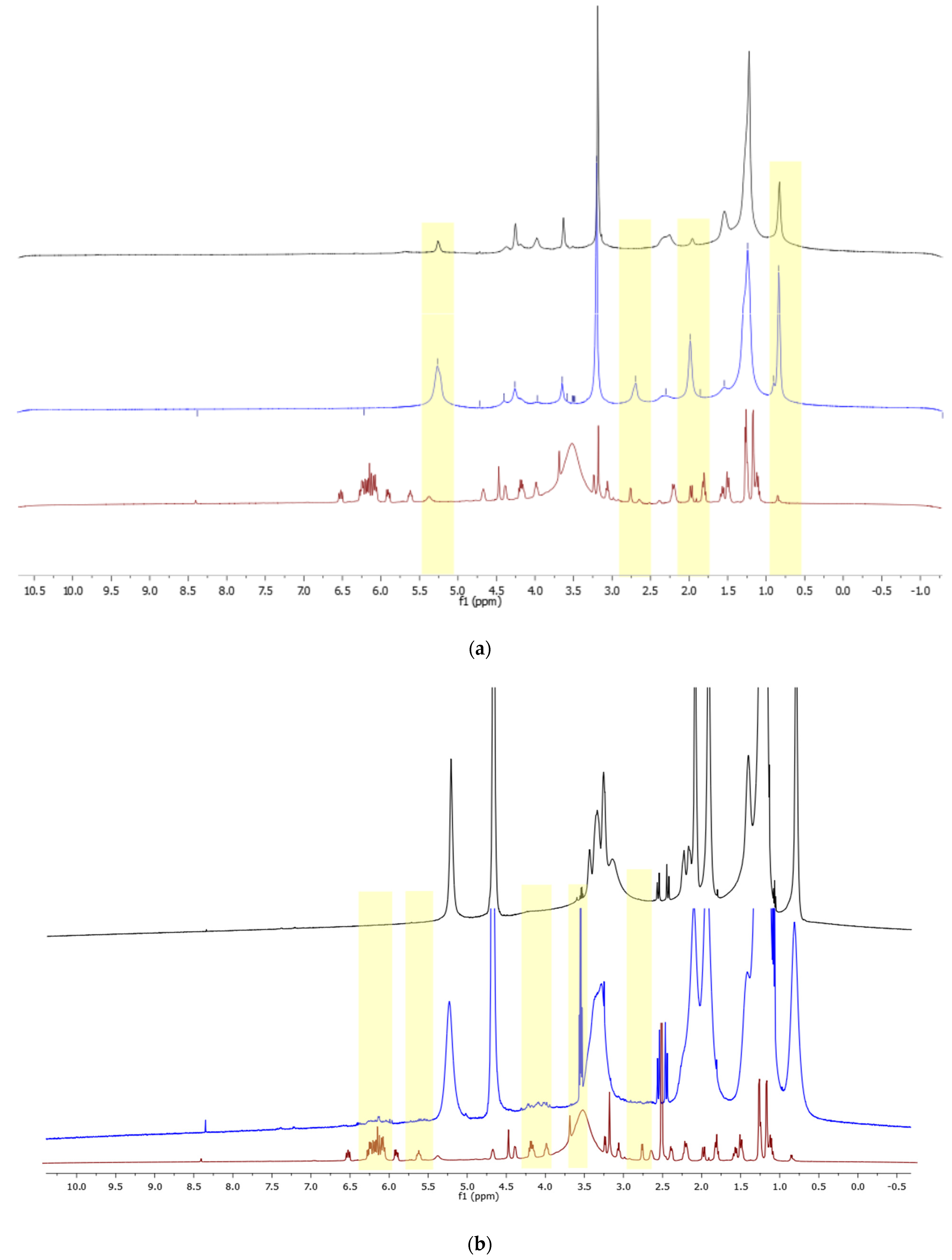
3.2. NMR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The Global Incidence and Diagnosis of Fungal Keratitis. Lancet Infect. Dis. 2021, 21, e49–e57. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.A.; Leck, A.K.; Myatt, M. Characteristic Clinical Features as an Aid to the Diagnosis of Suppurative Keratitis Caused by Filamentous Fungi. Br. J. Ophthalmol. 2005, 89, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.L.; Jones, D.B.; Wilhelmus, K.R. Clinical Characteristics and Outcome of Candida Keratitis. Am. J. Ophthalmol. 2007, 143, 1043–1045.e1. [Google Scholar] [CrossRef] [PubMed]
- Mselle, J. Fungal Keratitis as an Indicator of HIV Infection in Africa. Trop. Doct. 1999, 29, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Bartimote, C.; Foster, J.; Watson, S. The Spectrum of Microbial Keratitis: An Updated Review. Open Ophthalmol. J. 2020, 13, 100–130. [Google Scholar] [CrossRef]
- Deorukhkar, S.; Katiyar, R.; Saini, S. Epidemiological Features and Laboratory Results of Bacterial and Fungal Keratitis: A Five-Year Study at a Rural Tertiary-Care Hospital in Western Maharashtra, India. Singap. Med. J. 2012, 53, 264–267. [Google Scholar]
- Hoffman, J.J.; Yadav, R.; Das Sanyam, S.; Chaudhary, P.; Roshan, A.; Singh, S.K.; Arunga, S.; Matayan, E.; Macleod, D.; Weiss, H.A.; et al. Topical Chlorhexidine 0.2% versus Topical Natamycin 5% for Fungal Keratitis in Nepal: Rationale and Design of a Randomised Controlled Non-Inferiority Trial. BMJ Open 2020, 10, e038066. [Google Scholar] [CrossRef] [PubMed]
- Prajna, N.V.; Krishnan, T.; Rajaraman, R.; Patel, S.; Srinivasan, M.; Das, M.; Ray, K.J.; O’Brien, K.S.; Oldenburg, C.E.; McLeod, S.D.; et al. Effect of Oral Voriconazole on Fungal Keratitis in the Mycotic Ulcer Treatment Trial II (MUTT II). JAMA Ophthalmol. 2016, 134, 1365. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.S.; Meletiadis, J.; Curfs-Breuker, I.; Bonifaz, A.; Meis, J.F.; De Hoog, G.S. In Vitro Combinations of Natamycin with Voriconazole, Itraconazole and Micafungin against Clinical Fusarium Strains Causing Keratitis: Table 1. J. Antimicrob. Chemother. 2016, 71, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, P.; Patil, A.; Majumdar, S. Challenges in the Polyene- and Azole-Based Pharmacotherapy of Ocular Fungal Infections. J. Ocul. Pharmacol. Ther. 2019, 35, 6–22. [Google Scholar] [CrossRef]
- Medina, Á.; Jiménez, M.; Mateo, R.; Magan, N. Efficacy of Natamycin for Control of Growth and Ochratoxin A Production by Aspergillus Carbonarius Strains under Different Environmental Conditions. J. Appl. Microbiol. 2007, 103, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Prajapati, P.; Ravichandran, C.; Sehrawat, R. Natamycin: A Natural Preservative for Food Applications—A Review. Food Sci. Biotechnol. 2021, 30, 1481–1496. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, P.; Vijaykumar, R.; Prajna, N.V.; Fothergill, A.W. In Vitro Natamycin Susceptibility of Ocular Isolates of Fusarium and Aspergillus Species: Comparison of Commercially Formulated Natamycin Eye Drops to Pharmaceutical-Grade Powder. J. Clin. Microbiol. 2008, 46, 3477–3478. [Google Scholar] [CrossRef] [PubMed]
- te Welscher, Y.M.; ten Napel, H.H.; Balagué, M.M.; Souza, C.M.; Riezman, H.; de Kruijff, B.; Breukink, E. Natamycin Blocks Fungal Growth by Binding Specifically to Ergosterol without Permeabilizing the Membrane. J. Biol. Chem. 2008, 283, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- Munn, A.L.; Heese-Peck, A.; Stevenson, B.J.; Pichler, H.; Riezman, H. Specific Sterols Required for the Internalization Step of Endocytosis in Yeast. Mol. Biol. Cell 1999, 10, 3943–3957. [Google Scholar] [CrossRef] [PubMed]
- Szomek, M.; Reinholdt, P.; Walther, H.-L.; Scheidt, H.A.; Müller, P.; Obermaier, S.; Poolman, B.; Kongsted, J.; Wüstner, D. Natamycin Sequesters Ergosterol and Interferes with Substrate Transport by the Lysine Transporter Lyp1 from Yeast. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184012. [Google Scholar] [CrossRef]
- Brik, H. Natamycin. In Analytical Profiles of Drug Substances; Academic Press: New York, NY, USA, 1981; pp. 513–561. ISBN 0122608100. [Google Scholar]
- Koontz, J.L.; Marcy, J.E.; Barbeau, W.E.; Duncan, S.E. Stability of Natamycin and Its Cyclodextrin Inclusion Complexes in Aqueous Solution. J. Agric. Food Chem. 2003, 51, 7111–7114. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, P.; Patil, A.; Wu, K.-W.; Sweeney, C.; Tripathi, S.; Avula, B.; Taskar, P.; Khan, S.; Majumdar, S. Optimization, Stabilization, and Characterization of Amphotericin B Loaded Nanostructured Lipid Carriers for Ocular Drug Delivery. Int. J. Pharm. 2019, 572, 118771. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for Ocular Drug Delivery: Current Status and Translational Opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef] [PubMed]
- Kumar Mishra, K.; Deep Kaur, C.; Verma, S.; Kumar Sahu, A.; Kumar Dash, D.; Kashyap, P.; Prasad Mishra, S. Transethosomes and Nanoethosomes: Recent Approach on Transdermal Drug Delivery System. In Nanomedicines; IntechOpen: London, UK, 2019; Volume 32, pp. 137–144. ISBN 9781626239777. [Google Scholar]
- Ahmed, T.A.; Alzahrani, M.M.; Sirwi, A.; Alhakamy, N.A. Study the Antifungal and Ocular Permeation of Ketoconazole from Ophthalmic Formulations Containing Trans-Ethosomes Nanoparticles. Pharmaceutics 2021, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Yu, J.M.; Ko, J.H. Analysis of Ethanol Effects on Corneal Epithelium. Investig. Opthalmology Vis. Sci. 2013, 54, 3852. [Google Scholar] [CrossRef] [PubMed]
- Song, C.K.; Balakrishnan, P.; Shim, C.-K.; Chung, S.-J.; Chong, S.; Kim, D.-D. A Novel Vesicular Carrier, Transethosome, for Enhanced Skin Delivery of Voriconazole: Characterization and in Vitro/in Vivo Evaluation. Colloids Surf. B Biointerfaces 2012, 92, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Ferrara, F.; Hallan, S.S.; Baldisserotto, A.; Drechsler, M.; Malatesta, M.; Costanzo, M.; Cortesi, R.; Puglia, C.; Valacchi, G.; et al. Ethosomes and Transethosomes for Mangiferin Transdermal Delivery. Antioxidants 2021, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Benedusi, M.; Sguizzato, M.; Cortesi, R.; Baldisserotto, A.; Buzzi, R.; Valacchi, G.; Esposito, E. Ethosomes and Transethosomes as Cutaneous Delivery Systems for Quercetin: A Preliminary Study on Melanoma Cells. Pharmaceutics 2022, 14, 1038. [Google Scholar] [CrossRef]
- Allam, A.A.; Fathalla, D.; Safwat, M.A.; Soliman, G.M. Transferosomes versus Transethosomes for the Dermal Delivery for Minoxidil: Preparation and in Vitro/Ex Vivo Appraisal. J. Drug Deliv. Sci. Technol. 2022, 76, 103790. [Google Scholar] [CrossRef]
- Sebastiani, F.; Yanez Arteta, M.; Lerche, M.; Porcar, L.; Lang, C.; Bragg, R.A.; Elmore, C.S.; Krishnamurthy, V.R.; Russell, R.A.; Darwish, T.; et al. Apolipoprotein E Binding Drives Structural and Compositional Rearrangement of MRNA-Containing Lipid Nanoparticles. ACS Nano 2021, 15, 6709–6722. [Google Scholar] [CrossRef]
- Verma, A.; Jain, A.; Tiwari, A.; Saraf, S.; Panda, P.K.; Jain, S.K. Promising Antifungal Potential of Engineered Non-Ionic Surfactant-Based Vesicles: In Vitro and In Vivo Studies. AAPS PharmSciTech 2021, 22, 19. [Google Scholar] [CrossRef]
- Pabst, G.; Rappolt, M.; Amenitsch, H.; Laggner, P. Structural Information from Multilamellar Liposomes at Full Hydration: Full q -Range Fitting with High Quality x-Ray Data. Phys. Rev. E 2000, 62, 4000–4009. [Google Scholar] [CrossRef]
- Pabst, G.; Katsaras, J.; Raghunathan, V.A.; Rappolt, M. Structure and Interactions in the Anomalous Swelling Regime of Phospholipid Bilayers. Langmuir 2003, 19, 1716–1722. [Google Scholar] [CrossRef]
- Caillé, A. Remarques Sur La Diffusion Des Rayons X Dans Les Smectiques. CR Acad. Sci. 1972, 274, 891–893. [Google Scholar]
- Zhang, R.; Suter, R.M.; Nagle, J.F. Theory of the Structure Factor of Lipid Bilayers. Phys. Rev. E 1994, 50, 5047–5060. [Google Scholar] [CrossRef] [PubMed]
- Talarico, L.; Consumi, M.; Leone, G.; Tamasi, G.; Magnani, A. Solid Lipid Nanoparticles Produced via a Coacervation Method as Promising Carriers for Controlled Release of Quercetin. Molecules 2021, 26, 2694. [Google Scholar] [CrossRef] [PubMed]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Rostom, H.; Leone, G.; Lamponi, S.; Consumi, M.; Magnani, A.; Rossi, C. Chemical Characterization of Liposomes Containing Nutraceutical Compounds: Tyrosol, Hydroxytyrosol and Oleuropein. Biophys. Chem. 2019, 246, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Tilley, A.; Dong, Y.-D.; Amenitsch, H.; Rappolt, M.; Boyd, B.J. Transfer of Lipid and Phase Reorganisation in Self-Assembled Liquid Crystal Nanostructured Particles Based on Phytantriol. Phys. Chem. Chem. Phys. 2011, 13, 3026. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, O.; Mannina, L.; Sobolev, A.; Cametti, C.; Segre, A. An Improved NMR Study of Liposomes Using 1-Palmitoyl-2-Oleoyl-Sn-Glycero-3-Phospatidylcholine as Model. Molecules 2006, 11, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Ijare, O.B.; Somashekar, B.S.; Jadegoud, Y.; Gowda, G.A.N. 1H and 13C NMR Characterization and Stereochemical Assignments of Bile Acids in Aqueous Media. Lipids 2005, 40, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Lancelin, J.M.; Beau, J.M. Stereostructure of Pimaricin. J. Am. Chem. Soc. 1990, 112, 4060–4061. [Google Scholar] [CrossRef]
- Bonechi, C.; Ristori, S.; Martini, G.; Martini, S.; Rossi, C. Study of Bradykinin Conformation in the Presence of Model Membrane by Nuclear Magnetic Resonance and Molecular Modelling. Biochim. Biophys. Acta Biomembr. 2009, 1788, 708–716. [Google Scholar] [CrossRef]
- Yang, Y.; Huan, C.; Liang, X.; Fang, S.; Wang, J.; Chen, J. Development of Starch-Based Antifungal Coatings by Incorporation of Natamycin/Methyl-β-Cyclodextrin Inclusion Complex for Postharvest Treatments on Cherry Tomato against Botrytis Cinerea. Molecules 2019, 24, 3962. [Google Scholar] [CrossRef] [PubMed]
- Clemente, I.; D’Aria, F.; Giancola, C.; Bonechi, C.; Slouf, M.; Pavlova, E.; Rossi, C.; Ristori, S. Structuring and De-Structuring of Nanovectors from Algal Lipids. Part 1: Physico-Chemical Characterization. Colloids Surf. B Biointerfaces 2022, 220, 112939. [Google Scholar] [CrossRef] [PubMed]
- Halevas, E.G.; Avgoulas, D.I.; Katsipis, G.; Pantazaki, A.A. Flavonoid-Liposomes Formulations: Physico-Chemical Characteristics, Biological Activities and Therapeutic Applications. Eur. J. Med. Chem. Rep. 2022, 5, 100059. [Google Scholar] [CrossRef]
- Bonechi, C.; Martini, S.; Ciani, L.; Lamponi, S.; Rebmann, H.; Rossi, C.; Ristori, S. Using Liposomes as Carriers for Polyphenolic Compounds: The Case of Trans-Resveratrol. PLoS ONE 2012, 7, e41438. [Google Scholar] [CrossRef]
- Ciesielski, F.; Griffin, D.C.; Loraine, J.; Rittig, M.; Delves-Broughton, J.; Bonev, B.B. Recognition of Membrane Sterols by Polyene Antifungals Amphotericin B and Natamycin, A 13C MAS NMR Study. Front. Cell Dev. Biol. 2016, 4, 57. [Google Scholar] [CrossRef]
| Sample Name | Helper Lipid | Membrane Lipid 1 | Membrane Lipid 2 | Composition (Helper:Lipid1:Lipid2) | NAT (mg) |
|---|---|---|---|---|---|
| TE | Sodium Cholate | Soy PC | - | 1:4 | - |
| TE-NAT | Sodium Cholate | Soy PC | - | 1:4 | 0.5 |
| LNP1 | Cholesterol | DODMA | DSPC | 9:6:5 | - |
| LNP2 | Cholesterol | DODMA | DSPC | 9:5:6 | - |
| LNP3 | Cholesterol | DODMA | DSPC | 10:4:6 | - |
| LNP1-NAT | Cholesterol | DODMA | DSPC | 9:6:5 | 0.7 |
| LNP2-NAT | Cholesterol | DODMA | DSPC | 9:5:6 | 0.7 |
| LNP3-NAT | Cholesterol | DODMA | DSPC | 10:4:6 | 0.7 |
| Sample | Size (nm) ± St. Dev. | PDI ± St. Dev. | Zeta Potential (mV) ± St. Dev. |
|---|---|---|---|
| TE | 158.9 ± 1.3 | 0.16 ± 0.074 | −28.5 ± 0.6 |
| TE-NAT | 117.6 ± 1.6 | 0.25 ± 0.008 | −35.5 ± 1.4 |
| LNP1 | 212.5 ± 3 | 0.06 ± 0.05 | +34.7 ± 1 |
| LNP1-NAT | 155.8 ± 0.7 | 0.14 ± 0.02 | +62 ± 3.3 |
| LNP2 | 259.8 ± 1.7 | 0.15 ± 0.01 | +35.8 ± 1.2 |
| LNP2-NAT | 179.2 ± 1.7 | 0.22 ± 0.02 | +55.2 ± 0.25 |
| LNP3 | 252.2 ± 2.4 | 0.05 ± 0.04 | +26.5 ± 0.3 |
| LNP3-NAT | 146.5 ± 1.3 | 0.14 ± 0.008 | +58 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talarico, L.; Clemente, I.; Gennari, A.; Gabbricci, G.; Pepi, S.; Leone, G.; Bonechi, C.; Rossi, C.; Mattioli, S.L.; Detta, N.; et al. Physiochemical Characterization of Lipidic Nanoformulations Encapsulating the Antifungal Drug Natamycin. Nanomaterials 2024, 14, 726. https://doi.org/10.3390/nano14080726
Talarico L, Clemente I, Gennari A, Gabbricci G, Pepi S, Leone G, Bonechi C, Rossi C, Mattioli SL, Detta N, et al. Physiochemical Characterization of Lipidic Nanoformulations Encapsulating the Antifungal Drug Natamycin. Nanomaterials. 2024; 14(8):726. https://doi.org/10.3390/nano14080726
Chicago/Turabian StyleTalarico, Luigi, Ilaria Clemente, Alessandro Gennari, Giulia Gabbricci, Simone Pepi, Gemma Leone, Claudia Bonechi, Claudio Rossi, Simone Luca Mattioli, Nicola Detta, and et al. 2024. "Physiochemical Characterization of Lipidic Nanoformulations Encapsulating the Antifungal Drug Natamycin" Nanomaterials 14, no. 8: 726. https://doi.org/10.3390/nano14080726







