Mixed Metal Oxide W-TiO2 Nanopowder for Environmental Process: Synergy of Adsorption and Photocatalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis Procedure
2.2. Characterization Techniques
3. Results and Discussion
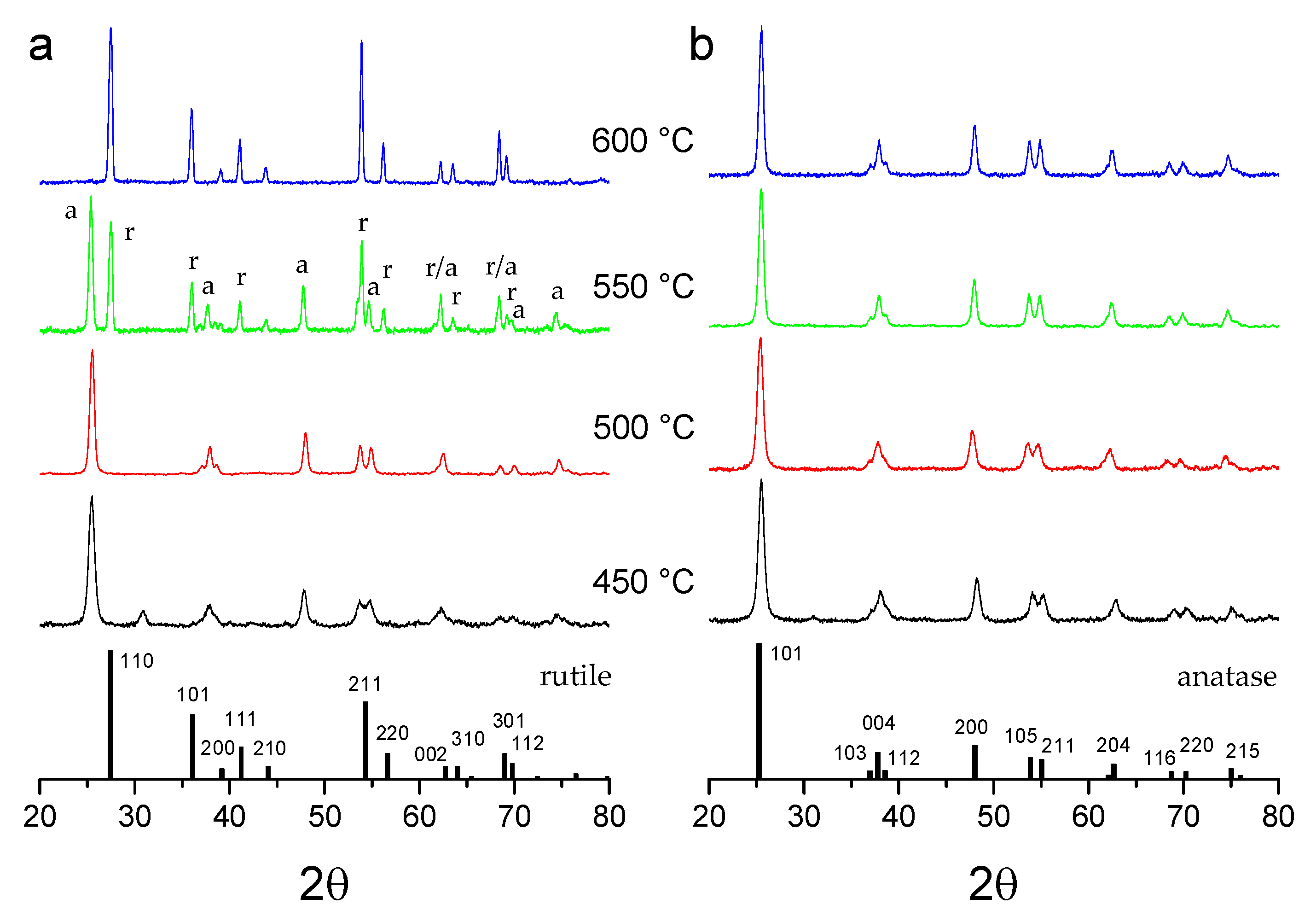
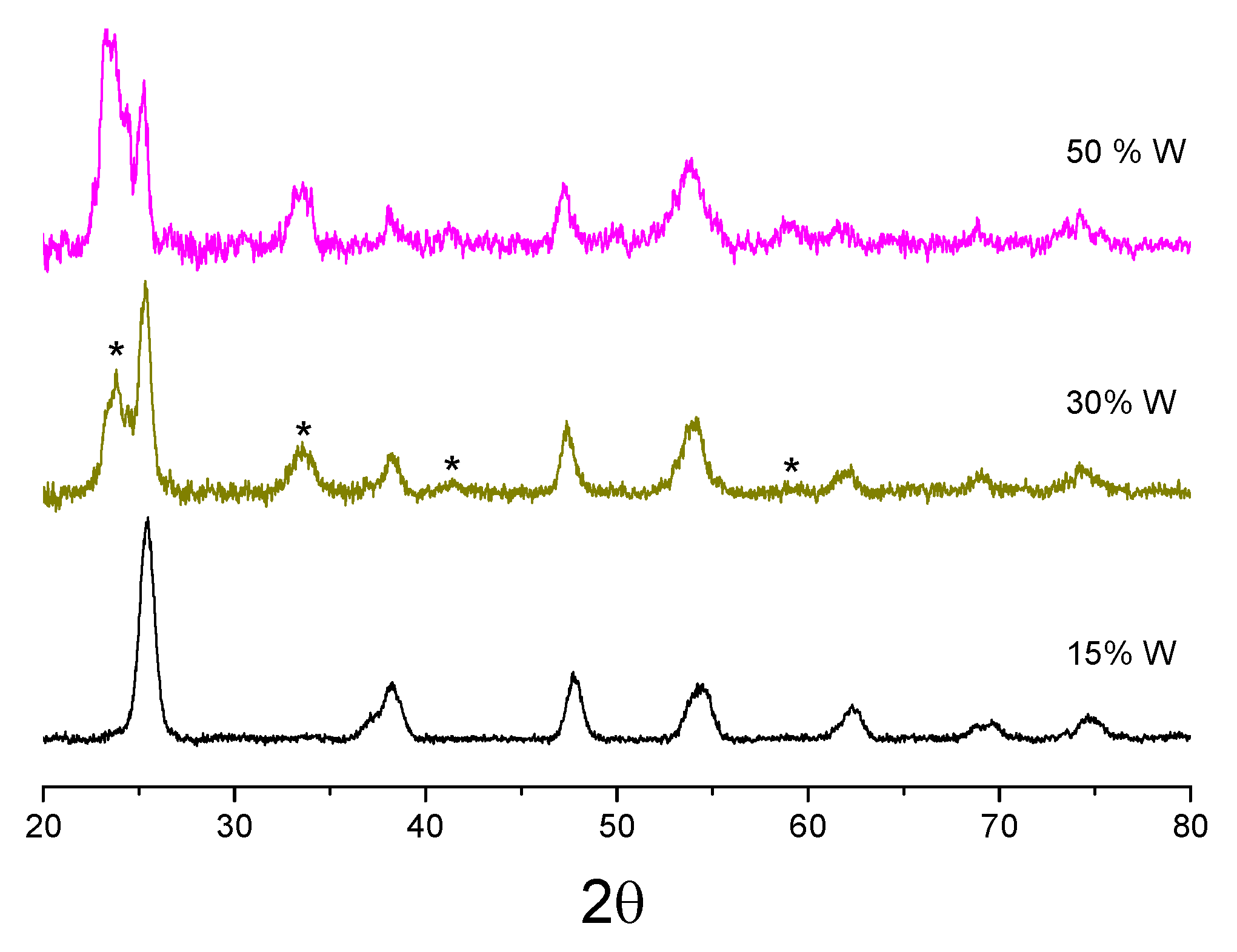
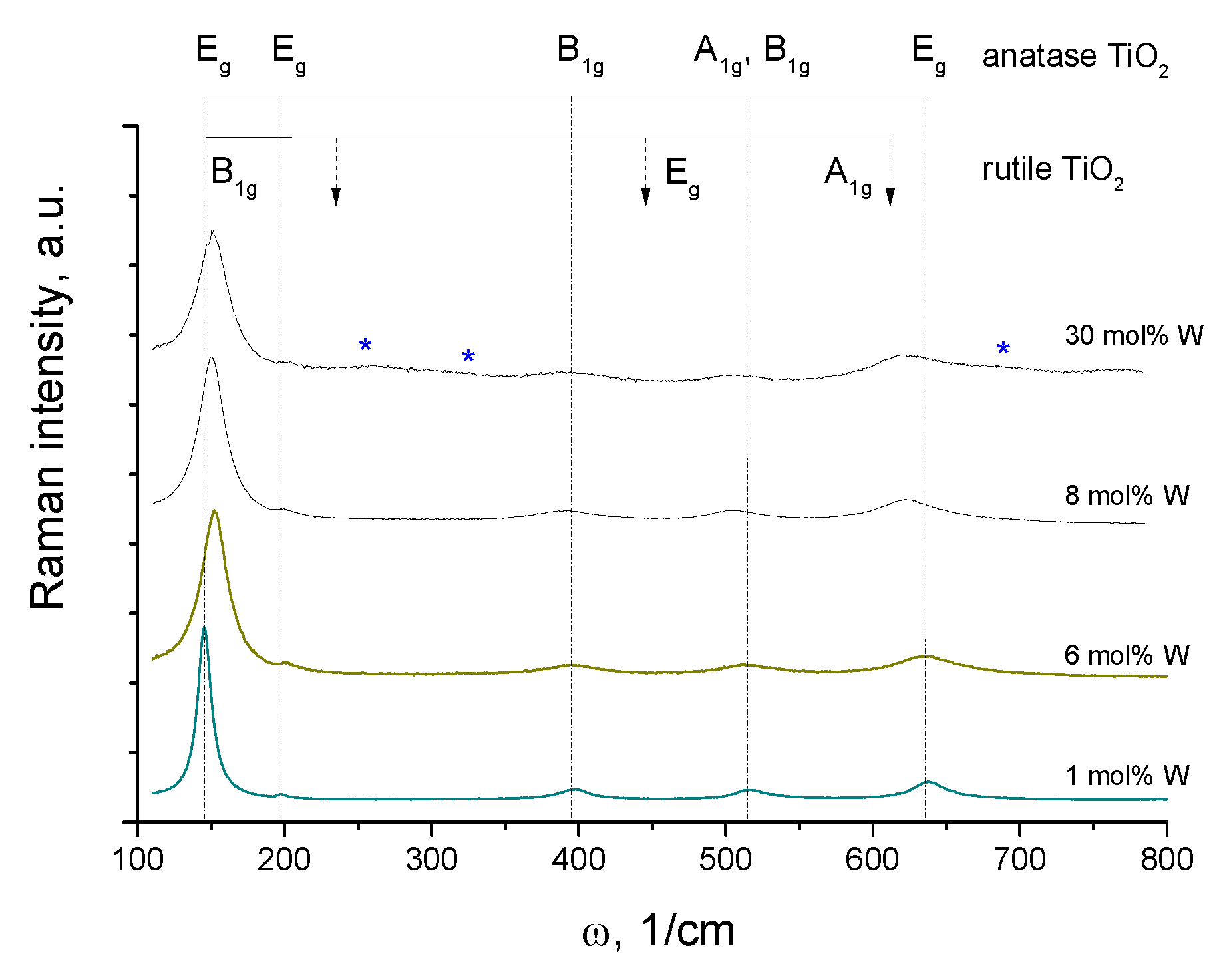
3.1. Structural Properties
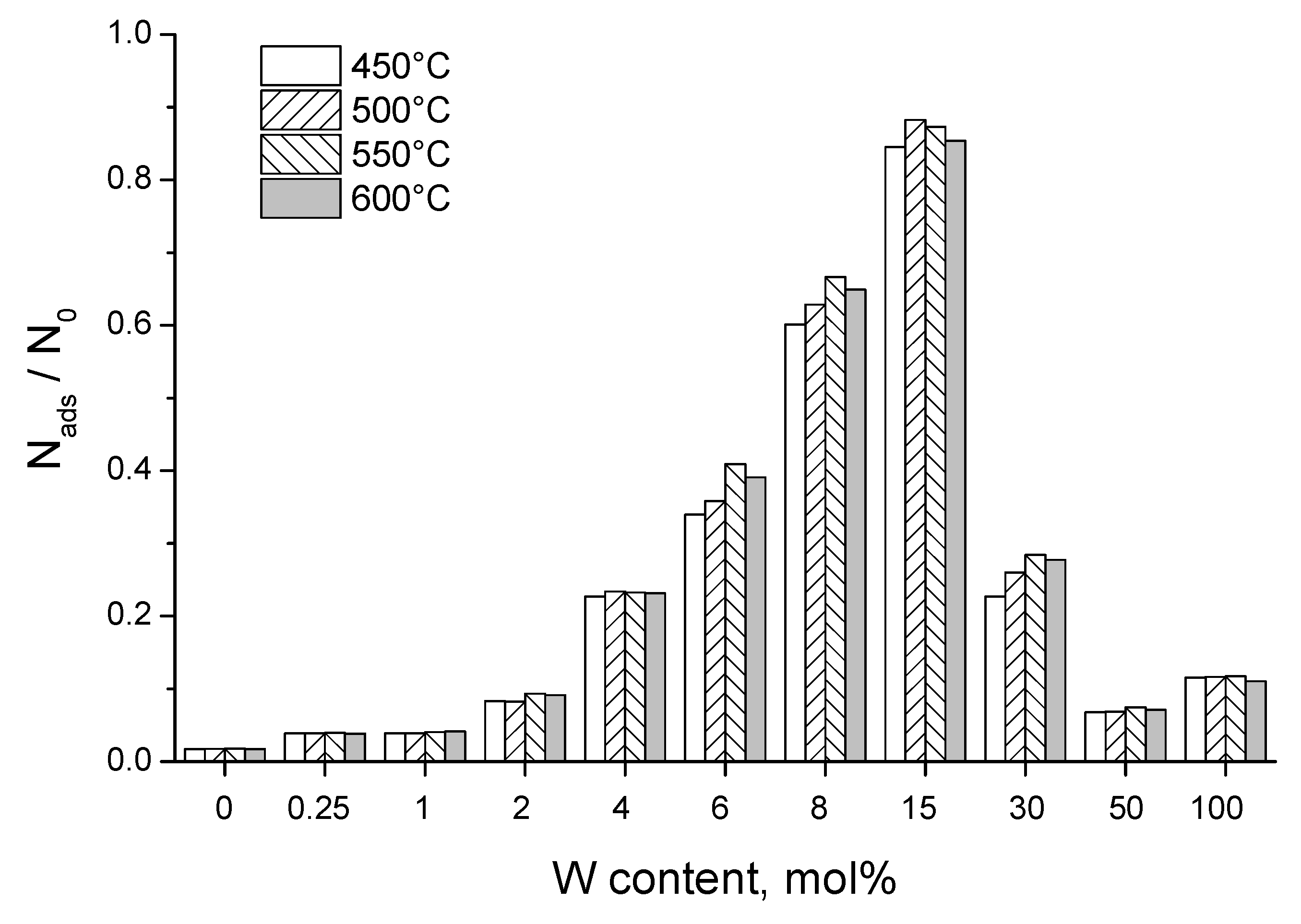
3.2. Adsorption Capacity
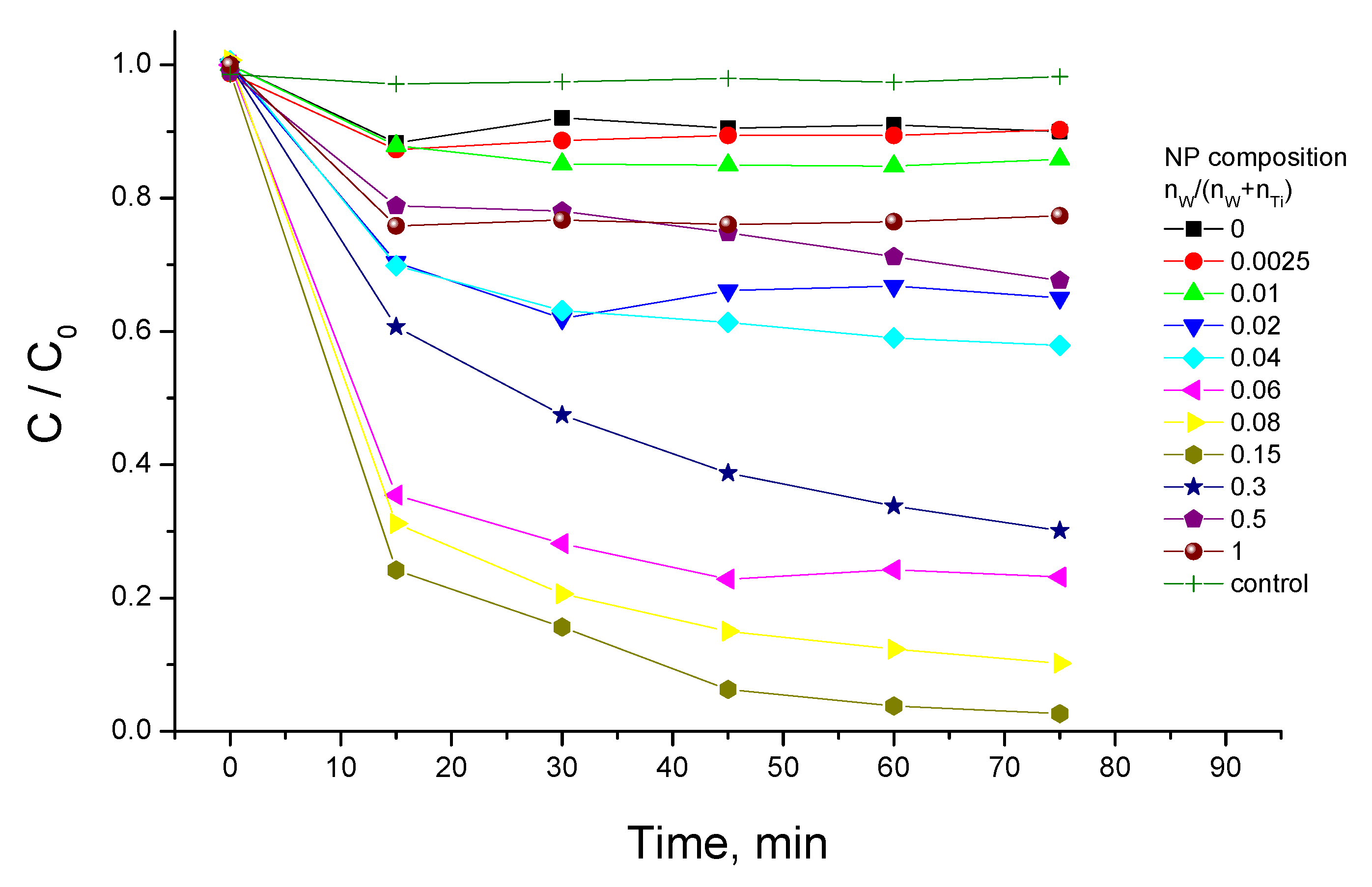
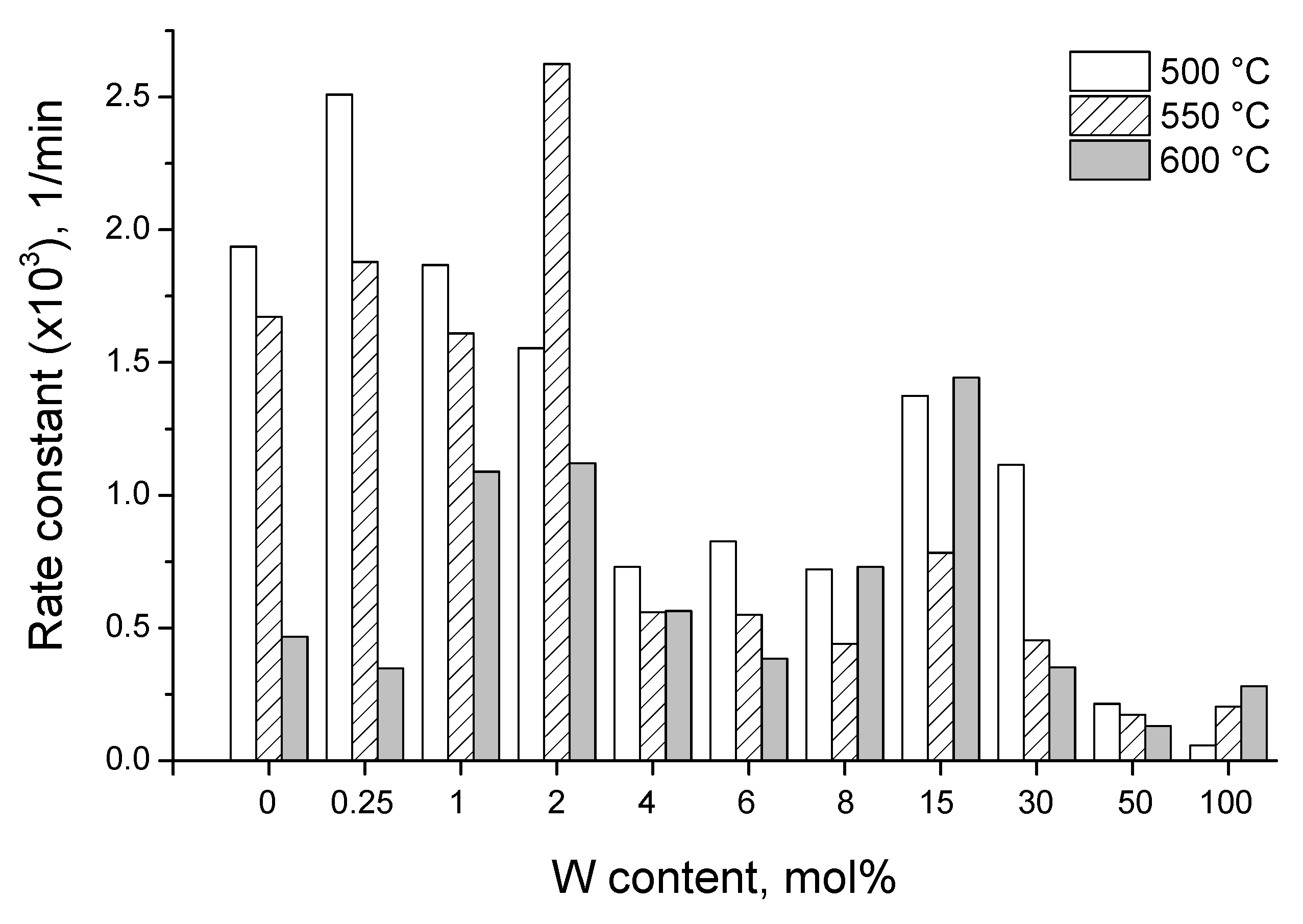
3.3. Photocatalytic Activity
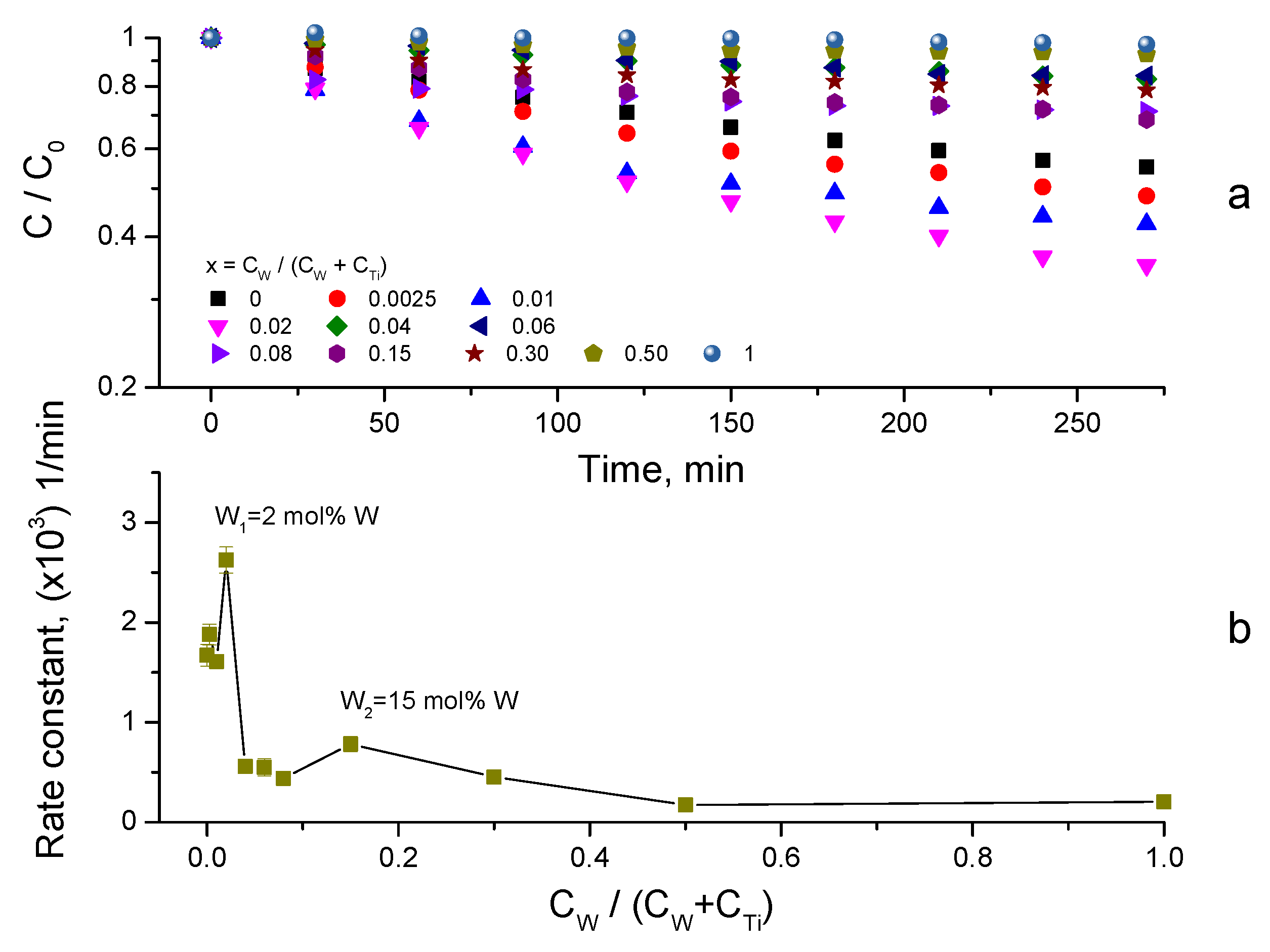
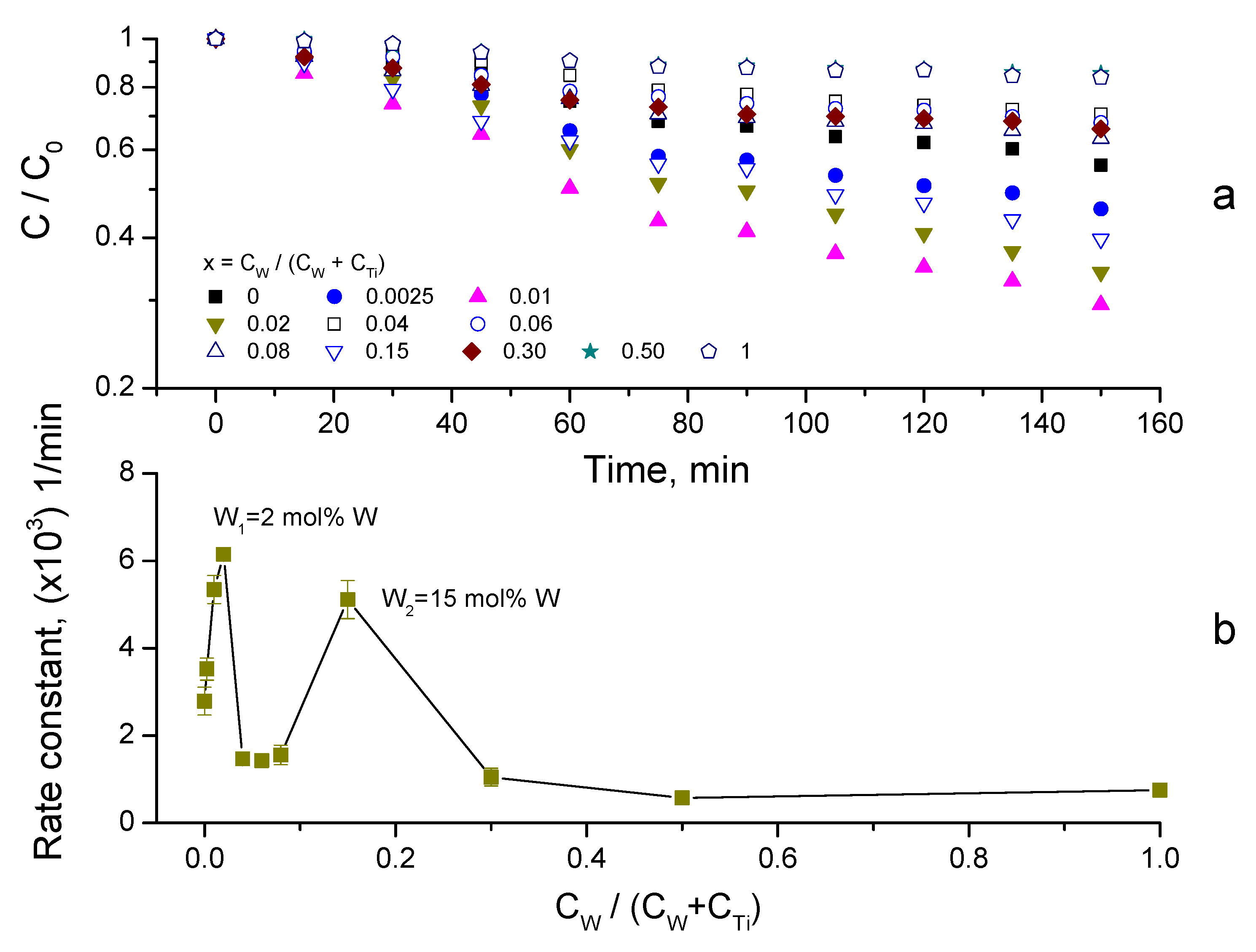
3.4. Synergy of Adsorption and Photocatalysis
- –
- kNORM was greatly different for 0 ≤ CW/(CW + CTi) ≤ 0.02 and 0.04 ≤ CW/(CW + CTi) ≤ 1;
- –
- kNORM was stronger under sunlight illumination compared to UV-A, attaining the peak value at 15 mol% W.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Hashimoto, K.; Watanabe, T. TiO2 Photocatalysis: Fundamentals and Applications; BKC: Tokyo, Japan, 1999. [Google Scholar]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jap. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Ochiai, T.; Murakami, T.; Fujishima, A. Photoenergy conversion with TiO2 photocatalysis: New materials and recent applications. Electrochim. Acta 2012, 84, 103–111. [Google Scholar] [CrossRef]
- Dharma, H.N.C.; Jaafar, J.; Widiastuti, N.; Matsuyama, H.; Rajabsadeh, S.; Othman, M.H.D.; Rahman, M.A.; Jafri, N.N.M.; Suhaimin, N.S.; Nasir, A.M.; et al. A review of titanium dioxide (TiO2)-based photocatalyst for oilfield-produced water treatment. Membranes 2022, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Baia, L.; Orbán, E.; Fodor, S.; Hampel, B.; Kedves, E.Z.; Saszet, K.; Székely, I.; Karácsonyi, É.; Réti, B.; Berki, P.; et al. Preparation of TiO2/WO3 composite photocatalysts by the adjustment of the semiconductors’ surface charge. Mater. Sci. Semicond. Process 2016, 42, 66–71. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Yue, X.; Xiang, J.; Chen, J.; Li, H.; Qiu, Y.; Yu, X. High surface area, high catalytic activity titanium dioxide aerogels prepared by solvothermal crystallization. J. Mater. Sci. Technol. 2020, 47, 223–230. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H.M. Review on: Titanium dioxide applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Lapertot, M.; Pichat, P.; Parra, S.; Guillard, C.; Pulgarin, C. Photocatalytic degradation of p-halophenols in TiO2 aqueous suspensions: Halogen effect on removal rate, aromatic intermediates and toxicity variations. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2006, 41, 1009–1025. [Google Scholar] [CrossRef] [PubMed]
- Hadei, M.; Mesdaghinia, A.; Nabizadeh, R.; Mahvi, A.H.; Rabbani, S.; Naddafi, K. A comprehensive systematic review of photocatalytic degradation of pesticides using nano TiO2. Environ. Sci. Pollut. Res. 2021, 28, 13055–13071. [Google Scholar] [CrossRef] [PubMed]
- Lee, Q.Y.; Li, H. Photocatalytic degradation of plastic waste: A mini review. Micromachines 2021, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Lizama, C.; Bravo, C.; Caneo, C.; Ollino, M. Photocatalytic degradation of surfactants with immobilized TIO2: Comparing two reaction systems. Environ. Technol. 2005, 26, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Peral, J.; Ollis, D.F. TiO2 photocatalyst deactivation by gas-phase oxidation of heteroatom organics. J. Mol. Catal. A Chem. 1997, 115, 347–354. [Google Scholar] [CrossRef]
- Niu, W.; Wang, G.; Liu, X.D.; Tang, J.; Bi, X.G. Preparation of WO3-TiO2 photo-anode and its performance of photocatalytic hydrogen production by water splitting. Int. J. Electrochem. Sci. 2015, 10, 8513–8521. [Google Scholar] [CrossRef]
- Lim, T.-T.; Yap, P.-S.; Srinivasan, M.; Fane, A.G. TiO2/AC composites for synergistic adsorption-photocatalysis processes: Present challenges and further developments for water treatment and reclamation. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1173–1230. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Marzorati, S.; Longhi, M.; Coduri, M.; Artiglia, L.; Selli, E. Photocatalytic activity of TiO2-WO3 mixed oxides in relation to electron transfer efficiency. Appl. Catal. B Environ. 2016, 186, 157–165. [Google Scholar] [CrossRef]
- Sanchez Mendez, M.; Lemarchand, A.; Traore, M.; Perruchot, C.; Sassoye, C.; Selmane, M.; Nikravech, M.; Ben Amar, M.; Kanaev, A. Photocatalytic activity of nanocoatings based on mixed oxide V-TiO2 nanoparticles with controlled composition and size. Catalysts 2021, 11, 1457. [Google Scholar] [CrossRef]
- Cheng, K.; Chhor, K.; Passarello, J.-P.; Colbeau-Justin, C.; Kanaev, A. Photocatalytic nanoparticulate ZrxTi1−xO2 coatings with controlled homogeneity of elemental composition. Chem. Select 2018, 3, 11118–11126. [Google Scholar] [CrossRef]
- Tieng, S.; Kanaev, A.; Chhor, K. New homogeneously doped Fe(III)-TiO2 photocatalyst for gaseous pollutant degradation. Appl. Catal. A 2011, 399, 191–197. [Google Scholar] [CrossRef]
- Mingmongkol, Y.; Trinh, D.T.T.; Channei, D.; Khanitchaidecha, W. Decomposition of dye pigment via photocatalysis process using CuO-TiO2 nanocomposite. Mater. Today Proceed. 2021, 47, 3441–3444. [Google Scholar] [CrossRef]
- Akhter, P.; Nawaz, S.; Shafiq, I.; Nazir, A. Efficient visible light assisted photocatalysis using ZnO/TiO2 nanocomposites. Mol. Catal. 2023, 535, 112896. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.Y.; Hao, X.D.; Zhang, Y.X. Facile biphasic synthesis of TiO2-MnO2 nanocomposites for photocatalysis. Ceram. Int. 2016, 42, 19425–19428. [Google Scholar] [CrossRef]
- Anandan, S.; Sivasankar, T.; Lana-Villarreal, T. Synthesis of TiO2/WO3 nanoparticles via sonochemical approach for the photocatalytic degradation of methylene blue under visible light illumination. Ultrason. Sonochem. 2014, 21, 1964–1968. [Google Scholar] [CrossRef]
- Khan, H.; Rigamonti, M.G.; Patience, G.S.; Boffito, D.C. Spray dried TiO2/WO3 heterostructure for photocatalytic applications with residual activity in the dark. Appl. Catal. B Environ. 2018, 226, 311–323. [Google Scholar] [CrossRef]
- Alhaddad, M.; Ismail, A.A.; Alkhatham, N.; Alghamdi, Y.G. Co3O4 nanoparticles accommodated mesoporous TiO2 framework as an excellent photocatalyst with enhanced photocatalytic properties. Opt. Mater. 2022, 131, 112643. [Google Scholar] [CrossRef]
- Tahir, M.B.; Nabi, G.; Rafique, M.; Khalid, N.R. Nanostructured-based WO3 photocatalysts: Recent development, activity enhancement, perspectives and applications for wastewater treatment. Int. J. Environ. Sci. Technol. 2017, 14, 2519–2542. [Google Scholar] [CrossRef]
- Santos, L.; Silveira, C.M.; Elangovan, E.; Neto, J.P.; Nunes, D.; Pereira, L.; Martins, R.; Viegas, J.; Moura, J.J.G.; Todorovic, S.; et al. Synthesis of WO3 nanoparticles for biosensing applications. Sens. Actuators B Chem. 2016, 223, 186–194. [Google Scholar] [CrossRef]
- Rao, M.C. Structure and properties of WO3 thin films for electrochromic device application. J. Non-Oxide Glass. 2013, 5, 1–8. [Google Scholar]
- Stapinski, T.; Marszalek, K.; Swatowska, B.; Stanco, A. Characterisation and application of WO3 films for electrochromic devices. In Proceedings of the SPIE 8902, Conference on Electron Technology, Ryn Castle, Poland, 16–20 April 2013; p. 890224. [Google Scholar]
- Riboni, F.; Bettini, L.G.; Bahnemann, D.W.; Selli, E. WO3-TiO2 vs. TiO2 photocatalysts: Effect of the W precursor and amount on the photocatalytic activity of mixed oxides. Catal. Today 2013, 209, 28–34. [Google Scholar] [CrossRef]
- Riboni, F.; Dozzi, M.V.; Paganini, M.C.; Giamello, E.; Selli, E. Photocatalytic activity of TiO2-WO3 mixed oxides in formic acid oxidation. Catal. Today 2017, 287, 176–181. [Google Scholar] [CrossRef]
- Qu, X.; Xie, D.; Gao, L.; Cao, L.; Du, F. Synthesis and characterization of TiO2/WO3 composite nanotubes for photocatalytic applications. J. Mater. Sci. 2015, 50, 21–27. [Google Scholar] [CrossRef]
- Ramos-Delgado, N.A.; Gracia-Pinilla, M.A.; Maya-Treviño, L.; Hinojosa-Reyes, L.; Guzman-Mar, J.L.; Hernández-Ramírez, A. Solar photocatalytic activity of TiO2 modified with WO3 on the degradation of an organophosphorus pesticide. J. Hazard. Mater. 2013, 263, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Patrocinio, A.O.T.; Paula, L.F.; Paniago, R.M.; Freitag, J.; Bahnemann, D.W. Layer-by-layer TiO2/WO3 thin films as efficient photocatalytic self-cleaning surfaces. ACS Appl. Mater. Interfaces 2014, 6, 16859–16866. [Google Scholar] [CrossRef] [PubMed]
- Arce-Sarria, A.; Machuca-Martínez, F.; Bustillo-Lecompte, C.; Hernández-Ramírez, A.; Colina-Márquez, J. Degradation and loss of antibacterial activity of commercial amoxicillin with TiO2/WO3-assisted solar photocatalysis. Catalysts 2018, 8, 222. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Hu, X.; Wong, K.S.; Yu, J.C. WO3/TiO2 microstructures for enhanced photocatalytic oxidation. Separ. Purif. Technol. 2012, 91, 67–72. [Google Scholar] [CrossRef]
- Žerjav, G.; Arshad, M.S.; Djinović, P.; Zavašnik, J.; Pintar, A. Electron trapping energy states of TiO2-WO3 composites and their influence on photocatalytic degradation of bisphenol A. Appl. Catal. B Environ. 2017, 209, 273–284. [Google Scholar] [CrossRef]
- Shinguu, H.; Bhuiyan, M.M.H.; Ikegami, T.; Ebihara, K. Preparation of TiO2/WO3 multilayer thin film by PLD method and its catalytic response to visible light. Thin Solid Films 2006, 506–507, 111–114. [Google Scholar] [CrossRef]
- Oliveira, H.; Silva, E.; Longo, C. Electrochemical and photocatalytic properties of TiO2/WO3 photoelectrodes. In Proceedings of the SPIE 7770, Solar Hydrogen and Nanotechnology V, San Diego, CA, USA, 24 August 2010; p. 777006. [Google Scholar]
- Pan, J.H.; Lee, W.I. Preparation of highly ordered cubic mesoporous WO3/TiO2 films and their photocatalytic properties. Chem. Mater. 2006, 18, 847–853. [Google Scholar] [CrossRef]
- Akurati, K.K.; Vital, A.; Dellemann, J.P.; Michalow, K.; Graule, T.; Ferri, D.; Baiker, A. Flame-made WO3/TiO2 nanoparticles: Relation between surface acidity, structure and photocatalytic activity. Appl. Catal. B Environ. 2008, 79, 53–62. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, M.; Yu, W.; Zhang, Q.; Xie, H.; Sun, Z.; Shao, Q.; Guo, X.; Hao, L.; Zheng, Y.; et al. Heterostructured TiO2/WO3 nanocomposites for photocatalytic degradation of toluene under visible light. J. Electrochem. Soc. 2017, 164, H1086. [Google Scholar] [CrossRef]
- Tahir, M.B.; Farman, S.; Rafique, M.; Shakil, M.; Khan, M.I.; Ijaz, M.; Mubeen, I.; Ashraf, M.; Riaz, K.N. Photocatalytic performance of hybrid WO3/TiO2 nanomaterials for the degradation of methylene blue under visible light irradiation. Int. J. Environ. Analyt. Chem. 2021, 101, 1448–1460. [Google Scholar] [CrossRef]
- Jaritkaun, N.; Wootthikanokkhan, J.; Piewnuan, C.; Ngaotrakanwiwat, P.; Chiarakorn, S. Inducing catalytic activity in the dark of TiO2/WO3 mixed metal oxides by using an in situ polymerized semiconductive polymeric binder. Synth. React. Inorg. Metal-Org. Nano-Metal Chem. 2016, 46, 1705–1714. [Google Scholar] [CrossRef]
- Singh, S.A.; Madras, G. Photocatalytic degradation with combustion synthesized WO3 and WO3-TiO2 mixed oxides under UV and visible light. Sep. Purif. Technol. 2013, 105, 79–89. [Google Scholar] [CrossRef]
- Chai, S.Y.; Kim, Y.J.; Lee, W.I. Photocatalytic WO3/TiO2 nanoparticles working under visible light. J. Electroceram. 2006, 17, 909–912. [Google Scholar] [CrossRef]
- Liu, S.; Huang, J.; Cao, L.; Li, J.; Ouyang, H.; Tao, X.; Liu, C. One-pot synthesis of TiO2-WO3 composite nanocrystallites with improved photocatalytic properties under natural sunlight irradiation. Mater. Sci. Semicond. Process. 2014, 25, 106–111. [Google Scholar] [CrossRef]
- Dohćević-Mitrović, Z.; Stojadinović, S.; Lozzi, L.; Aškrabić, S.; Rosić, M.; Tomić, N.; Paunović, N.; Lazović, S.; Nikolić, M.G.; Santucci, S. WO3/TiO2 composite coatings: Structural, optical and photocatalytic properties. Mater. Res. Bull. 2016, 83, 217–224. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Liu, B.; Terashima, C.; Katsumata, K.I.; Suzuki, N.; Fujishima, A.; Sakai, H.; Nakata, K. Fabrication of efficient visible-light-responsive TiO2-WO3 hollow particle photocatalyst by electrospray method. Chem. Lett. 2017, 46, 122–124. [Google Scholar] [CrossRef]
- Pal, B.; Vijayan, B.L.; Krishnan, S.G.; Harilal, M.; Basirun, W.J.; Lowe, A.; Yusoff, M.M.; Jose, R. Hydrothermal syntheses of tungsten doped TiO2 and TiO2/WO3 composite using metal oxide precursors for charge storage applications. J. Alloys Compd. 2018, 740, 703–710. [Google Scholar] [CrossRef]
- Kathirvelan, J.; Vijayaraghavan, R.; Thomas, A. Ethylene detection using TiO2-WO3 composite sensor for fruit ripening applications. Sens. Rev. 2017, 37, 147–154. [Google Scholar] [CrossRef]
- Georgieva, J. TiO2/WO3 photoanodes with enhanced photocatalytic activity for air treatment in a polymer electrolyte cell. J. Solid State Electrochem. 2012, 16, 1111–1119. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Chen, Y.-W. Anticorrosion of WO3-modified TiO2 thin film prepared by peroxo sol-gel method. Mod. Res. Catal. 2020, 9, 35–46. [Google Scholar] [CrossRef]
- Hu, J.; Wang, L.; Zhang, P.; Liang, C.; Shao, G. Construction of solid-state z-scheme carbon-modified TiO2/WO3 nanofibers with enhanced photocatalytic hydrogen production. J. Pow. Sour. 2016, 328, 28–36. [Google Scholar] [CrossRef]
- Guaraldo, T.T.; Gonçales, V.R.; Silva, B.F.; de Torresi, S.I.C.; Zanoni, M.V.B. Hydrogen production and simultaneous photoelectrocatalytic pollutant oxidation using a TiO2/WO3 nanostructured photoanode under visible light irradiation. J. Electroanalyt. Chem. 2016, 765, 188–196. [Google Scholar] [CrossRef]
- Reyes-Gil, K.R.; Stephens, Z.D.; Stavila, V.; Robinson, D.B. Composite WO3/TiO2 nanostructures for high electrochromic activity. ACS Appl. Mater. Interfaces 2015, 7, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gan, W.; Qiu, Z.; Zhan, X.; Qiang, T.; Li, J. Preparation of heterostructured WO3/TiO2 catalysts from wood fibers and its versatile photodegradation abilities. Sci. Rep. 2017, 7, 1102. [Google Scholar] [CrossRef]
- Cao, L.; Yuan, J.; Chen, M.; Shangguan, W. Photocatalytic energy storage ability of TiO2-WO3 composite prepared by wet-chemical technique. J. Environ. Sci. 2010, 22, 454–459. [Google Scholar] [CrossRef]
- Ngaotrakanwiwat, P.; Tatsuma, T.; Saitoh, S.; Ohko, Y.; Fujishima, A. Charge-discharge behavior of TiO2-WO3 photocatalysis systems with energy storage ability. Phys. Chem. Chem. Phys. 2003, 5, 3234–3237. [Google Scholar] [CrossRef]
- Tatsuma, T.; Takeda, S.; Saitoh, S.; Ohko, Y.; Fujishima, A. Bactericidal effect of an energy storage TiO2-WO3 photocatalyst in dark. Electrochem. Commun. 2003, 5, 793–796. [Google Scholar] [CrossRef]
- Zanetti, S.; Rocha, K.O.; Rodrigues, J.A.J.; Longo, E. Soft-chemical synthesis, characterization and humidity sensing behavior of WO3/TiO2 nanopowders. Sens. Actuat. B 2014, 190, 40–47. [Google Scholar] [CrossRef]
- Palmas, S.; Castresana, P.A.; Mais, L.; Vacca, A.; Mascia, M.; Ricci, P.C. TiO2-WO3 nanostructured systems for photoelectrochemical applications. RSC Adv. 2016, 6, 101671–101682. [Google Scholar] [CrossRef]
- Boga, B.; Székely, I.; Pap, Z.; Baia, L.; Baia, M. Detailed spectroscopic and structural analysis of TiO2/WO3 composite semiconductors. J. Spectrosc. 2018, 2018, 6260458. [Google Scholar] [CrossRef]
- Luo, X.; Deng, F.; Min, L.; Luo, S.; Guo, B.; Zeng, G.; Au, C. Facile one-step synthesis of inorganic-framework molecularly imprinted TiO2/WO3 nanocomposite and its molecular recognitive photocatalytic degradation of target contaminant. Environ. Sci. Technol. 2013, 47, 7404–7412. [Google Scholar] [CrossRef]
- Hai, G.; Zhang, H. The photocatalytic applications of TiO2-WO3 heterostructure in methylene blue. Am. Sci. Res. J. Eng. Technol. Sci. 2019, 61, 135–142. [Google Scholar]
- Paula, L.F.; Hofer, M.; Lacerda, V.P.B.; Bahnemann, D.W.; Patrocinio, A.O.T. Unraveling the photocatalytic properties of TiO2/WO3 mixed oxides. Photochem. Photobiol. Sci. 2019, 18, 2469–2483. [Google Scholar] [CrossRef]
- Arce-Sarria, A.; Caicedo-Rosero, C.L.; Lara-Ramos, J.A.; Diaz-Angulo, J.; Machuca-Martínez, F. Experimental data on synthesis and characterization of WO3/TiO2 as catalyst. Data Brief 2019, 25, 104151. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, R.; Li, M.; Pei, J.; Guo, F.; Zhang, S. Photocatalytic efficiencies of WO3/TiO2 nanoparticles for exhaust decomposition under UV and visible light irradiation. Mater. Res. Express 2018, 5, 095029. [Google Scholar] [CrossRef]
- Yang, H.; Shi, R.; Zhang, K.; Hu, Y.; Tang, A.; Li, X. Synthesis of WO3/TiO2 nanocomposites via sol-gel method. J. Alloys Compd. 2005, 398, 200–202. [Google Scholar] [CrossRef]
- Zhan, W.T.; Ni, H.W.; Chen, R.S.; Wang, Z.Y.; Li, Y.W.; Li, J.H. One-step hydrothermal preparation of TiO2/WO3 nanocomposite films on anodized stainless steel for photocatalytic degradation of organic pollutants. Thin Solid Films 2013, 548, 299–305. [Google Scholar] [CrossRef]
- Puddu, V.; Mokaya, R.; Puma, G.L. Novel one step hydrothermal synthesis of TiO2/WO3 nanocomposites with enhanced photocatalytic activity. Chem. Commun. 2007, 45, 4749–4751. [Google Scholar] [CrossRef] [PubMed]
- Mugunthan, E.; Saidutta, M.B.; Jagadeeshbabu, P.E. Visible light assisted photocatalytic degradation of diclofenac using TiO2-WO3 mixed oxide catalysts. Environ. Nanotechnol. Monitor. Manag. 2018, 10, 322–330. [Google Scholar]
- Soares, L.; Alves, A. Photocatalytic properties of TiO2 and TiO2/WO3 films applied as semiconductors in heterogeneous photocatalysis. Mater. Lett. 2018, 211, 339–342. [Google Scholar] [CrossRef]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on sol-gel synthesis of perovskite and oxide nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef]
- Saepurahman, A.M.A.; Abdullah, M.A.; Chong, F.K. Dual-effects of adsorption and photodegradation of methylene blue by tungsten-loaded titanium dioxide. Chem. Eng. J. 2010, 158, 418–425. [Google Scholar] [CrossRef]
- Couselo, N.; Einschlag, F.S.G.; Candal, R.J.; Jobbagy, M. Tungsten-doped TiO2 vs. pure TiO2 photocatalysts: Effects on photobleaching kinetics and mechanism. J. Phys. Chem. C 2008, 112, 1094–1100. [Google Scholar] [CrossRef]
- Azouani, R.; Soloviev, A.; Benmami, M.; Chhor, K.; Bocquet, J.-F.; Kanaev, A. Stability and growth of titanium-oxo-alcoxy TixOy(OiPr)z clusters. J. Phys. Chem. C 2007, 111, 16243–16248. [Google Scholar] [CrossRef]
- Colbeau-Justin, C.; Kunst, M.; Huguenin, D. Structural influence on charge-carrier lifetimes in TiO2 powders studied by microwave absorption. J. Mater. Sci. 2003, 38, 2429–2437. [Google Scholar] [CrossRef]
- Cheng, K.; Chhor, K.; Kanaev, A. Solvent effect on nucleation-growth of titanium-oxo-alkoxy nanoparticles. Chem. Phys. Lett. 2017, 672, 119–123. [Google Scholar] [CrossRef]
- Sanchez Mendez, M.; Jia, Z.; Traore, M.; Ben Amar, M.; Nikravech, M.; Kanaev, A. Nucleation and growth of mixed vanadium-titanium oxo-alkoxy nanoparticles in sol-gel synthesis. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125636. [Google Scholar] [CrossRef]
- Gribb, A.A.; Banfield, J.F. Particle size effects on transformation kinetics and phase stability in nanocrystalline TiO2. Am. Miner. 1997, 82, 717–728. [Google Scholar] [CrossRef]
- Peng, F.; Wang, S.; Yu, W.; Huang, T.; Sun, Y.; Cheng, C.; Chen, X.; Hao, J.; Dai, N. Ultrasensitive ppb-level H2S gas sensor at room temperature based on WO3/rGO hybrids. J. Mater. Sci. Mater. Electron. 2020, 31, 5008–5016. [Google Scholar] [CrossRef]
- Criado, J.; Real, C. Mechanism of the inhibiting effect of phosphate on the anatase → rutile transformation induced by thermal and mechanical treatment of TiO2. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1983, 79, 2765–2771. [Google Scholar] [CrossRef]
- Abdennouri, M.; Elmoubarki, R.; Elmhammedi, A.; Galadi, A.; Baâlala, M.; Bensitel, M.; Boussaoud, A.; El Hafiane, Y.; Smith, A.; Barka, N. Influence of tungsten on the anatase-rutile phase transition of sol-gel synthesized TiO2 and on its activity in the photocatalytic degradation of pesticides. J. Mater. Environ. Sci. 2013, 4, 953–960. [Google Scholar]
- Pham, H.Q.; Huynh, T.T.; Bich, H.N.; Pham, T.M.; Nguyen, S.T.; Lu, L.T.; Ho, V.T.T. Tungsten-doped titanium-dioxide-supported low-Pt-loading electrocatalysts for the oxidation reaction of ethanol in acidic fuel cells. Comptes Rendus Chim. 2019, 22, 829–837. [Google Scholar] [CrossRef]
- Sathasivam, S.; Bhachu, D.S.; Lu, Y.; Chadwick, N.; Althabaiti, S.A.; Alyoubi, A.O.; Basahel, S.N.; Carmalt, C.J.; Parkin, I.P. Tungsten doped TiO2 with enhanced photocatalytic and optoelectrical properties via aerosol assisted chemical vapor deposition. Sci. Rep. 2015, 5, 10952. [Google Scholar] [CrossRef]
- Bhargava, R.; Khan, S. Fabrication of WO3-reduced graphene oxide (WO3-G) nanocomposite for enhanced optical and electrical properties. J. Mater. Sci. Mater. Electron. 2020, 31, 8370–8384. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 photocatalyst (Degussa, P-25) consisting of anatase and rutile crystalline phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Sun, Y. Adsorption of methylene blue by coal-based activated carbon in high-salt wastewater. Water 2022, 14, 3576. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Léonard, A.; Lemaire, A.; Tian, G.; Su, B.-L. Self-formation phenomenon to hierarchically structured porous materials: Design, synthesis, formation mechanism and applications. Chem. Commun. 2011, 47, 2763–2786. [Google Scholar] [CrossRef] [PubMed]
- Bouslama, M.; Amamra, M.C.; Jia, Z.; Ben Amar, M.; Brinza, O.; Chhor, K.; Abderrabba, M.; Vignes, J.-L.; Kanaev, A. New nanoparticulate TiO2-Al2O3 photocatalytic media: Effect of particle size and polymorphism on photocatalytic activity. ACS Catal. 2012, 2, 1884–1892. [Google Scholar] [CrossRef]
- Ohtani, B. Titania photocatalysis beyond recombination: A critical review. Catalysts 2013, 3, 942–953. [Google Scholar] [CrossRef]
- Ruan, Q.; Miao, T.; Wang, H.; Tang, J. Insight on shallow trap states-introduced photocathodic performance in n-type polymer photocatalysts. J. Am. Chem. Soc. 2020, 142, 2795–2802. [Google Scholar] [CrossRef]




| Method | Optimal W Loading mol% | Illumination | Enhancement | Pollutant (1) | Reference |
|---|---|---|---|---|---|
| Sol–gel | 3 | UV-A | 2.1 | Formic acid | [35] |
| Hydrothermal | 1 | Sunlight | ~3 | Rhodamine B | [52] |
| Sol–gel | 2 | Sunlight | 2.1 | Malathion pesticide | [38] |
| Physical mixing | 10–15 | UV-A/visible | 2.0 | Methylene blue and orange G | [50] |
| Sol–gel | 1 | UV-A | 2.5 | Formic acid | [36] |
| Sol–gel | 0.02–1 | UV-A | 2.3 | Cr2O72− | [21] |
| Ultrasound- assisted | 5.4 | Visible | 3.9 | Methylene blue | [28] |
| PLD | - (2) | Visible | - | Methylene blue | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, K.; Heng, S.; Tieng, S.; David, F.; Dine, S.; Haddad, O.; Colbeau-Justin, C.; Traore, M.; Kanaev, A. Mixed Metal Oxide W-TiO2 Nanopowder for Environmental Process: Synergy of Adsorption and Photocatalysis. Nanomaterials 2024, 14, 765. https://doi.org/10.3390/nano14090765
Cheng K, Heng S, Tieng S, David F, Dine S, Haddad O, Colbeau-Justin C, Traore M, Kanaev A. Mixed Metal Oxide W-TiO2 Nanopowder for Environmental Process: Synergy of Adsorption and Photocatalysis. Nanomaterials. 2024; 14(9):765. https://doi.org/10.3390/nano14090765
Chicago/Turabian StyleCheng, Khley, Socheata Heng, Siteng Tieng, Ford David, Sarah Dine, Oriana Haddad, Christophe Colbeau-Justin, Mamadou Traore, and Andrei Kanaev. 2024. "Mixed Metal Oxide W-TiO2 Nanopowder for Environmental Process: Synergy of Adsorption and Photocatalysis" Nanomaterials 14, no. 9: 765. https://doi.org/10.3390/nano14090765






