The Effectiveness Mechanisms of Carbon Nanotubes (CNTs) as Reinforcements for Magnesium-Based Composites for Biomedical Applications: A Review
Abstract
:1. Introduction
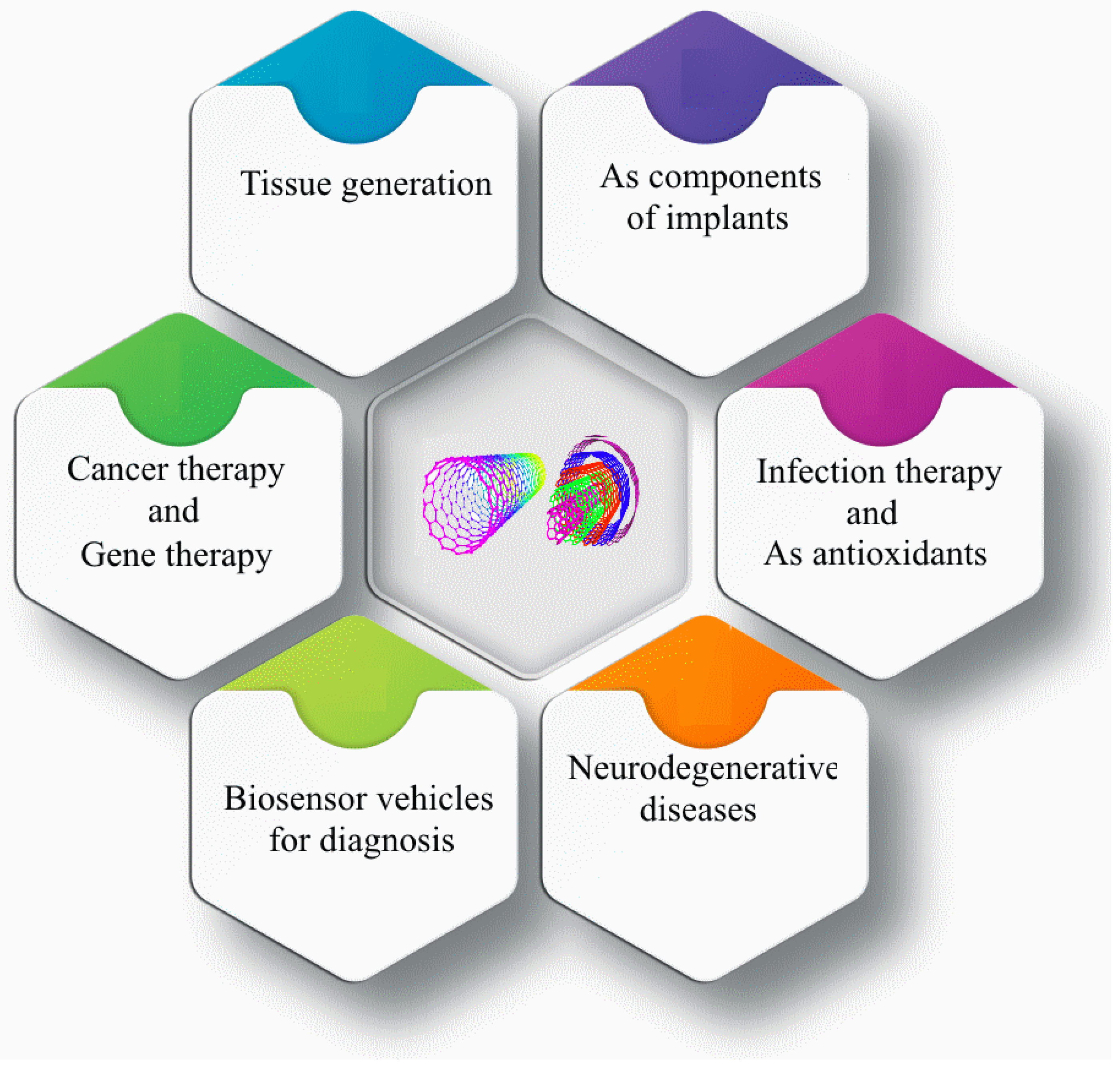
2. Carbon Nanotubes (CNTs)
3. Mg/CNT Biocomposites
4. Strengthening Mechanisms and Mechanical Properties
4.1. Strengthening Mechanisms of Mg Matrix Alloys
4.2. Strengthening Mechanisms of Mg/CNT Biocomposites

5. Degradation Behavior of Mg/CNT Biocomposites
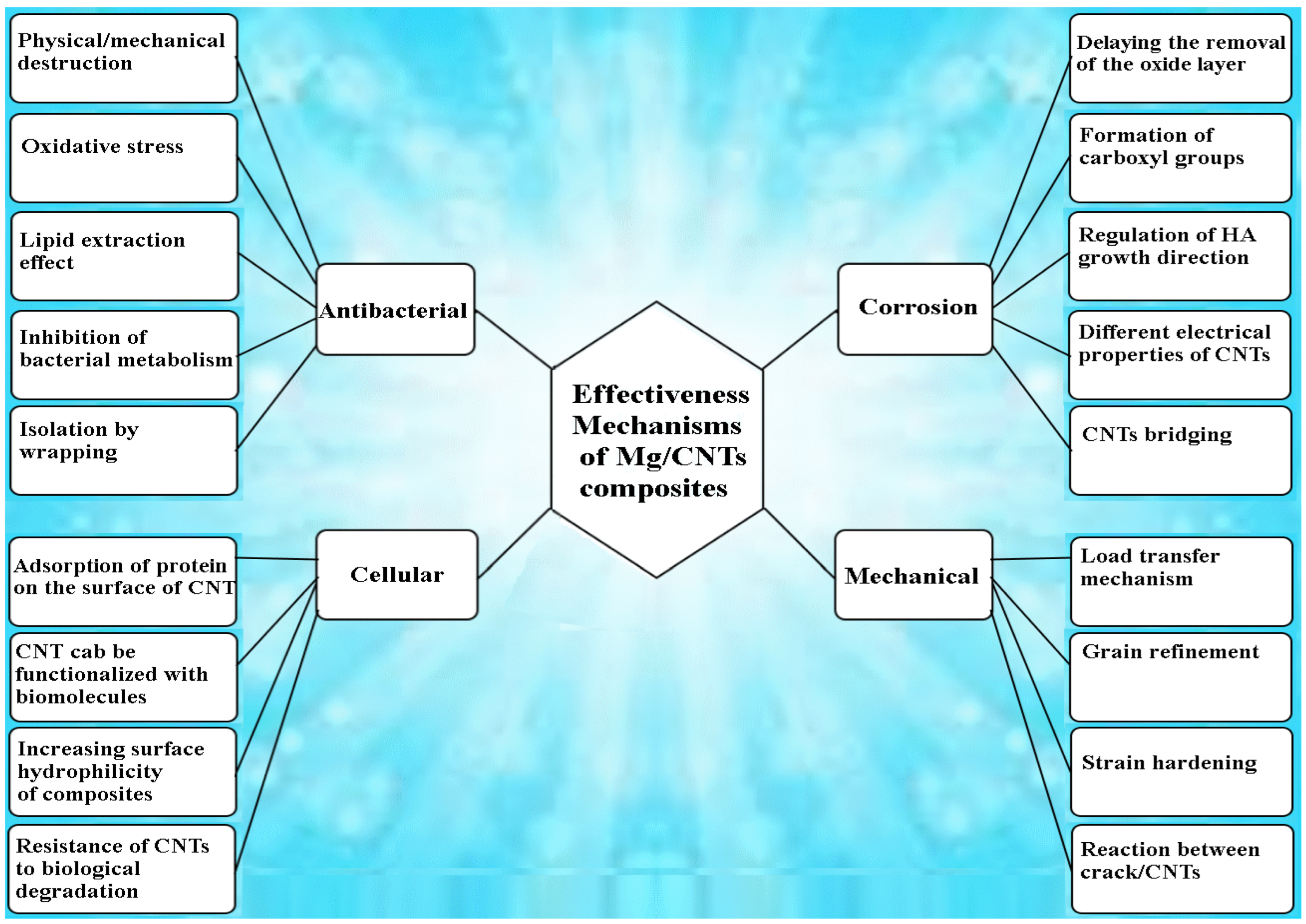
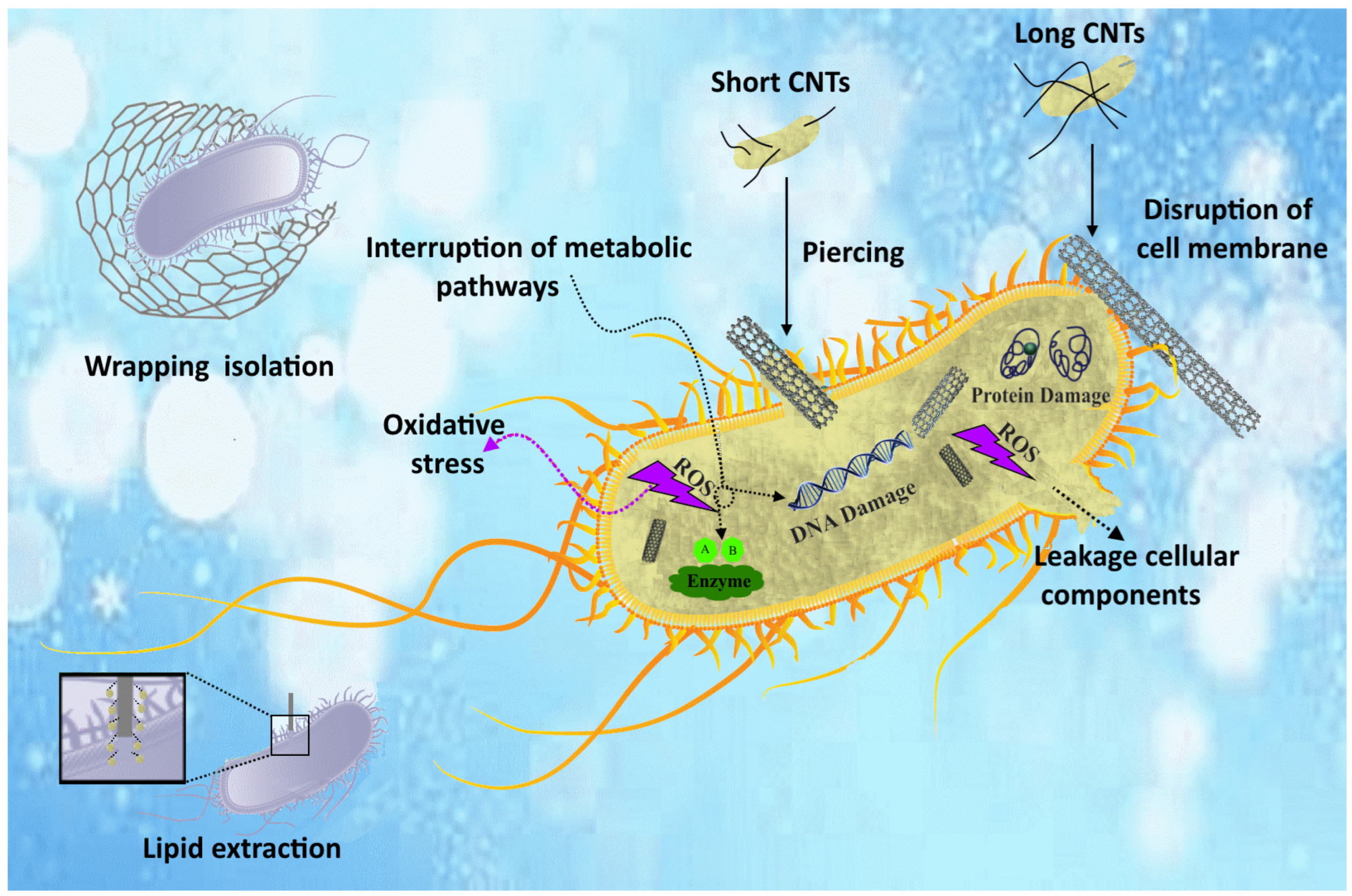
6. Antibacterial Performance of Mg/CNT Biocomposites
7. Cellular Response of Mg/CNT-Based Composites
8. Summary and Future Road Maps
Author Contributions
Funding
Conflicts of Interest
References
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Heydari, Z.; Lami, G.H.; Saberi, A.; Baltatu, M.S.; Vizureanu, P. A Comprehensive Review of the Current Research Status of Biodegradable Zinc Alloys and Composites for Biomedical Applications. Materials 2023, 16, 4797. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.F.; Gu, X.N.N.; Witte, F. Biodegradable Metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Somasundaram, M.; Uttamchand, N.K.; Annamalai, A.R.; Jen, C.P. Insights on Spark Plasma Sintering of Magnesium Composites: A Review. Nanomaterials 2022, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.; Bakhsheshi-Rad, H.R.R.; Karamian, E.; Kasiri-Asgarani, M.; Ghomi, H. Magnesium-Graphene Nano-Platelet Composites: Corrosion Behavior, Mechanical and Biological Properties. J. Alloys Compd. 2020, 821, 153379. [Google Scholar] [CrossRef]
- Jaiswal, S.; Kumar, R.M.; Gupta, P.; Kumaraswamy, M.; Roy, P.; Lahiri, D. Mechanical, Corrosion and Biocompatibility Behaviour of Mg-3Zn-HA Biodegradable Composites for Orthopaedic Fixture Accessories. J. Mech. Behav. Biomed. Mater. 2018, 78, 442–454. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Florido-Suarez, N.; Saceleanu, M.V.; Mirza-Rosca, J.C. New Titanium Alloys, Promising Materials for Medical Devices. Materials 2021, 14, 5934. [Google Scholar] [CrossRef] [PubMed]
- Simona Baltatu, M.; Andrei Tugui, C.; Cristina Perju, M.; Benchea, M.; Claudia Spataru, M.; Victor Sandu, A.; Vizureanu, P. Biocompatible Titanium Alloys Used in Medical Applications. Rev. Chim. 2019, 70, 1302–1306. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Munteanu, C.; Istrate, B. Microstructural Analysis and Tribological Behavior of Ti-Based Alloys with a Ceramic Layer Using the Thermal Spray Method. Coatings 2020, 10, 1216. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Sandu, A.V.; Nabialek, M.; Vizureanu, P.; Ciobanu, G. Biomimetic Deposition of Hydroxyapatite Layer on Titanium Alloys. Micromachines 2021, 12, 1447. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Zhou, J.; Li, H.; Chang, J.; Huan, Z. In Vitro Degradation and Surface Bioactivity of Iron-Matrix Composites Containing Silicate-Based Bioceramic. Bioact. Mater. 2017, 2, 10–18. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, S.; Torres, B.; Lopez, A.J.; Rainforth, W.M.; Otero, E.; Muñoz, M.; Rams, J. Wear Resistance of Stainless Steel Coatings on ZE41 Magnesium Alloy. J. Therm. Spray Technol. 2018, 27, 1615–1631. [Google Scholar] [CrossRef]
- Torkaman, R.; Darvishi, S.; Jokar, M.; Kharaziha, M.; Karbasi, M. Electrochemical and in Vitro Bioactivity of Nanocomposite Gelatin-Forsterite Coatings on AISI 316 L Stainless Steel. Prog. Org. Coatings 2017, 103, 40–47. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Karamian, E.; Kasiri-Asgarani, M.; Ghomi, H.; Omidi, M.; Abazari, S.; Ismail, A.F.; Sharif, S.; Berto, F. Synthesis and Characterization of Hot Extruded Magnesium-Zinc Nano-Composites Containing Low Content of Graphene Oxide for Implant Applications. Phys. Mesomech. 2021, 24, 486–502. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; Ramakrishna, S.; Berto, F. The Effect of Co-Encapsulated GO-Cu Nanofillers on Mechanical Properties, Cell Response, and Antibacterial Activities of Mg-Zn Composite. Metals 2022, 12, 207. [Google Scholar] [CrossRef]
- Zhang, S.; Bi, Y.; Li, J.; Wang, Z.; Yan, J.; Song, J.; Sheng, H.; Guo, H.; Li, Y. Biodegradation Behavior of Magnesium and ZK60 Alloy in Artificial Urine and Rat Models. Bioact. Mater. 2017, 2, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-N.N.; Hou, Z.-T.T.; Ye, X.; Xu, Z.-B.; Bai, X.-L.L.; Shang, P. The Effect of Selected Alloying Element Additions on Properties of Mg-Based Alloy as Bioimplants: A Literature Review. Front. Mater. Sci. 2013, 7, 227–236. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Majdi, A.; Rani, A.; Baig, Z.; Ahmed, S.W.; Hussain, G. Biocompatibility and Corrosion Resistance of Metallic Biomaterials. Corros. Rev. 2020, 38. [Google Scholar] [CrossRef]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent Research and Progress of Biodegradable Zinc Alloys and Composites for Biomedical Applications: Biomechanical and Biocorrosion Perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- Baltatu, I.; Sandu, A.V.; Vlad, M.D.; Spataru, M.C.; Vizureanu, P.; Baltatu, M.S. Mechanical Characterization and In Vitro Assay of Biocompatible Titanium Alloys. Micromachines 2022, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, B.; Wu, Y.H.; Zheng, Y.F. Comparative in Vitro Study on Pure Metals (Fe, Mn, Mg, Zn and W) as Biodegradable Metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable Magnesium Alloys for Orthopaedic Applications: A Review on Corrosion, Biocompatibility and Surface Modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I. Fabrication of Zinc–Tungsten Carbide Nanocomposite Using Cold Compaction Followed by Melting. J. Manuf. Sci. Eng. 2018, 140, 084503. [Google Scholar] [CrossRef]
- Pulido-González, N.; Hidalgo-Manrique, P.; García-Rodríguez, S.; Torres, B.; Rams, J. Effect of Heat Treatment on the Mechanical and Biocorrosion Behaviour of Two Mg-Zn-Ca Alloys. J. Magnes. Alloys 2022, 10, 540–554. [Google Scholar] [CrossRef]
- Tayebi, M.; Bizari, D.; Hassanzade, Z. Investigation of Mechanical Properties and Biocorrosion Behavior of in Situ and Ex Situ Mg Composite for Orthopedic Implants. Mater. Sci. Eng. C 2020, 113, 110974. [Google Scholar] [CrossRef] [PubMed]
- Voicu, M.E.; Demetrescu, I.; Dorobantu, A.; Enachescu, M.; Buica, G.O.; Ionita, D. Interaction of Mg Alloy with PLA Electrospun Nanofibers Coating in Understanding Changes of Corrosion, Wettability, and PH. Nanomaterials 2022, 12, 1369. [Google Scholar] [CrossRef] [PubMed]
- AbdelGawad, M.; Usman, C.A.; Shunmugasamy, V.C.; Karaman, I.; Mansoor, B. Corrosion Behavior of Mg-Zn-Zr-RE Alloys under Physiological Environment—Impact on Mechanical Integrity and Biocompatibility. J. Magnes. Alloys 2021, 10, 1542–1572. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg Alloys for Orthopedic Implants—A Review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.P.; Vega-Jiménez, A.L.; Vázquez-Olmos, A.R.; Ortega-Maldonado, M.; Ximenez-Fyvie, L.A. Antibacterial Properties In Vitro of Magnesium Oxide Nanoparticles for Dental Applications. Nanomaterials 2023, 13, 502. [Google Scholar] [CrossRef]
- Shao, Y.; Zeng, R.C.; Li, S.Q.; Cui, L.Y.; Zou, Y.H.; Guan, S.K.; Zheng, Y.F. Advance in Antibacterial Magnesium Alloys and Surface Coatings on Magnesium Alloys: A Review. Acta Metall. Sin. 2020, 33, 615–629. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Mat Yajid, M.A.; Kumar Gupta, R.; Mohd Yusof, N.; Bakhsheshi-Rad, H.R.; Ghandvar, H.; Ghasemi, E. Antibacterial Activities and Corrosion Behavior of Novel PEO/Nanostructured ZrO2 Coating on Mg Alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 1571–1581. [Google Scholar] [CrossRef]
- Ghasali, E.; Bordbar-Khiabani, A.; Alizadeh, M.; Mozafari, M.; Niazmand, M.; Kazemzadeh, H.; Ebadzadeh, T. Corrosion Behavior and In-Vitro Bioactivity of Porous Mg/Al2O3 and Mg/Si3N4 Metal Matrix Composites Fabricated Using Microwave Sintering Process. Mater. Chem. Phys. 2019, 225, 331–339. [Google Scholar] [CrossRef]
- Kasaeian-Naeini, M.; Sedighi, M.; Hashemi, R.; Delavar, H. Microstructure, Mechanical Properties and Fracture Toughness of ECAPed Magnesium Matrix Composite Reinforced with Hydroxyapatite Ceramic Particulates for Bioabsorbable Implants. Ceram. Int. 2023, 49, 17074–17090. [Google Scholar] [CrossRef]
- Meenashisundaram, G.; Nai, M.; Gupta, M. Effects of Primary Processing Techniques and Significance of Hall-Petch Strengthening on the Mechanical Response of Magnesium Matrix Composites Containing TiO2 Nanoparticulates. Nanomaterials 2015, 5, 1256–1283. [Google Scholar] [CrossRef]
- Kondoh, K.; Fukuda, H.; Umeda, J.; Imai, H.; Fugetsu, B.; Endo, M. Microstructural and Mechanical Analysis of Carbon Nanotube Reinforced Magnesium Alloy Powder Composites. Mater. Sci. Eng. A 2010, 527, 4103–4108. [Google Scholar] [CrossRef]
- Chen, B.; Li, S.; Imai, H.; Jia, L.; Umeda, J.; Takahashi, M.; Kondoh, K. Load Transfer Strengthening in Carbon Nanotubes Reinforced Metal Matrix Composites via In-Situ Tensile Tests. Compos. Sci. Technol. 2015, 113, 1–8. [Google Scholar] [CrossRef]
- Du, W.B.; Han, G.Q.; Li, S.B.; Liu, K.; Wang, Z.H.; Du, X. Effects of Carbon Nanotubes on Twin and Texture Evolution of Magnesium Matrix Composite during Compression Process. Mater. Charact. 2018, 141, 398–405. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Wen, C. Recent Progress on Nanocrystalline Metallic Materials for Biomedical Applications. Nanomaterials 2022, 12, 2111. [Google Scholar] [CrossRef]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; Ramakrishna, S.; Berto, F. Carbon Nanotubes (CNTs)-Reinforced Magnesium-Based Matrix Composites: A Comprehensive Review. Materials 2020, 13, 4421. [Google Scholar] [CrossRef]
- Zhao, J.; Haowei, M.; Saberi, A.; Heydari, Z.; Baltatu, M.S. Carbon Nanotube (CNT) Encapsulated Magnesium-Based Nanocomposites to Improve Mechanical, Degradation and Antibacterial Performances for Biomedical Device Applications. Coatings 2022, 12, 1589. [Google Scholar] [CrossRef]
- Pillari, L.K.; Lessoway, K.; Bichler, L. Carbon Nanotube and Graphene Reinforced Magnesium Matrix Composites: A State-of-the-Art Review. J. Magnes. Alloys 2023, 11, 1825–1905. [Google Scholar] [CrossRef]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon Nanotube—A Review on Synthesis, Properties and Plethora of Applications in the Field of Biomedical Science. Sensors Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Zhao, M.; Su, R.; Ji, L.; Zhang, Y.; Wu, H.; Wen, Z.; Dai, C. The Biocompatibility and Antibacterial Properties of the CNTs-Doped Magnesium Based Composite Implants in a Long-Term Biodegradation Process. J. Nanomater. 2023, 2023, 5012576. [Google Scholar] [CrossRef]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon Nanotubes: Applications in Pharmacy and Medicine. Biomed. Res. Int. 2013, 2013, 578290. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.A.; Abdel-Gawad, S.A.; Shoeib, M.A. Toward CNT-Reinforced Chitosan-Based Ceramic Composite Coatings on Biodegradable Magnesium for Surgical Implants. J. Coat. Technol. Res. 2021, 18, 971–988. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Jia, C.; Xu, M.; Kaseem, M.; Tayebi, M. Microstructural Changes Caused by the Creep Test in ZK60 Alloy Reinforced by SiCp at Intermediate Temperature after KOBO Extrusion and Aging. Materials 2023, 16, 3885. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.F.; Sharifi, H.; Tayebi, M.; Hamawandi, B.; Behnamian, Y. Thermal Cycles Behavior and Microstructure of AZ31/SiC Composite Prepared by Stir Casting. Sci. Rep. 2022, 12, 15191. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Antoniac, I.V.; Lupescu, Ș.C. Current Research Studies of Mg–Ca–Zn Biodegradable Alloys Used as Orthopedic Implants—Review. Crystals 2022, 12, 1468. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Bakhsheshi-Rad, H.R.; Saberi, A.; Razzaghi, M.; Kasar, A.K.; Ramakrishna, S.; Menezes, P.L.; Misra, M.; Ismail, A.F.; Sharif, S.; et al. Surface Modification of Magnesium Alloys Using Thermal and Solid-State Cold Spray Processes: Challenges and Latest Progresses. J. Magnes. Alloys 2022, 10, 2025–2061. [Google Scholar] [CrossRef]
- Tayebi, M.; Najafi, H.; Nategh, S.; Khodabandeh, A. Creep Behavior of ZK60 Alloy and ZK60/SiCw Composite After Extrusion and Precipitation Hardening. Met. Mater. Int. 2021, 27, 3905–3917. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Chi, P.; Bahonar, E.; Tayebi, M. Effects of the Microstructure and Precipitation Hardening on the Thermal Expansion Behavior of ZK60 Magnesium Alloy. J. Alloys Compd. 2022, 901, 163422. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Yarigarravesh, M.; Tayyebi, M.; Tayebi, M. Evaluation of Whisker Alignment and Anisotropic Mechanical Properties of ZK60 Alloy Reinforced with SiCw during KOBO Extrusion Method. J. Manuf. Process. 2022, 84, 344–356. [Google Scholar] [CrossRef]
- Du, X.; Du, W.; Wang, Z.; Liu, K.; Li, S. Ultra-High Strengthening Efficiency of Graphene Nanoplatelets Reinforced Magnesium Matrix Composites. Mater. Sci. Eng. A 2018, 711, 633–642. [Google Scholar] [CrossRef]
- JunRu Liu, J.; Wang, X.; Saberi, A.; Heydari, Z.H. The Effect of Co-Encapsulated GNPs-CNTs Nanofillers on Mechanical Properties, Degradation and Antibacterial Behavior of Mg-Based Composite. J. Mech. Behav. Biomed. Mater. 2023, 138, 105601. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Wong, W.L.E. Magnesium-Based Nanocomposites: Lightweight Materials of the Future. Mater. Charact. 2015, 105, 30–46. [Google Scholar] [CrossRef]
- Nai, M.H.; Wei, J.; Gupta, M. Interface Tailoring to Enhance Mechanical Properties of Carbon Nanotube Reinforced Magnesium Composites. Mater. Des. 2014, 60, 490–495. [Google Scholar] [CrossRef]
- Huang, S.J.; Abbas, A.; Ballóková, B. Effect of CNT on Microstructure, Dry Sliding Wear and Compressive Mechanical Properties of AZ61 Magnesium Alloy. J. Mater. Res. Technol. 2019, 8, 4273–4286. [Google Scholar] [CrossRef]
- Upadhyay, G.; Saxena, K.K.; Sehgal, S.; Mohammed, K.A.; Prakash, C.; Dixit, S.; Buddhi, D. Development of Carbon Nanotube (CNT)-Reinforced Mg Alloys: Fabrication Routes and Mechanical Properties. Metals 2022, 12, 1392. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Lu, Y.; Asif, M.; Hussain, S.; She, J.; Gou, J.; Mao, J. Effect of Graphene Nanoplatelets (GNPs) Addition on Strength and Ductility of Magnesium-Titanium Alloys. J. Magnes. Alloys 2013, 1, 242–248. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M.; Aamir, M. Synergetic Effect of Graphene Nanoplatelets (GNPs) and Multi-Walled Carbon Nanotube (MW-CNTs) on Mechanical Properties of Pure Magnesium. J. Alloys Compd. 2014, 603, 111–118. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M.; Hussain, S.; Gou, J.; Mao, J. Improved Strength and Ductility of Magnesium with Addition of Aluminum and Graphene Nanoplatelets (Al+GNPs) Using Semi Powder Metallurgy Method. J. Ind. Eng. Chem. 2015, 23, 243–250. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Asif, M.; Tang, A. Powder Metallurgy of Mg-1%Al-1%Sn Alloy Reinforced with Low Content of Graphene Nanoplatelets (GNPs). J. Ind. Eng. Chem. 2014, 20, 4250–4255. [Google Scholar] [CrossRef]
- Wang, L.; Qin, G.; Sun, S.; Ren, Y.; Li, S. Effect of Solid Solution Treatment on in Vitro Degradation Rate of As-Extruded Mg-Zn-Ag Alloys. Trans. Nonferrous Met. Soc. China 2017, 27, 2607–2612. [Google Scholar] [CrossRef]
- Hassan, S.F.; Islam, M.T.; Nouari, S.; Baig, M.M.A.; Patel, F.; Al-Aqeeli, N. Extraordinary Strengthening of Magnesium by Solid-State Diffusion of Copper in Mg-0.5Cu Alloy. JOM 2020, 72, 1597–1606. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, Y.; Chen, T.; Wu, L.; Chen, X.; Jiang, B.; Wang, J. Multi-Solute Solid Solution Behavior and Its Effect on the Properties of Magnesium Alloys. J. Magnes. Alloys 2022, 10, 1786–1820. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y. Research on an Mg–Zn Alloy as a Degradable Biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Lotfpour, M.; Dehghanian, C.; Emamy, M.; Bahmani, A.; Malekan, M.; Saadati, A.; Taghizadeh, M.; Shokouhimehr, M. In- Vitro Corrosion Behavior of the Cast and Extruded Biodegradable Mg-Zn-Cu Alloys in Simulated Body Fluid (SBF). J. Magnes. Alloys 2021, 9, 2078–2096. [Google Scholar] [CrossRef]
- Huan, Z.G.; Leeflang, M.A.; Zhou, J.; Fratila-Apachitei, L.E.; Duszczyk, J.; Zhou, J.; Fratila-Apachitei, L.E.; Duszczyk, J. In Vitro Degradation Behavior and Cytocompatibility of Mg-Zn-Zr Alloys. J. Mater. Sci. Mater. Med. 2010, 21, 2623–2635. [Google Scholar] [CrossRef]
- Ramkumar, R.; Arunkumar, G.; Radhakrishnan, K.; Kajendra Kumar, S.V. Studies on Mechanical, Microstructure and Corrosion Properties on Bio-Degradable Mg-Zn Alloys. Mater. Today Proc. 2021, 37, 3550–3553. [Google Scholar] [CrossRef]
- Jin, Y.; Blawert, C.; Feyerabend, F.; Bohlen, J.; Silva Campos, M.; Gavras, S.; Wiese, B.; Mei, D.; Deng, M.; Yang, H.; et al. Time-Sequential Corrosion Behaviour Observation of Micro-Alloyed Mg-0.5Zn-0.2Ca Alloy via a Quasi-in Situ Approach. Corros. Sci. 2019, 158, 108096. [Google Scholar] [CrossRef]
- Cho, D.H.; Lee, B.W.; Park, J.Y.; Cho, K.M.; Park, I.M. Effect of Mn Addition on Corrosion Properties of Biodegradable Mg-4Zn-0.5Ca-XMn Alloys. J. Alloys Compd. 2017, 695, 1166–1174. [Google Scholar] [CrossRef]
- Liu, W.; Cao, F.; Chen, A.; Chang, L.; Zhang, J.; Cao, C. Corrosion Behaviour of AM60 Magnesium Alloys Containing Ce or La under Thin Electrolyte Layers. Part 1: Microstructural Characterization and Electrochemical Behaviour. Corros. Sci. 2010, 52, 627–638. [Google Scholar] [CrossRef]
- KUBÁSEK, J.; VOJTĚCH, D. Structural and Corrosion Characterization of Biodegradable Mg–RE (RE=Gd, Y, Nd) Alloys. Trans. Nonferrous Met. Soc. China 2013, 23, 1215–1225. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Khobaib, M.; Robertson, E.; Froes, F.H. Corrosion Behavior of Rapidly Solidified Mg Nd and Mg Y Alloys. Mater. Sci. Eng. 1988, 99, 507–511. [Google Scholar] [CrossRef]
- Nie, J.F. Precipitation and Hardening in Magnesium Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 3891–3939. [Google Scholar] [CrossRef]
- Mendis, C.L.; Hono, K. Understanding Precipitation Processes in Magnesium Alloys; Woodhead Publishing Limited: Cambridge, MA, USA, 2013; ISBN 9780857090881. [Google Scholar]
- Zhao, J.; Du, G.; Ghanaei, A.; Rajaee, A.; Davoodi, D.; Miri, R.; Tayebi, M. Effect of Adding Ta on the Tribological Behavior of Zr Alloy after β-Quenching and Annealing. Tribol. Int. 2023, 189, 108961. [Google Scholar] [CrossRef]
- Momeni, E.; Sharifi, H.; Tayebi, M.; Keyvani, A.; Aghaie, E.; Behnamian, Y. Tribological Behavior of ZK60Gd Alloy Reinforced by SiC Particles after Precipitation Hardening. J. Magnes. Alloys 2023, 11, 3362–3381. [Google Scholar] [CrossRef]
- Moheimani, S.K.; Keshtgar, A.; Khademzadeh, S.; Tayebi, M.; Rajaee, A.; Saboori, A. Tribological Behaviour of AZ31 Magnesium Alloy Reinforced by Bimodal Size B4C after Precipitation Hardening. J. Magnes. Alloys 2022, 10, 3267–3280. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Xin, Y.; Cai, Y.; Han, J. Effect of Equal Channel Angular Pressing on Microstructure and Mechanical Performance of Innovative Nano MgO-Added Mg-Zn-Ca Composite as a Biomaterial. Mater. Lett. 2021, 304, 130604. [Google Scholar] [CrossRef]
- Lu, Y.; Bradshaw, A.R.; Chiu, Y.L.; Jones, I.P. Effects of Secondary Phase and Grain Size on the Corrosion of Biodegradable Mg-Zn-Ca Alloys. Mater. Sci. Eng. C 2015, 48, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Conrad, H.; Narayan, J. On the Grain Size Softening in Nanocrystalline Materials. Scr. Mater. 2000, 42, 1025–1030. [Google Scholar] [CrossRef]
- Nardone, V.C.; Prewo, K.M. On the Strength of Discontinuous Silicon Carbide Reinforced Aluminum Composites. Scr. Metall. 1986, 20, 43–48. [Google Scholar] [CrossRef]
- Chen, B.; Shen, J.; Ye, X.; Jia, L.; Li, S.; Umeda, J.; Takahashi, M.; Kondoh, K. Length Effect of Carbon Nanotubes on the Strengthening Mechanisms in Metal Matrix Composites. Acta Mater. 2017, 140, 317–325. [Google Scholar] [CrossRef]
- Hou, J.; Du, W.; Parande, G.; Gupta, M.; Li, S. Significantly Enhancing the Strength + Ductility Combination of Mg-9Al Alloy Using Multi-Walled Carbon Nanotubes. J. Alloys Compd. 2019, 790, 974–982. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Asif, M. Exploring Mechanical Behavior of Mg–6Zn Alloy Reinforced with Graphene Nanoplatelets. Mater. Sci. Eng. A 2016, 649, 263–269. [Google Scholar] [CrossRef]
- Han, G.; Wang, Z.; Liu, K.; Li, S.; Du, X.; Du, W. Synthesis of CNT-Reinforced AZ31 Magnesium Alloy Composites with Uniformly Distributed CNTs. Mater. Sci. Eng. A 2015, 628, 350–357. [Google Scholar] [CrossRef]
- Carneiro, Í.; Simões, S. Strengthening Mechanisms in Carbon Nanotubes Reinforced Metal Matrix Composites: A Review. Metals 2021, 11, 1613. [Google Scholar] [CrossRef]
- Park, J.G.; Keum, D.H.; Lee, Y.H. Strengthening Mechanisms in Carbon Nanotube-Reinforced Aluminum Composites. Carbon N. Y. 2015, 95, 690–698. [Google Scholar] [CrossRef]
- Mirza, F.; Chen, D. A Unified Model for the Prediction of Yield Strength in Particulate-Reinforced Metal Matrix Nanocomposites. Materials 2015, 8, 5138–5153. [Google Scholar] [CrossRef]
- Ma, P.; Jia, Y.; Konda Gokuldoss, P.; Yu, Z.; Yang, S.; Zhao, J.; Li, C. Effect of Al2O3 Nanoparticles as Reinforcement on the Tensile Behavior of Al-12Si Composites. Metals 2017, 7, 359. [Google Scholar] [CrossRef]
- Amirzade-Iranaq, M.T.; Omidi, M.; Bakhsheshi-Rad, H.R.; Saberi, A.; Abazari, S.; Teymouri, N.; Naeimi, F.; Sergi, C.; Ismail, A.F.; Sharif, S.; et al. MWCNTs-TiO2 Incorporated-Mg Composites to Improve the Mechanical, Corrosion and Biological Characteristics for Use in Biomedical Fields. Materials 2023, 16, 1919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Saberi, A.; Heydari, Z.; Baltatu, M.S. Bredigite-CNTs Reinforced Mg-Zn Bio-Composites to Enhance the Mechanical and Biological Properties for Biomedical Applications. Materials 2023, 16, 1681. [Google Scholar] [CrossRef] [PubMed]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Berto, F. Functionalized Carbon Nanotube-Encapsulated Magnesium-Based Nanocomposites with Outstanding Mechanical and Biological Properties as Load-Bearing Bone Implants. Mater. Des. 2022, 213, 110354. [Google Scholar] [CrossRef]
- Habibi, M.K.; Paramsothy, M.; Hamouda, A.M.S.; Gupta, M. Enhanced Compressive Response of Hybrid Mg–CNT Nano-Composites. J. Mater. Sci. 2011, 46, 4588–4597. [Google Scholar] [CrossRef]
- Paramsothy, M.; Chan, J.; Kwok, R.; Gupta, M. Addition of CNTs to Enhance Tensile/Compressive Response of Magnesium Alloy ZK60A. Compos. Part A Appl. Sci. Manuf. 2011, 42, 180–188. [Google Scholar] [CrossRef]
- Abbas, A.; Huang, S.J.; Ballóková, B.; Sülleiová, K. Tribological Effects of Carbon Nanotubes on Magnesium Alloy AZ31 and Analyzing Aging Effects on CNTs/AZ31 Composites Fabricated by Stir Casting Process. Tribol. Int. 2020, 142, 105982. [Google Scholar] [CrossRef]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Keshavarz, M.; Kehtari, M.; Ramakrishna, S.; Berto, F. MgO-Incorporated Carbon Nanotubes-Reinforced Mg-Based Composites to Improve Mechanical, Corrosion, and Biological Properties Targeting Biomedical Applications. J. Mater. Res. Technol. 2022, 20, 976–990. [Google Scholar] [CrossRef]
- Handayani, M.; Ganta, M.; Darsono, N.; Sulistiyono, E.; Lestari, F.P.; Erryani, A.; Astawa, I.N.G.P.; Lusiana; Azhari, S.N. Multi-Walled Carbon Nanotubes Reinforced-Based Magnesium Metal Matrix Composites Prepared by Powder Metallurgy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 578, 012041. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Abdul-Kadir, M.R.; Idris, M.H.; Farahany, S. Relationship between the Corrosion Behavior and the Thermal Characteristics and Microstructure of Mg-0.5Ca-XZn Alloys. Corros. Sci. 2012, 64, 184–197. [Google Scholar] [CrossRef]
- Xia, D.; Yang, F.; Zheng, Y.; Liu, Y.; Zhou, Y. Research Status of Biodegradable Metals Designed for Oral and Maxillofacial Applications: A Review. Bioact. Mater. 2021, 6, 4186–4208. [Google Scholar] [CrossRef]
- Lin, J.; Nguyen, N.Y.T.; Zhang, C.; Ha, A.; Liu, H.H. Antimicrobial Properties of MgO Nanostructures on Magnesium Substrates. ACS Omega 2020, 5, 24613–24627. [Google Scholar] [CrossRef]
- Maqsood, M.F.; Raza, M.A.; Rehman, Z.U.; Tayyeb, A.; Makhdoom, M.A.; Ghafoor, F.; Latif, U.; Khan, M.F. Role of Solvent Used in Development of Graphene Oxide Coating on AZ31B Magnesium Alloy: Corrosion Behavior and Biocompatibility Analysis. Nanomaterials 2022, 12, 3745. [Google Scholar] [CrossRef] [PubMed]
- Roche, V.; Koga, G.Y.; Matias, T.B.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J.; Nogueira, R.P.; Jorge Junior, A.M. Degradation of Biodegradable Implants: The Influence of Microstructure and Composition of Mg-Zn-Ca Alloys. J. Alloys Compd. 2019, 774, 168–181. [Google Scholar] [CrossRef]
- Dong, J.; Lin, T.; Shao, H.; Wang, H.; Wang, X.; Song, K.; Li, Q. Advances in Degradation Behavior of Biomedical Magnesium Alloys: A Review. J. Alloys Compd. 2022, 908, 164600. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Abdal Dayem, A.; Eppakayala, V.; Kim, J.H. Oxidative Stress-Mediated Antibacterial Activity of Graphene Oxide and Reduced Graphene Oxide in Pseudomonas Aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Maksimova, Y.G. Microorganisms and Carbon Nanotubes: Interaction and Applications (Review). Appl. Biochem. Microbiol. 2019, 55, 1–12. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mater. 2019, 31, 1–15. [Google Scholar] [CrossRef]
- Khorashadizade, F.; Abazari, S.; Rajabi, M.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Berto, F. Overview of Magnesium-Ceramic Composites: Mechanical, Corrosion and Biological Properties. J. Mater. Res. Technol. 2021, 15, 6034–6066. [Google Scholar] [CrossRef]
- Emily Walker, M.H. Magnesium, Iron and Zinc Alloys, the Trifecta of Bioresorbable Orthopaedic and Vascular Implantation—A Review. J. Biotechnol. Biomater. 2015, 5, 178. [Google Scholar] [CrossRef]
- Dezfuli, S.N.; Huan, Z.; Mol, A.; Leeflang, S.; Chang, J.; Zhou, J. Advanced Bredigite-Containing Magnesium-Matrix Composites for Biodegradable Bone Implant Applications. Mater. Sci. Eng. C 2017, 79, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, A.; Menaszek, E.; Paluszkiewicz, C.; Blazewicz, M. Comparative In Vivo Biocompatibility Study of Single- and Multi-Wall Carbon Nanotubes. Acta Biomater. 2008, 4, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Luo, J.; Zhou, Q.; Wang, H. Pharmacokinetics, Metabolism and Toxicity of Carbon Nanotubes for Biomedical Purposes. Theranostics 2012, 2, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Abouei, E.; Hatamie, S.; Ghasemi, E. Accelerated Differentiation of Neural Stem Cells into Neurons on Ginseng-Reduced Graphene Oxide Sheets. Carbon N. Y. 2014, 66, 395–406. [Google Scholar] [CrossRef]
- Serrano, M.C.; Gutiérrez, M.C.; del Monte, F. Role of Polymers in the Design of 3D Carbon Nanotube-Based Scaffolds for Biomedical Applications. Prog. Polym. Sci. 2014, 39, 1448–1471. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S.; Metaxa, Z.S.; Shah, S.P. Highly Dispersed Carbon Nanotube Reinforced Cement Based Materials. Cem. Concr. Res. 2010, 40, 1052–1059. [Google Scholar] [CrossRef]
- Belyaeva, L.A.; Schneider, G.F. Wettability of Graphene. Surf. Sci. Rep. 2020, 75, 100482. [Google Scholar] [CrossRef]
- Sun, L.; Guo, J.; Chen, H.; Zhang, D.; Shang, L.; Zhang, B.; Zhao, Y. Tailoring Materials with Specific Wettability in Biomedical Engineering. Adv. Sci. 2021, 8, 2100126. [Google Scholar] [CrossRef]


| Material Type | Quantitative Parameters | Qualitative Parameters | Refs. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D (g/cm3) | E (GPa) | ε (%) | UTS (MPa) | Y.S | UCS (MPa) | D.R (mm y−1) | Advantages | Disadvantages | ||
| Cortical human bone | 1.8–2.1 | 3–30 | 1–4 | 35–283 | 70–100 | 164–200 | NBR | - | - | [17,18] |
| S.S | 7.9–8.1 | 170–205 | 10–40 | 460–1700 | 300–400 | 500–1000 | No |
|
| [17,19] |
| Ti alloys | 4.4–4.5 | 110–127 | 10–15 | 800–1200 | 700–900 | 900 | No |
|
| [20,21] |
| Co–Cr alloys | 7.9–9.2 | 200–240 | 8–20 | 700–1300 | 480–550 | 450–1000 | No |
|
| [2,20] |
| Mg alloys | 1.74 | 41–45 | 5–40 | 135–285 | 130–250 | 65–100 | 0.8–2.7 |
|
| [17,22,23] |
| Zn | 7.1 | 78–121 | 0.3–2 | 100–150 | 21–27 | 30–100 | 0.1–0.3 |
|
| [2,24] |
| Fe | 7.8 | 213 | 37.5 | 300 | 120–150 | 560 | 0.1 |
|
| [8,22] |
| Sample | Processing Route | Applications | UCS (MPa) | Hardness (HV) | Years | Ref. |
|---|---|---|---|---|---|---|
| Mg-3Zn | SPM + SPS | Biodegradable implants | 122 ± 6 | 59 ± 2.3 | 2023 | [94] |
| (Mg-3Zn)94.5/Br5-CNTs0.5 | SPM + SPS | Biodegradable implants | 185 ± 9 | 79 ± 3.1 | 2023 | [94] |
| (Mg-3Zn)89/Br10-CNTs1 | SPM + SPS | Biodegradable implants | 210 ± 10 | 93 ± 3.6 | 2023 | [94] |
| (Mg-3Zn)83.5/Br15-CNTs1.5 | SPM + SPS | Biodegradable implants | 110 ± 5.5 | 65 ± 2.4 | 2023 | [94] |
| Mg-6Zn | SPM + SPS | OFFD | 156 | 49.5 | 2023 | [93] |
| Mg-6Zn/5TiO2-0.5MWCNTs | SPM + SPS | OFFD | 221 | 68 | 2023 | [93] |
| Mg-6Zn/10TiO2-1MWCNTs | SPM + SPS | OFFD | 269 | 79 | 2023 | [93] |
| Mg-6Zn/15TiO2-0.5MWCNTs | SPM + SPS | OFFD | 233 | 82 | 2023 | [93] |
| Mg-6Zn | SPM + SPS | Implants | 145 ± 7 | 50 ± 1.7 | 2023 | [55] |
| Mg-6Zn/0.25GNPs-0.25CNTs | SPM + SPS | Implants | 210 ± 10 | 68 ± 2.3 | 2023 | [55] |
| Mg-6Zn/0.5GNPs-0.5CNTs | SPM + SPS | Implants | 255 ± 12 | 76 ± 2.6 | 2023 | [55] |
| Mg-6Zn/1GNPs-1CNTs | SPM + SPS | Implants | 115 ± 6 | 78 ± 2.7 | 2023 | [55] |
| Mg-2.5Zn-0.5Zr | SPM | Biomedical devices | 151 ± 7.5 | 58 | 2022 | [41] |
| Mg-2.5Zn-0.5Zr/0.3CNTs | SPM | Biomedical devices | 196 ± 9.8 | 67 | 2022 | [41] |
| Mg-2.5Zn-0.5Zr/0.6CNTs | SPM | Biomedical devices | 237 ± 12 | 78 | 2022 | [41] |
| Mg-2.5Zn-0.5Zr/0.9CNTs | SPM | Biomedical devices | 148 ± 7 | 80 | 2022 | [41] |
| Mg-3Zn | SPM + HTE | Load-bearing bone implants | 289.6 ± 13 | 66 ± 2 | 2022 | [95] |
| Mg-3Zn/0.2fCNT | SPM + HTE | Load-bearing bone implants | 368.2 ± 12 | 70 ± 2 | 2022 | [95] |
| Mg-3Zn/0.4fCNT | SPM + HTE | Load-bearing bone implants | 390 ± 15 | 74 ± 2.5 | 2022 | [95] |
| Mg-3Zn/0.8fCNT | SPM + HTE | Load-bearing bone implants | 320.2 ± 14 | 76 ± 3 | 2022 | [95] |
| Mg-3Zn-1Mn | SPM + HTE | Medical implant | 295.6 ± 20.4 | 51.6 | 2022 | [99] |
| Mg-3Zn-1Mn/CNTs | SPM + HTE | Medical implant | 404.8 ± 16.1. | 74.5 | 2022 | [99] |
| Mg-3Zn-1Mn/CNTs-MgO | SPM + HTE | Medical implant | 429 ± 15 | 83.4 | 2022 | [99] |
| Mg | PM | Biodegradable implants | - | 36.9 | 2019 | [100] |
| Mg/0.1% MWCNTs | PM | Biodegradable implants | - | 43.6 | 2019 | [100] |
| Mg/0.2% MWCNTs | PM | Biodegradable implants | - | 43 | 2019 | [100] |
| Mg/0.3% MWCNTs | PM | Biodegradable implants | - | 46.5 | 2019 | [100] |
| Mg/0.5% MWCNTs | PM | Biodegradable implants | - | 37.5 | 2019 | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saberi, A.; Baltatu, M.S.; Vizureanu, P. The Effectiveness Mechanisms of Carbon Nanotubes (CNTs) as Reinforcements for Magnesium-Based Composites for Biomedical Applications: A Review. Nanomaterials 2024, 14, 756. https://doi.org/10.3390/nano14090756
Saberi A, Baltatu MS, Vizureanu P. The Effectiveness Mechanisms of Carbon Nanotubes (CNTs) as Reinforcements for Magnesium-Based Composites for Biomedical Applications: A Review. Nanomaterials. 2024; 14(9):756. https://doi.org/10.3390/nano14090756
Chicago/Turabian StyleSaberi, Abbas, Madalina Simona Baltatu, and Petrica Vizureanu. 2024. "The Effectiveness Mechanisms of Carbon Nanotubes (CNTs) as Reinforcements for Magnesium-Based Composites for Biomedical Applications: A Review" Nanomaterials 14, no. 9: 756. https://doi.org/10.3390/nano14090756








